Genetic Networks of Alzheimer’s Disease, Aging, and Longevity in Humans
Abstract
1. Introduction
2. Results
2.1. AD Genes
2.2. AR Genes
2.3. Longevity Genes
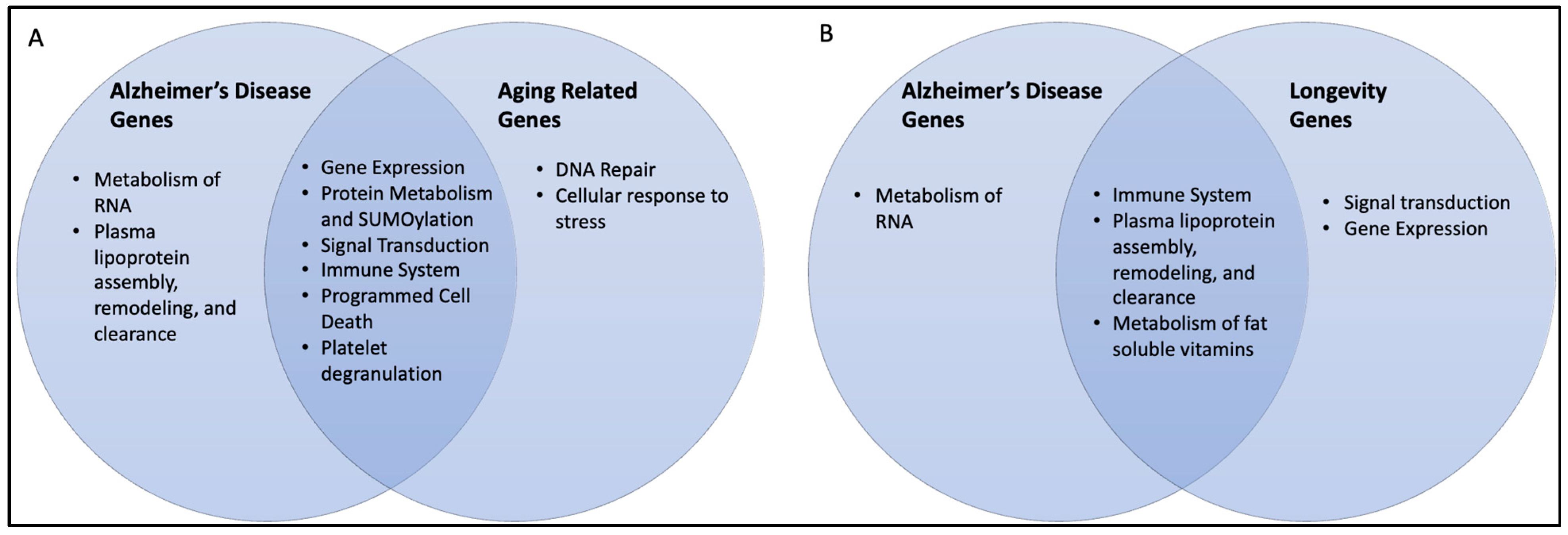
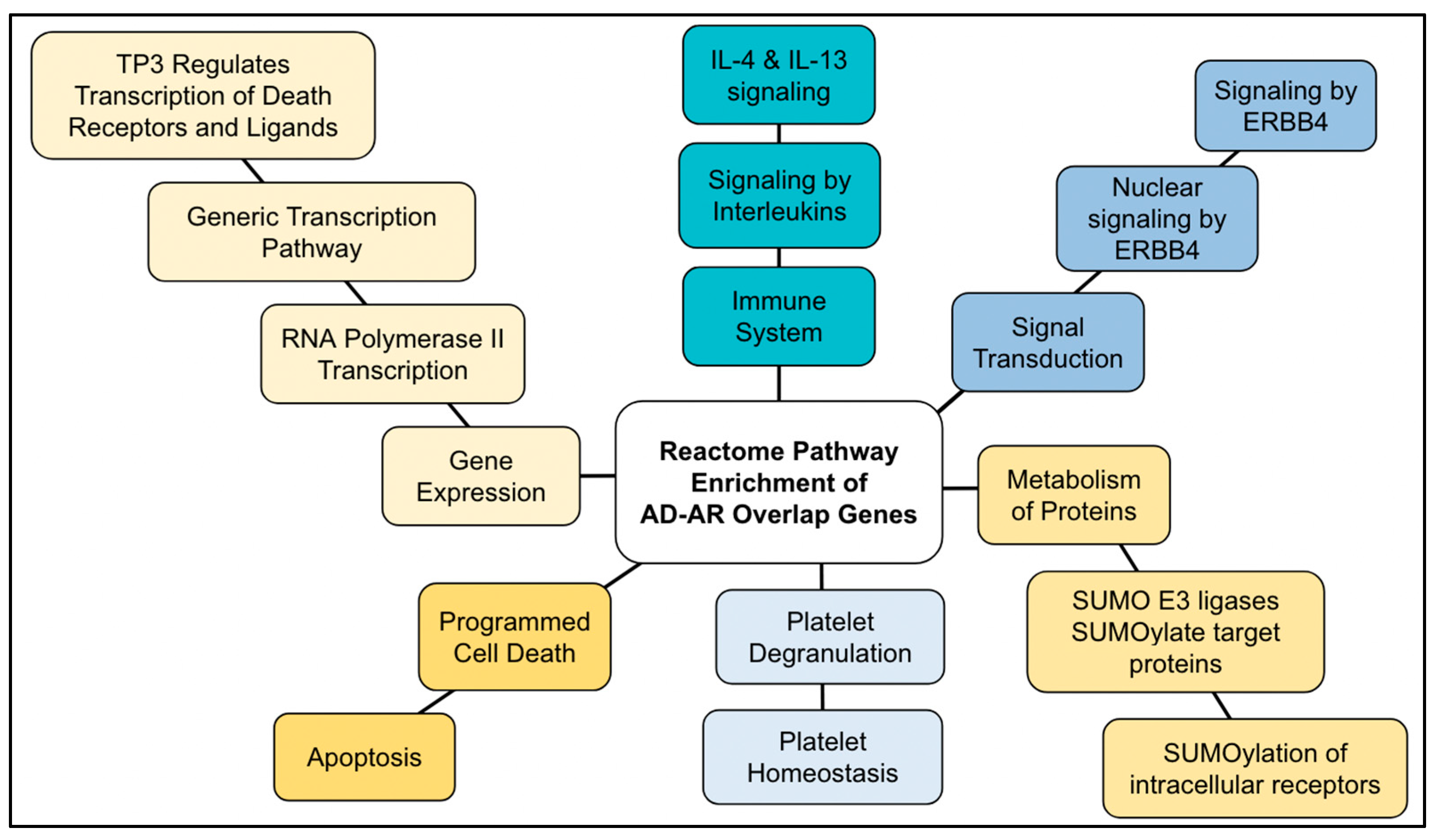
