Zinc and Strontium-Substituted Bioactive Glass Nanoparticle/Alginate Composites Scaffold for Bone Regeneration
Abstract
1. Introduction
2. Results and Discussion
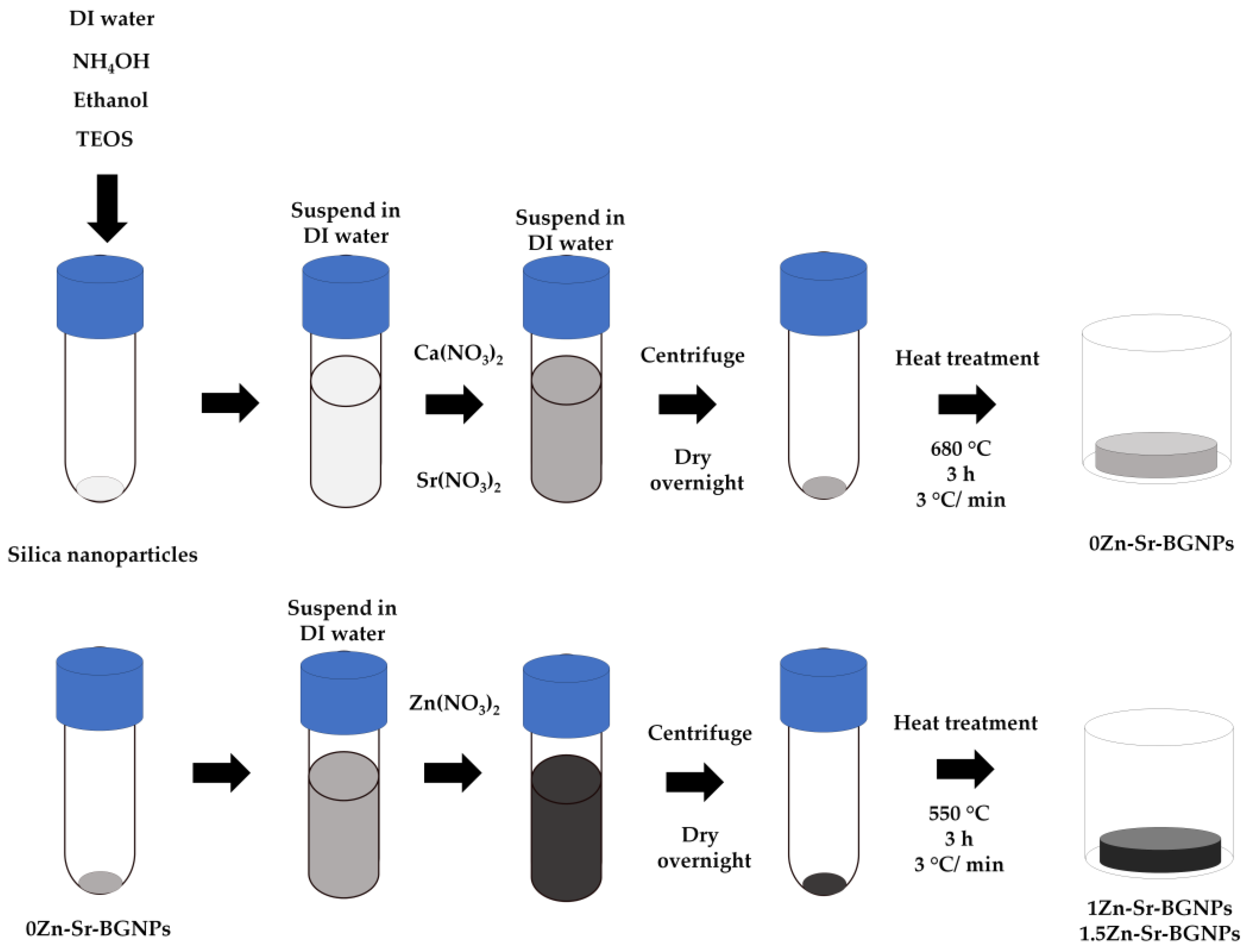
2.1. Zn-Sr-BGNPs Characterization
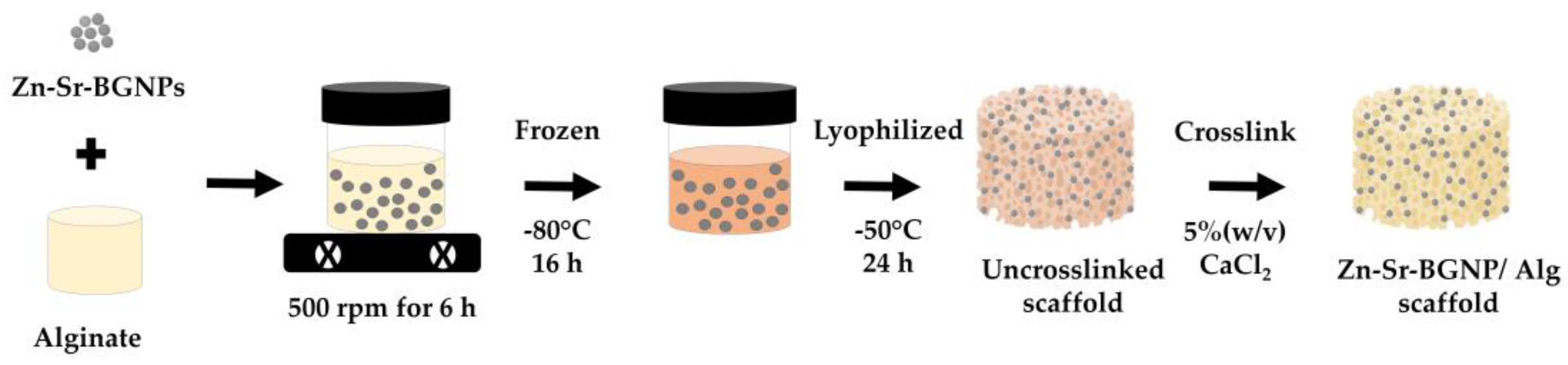
2.2. Zn-Sr-BGNP: Alg Scaffold Characterization
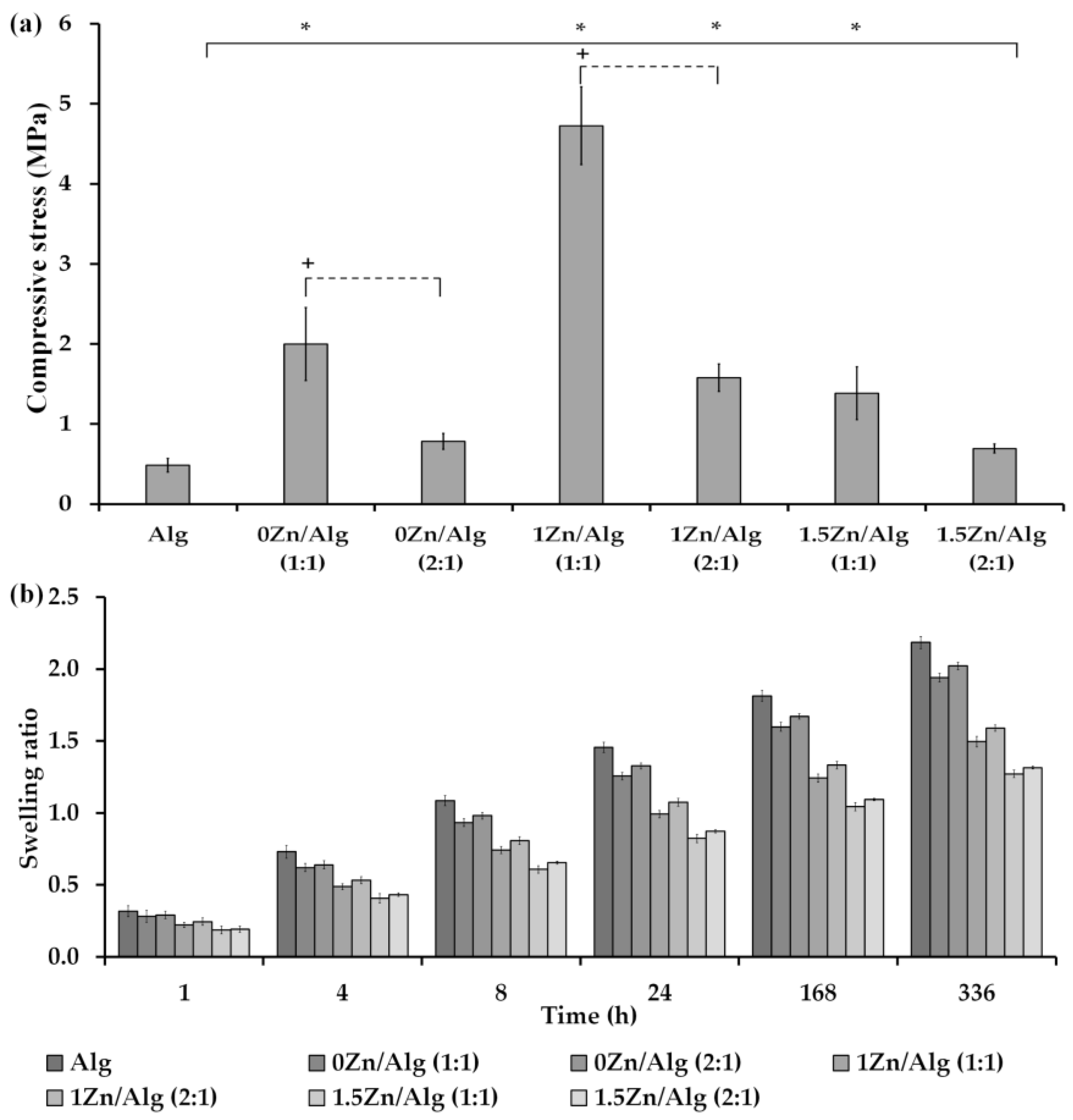
2.3. Compression Testing of Scaffolds
2.4. In Vitro Swelling
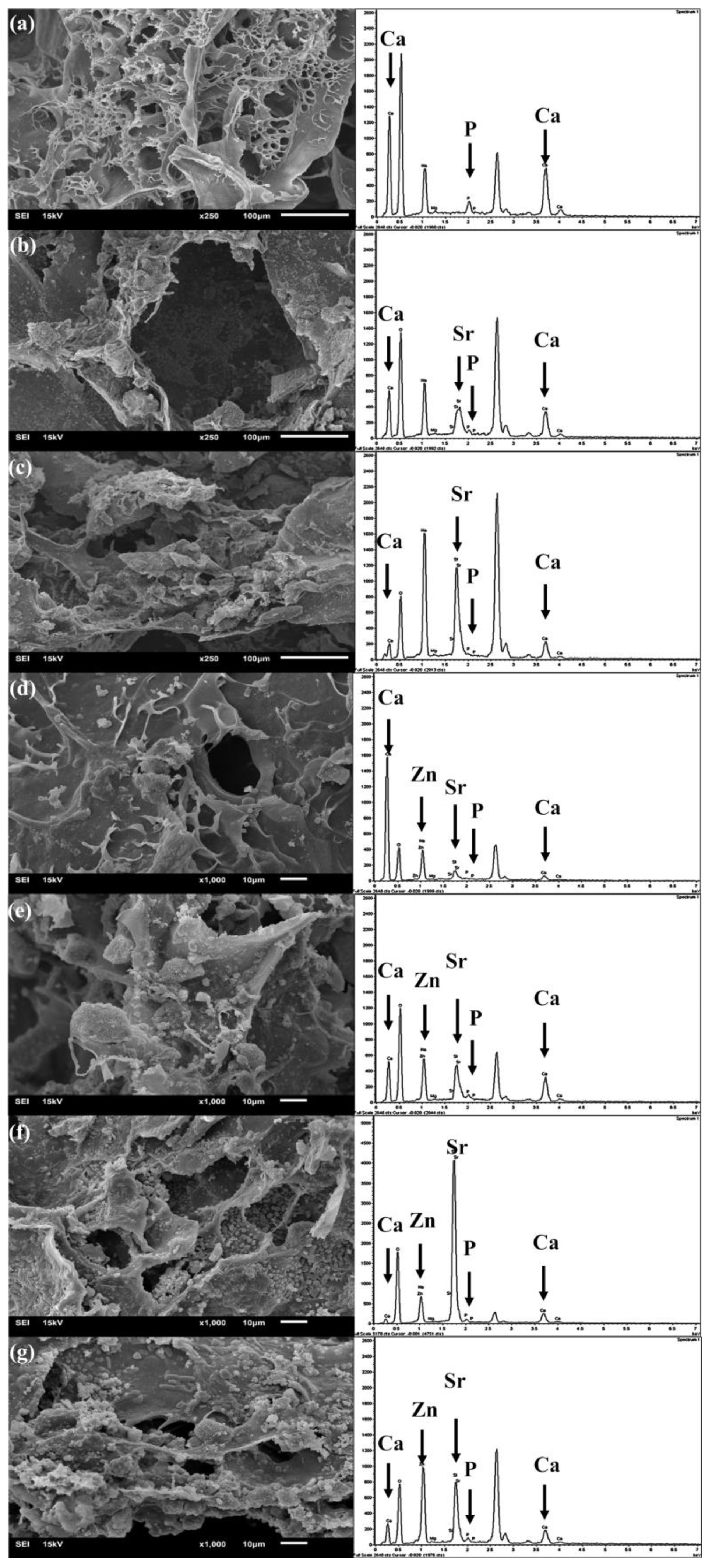
2.5. In Vitro Bioactivity Assessment
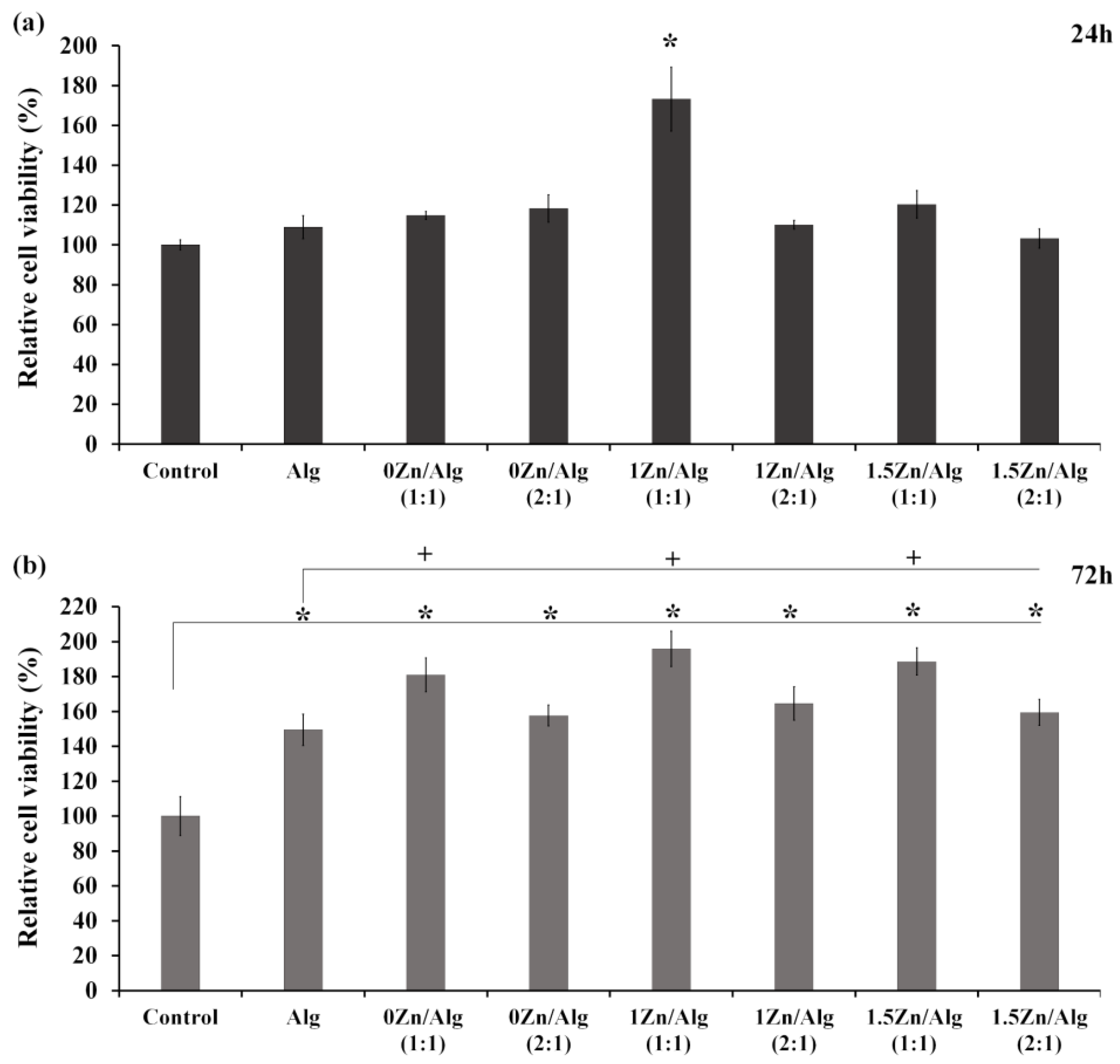
2.6. Cell Viability Study
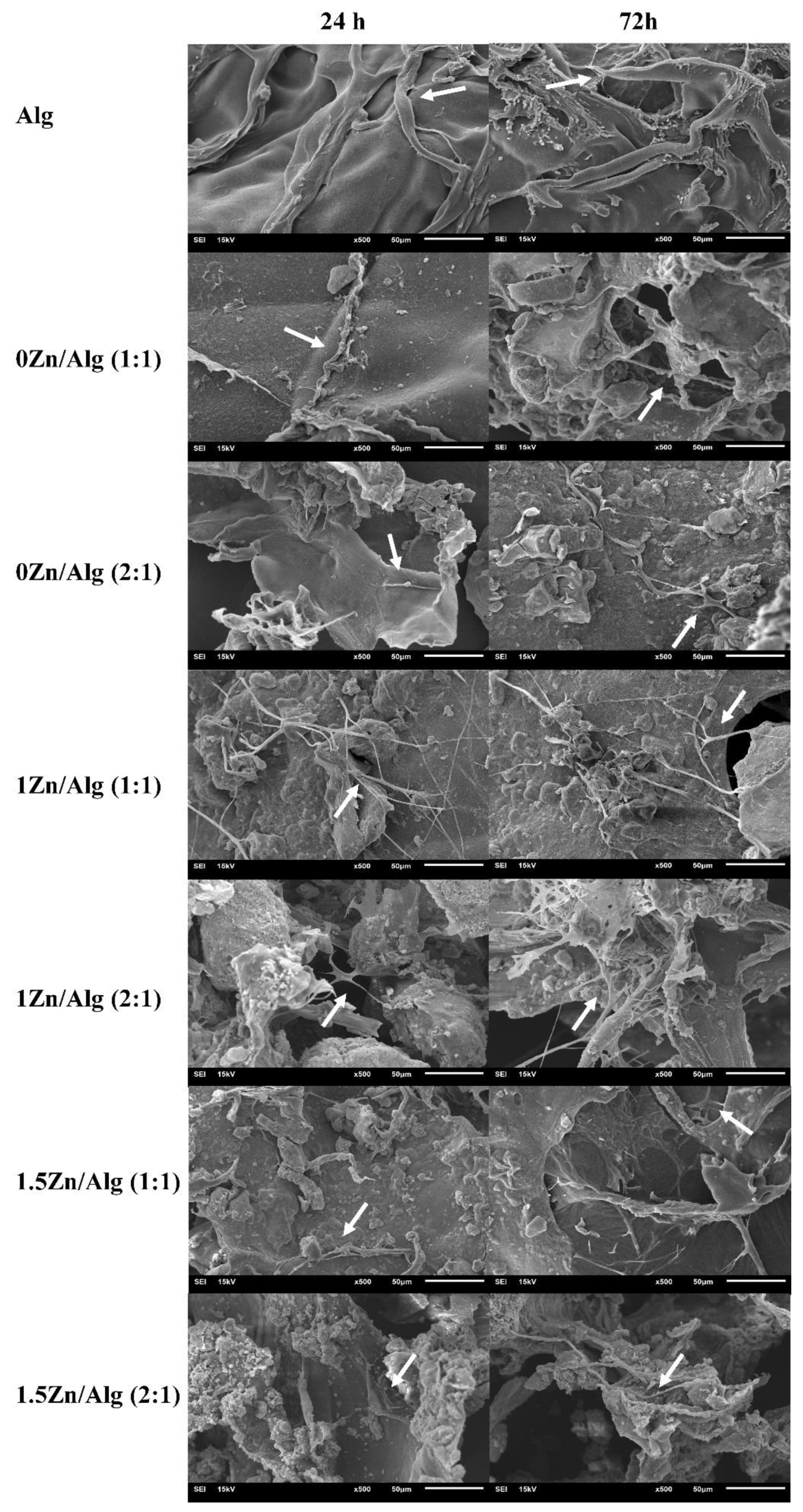
2.7. Cell Attachment
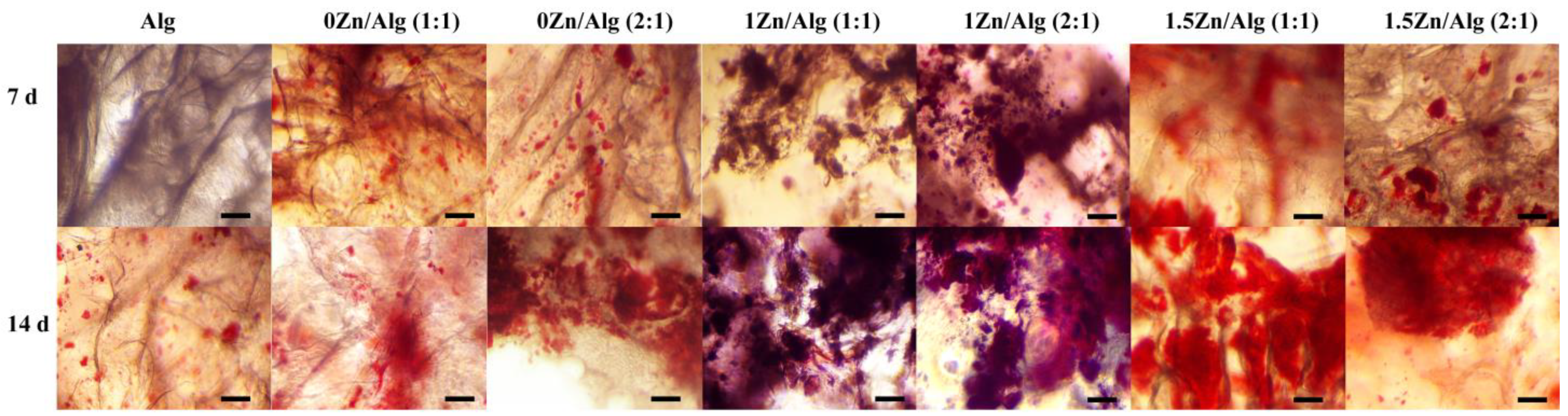
2.8. Calcium Deposition
3. Materials and Methods
3.1. Zn-Sr-BGNPs Synthesis and Characterization
3.2. Zn-Sr-BGNP: Alg Scaffold Fabrication and Characterization
3.3. Compression Testing of Scaffolds
3.4. In Vitro Swelling
3.5. In Vitro Bioactivity Assessment
3.6. Cell Viability Study
3.6.1. Cell Culture
3.6.2. Cell Viability
3.7. Cell Attachment and Morphology Study
3.8. Calcium Deposition
3.9. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michel, J.-P.; Leonardi, M.; Martin, M.; Prina, M. WHO’s report for the decade of healthy ageing 2021–2030 sets the stage for globally comparable data on healthy ageing. Lancet Healthy Longev. 2021, 2, e121–e122. [Google Scholar] [CrossRef] [PubMed]
- Im, G.-I. Biomaterials in orthopaedics: The past and future with immune modulation. Biomater. Res. 2020, 24, 7. [Google Scholar] [CrossRef] [PubMed]
