1. Introduction
Cervical cancer (CC) is the fourth most frequently detected cancer in females and presents a considerable health burden to women globally [
1], warranting more effective prevention, management, and control strategies [
2]. Orthodox CC interventions, such as surgical removal, chemotherapy, and radiotherapy, may manifest adverse effects and are intrusive [
3]. Notwithstanding notable medical advances, nearly 70% of late-stage CC sufferers experience metastasis, due to resistance to repeated therapies and disease progression [
4]. This therefore necessitates the need for novel therapeutic research.
Photodynamic therapy (PDT) is an alternative treatment modality, which has presented support of CC primary abolition [
5]. The mechanism of PDT is orchestrated by the interplay of red light, oxygen, and a photosensitizer (PS). The PS is activated using a red light and in turn yields cytotoxic reactive oxygen species (ROS) and singlet oxygen (
1O
2). The generation of these cytotoxic species induces oxidative stress, which can cause primary CC cell death via apoptosis [
6]. The anti-cancer effects of PDT are derived from its direct cytotoxic effects on malignant cells, damage to the tumor vasculature and induction of inflammatory reaction, which can manifest in the development of systemic immunity, but exercise negligible effects in normal cells [
7].
Cancer research into the prospective use of phthalocyanines (Pcs) as PSs in PDT is favorable. This is attributed to their superior tumor passive uptake, high ROS production, and potent absorption in the 680 nm red wavelength of light [
8]. Studies have reported that zinc phthalocyanines (ZnPcs) have a strong absorption in the NIR and exhibit low absorption at wavelengths between 400 and 600 nm, potentially leading to a lower skin photosensitization when exposed to sunlight [
9]. Moreover, the presence of a diamagnetic central metal, such as Zn
2+ in the Pc nucleus, seems to improve the triplet state lifetime, as well as its yield and singlet oxygen yields compared to paramagnetic metals, however, the metalation is not required for its photodynamic activity [
9].
A review by Carobeli et al. (2021) indicates that Pc PSs have potential as PDT pharmaceutical agents for anti-CC therapy. The authors firmly believe that Pc-based PS formulations could allow for the development of lead PDT compounds for the primary treatment of CC [
10]. However, this review went on to note that even though Pcs PSs have excellent optical properties for in vitro PDT, the applications of unsubstituted Pc PSs within in vivo and clinical applications have a shortfall [
10]. Since, unsubstituted Pc PSs are hydrophobic planar molecules, they have poor solubility and tend to aggregate in vivo, limiting their abilities to effectively reach target tissues for desired therapeutic outcomes [
10]. Nonetheless, Pc PSs exhibit unique chemical structures, which allow for the introduction of various peripheral (macrocycle) and axial (central metal ion coordination) substitutions, which control the tendency for aggregation, pharmacokinetics, biodistribution, and solubility, as well fine-tuning of near-infrared spectroscopy absorbance [
10,
11]. Typical substitutions to improve solubility and reduce the aggregation of Pc PSs include sulfonation of the periphery of its macrocycle to make it more hydrophilic [
12]. The advantage of increasing solubility of Pc PSs through sulfonation is that they can sometimes allow for direct biological administration, without the need for an additional carrier [
11,
13]. These attempts have led to the development of easy synthetic routes for the new generation of Pcs [
10,
11,
14].
The potential of sulfonated Pcs for clinical PDT has prompted a search for alternate synthetic approaches to yield well-characterized compounds as single isomeric products [
12]. Hydrophilic, sulfonated ZnPcS
n PSs have received particular attention as PDT agents over the years, since sulfonation of the substituted ZnPc enhances its solubility to aggregate less and promote its uptake in tumor tissues, with improved in vitro and in vivo PDT outcomes [
12]. However, the degree of sulfonation can also make Pc PSs bulky for direct tumor localization, and so, occasionally, the incorporation of a target nanocarrier is necessary. However, as long as the Pc PS molecules remain in their monomeric form, the quantum yield for singlet oxygen will remain unaffected [
12,
14,
15].
Zinc phthalocyanine tetrasulfonate (ZnPcS
4) has been designed as an amphiphilic PS agent to promote uptake, as well as circumvent any issues associated with solubility and aggregation [
16]. Studies by Pashkovskaya et al. (2007) and Montaseri et al. (2022) noted that Pc complexes, such as anionic ZnPcS
4 PSs, can efficiently bind to cancer tumor phospholipid membranes, through metal–phosphate coordination [
16,
17]. Therefore, ZnPcS
4 PSs can spontaneously accumulate in tumors through an enhanced permeability and retention (EPR) effect, due to the binding of its tetrasulfonated groups to cancer cell membranes (which have high phospholipid contents), and so allow for passively selective accumulation [
16,
17,
18,
19].
Studies by Hodgkinson et al. (2017) demonstrated that sulphonated ZnPc, containing a mixture of differently sulfonated derivatives (called ZnPcS
mix), was an effective PS within in-vitro-cultured HeLa cells, when treated with 4 J/cm
2 at a wavelength of 673 nm, producing 50% cytotoxicity [
20]. The study also noted that the PS was located in the cytoplasm and perinuclear region of HeLa cells [
20]. More recently, a study performed by Pola et al. (2020) investigated the PDT effects of a disulfonated zinc phthalocyanine (ZnPcS
2) PS within in vitro HeLa cells, and noted that the PS was capable of subcellular localization and that this PDT inhibited mitochondrial respiration in hypoxic conditions [
21]. Additionally, Brozek-Pluska et al. (2020) analyzed the fluorescence/Raman signals of ZnPcS
4 PS within in vivo human normal versus cancerous colon tissue samples, and demonstrated that this PS had a lower affinity for normal tissues [
15]. Moreover, this same study reported that ZnPcS
4 PS had a monomer region signal, confirming its ideal PS properties within PDT applications [
15]. Lastly, this study noted that lower concentrations of ZnPcS
4 PS were capable of endoplasmic reticulum (ER) localization, with mid concentrations capable of mitochondrial and/or Golgi apparatus lysosome localizations, while reporting preferential localization in the nucleus of colon cancer tissues at higher concentrations [
15]. This was of importance, since the nucleus is the largest cellular organelle, which stores genetic information, and should PDT-induced singlet oxygen be able to destroy it in cancer tissues, enhanced therapeutic outcomes can be achieved [
15]. Similarly, in vitro studies performed by Chekwube et al. (2020) successfully investigated the phototoxic effectiveness of ZnPcS
4 PS in MCF-7 breast cancer cells, with significant cytotoxic effects reported [
22].
According to Rak et al. (2019), other options to increase the bioavailability and solubility of metallo-phthalocyanine (MPc) PSs in cancer PDT to translate it into future in vivo and clinical trial research is by using various carrier delivery systems, such as nanoparticles (NPs), nanoemulsions, and liposomes, among others [
13]. De Toldeo et al. (2020) demonstrated the improved uptake of ZnPcS
4 PS when it was encapsulated in poly (lactic acid-glycolic acid) (PLGA) NPs and research by Naidoo et al. (2019) showed excellent active targeting subcellular uptake, with enhanced PDT treatment outcomes when conjugating ZnPcS
4 PSs and an antibody PLGA gold NP delivery carrier, within in vitro melanoma cancer cultured cells, suggesting that both carriers could potentially serve as bioactive models [
23,
24]. Similarly, studies by Simelane et al. (2021) and Montaseri et al. (2022) showed improved subcellular localization and enhanced ZnPcS
4 PS PDT treatment outcomes within in-vitro-cultured colorectal cancer cells, when conjugating to an antibody actively targeted PLGA-loaded gold NP or loading it in core/shell Ag@mSiO
2 NP with folic acid, respectively [
16,
25]. In a study performed by Portilho et al. (2013), an albumin nanosphere (AN), containing ZnPcS
4 PSs, was developed. Results reported excellent PDT anti-tumor activity within in vivo Swiss albino mice, using an Ehrlich solid tumor as an experimental model for breast cancer [
26]. Intratumorally, the ZnPcS
4-AN was capable of mediating PDT to refrain tumor aggressiveness, as well as induce regression [
26]. Moreover, the use of this ZnPcS
4-AN-mediating PDT exposed anti-neoplastic activity, such as that obtained while using intra-tumoral conventional chemo-Dox therapy [
26]. More recently, Dias et al. (2022) investigated targeted liposomes (ITLs) encapsulating ZnPcS
4 PSs in vivo interstitially, to attempt to bring this platform closer to clinical investigations [
27]. The key findings from this study were that the ZnPcS
4 PS did not elicit notable systemic toxicity in zebrafish and chicken embryos, and in human tumor breast cancer xenografts, it produced a significant tumor reduction. However, it did report skin phototoxicity in mouse models [
27]. This study concluded that more effective and safer carrier delivery models must be developed to integrate this PS into a comprehensive tumor-targeting and delivery platform, so that future in vivo research can possibly translate into clinical applications [
27].