2.4. AD–AR Overlap Genes
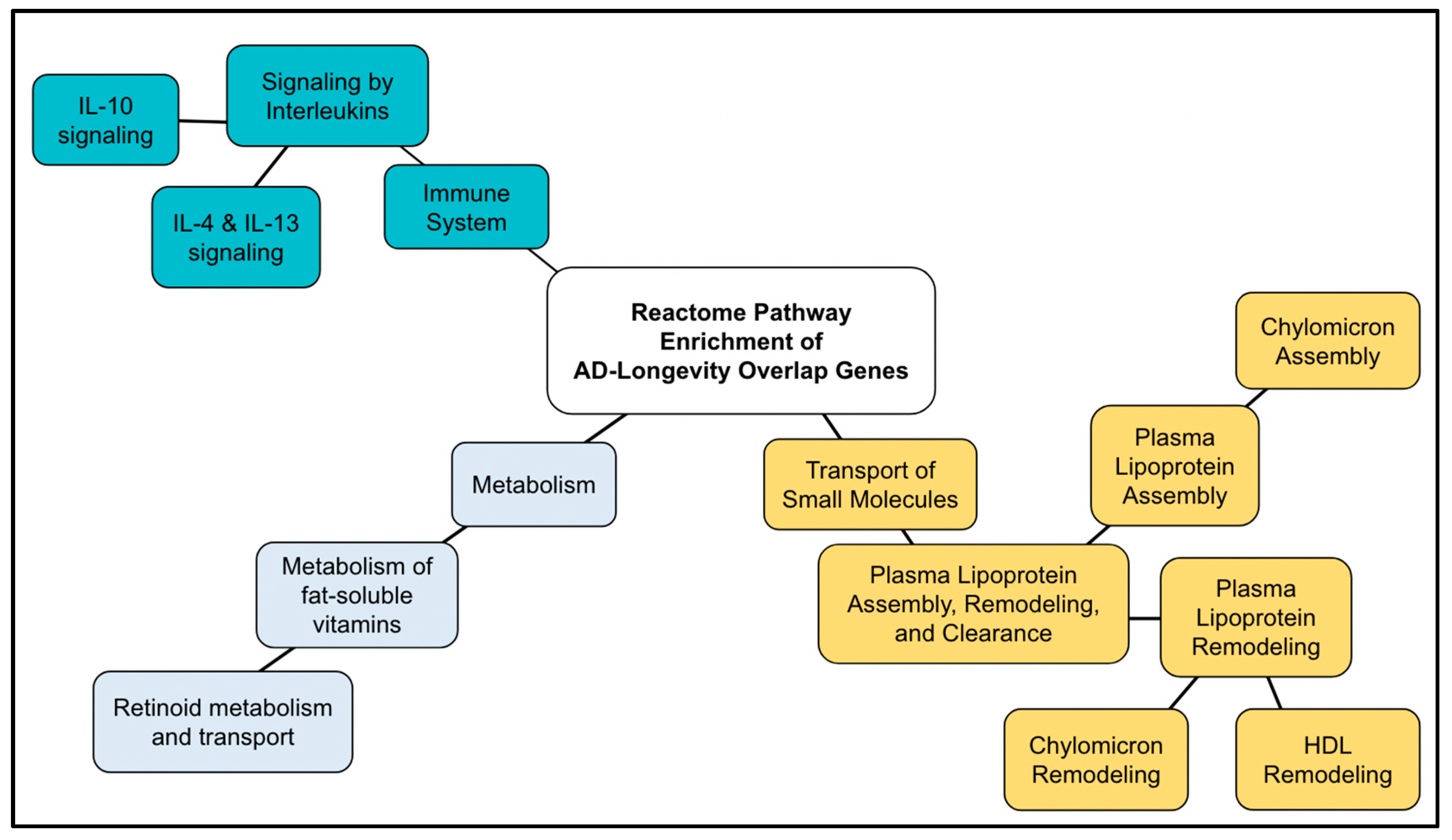
2.5. AD–Longevity Overlap Genes