- Coenen, A.M.; Bernaerts, K.V.; Harings, J.A.; Jockenhoevel, S.; Ghazanfari, S. Elastic materials for tissue engineering applications: Natural, synthetic, and hybrid polymers. Acta Biomater. 2018, 79, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.; Valente, T.; Alves, P.; Ferreira, P.; Silva, A.; Correia, I. Alginate based scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2012, 32, 2596–2603. [Google Scholar] [CrossRef]
- Yan, J.; Miao, Y.; Tan, H.; Zhou, T.; Ling, Z.; Chen, Y.; Xing, X.; Hu, X. Injectable alginate/hydroxyapatite gel scaffold combined with gelatin microspheres for drug delivery and bone tissue engineering. Mater. Sci. Eng. C 2016, 63, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Mohanta, B.C.; Hasnain, M.S.; Hoda, M.N.; Tripathi, G. Chapter 14—Alginate-based scaffolds for drug delivery in tissue engineering. In Alginates in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 359–386. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Skallevold, H.E.; Rokaya, D.; Khurshid, Z.; Zafar, M.S. Bioactive glass applications in dentistry. Int. J. Mol. Sci. 2019, 20, 5960. [Google Scholar] [CrossRef]
- Nam, H.-J.; Kim, Y.-M.; Kwon, Y.H.; Yoo, K.-H.; Yoon, S.-Y.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Lee, S.-M.; Kim, Y.-I. Fluorinated Bioactive Glass Nanoparticles: Enamel Demineralization Prevention and Antibacterial Effect of Orthodontic Bonding Resin. Materials 2019, 12, 1813. [Google Scholar] [CrossRef]
- Brauer, D.S. Bioactive glasses—Structure and properties. Angew. Chem. Int. Ed. 2015, 54, 4160–4181. [Google Scholar] [CrossRef]