The utilization of ZnPcS4 PS within PDT has, thus, been extensively researched in the cancers mentioned above, and has presented some promising results; however, it has not been researched within in vitro or in vivo models for possible shortcomings in CC. It was therefore decided to investigate this PS within this novel study, to provide a platform for future clinical development. Researchers do note that there is still a lot of in vitro work to be done to improve ZnPcS4 PS PDT in relation to its penetrability to tumor tissues, selectivity, stimulating methods, as well as promote its overall ability to overcome tumor hypoxia microenvironment to translate its use into future in vivo models.
Moreover, even though research has reported that PDT has emerged as a possible, effective, and tolerable treatment approach for the management of primary CC, it also requires improvement in terms of investigating combinative PDT treatments to stimulate specific immune responses to eliminate secondary spread [
7]. Overall, accumulating evidence indicates that the therapeutic efficacy of PDT, relies on its capacity to influence tumor–host interaction, and so by tipping the balance toward the activation of an immune response specific for malignant cells, it can eradicate metastasized cancer [
28]. Therefore, to make more robust conclusions about ZnPcS
4 PS PDT real clinical translational potential, investigations in combination with other treatments are needed. A study by Shams and colleagues (2015) suggested a need to develop PDT regimens which eradicate primary tumor growth, as well as inhibit metastasis through host immune system stimulation [
29]. Chota et al. (2022) investigated the in vitro cell death mechanisms induced by
Dicoma anomala root extract in combination with ZnPcS
4-PS-mediated PDT in A549 lung cancer cells. This combination therapy confirmed the cytotoxic and anti-proliferative effects of
Dicoma anomala extracts in monotherapy and in combination with ZnPcS
4-mediated PDT, through apoptosis and the upregulation of p38, p53, Bax, caspase 3, 8, and 9 apoptotic proteins, suggesting that combinative therapies are worth exploring [
30].
Cannabinoids are a group of naturally occurring metabolites located abundantly in the
Cannabis sativa L. plant [
31]. Research evidence denotes that the pharmacological anti-cancer therapeutic potential of the cannabinoid derivative, known as cannabidiol (CBD), from this plant can inhibit tumor cell growth and proliferation [
32]. The efficacy of CBD has been attributed to its capability of targeting several cellular pathways which control tumorigenesis via the stimulation of various immune system intracellular signaling pathways [
31]. The evidence for CBD’s various cancer therapeutic applications were reviewed by Zhelyazkova, Kirilov, and Momekov (2020), and its anti-proliferative and anti-invasive actions were highlighted, noting its capability to induce autophagy-mediated cancer cell death with its inherent chemotherapeutic abilities to prevent secondary spread [
33].
More recently, Nkune, Kruger, and Abrahamse (2022) also reported encouraging results, demonstrating that utilizing antibody-targeted PLGA-loaded gold NP ZnPcS
4 PS carrier as a PDT treatment for in-vitro-cultured colorectal cancer in combination with CBD allows for targeted primary tumor destruction, as well as activation of specific immune responses, which limit metastasis [
34]. Lukhele and Motadi (2016) reported that CBD was able to stimulate specific cellular responses that aid its anti-cancer efficiency within in-vitro-cultured CCs, since it lessens their proliferation, and so eliminates spread [
35]. Therefore, to fully understand the effectiveness of primary PDT CC treatment strategies, especially in relation to CC’s aggressive metastatic nature, there is a great demand to further explore the cytotoxic therapeutic effects of primary CC PDT in combination with secondary CBD treatments.
Thus, the aim of this study was to investigate the PDT-mediated effect of ZnPcS4 PS and CBD on the survival of in-vitro-cultured HeLa cells, to determine its potential application as a combinative complementary treatment form for CC. Since this is a novel study and the combinative effect of ZnPcS4 PS PDT and CBD has never been investigated within in vitro CC, the intention of this research was to lay the groundwork foundation in terms of investigating its potential effectiveness. These findings will possibly allow for future investigations in relation to enhancing the delivery of this combinative treatment with various drug targets and carriers, to promote its possible in vivo effectiveness.
2. Results
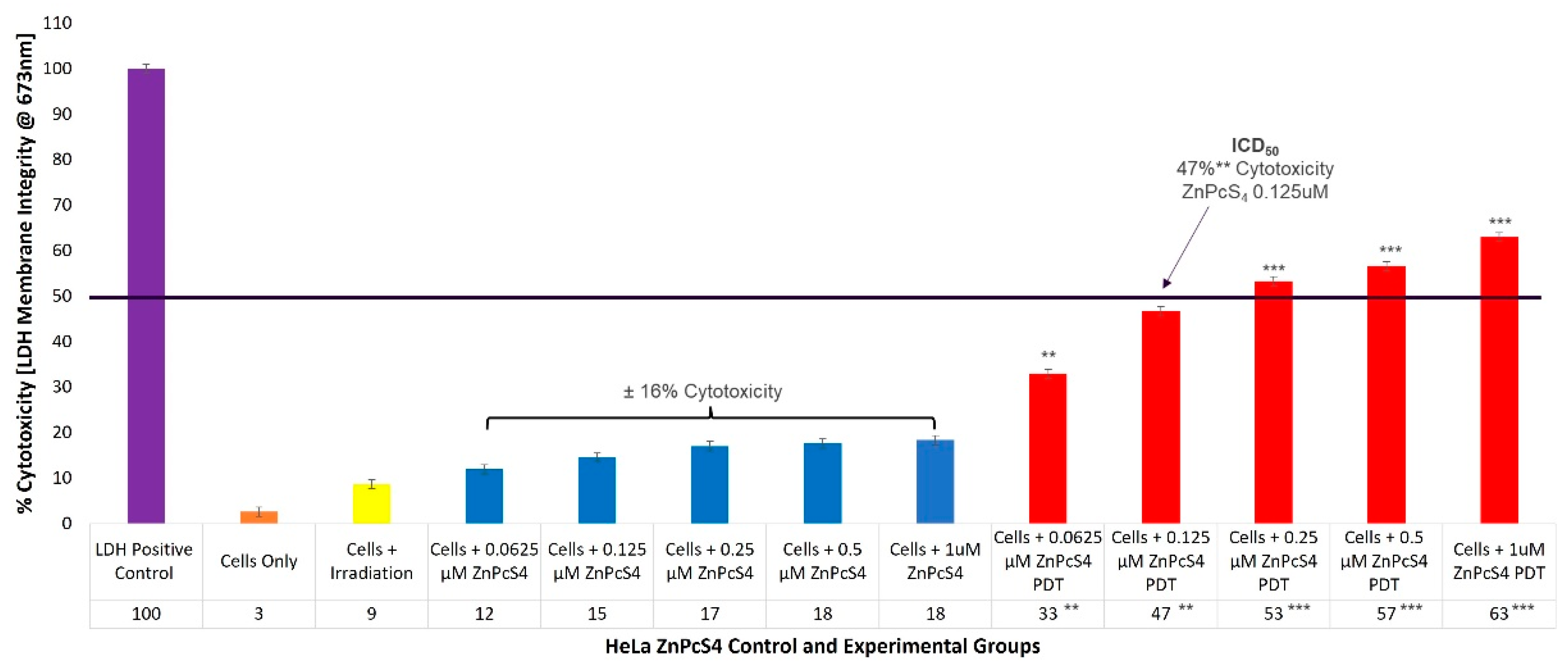
2.1. HeLa ZnPcS4 PS ICD50 PDT Irradiation and LDH Cellular Cytotoxicity Dose Response Assays
To evaluate the cytotoxicity of the ZnPcS
4 PS PDT on HeLa cells, experimental and control groups were treated with different concentrations of ZnPcS
4 PS (0.0625, 0.125, 0.25, 0.5, and 1 µM). After laser irradiation, culture plates were re-incubated for an additional 24 h prior to being subjected to lactate dehydrogenase (LDH) membrane damage integrity analysis. Cellular lysis was induced and 100% LDH release was noted in the positive control, whereas the cells-only control indicated 3% (±SEM 0.47) cytotoxicity, and were used for statistical comparisons. The control groups of HeLa CC cells, which received increasing concentrations of ZnPcS
4 PS (0.0625, 0.125, 0.25, 0.5, and 1 µM) alone, without any influence of irradiation, respectively, reported an insignificant incremental average increase (±16%) in cellular cytotoxicity (
Figure 1).