- Malucelli, G. Hybrid Organic/Inorganic Coatings Through Dual-Cure Processes: State of the Art and Perspectives. Coatings 2016, 6, 10. [Google Scholar] [CrossRef]
- Pajares-Chamorro, N.; Chatzistavrou, X. Bioactive Glass Nanoparticles for Tissue Regeneration. ACS Omega 2020, 5, 12716–12726. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Boccaccini, A.R. Sol-gel processing of bioactive glass nanoparticles: A review. Adv. Colloid Interface Sci. 2017, 249, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Schatkoski, V.M.; do Amaral Montanheiro, T.L.; de Menezes, B.R.C.; Pereira, R.M.; Rodrigues, K.F.; Ribas, R.G.; da Silva, D.M.; Thim, G.P. Current advances concerning the most cited metal ions doped bioceramics and silicate-based bioactive glasses for bone tissue engineering. Ceram. Int. 2021, 47, 2999–3012. [Google Scholar] [CrossRef]
- Cacciotti, I. Bivalent cationic ions doped bioactive glasses: The influence of magnesium, zinc, strontium and copper on the physical and biological properties. J. Mater. Sci. 2017, 52, 8812–8831. [Google Scholar] [CrossRef]
- Thanasrisuebwong, P.; Jones, J.R.; Eiamboonsert, S.; Ruangsawasdi, N.; Jirajariyavej, B.; Naruphontjirakul, P. Zinc-Containing Sol–Gel Glass Nanoparticles to Deliver Therapeutic Ions. Nanomaterials 2022, 12, 1691. [Google Scholar] [CrossRef]
- Sun, H.; Zheng, K.; Zhou, T.; Boccaccini, A.R. Incorporation of Zinc into Binary SiO2-CaO Mesoporous Bioactive Glass Nanoparticles Enhances Anti-Inflammatory and Osteogenic Activities. Pharmaceutics 2021, 13, 2124. [Google Scholar] [CrossRef]
- Miola, M.; Verné, E.; Ciraldo, F.E.; Cordero-Arias, L.; Boccaccini, A.R. Electrophoretic Deposition of Chitosan/45S5 Bioactive Glass Composite Coatings Doped with Zn and Sr. Front. Bioeng. Biotechnol. 2015, 3, 159. [Google Scholar] [CrossRef]
- Zamani, D.; Moztarzadeh, F.; Bizari, D. Alginate-bioactive glass containing Zn and Mg composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2019, 137, 1256–1267. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Review of Bioactive Glass/Natural Polymer Composites: State of the Art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef]
- Guduric, V.; Belton, N.; Richter, R.; Bernhardt, A.; Spangenberg, J.; Wu, C.; Lode, A.; Gelinsky, M. Tailorable Zinc-Substituted Mesoporous Bioactive Glass/Alginate-Methylcellulose Composite Bioinks. Materials 2021, 14, 1225. [Google Scholar] [CrossRef]
- Erol, M.; Mouriňo, V.; Newby, P.; Chatzistavrou, X.; Roether, J.; Hupa, L.; Boccaccini, A.R. Copper-releasing, boron-containing bioactive glass-based scaffolds coated with alginate for bone tissue engineering. Acta Biomater. 2011, 8, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Hatton, J.; Davis, G.R.; Mourad, A.-H.I.; Cherupurakal, N.; Hill, R.G.; Mohsin, S. Fabrication of Porous Bone Scaffolds Using Alginate and Bioactive Glass. J. Funct. Biomater. 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Neščáková, Z.; Zheng, K.; Liverani, L.; Nawaz, Q.; Galusková, D.; Kaňková, H.; Michálek, M.; Galusek, D.; Boccaccini, A.R. Multifunctional zinc ion doped sol–gel derived mesoporous bioactive glass nanoparticles for biomedical applications. Bioact. Mater. 