The experimental groups of HeLa cells which received increasing concentrations of ZnPcS4 PS and irradiation, showed significant linear increases in cellular cytotoxicity when compared to their respective cells, only within control groups. Experimental groups, which received 0.0625 µM ZnPcS4 PS and PDT, reported 33%** (±SEM 0.82) cytotoxicity, while for experimental groups which received 0.125 µM ZnPcS4 PS and PDT, 47%** (±SEM 1.25) cytotoxicity was somewhat significant. However, experimental groups which received 0.25 µM and 0.5 µM ZnPcS4 PS, as well as PDT, produced highly significant increases of 53%*** (±SEM 0.47) and 57%*** (±SEM 0.48) in their respective cytotoxicity assays. The most significant increase in cytotoxicity of 63%*** (±SEM 0.82) was reported in experimental groups, which received 1 µM ZnPcS4 PS and PDT.
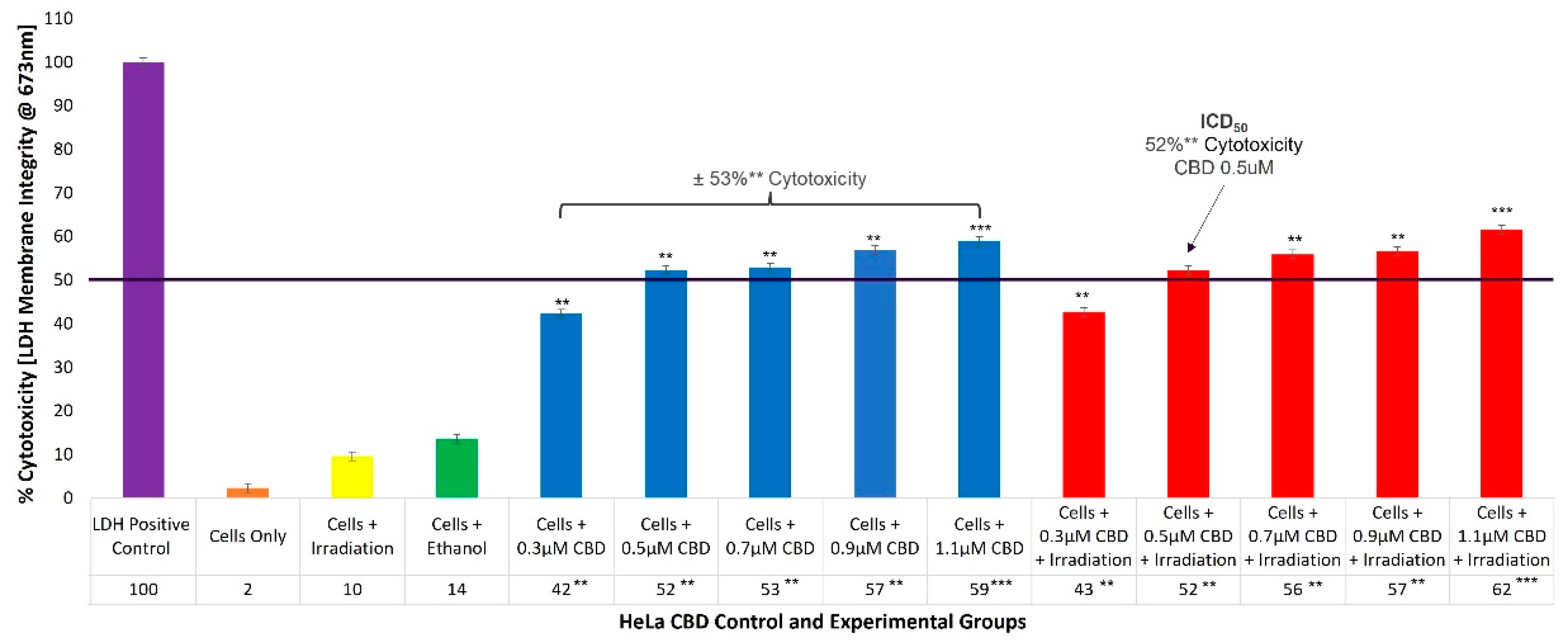
2.2. HeLa CBD ICD50 Irradiation and LDH Cellular Cytotoxicity Quantitative Dose Response Assays
To evaluate the cellular cytotoxicity of CBD on HeLa cells, experimental and control groups were treated with different concentrations of CBD (0.3, 0.5, 0.7, 0.9, and 1.1 µM). The control groups of HeLa cells which received 99.8% (
v/
v) ethanol only, generated an incremental increase of 14% (±SEM 0.34) in cellular cytotoxicity. The experimental group, which received 0.3 µM CBD and irradiation, reported 43%** (±SEM 1.05) cytotoxicity, which was somewhat significant. However, experimental groups which received 0.5 µM and 0.7 µM CBD, as well as irradiation, denoted significantly increased values of 52%** (±SEM 0.75) and 56%** (±SEM 0.78), with reference to their respective cytotoxicity assays. The most significant increases in cytotoxicity of 57%** (±SEM 0.86) and 62%*** (±SEM 0.42) were reported in the experimental groups which received 0.9 µM and 1.1 µM CBD plus irradiation, respectively. Similar overall average cytotoxicity results were reported for control groups consisting of cells plus CBD of ±53%** (
Figure 2).