2019, 4, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Greasley, S.L.; Page, S.J.; Sirovica, S.; Chen, S.; Martin, R.A.; Riveiro, A.; Hanna, J.V.; Porter, A.E.; Jones, J.R. Controlling particle size in the Stöber process and incorporation of calcium. J. Colloid Interface Sci. 2016, 469, 213–223. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Dev. 2020, 5, 1. [Google Scholar] [CrossRef]
- Putra, R.U.; Basri, H.; Prakoso, A.T.; Chandra, H.; Ammarullah, M.I.; Akbar, I.; Syahrom, A.; Kamarul, T. Level of Activity Changes Increases the Fatigue Life of the Porous Magnesium Scaffold, as Observed in Dynamic Immersion Tests, over Time. Sustainability 2023, 15, 823. [Google Scholar] [CrossRef]
- Sowjanya, J.; Singh, J.; Mohita, T.; Sarvanan, S.; Moorthi, A.; Srinivasan, N.; Selvamurugan, N. Biocomposite scaffolds containing chitosan/alginate/nano-silica for bone tissue engineering. Coll. Surf. B Biointerfaces 2013, 109, 294–300. [Google Scholar] [CrossRef]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Chae, T.; Yang, H.; Leung, V.; Ko, F.; Troczynski, T. Novel biomimetic hydroxyapatite/alginate nanocomposite fibrous scaffolds for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2013, 24, 1885–1894. [Google Scholar] [CrossRef]
- Srinivasan, S.; Jayasree, R.; Chennazhi, K.; Nair, S.; Jayakumar, R. Biocompatible alginate/nano bioactive glass ceramic composite scaffolds for periodontal tissue regeneration. Carbohydr. Polym. 2012, 87, 274–283. [Google Scholar] [CrossRef]
- Bargavi, P.; Ramya, R.; Chitra, S.; Vijayakumari, S.; Chandran, R.R.; Durgalakshmi, D.; Rajashree, P.; Balakumar, S. Bioactive, degradable and multi-functional three-dimensional membranous scaffolds of bioglass and alginate composites for tissue regenerative applications. Biomater. Sci. 2020, 8, 4003–4025. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, Z.; Parvin, N.; Sharifianjazi, F. Formation of hydroxyapatite on surface of SiO2–P2O5–CaO–SrO–ZnO bioactive glass synthesized through sol-gel route. Ceram. Int. 2019, 45, 19323–19330. [Google Scholar] [CrossRef]
- Asadi, N.; Alizadeh, E.; Del Bakhshayesh, A.R.; Mostafavi, E.; Akbarzadeh, A.; Davaran, S. Fabrication and in Vitro Evaluation of Nanocomposite Hydrogel Scaffolds Based on Gelatin/PCL–PEG–PCL for Cartilage Tissue Engineering. ACS Omega 2019, 4, 449–457. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. Enhanced mechanical, biomineralization, and cellular response of nanocomposite hydrogels by bioactive glass and halloysite nanotubes for bone tissue regeneration. Mater. Sci. Eng. C 2021, 128, 112236. [Google Scholar] [CrossRef] [PubMed]
- Tulyaganov, D.U.; Fiume, E.; Akbarov, A.; Ziyadullaeva, N.; Murtazaev, S.; Rahdar, A.; Massera, J.; Verné, E.; Baino, F. In Vivo Evaluation of 3D-Printed Silica-Based Bioactive Glass Scaffolds for Bone Regeneration. J. Funct. Biomater. 2022, 13, 74. [Google Scholar] [CrossRef]
- Sanz-Herrera, J.; Boccaccini, A. Modelling bioactivity and degradation of bioactive glass based tissue engineering scaffolds. Int. J. Solids Struct. 2011, 48, 257–268. [Google Scholar] [CrossRef]