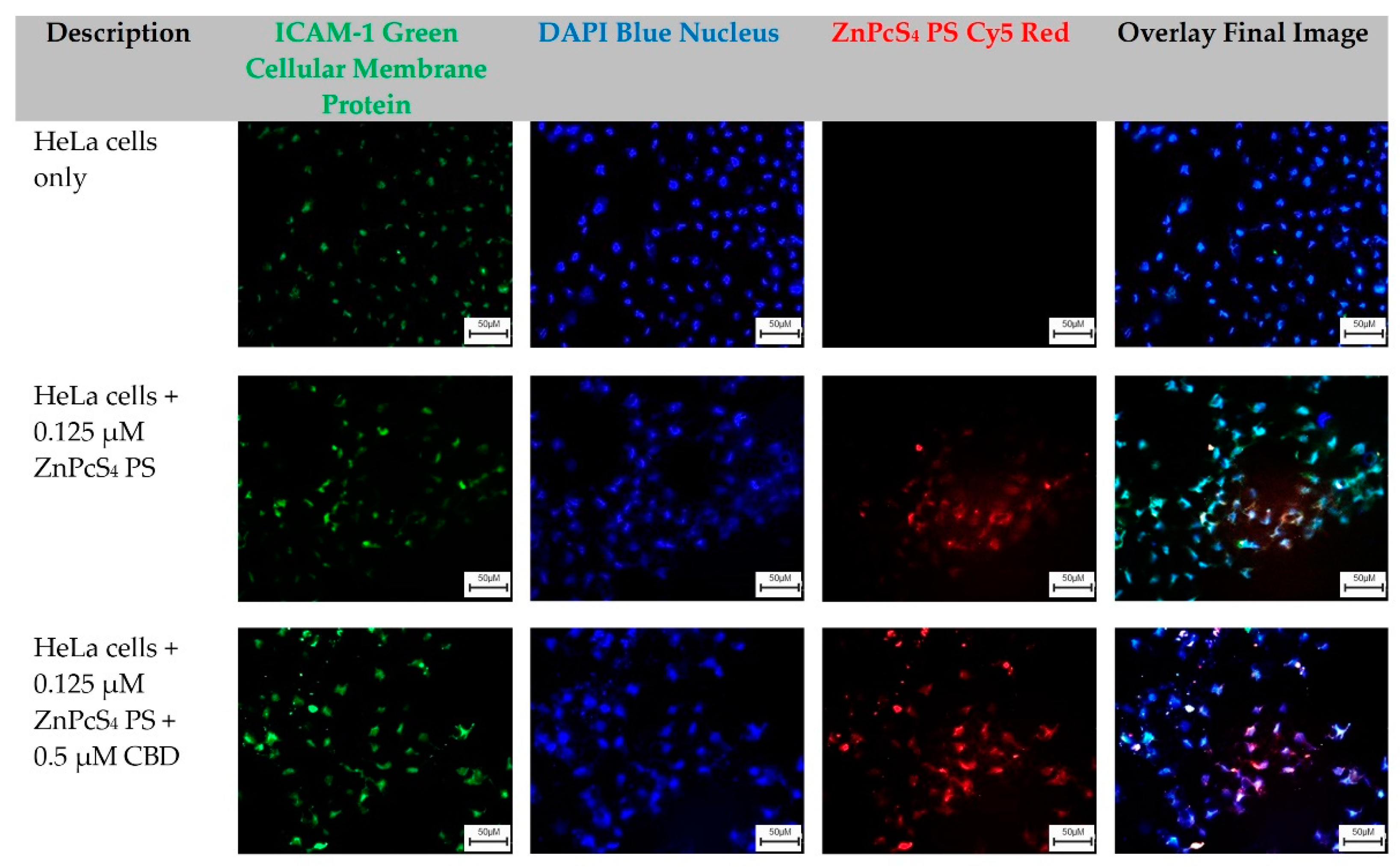
2.3. Qualitative Subcellular Localization Immunofluorescent Staining Confirmation of ZnPcS4 PS Uptake in HeLa and WS1
The HeLa-cells-only control group, which received no forms of treatment, was utilized as the control comparator for result interpretation outcomes (
Figure 3).
From these images, cellular green membrane staining, and blue cellular nuclei of the HeLa-cells-only were identified and collated into an overlay image. Control groups of HeLa cells which received 0.125 µM ZnPcS
4 PS, and experimental groups which received 0.125 µM ZnPcS
4 PS + 0.5 µM CBD, also had their green cellular membrane proteins and blue-stained nuclei, as well as their red fluorescent ZnPcS
4 PS signal identified clearly. When these three images were overlayed, it was possible to potentially identify and locate where the ZnPcS
4 PS had localized within HeLa cells (
Figure 3).
The WS1-cells-only control group, which received no forms of treatment, was utilized as the benchmark comparator for result interpretation outcomes. From these images, the cellular green membrane staining, and blue cellular nuclei of the WS1 fibroblast cells only were identified and collated into an overlay image. Control groups of WS1 fibroblast cells which received 0.125 µM ZnPcS
4 PS, and experimental groups which received 0.125 µM ZnPcS
4 PS + 0.5 µM CBD, also had their green cellular membrane proteins and blue-stained nuclei, as well as their red fluorescent ZnPcS
4 PS signal identified clearly. When these three images were overlayed, it was possible to potentially identify and locate where the ZnPcS
4 PS had localized within the WS1 fibroblast cells (
Figure 4).
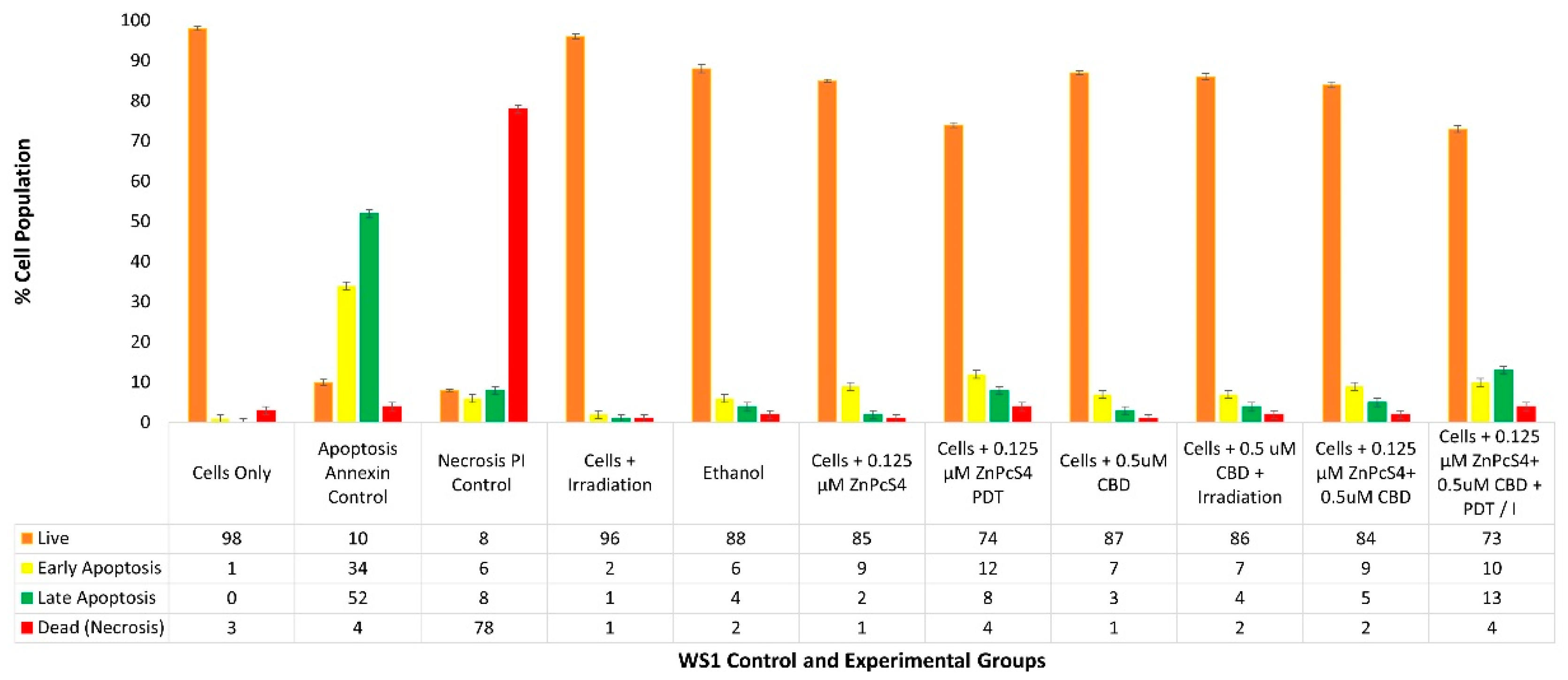
2.4. HeLa Flow Cytometry Cell Death Pathway Quantitative Analysis of ZnPcS4 PS and CBD PDT/Irradiation in Combinative Assays
With reference to
Figure 5, the percentage of different stages of cell death, using the flow cytometry Annexin V-FITC/PI staining method on various HeLa control and experimental groups within ZnPcS
4 PS and CBD PDT/irradiation combinative assays, has been shown.
The cells-only positive control, which received no forms of treatment produced a 96% (±SEM 0.68) viable population of cells, with negligible cell death. The positive Annexin-V-FITC-stained control of apoptotic-induced cell death denoted only 12% (±SEM 0.45) of viable cells, a significant 49% (±SEM 0.48) of early apoptosis and 37% (±SEM 0.41) of late apoptosis, as well as a minor 2% (±SEM 0.51) of necrotic cell death. The negative PI-stained control of necrotic-induced cell death denoted only 10% (±SEM 0.57) of viable cells, a minor 8% (±SEM 0.61) of early apoptosis, and 6% (±SEM 0.56) of late apoptosis; however, a significant 76% (±SEM 0.49) necrotic cell death was also observed. Thus, these positive controls were considered as acceptable standards for result comparisons and statistical data interpretations.