- Hsin, I.C.; Yiwei, W. Cell Responses to Surface and Architecture of Tissue Engineering Scaffolds. In Regenerative Medicine and Tissue Engineering; Daniel, E., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Ravanbakhsh, M.; Labbaf, S.; Karimzadeh, F.; Pinna, A.; Houreh, A.B.; Nasr-Esfahani, M. Mesoporous bioactive glasses for the combined application of osteosarcoma treatment and bone regeneration. Mater. Sci. Eng. C 2019, 104, 109994. [Google Scholar] [CrossRef]
- Haider, A.; Waseem, A.; Karpukhina, N.; Mohsin, S. Strontium- and Zinc-Containing Bioactive Glass and Alginates Scaffolds. Bioengineering 2020, 7, 10. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, W.; Fu, X.; Xie, W.; Chen, X. Fabrication and characterization of bioactive glass/alginate composite scaffolds by a self-crosslinking processing for bone regeneration. RSC Adv. 2016, 6, 91201–91208. [Google Scholar] [CrossRef]
- Diomede, F.; Marconi, G.D.; Fonticoli, L.; Pizzicanella, J.; Merciaro, I.; Bramanti, P.; Mazzon, E.; Trubiani, O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 3242. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
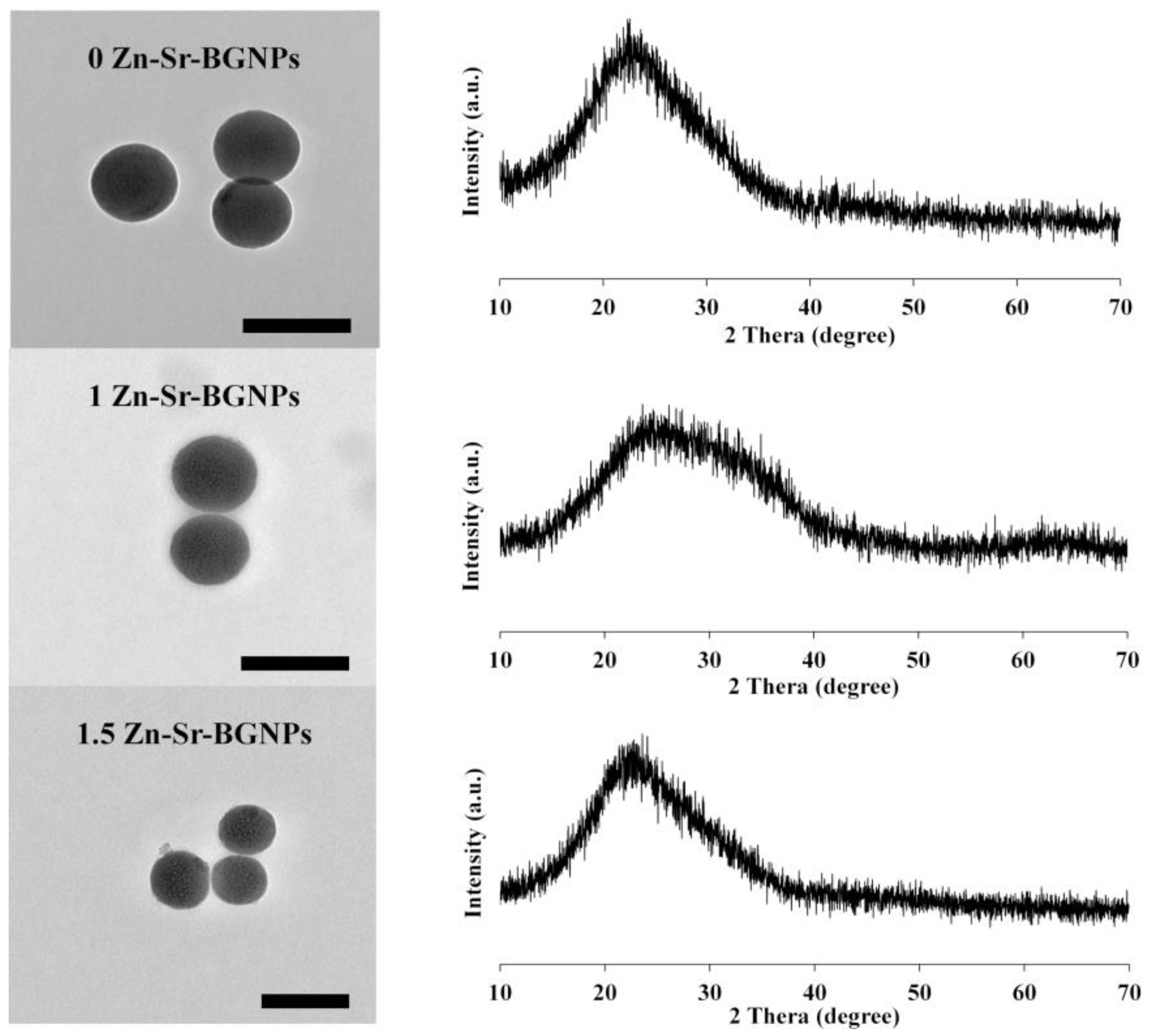
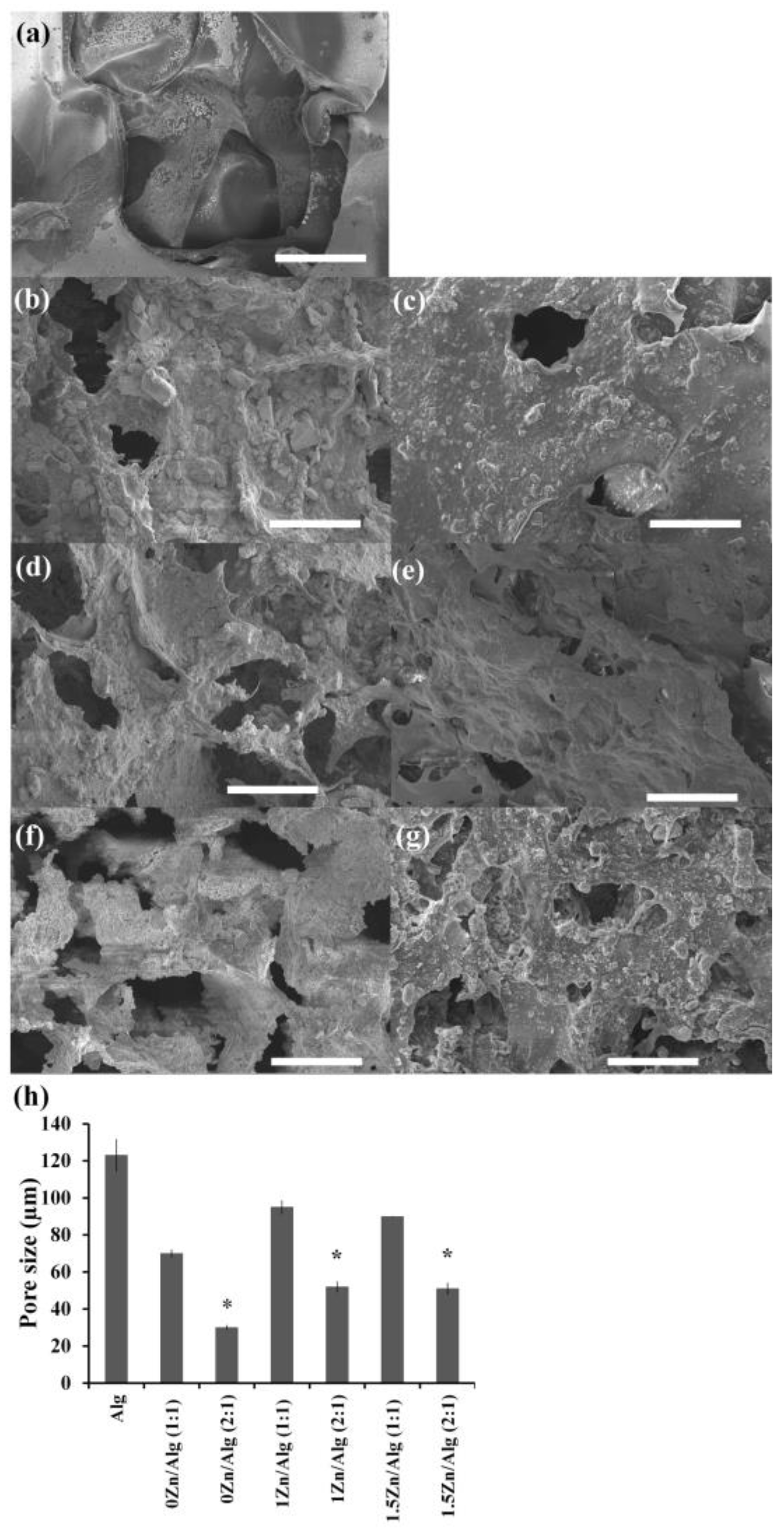
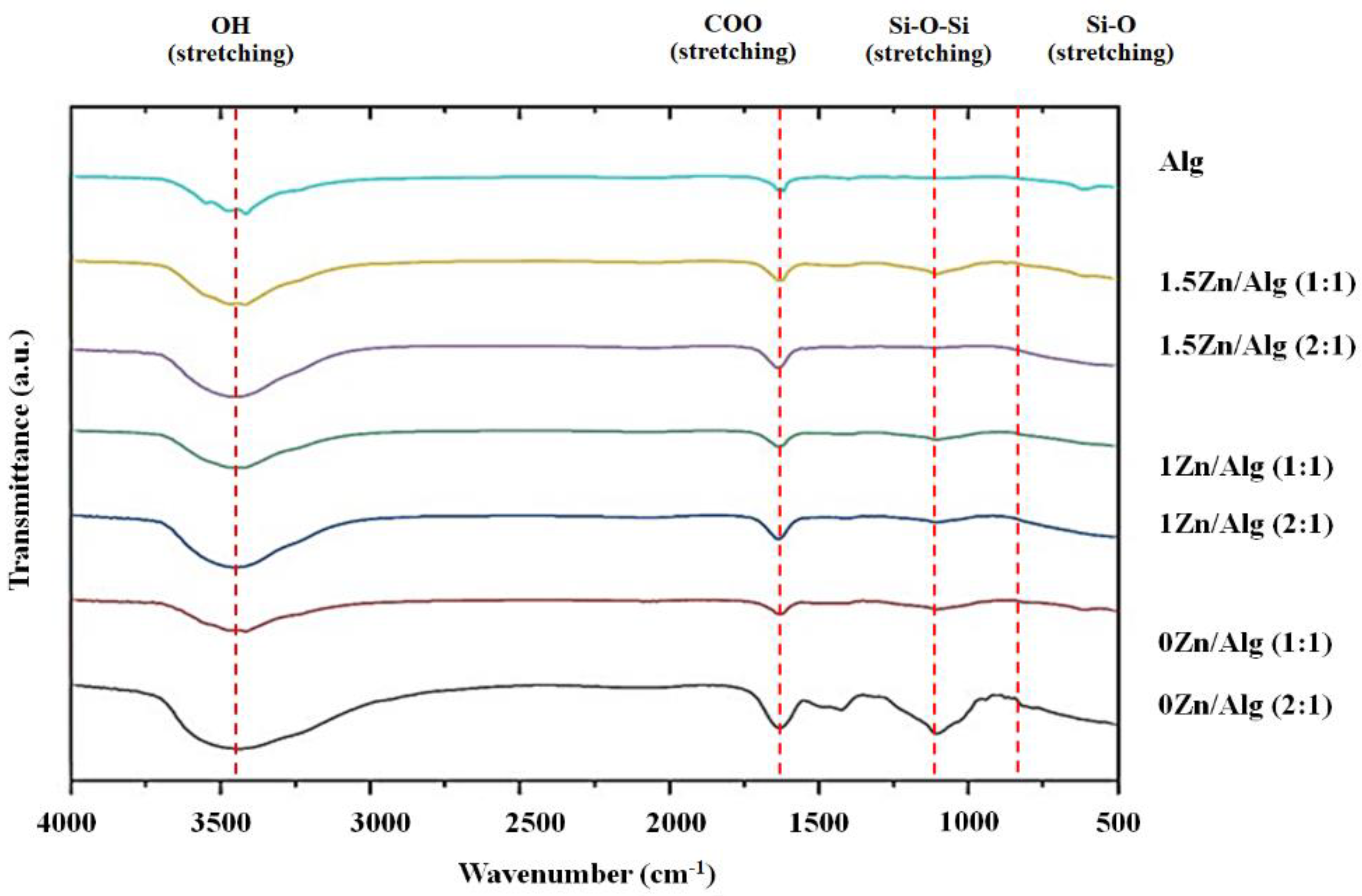
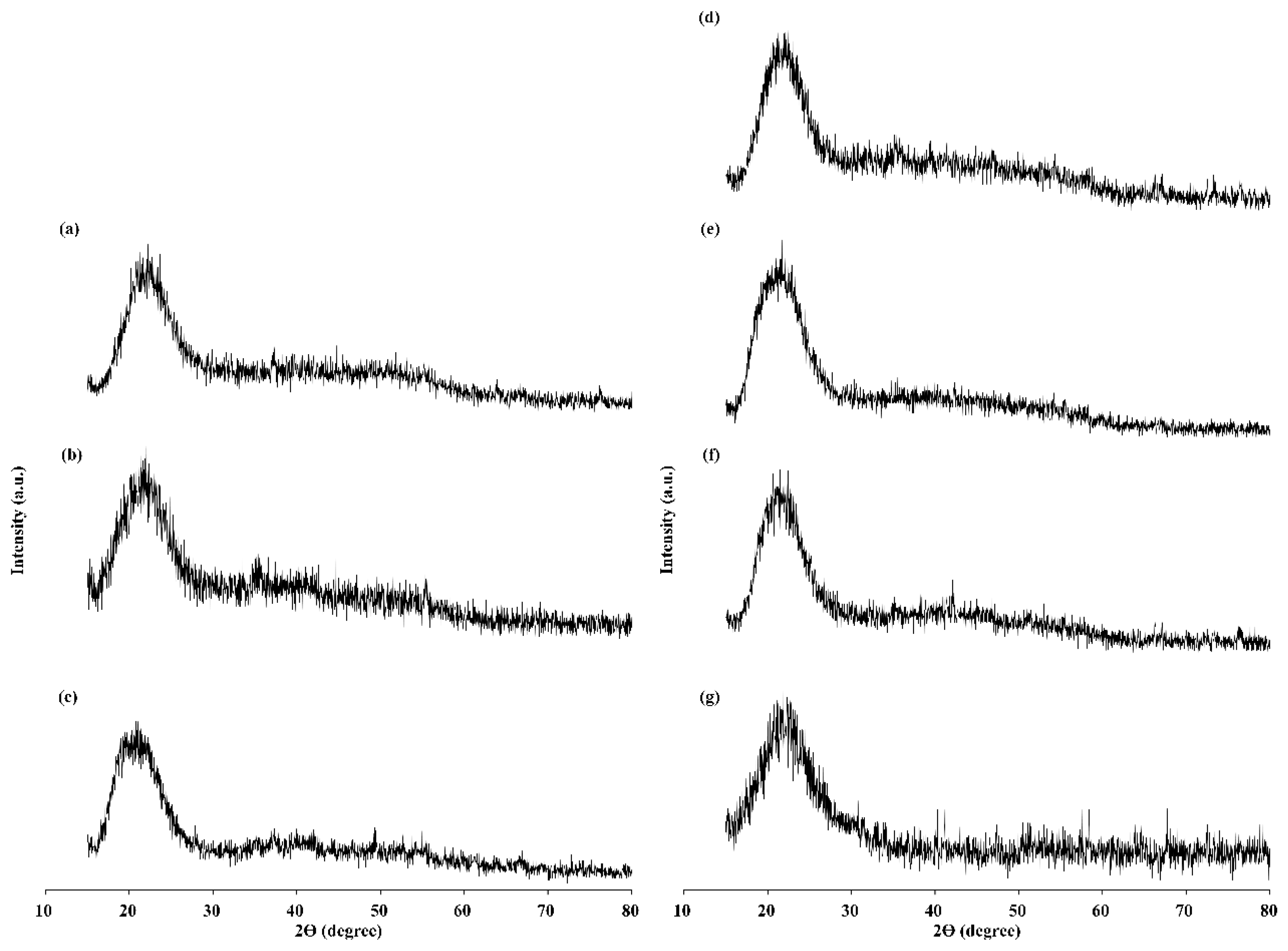
| Samples | %Weight ± S.D. | |||
|---|---|---|---|---|
| Si | Ca | Sr | Zn | |
| 0Zn-Sr-BGNPs | 45.20 ± 1.09 | 17.50 ± 0.81 | 37.40 ± 1.56 | N/A |
| 1Zn-Sr-BGNPs | 37.80 ± 0.77 | 10.13 ± 0.09 | 28.13 ± 0.80 | 23.94 ± 0.28 |
| 1.5Zn-Sr-BGNPs | 36.03 ± 1.20 | 10.51 ± 0.42 | 27.89 ± 0.26 | 25.57 ± 1.22 |
| Samples | BET Surface Area, m2/g | Pore Volume, cm3/g |
|---|---|---|
| Alg | 21.77 | 0.046 |
| 0Zn/Alg (1:1) | 30.64 | 0.051 |