The control group of HeLa cells plus irradiation reported a substantial 92% (±SEM 0.92) of viable cells, without any other significant forms of cell death. The control group of HeLa cells, which received 99.8% (v/v) of ethanol only, reported a majority of 87% (±SEM 0.53) of viable cells, without any other significant forms of cell death.
The control group of HeLa cells which received 0.125 µM ZnPcS4 PS alone, reported a noteworthy 84% (±SEM 0.96) of viable cells, without any other significant forms of cell death. The HeLa cells which received 0.125 µM ZnPcS4 PS and irradiation reported a significant reduction in viability, whereby only 52%* (±SEM 0.87) of cells were noted as viable. Furthermore, 15% (±SEM 0.92) of these cells were undergoing early apoptosis, and an even more significant 24%** (±SEM 0.85) were in a late apoptotic form of cell death, with an incremental amount of 9% (±SEM 0.56) necrosis.
The control and experimental groups of HeLa cells which received 0.5 µM CBD alone, with or without laser irradiation, respectively, reported similar significant forms of cell death. The control group which received 0.5 µM CBD without irradiation noted a significant decrease of only 46%** (±SEM 0.44) of cells being viable, as well as a significant increase of 38%** (±SEM 0.46) of cells undergoing late apoptosis, with incremental amounts of early apoptosis and necrosis. Similarly, the experimental group which received 0.5 µM CBD with irradiation noted a significant decrease of only 50%** (±SEM 0.56) of cells being viable, as well as a significant increase of 42%** (±SEM 0.62) of cells undergoing late apoptosis, with incremental amounts of early apoptosis and necrosis.
The experimental groups of HeLa cells which received 0.125 µM ZnPcS4 PS plus 0.5 µM CBD, without PDT, reported a significant reduction, whereby only 35%** (±SEM 0.76) of cells were viable. Furthermore, 11% (±SEM 0.66) of these cells were undergoing early apoptosis and an even more significant 45%** (±SEM 0.72) of these cells were in a late apoptotic form of cell death, with an incremental amount of 9% (±SEM 0.55) necrosis.
In contrast, HeLa cells which received 0.125 µM ZnPcS4 PS plus 0.5 µM CBD and irradiation reported the most highly significant and favorable forms of cell death, with only 13%*** (±SEM 1.04) of the cells being viable. A further 7% (±SEM 0.98) of the cells were in early apoptosis and a confounding 64%*** (±SEM 0.93) were in late forms of favorable apoptotic cell death, with a minor 16% (±SEM 1.12) of necrosis post-PDT. In comparison to the experimental groups of HeLa cells which received 0.125 µM ZnPcS4 PS plus 0.5 µM CBD without PDT, the percentages of cellular viability were significantly decreased (35%** vs. 13%**) and the percentages of late apoptotic cell death were also significantly increased (45%** vs. 64%***).
2.5. WS1 Normal Fibroblast Flow Cytometry Cell Death Pathway Quantitative Analysis of ZnPcS4 PS and CBD PDT/Irradiation in Combinative Assays
With reference to
Figure 6, the percentage of different stages of cell death using the flow cytometry Annexin V-FITC/PI staining method on various WS1 normal human fibroblast control and experimental groups within ZnPcS
4 PS and CBD PDT/irradiation combinative assays, are shown.
The cells-only positive control noted a 98% (±SEM 0.51) viable population of cells, with negligible cell death. The positive Annexin-V-FITC-stained control of apoptotic-induced cell death noted only 10% (±SEM 0.73) of viable cells, a significant 34% (±SEM 0.81) of early apoptosis and 52% (±SEM 0.79) of late apoptosis, as well as a minor 4% (±SEM 0.84) necrotic cell death. The negative PI-stained control of necrotic-induced cell death noted only 8% (±SEM 0.32) of viable cells, a minor 6% (±SEM 0.46) of early apoptosis and 8% (±SEM 0.39) of late apoptosis; however, a significant 78% (±SEM 0.41) necrotic cell death was also observed.
The control group of WS1 cells plus irradiation reported a substantial 96% (±SEM 0.60) of viable cells, without any other significant forms of cell death. The control group of WS1 cells which received 99.8% (v/v) of ethanol only, reported a majority of 88% (±SEM 1.01) of viable cells, without any other significant forms of cell death.
The control group of WS1 cells which received 0.125 µM ZnPcS4 PS without any influence of irradiation, reported a noteworthy 85% of viable cells, without any other significant forms of cell death. The control groups of WS1 (±SEM 0.44) cells which received 0.125 µM ZnPcS4 PS and irradiation, did not report significant forms of cell death, however, in comparison to normal cells, only 74% (±SEM 0.63) remained viable, with an 8% (±SEM 0.67) increase in late apoptosis.
The control and experimental groups of WS1 cells which received 0.5 µM CBD with or without laser irradiation, respectively, reported no significant forms of cell death. The control group which received 0.5 µM CBD without irradiation noted 87% (±SEM 0.58) of cells being viable, whereas the experimental group which received 0.5 µM CBD with irradiation similarly noted 86% (±SEM 0.87) of cells being viable.
The experimental groups of WS1 cells which received 0.125 µM ZnPcS4 PS plus 0.5 µM CBD without irradiation also reported no significant reduction in cell viability, with incremental amounts of cell death. Similarly, the same results were reported in control groups which received 0.125 µM ZnPcS4 PS alone or 0.5 µM CBD alone.
The experimental group of WS1 cells which received 0.125 µM ZnPcS4 PS plus 0.5 µM CBD with laser irradiation noted no significant forms of cell death and 73% (±SEM 0.93) of the cell population remained viable. However, in comparison to the cells-only control group, there was a higher population of early (10% ±SEM 1.03) and late apoptosis (13% ±SEM 0.99) being noted. Similar findings were reported in control groups which received 0.125 µM ZnPcS4 PS and PDT, whereby 12% (±SEM 0.77) of early and 8% (±SEM 0.47) of late apoptosis was noted. Subsequently, control groups of WS1 cells which received 0.5 µM CBD with irradiation, respectively, showed slightly less (7% ±SEM 0.86) early apoptosis and 4% (±SEM 0.79) of late apoptosis.
Nonetheless, even though the cell death was insignificant, since minor early apoptosis/autophagy was observed, there was a possibility that WS1 post-PDT could recover, which required corroboration in ATP proliferation assays.
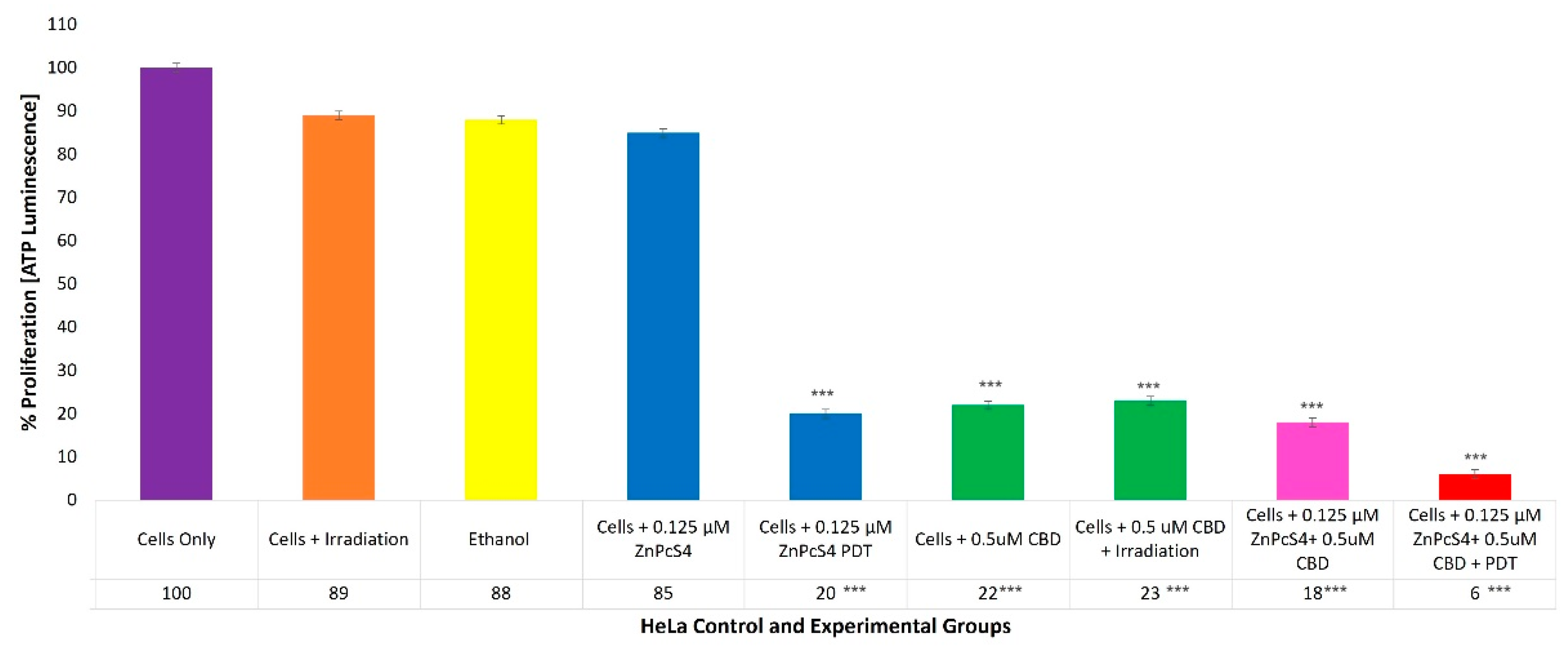
2.6. HeLa Adenosine Triphosphate (ATP) Quantitative Cell Proliferation Analysis of ZnPcS4 PS and CBD PDT/Irradiation in Combinative Assays
With reference to
Figure 7, the percentage of cell proliferation using the ATP illumination method among various HeLa control and experimental groups, within ZnPcS
4 PS and CBD PDT/irradiation combinative assays, has been shown.
The cells-only positive control, which received no forms of treatment, noted a 100% (±SEM 1.07) viable population of cells capable of cellular proliferation, and was thus considered an acceptable standard for result comparisons and statistical data interpretations.
The control groups of HeLa cells which received irradiation, 99.8% (v/v) of ethanol or 0.125 µM ZnPcS4 PS only, noted no significant decreases in cellular proliferation. However, the control groups of HeLa cells which received 0.125 µM ZnPcS4 PS and laser irradiation reported a significant decrease, as only 20%*** (±SEM 0.82) of the cell population post-PDT treatment was able to proliferate.
The control and experimental groups of HeLa cells which received 0.5 µM CBD, with or without laser irradiation, reported similar significant decreases, since only of 22%*** (±SEM 0.47) and 23%*** (±SEM 0.82) of the cell populations pre- and post-PDT treatment, respectively, were capable of proliferation.
The experimental groups of HeLa cells which received 0.125 µM ZnPcS4 PS plus 0.5 µM CBD without PDT reported a significant reduction, whereby only 18%*** (±SEM 0.82) of the cells maintained their ability to proliferate, whereas HeLa cells which received 0.125 µM ZnPcS4 PS plus 0.5 µM CBD and PDT reported the most significant decreases in cellular proliferation, noting that only 6%*** (±SEM 0.94) of the cellular population was capable of proliferation.
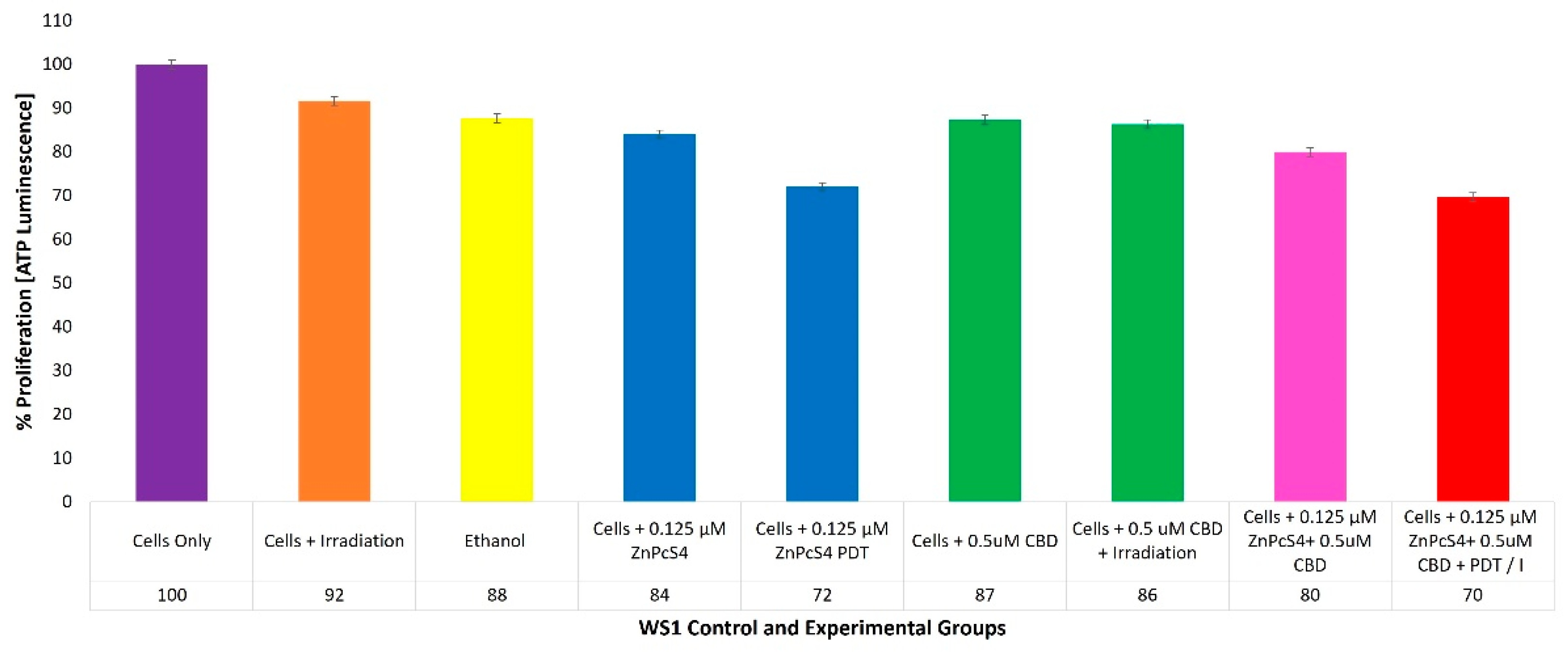
2.7. WS1 Normal Fibroblast ATP Quantitative Cell Proliferation Analysis of ZnPcS4 PS and CBD PDT/Irradiation in Combinative Assays
With reference to
Figure 8, the percentage of cell proliferation using the ATP illumination method whiathin various WS1 normal human control and experimental groups within ZnPcS
4 PS and CBD PDT/irradiation combinative assays, has been shown.
The positive control of cells only noted a 100% (±SEM 0.98) viable population of cells, capable of cellular proliferation and so was considered as an acceptable standard for result comparisons and statistical data interpretations.
4. Materials and Methods
4.1. Cell Culture
Commercially purchased CC cell line HeLa (Cellonex CAT SS1411) was used in this study as the CC-positive cell line, whereas WS1 human skin fibroblasts procured from the American Type Culture Collection (ATTC CRL-1502) were used for result comparison outcomes to represent normal human tissues. Cells were cultivated in a T25 culture flask containing supplemented pre-warmed media.