| 0Zn/Alg (2:1) | 24.38 | 0.029 |
| 1Zn/Alg (1:1) | 22.58 | 0.055 |
| 1Zn/Alg (2:1) | 11.64 | 0.018 |
| 1.5Zn/Alg (1:1) | 15.74 | 0.014 |
| 1.5Zn/Alg (2:1) | 11.57 | 0.012 |
| Samples | Zn-Sr-BGNPs | Zn-Sr-BGNP:Alg (wt./wt.) |
|---|---|---|
| Alg | - | 0:1 |
| 0Zn/Alg (1:1) | 0 Zn-Sr-BGNPs | 1:1 |
| 0Zn/Alg (2:1) | 2:1 | |
| 1Zn/Alg (1:1) | 1 Zn-Sr-BGNPs | 1:1 |
| 1Zn/Alg (2:1) | 2:1 | |
| 1.5Zn/Alg (1:1) | 1.5 Zn-Sr-BGNPs | 1:1 |
| 1.5Zn/Alg (2:1) | 2:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naruphontjirakul, P.; Panpisut, P.; Patntirapong, S. Zinc and Strontium-Substituted Bioactive Glass Nanoparticle/Alginate Composites Scaffold for Bone Regeneration. Int. J. Mol. Sci. 2023, 24, 6150. https://doi.org/10.3390/ijms24076150
Naruphontjirakul P, Panpisut P, Patntirapong S. Zinc and Strontium-Substituted Bioactive Glass Nanoparticle/Alginate Composites Scaffold for Bone Regeneration. International Journal of Molecular Sciences. 2023; 24(7):6150. https://doi.org/10.3390/ijms24076150
Chicago/Turabian StyleNaruphontjirakul, Parichart, Piyaphong Panpisut, and Somying Patntirapong. 2023. "Zinc and Strontium-Substituted Bioactive Glass Nanoparticle/Alginate Composites Scaffold for Bone Regeneration" International Journal of Molecular Sciences 24, no. 7: 6150. https://doi.org/10.3390/ijms24076150
APA StyleNaruphontjirakul, P., Panpisut, P., & Patntirapong, S. (2023). Zinc and Strontium-Substituted Bioactive Glass Nanoparticle/Alginate Composites Scaffold for Bone Regeneration. International Journal of Molecular Sciences, 24(7), 6150. https://doi.org/10.3390/ijms24076150