HeLa cells were cultivated in Dulbecco’s modified eagle medium (DMEM), enriched with 10% fetal bovine serum (FBS), 4 mM sodium pyruvate, 4 mM L-glutamine, 2.5 g/mL amphotericin β, and 100 U penicillin 100 g/mL streptomycin solution. WS1 cells were cultivated in minimum essential media (EMEM), enriched with 2 mM L-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 10% FBS, 2.5 g/mL amphotericin β and 100 U penicillin 100 g/mL streptomycin solution. These re-constituted cell cultures were then incubated at 37 °C in 5% CO2 and 85% humidity. Once 90% confluent monolayers of the cells were detached from the flasks using TrypLE Select™, consequent cellular pellets were re-suspended in fresh culture media.
Thereafter, HeLa cells were seeded at a density of 5 × 105 cells/mL and WS1 cells at 3 × 105 cells/mL of supplemented media into sterile 3.4 cm diameter cell culture plates. The plates were incubated for 4 h to allow for cellular attachment, prior to conducting experiments in the sterile tissue-culture-treated plates.
4.2. Cell-Culture Plate Groupings and Laser Irradiation
After 4 h, HeLa and WS1 culture plates were divided into the various control and experimental groups for CBD or ZnPcS4 PS PDT individual dose response assays, and combinative experiments.
The cell- and irradiation-only control groups had their media simply replaced with fresh media. The control or experimental groups which needed varying or concentrations of ZnPcS
4 PS or CBD alone, had these concentrations added to their fresh media. All culture plates were then re-incubated for an additional 20 h. Then, the PDT experimental and control groups were irradiated in the dark using a Roithner 1000 mA 673 nm high-power semiconductor diode laser, at a fluency of 10 J/cm
2 for average time of 16 min and 8 sec [
16]. After irradiation the old cell culture media from all culture plates was removed and replaced, following re-incubation of all culture plates for an additional 24 h before biochemical assessments.
4.3. Chemicals
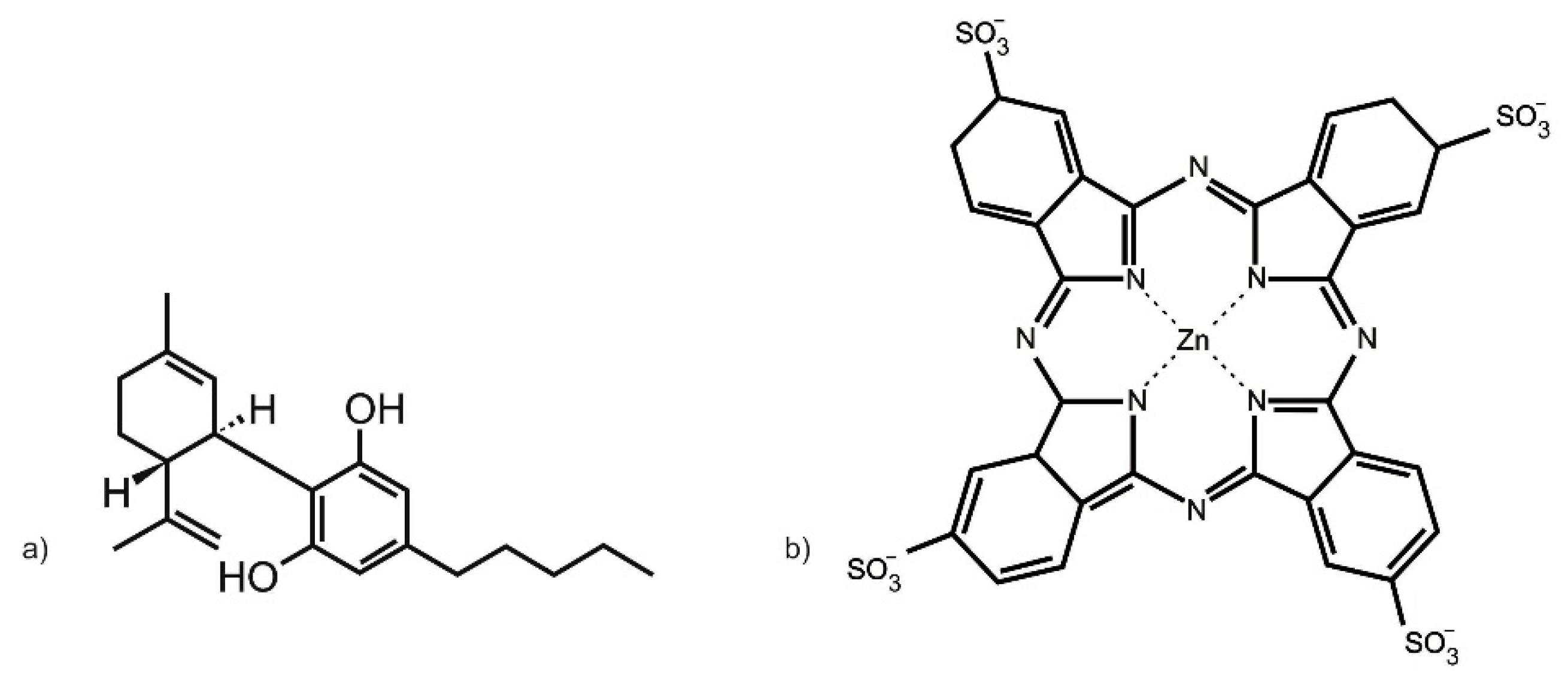
The zinc (II) phthalocyanine tetrasulfonic acid (ZnPcS
4) PS used in this study was purchased from Santa Cruz
® Biotechnology (Dallas, TX, USA) (sc-264509A, molecular weight 898.15 g/mol) (
Figure 9a). ZnPcS
4 powder was solubilized in 0.001 M phosphate buffered saline (PBS). It exhibits three major Q-bands at emissions of 583, 634, and 674 nm, within the far-red spectral range, considered ideal for primary PDT cancer treatment [
39]. To make a working stock concentration of 125 µM, 1 mL of 0.0005 M ZnPcS
4 PS stock solution was diluted with 4 mL of 0.001 M PBS. This working stock concentration of ZnPcS
4 PS was then further diluted in complete cell culture media, to acquire varying dose concentrations (0.0625, 0.125, 0.25, 0.5, and 1 µM), which were utilized within the below discussed assays [
34].
CBD was administered to CC cells pre-PDT treatment. The 10 mg/mL CBD solution solubilized in 1 mL 99.8% ethanol, with a molecular mass of 314.46 g/mol, was commercially obtained from Sigma-Aldrich (St. Louis, MO, USA) (90899, 1 mL) (
Figure 9b). The CBD was diluted with 19 mL of 99.8% of ethanol to make a stock concentration of 0.5 mg/mL. CBD working stock solution was stored in the dark in a refrigerator. This working stock concentration of CBD was then further diluted in complete cell culture media to acquire varying dose concentrations (0.3, 0.5, 0.7, 0.9, and 1.1 µM), which were utilized within the below-discussed assays [
35].
4.4. Individual ZnPcS4 PS PDT or CBD Irradiation LDH Cytoxicity Dose Response Assays
Individual dose response studies were performed using the inhibitory concentration dose (ICD) method to determine the lowest concentration dose of ZnPcS4 PS or CBD alone that could yield 50% cytotoxicity (ICD50), so that cellular viability could be ensured post-experimentation within combinative experimentation, to allow for biological result interpretations.
Following 4 h incubation, HeLa or WS1 cell control or experimental culture plate groups which required ZnPcS4 PS in varying doses received it in its diluted form through their culture media. Four hours post incubation, control or experimental culture plate groups which required CBD varying doses or ICD50 concentrations received it in its diluted 99.8% ethanol form through their culture media. The culture plates were then re-incubated in the dark for an additional 20 h, after which the control and experimental groups which required irradiation received it. All culture plates had their media replaced and they were re-incubated again for 24 h before conducting LDH dose response assays.
The CytoTox 96® non-radioactive cytotoxicity assay kit (Promega™ G1780, Madison, WI, USA) was used to quantitatively measure lactate dehydrogenase (LDH), which is a cytosolic enzyme that is released upon cell lysis, that is directly proportional to cellular cytotoxicity. Briefly, 24 h post PDT, 50 μL of complete cell culture media supernatant from each experimental and control culture plate was removed and mixed with 50 μL of LDH reconstituted substrate mix in flat bottom 96-well microplates. To determine cytotoxicity from the LDH cellular lysis that was produced post treatment, LDH absorbance was measured at 490 nm, using a spectrophotometer (Perkin Elmer, Victor3, 1420 Multilabel Counter, Waltham, MA, USA).
The above dose response assays reported that the ICD50 post PDT for ZnPcS4 PS and CBD was found to be 0.125 µM and 0.5 µM, respectively. Thus, 24 h post treatment, both of these concentrations were utilized in the below-combinative subcellular localization, flow cytometry cell death, and cellular proliferation assays, to determine the combined effects that ZnPcS4 PS and CBD had on CC and WS1 post treatment.
4.5. Quantitative Subcellular Localization Immunofluorescent Staining Confirmation of ZnPcS4 PS Uptake
HeLa cells or WS1 cells were detached from cell culture flasks and seeded in culture plates, which had sterile cover slips inserted into their base at previously mentioned densities. After 4 h incubation to allow for cellular attachment of the coverslips, the complete growth medium from the culture plates was replaced. The culture plates were then divided into various experimental and control groups, and received varying volumes of complete cell culture media and pre-determined ICD50 0.125 µM ZnPcS4 PS and/or 0.5 µM CBD to accommodate for the required combinative PDT assays when both were applied in combination. The culture plates were then re-incubated in the dark for an additional 20 h.
All experimentation was conducted in the dark, to ensure that the PS or fluorochromes used did not photo-bleach. Following incubation, the complete culture media in all culture plates were discarded, then placed on ice and rinsed with 1 mL ice-cold 0.01 M PBS. Then, 1 mL of 3.7% (v/v) formaldehyde solution was added for 10 min to allow for cellular fixation to the coverslips. Thereafter, 1 mL of rinsing solution consisting of ice-cold 0.01 M PBS in 1% (w/v) bovine serum albumin (BSA) and 0.01% (w/v) sodium azide buffer solution was briefly added and then discarded. The cells were then stained with 200 µL diluted primary antibody (2 µg/mL ICAM-1 mouse monoclonal IgG1: AbAB2213 AC Abcam) for 30 min on ice and washed with rinsing solution. Then, the cells were stained with 200 µL diluted secondary antibody (5 µg/mL goat anti-mouse IgG-FITC: AB6785 AC Abcam) for 30 min on ice and washed with rinsing solution. Next, 50 µL of 1 µg/mL 40-6-Diamidino-2-phenylindole (DAPI) was added, and the culture plates were incubated for 5 min at room temperature, after which they were rinsed with 1 mL HBSS. The coverslips were then removed from the culture plates and attached to glass microscope slides with 50 µL of mounting medium. The slides were examined using the filter settings of a Carl Zeiss Axio Z1 Observer immunofluorescent microscope at 40× magnification.
The 358Ex/461Em filter was used to detect blue-DAPI-counter-stained nuclei in cultured cells, while the 495Ex/519Em filter was used to detect any green-FITC-stained ICAM-1 membrane proteins in cultured cells. ICAM-1 proteins are cellular surface membrane markers that are expressed within almost all in-vitro-adherent cells [
54]. Thus, ICAM-1 proteins may be used to indirectly (when conjugated to a fluorochrome, such as FITC) counter-stain in-vitro-cultured cells membranes, to allow for identification and subcellular location of internal cellular contents when conducting immunofluorescent microscopy experiments. Lastly, the 589Ex/610Em filter was used to detect any Cy5 red fluorescent signals that were produced by the ZnPcS
4 PS within the various control and experimental groups, to determine if the PS was capable of subcellular localization in HeLa cells only, and whether it had no effective uptake in WS1 cells when combined with CBD.
4.6. Flow Cytometry Cell Death Pathway Analysis of ZnPcS4 PS and CBD PDT/Irradiation Combinative Assays
The Annexin V-FITC/PI cell death detection kit (BD Pharmingen™ Scientific: BD/556570) was used for the detection and quantitation of cells undergoing early or late apoptosis, cells dying from necrosis cells, or cells remaining viable within combinative ZnPcS4 PS and CBD PDT response assays 24 h post irradiation, using a BD Accuri™ C6 flow cytometer. All experimentation instructions and controls for gating were included as per the manufacturers’ protocol recommendations.
Briefly, with reference to
Table S1 cellular suspensions were centrifuged in microcentrifuge tubes at 2200 rpm for 4 min at 20 °C. Their supernatants and cell pellets were re-suspended in 1× (
v/
v) binding buffer to a 1 × 10
5 cells/mL. The samples were centrifuged, and 100 µL of each cellular suspension was stained with 5 µL of Annexin V-FITC solution and 5 µL of reconstituted propidium iodide staining solution. Before the acquisition, 400 μL of binding buffer 1× was added. For each sample, 20,000 events were acquired using a BD Accuri™ C6 flow cytometer, with a 488 nm solid-state laser (40 mW), and optimal photomultiplier (PMT) voltages were established for each channel. The matching BD Accuri C6 Plus Annexin V-FITC/PI software kit template was used for data acquisition. Debris and doublets were excluded from the analysis. Early and late apoptotic cells were identified for their positivity to Annexin V, and necrotic cells were identified for their positivity to PI, as well as live cells being identified as per non-stain controls, as shown in the FACS gating strategy represented in
Supplementary Material Figure S1. The FlowJo v 10.8.1 Software (BD Biosciences) was used for data analysis. To obtain comparable results, prior to sample analyses, all quality control procedures and calibrations were performed on the flow cytometer using BD Pharmingen™ Scientific Flow Maintenance and Flow Starter Kits, with tracking beads.
4.7. Adenosine Triphosphate (ATP) Cell Proliferation Analysis of ZnPcS4 PS and CBD PDT/Irradiation Combinative Assays
The CellTiter-Glo® luminescent cell viability assay (Promega™ PRG7571) was used to determine the number of metabolically active cells which can generate adenosine triphosphate (ATP) post irradiation. The assay utilizes the properties of a commercial thermostable luciferase, which generates a luminescent signal proportional to the amount of ATP released upon cell lysis, and so signifies their overall viability and abilities to recover/proliferate post treatment.
The cellular proliferation of HeLa and WS1 cells post-irradiation for the various control and experimental groups of combinative ZnPcS4 PS and CBD PDT response assays was measured to determine the combinative anti-proliferative effects this treatment had on HeLa, as well as determine if there were any unwanted side effects on normal cells.
Briefly, 100 μL of reconstituted reagent was added to an equivalent volume of cell culture plates cell suspension post treatment. The contents were then pipetted into a 96-well white-walled microplate, and stirred together on a shaker for 2 min to induce cell lysis. The microplate was then incubated at room temperature for 10 min to allow for the luminescent signal to stabilize. The luminescent signal was determined in relative light units (RLUs) using a Perkin Elmer, Victor3, multilabel high-performance multiplate counter (model 1420). The amount of ATP detected was quantified in direct proportion to the number of proliferating cells post treatment.
4.8. Statistical Analysis
Experimental assays were all performed in triplicate and repeated over three different days, and an average of these results was reported. Raw data were captured using Microsoft excel sheets and statistical analysis was done using the GraphPad Prism version 9 software to determine the mean, standard deviation (SD), standard error, and significant changes for the data when comparing the various control and experimental groups. The Student t-test and one-way analysis of variance (ANOVA) were used for comparing two or more means of normally distributed data, whereas the Mann–Whitney test was used for non-normally distributed data to determine the statistical difference between the various control and experimental groups. The error bars on graphs display the mean standard error (SEM), and if an experimental or control group reported a SD higher than 1 within a sample repeat, it was repeated to ensure data accuracy and precise statistical comparisons. Statistical differences between the various control and experimental groups were determined. Values within the 95% confidence interval (p < 0.05*, p < 0.01** or p < 0.001***) were accepted as statistically different and represented graphically.