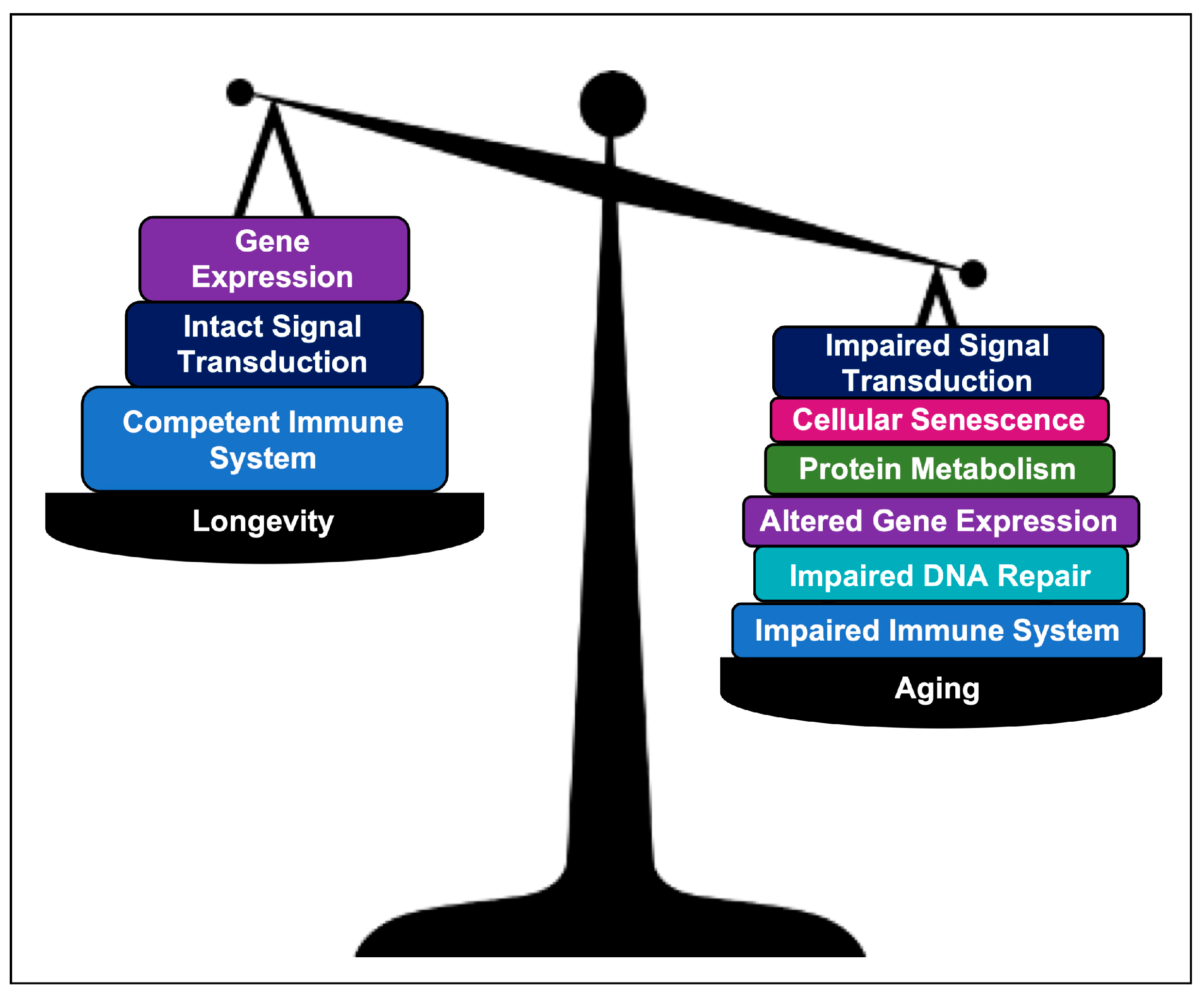
3. Discussion
3.1. AD Genes
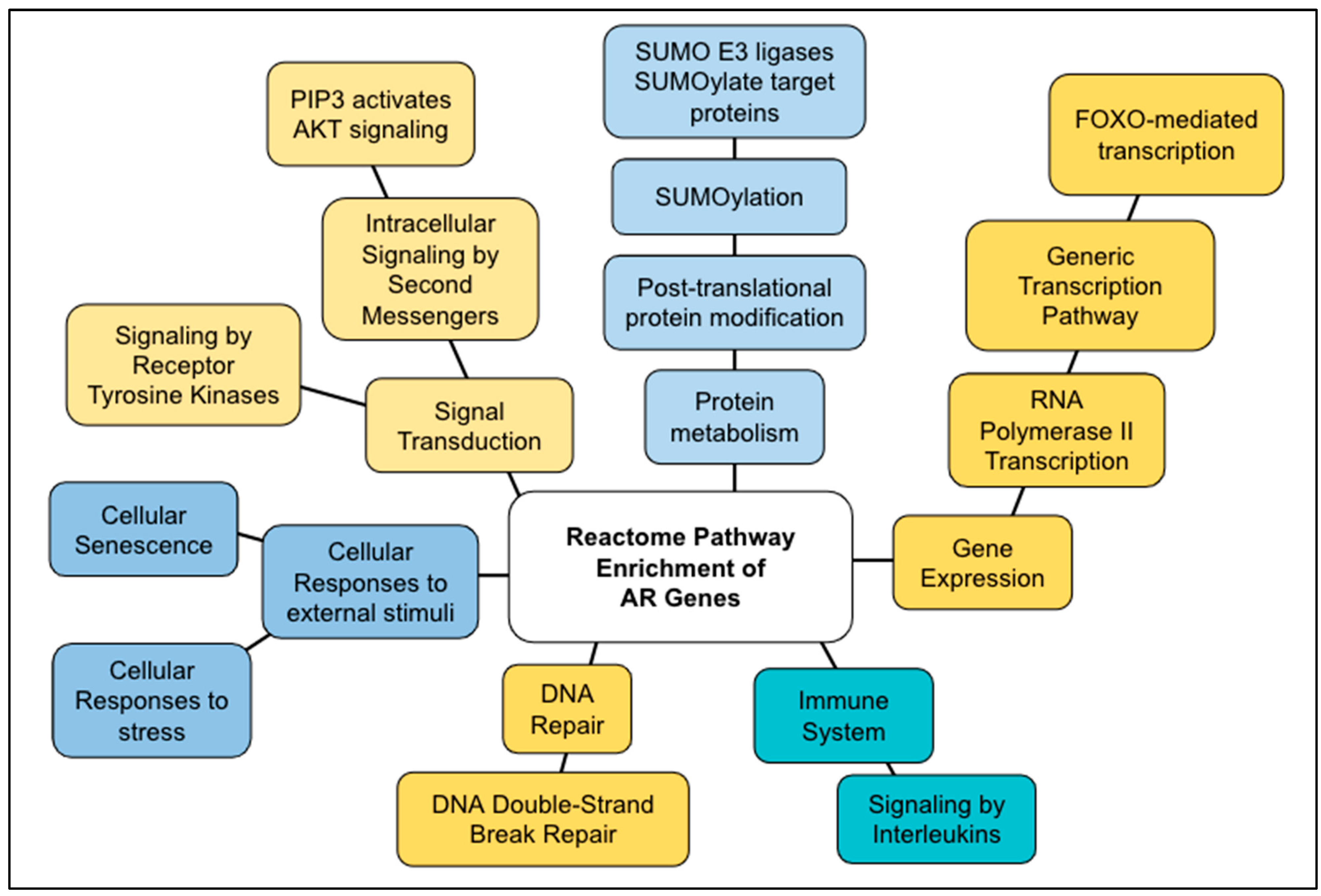
3.2. AR Genes
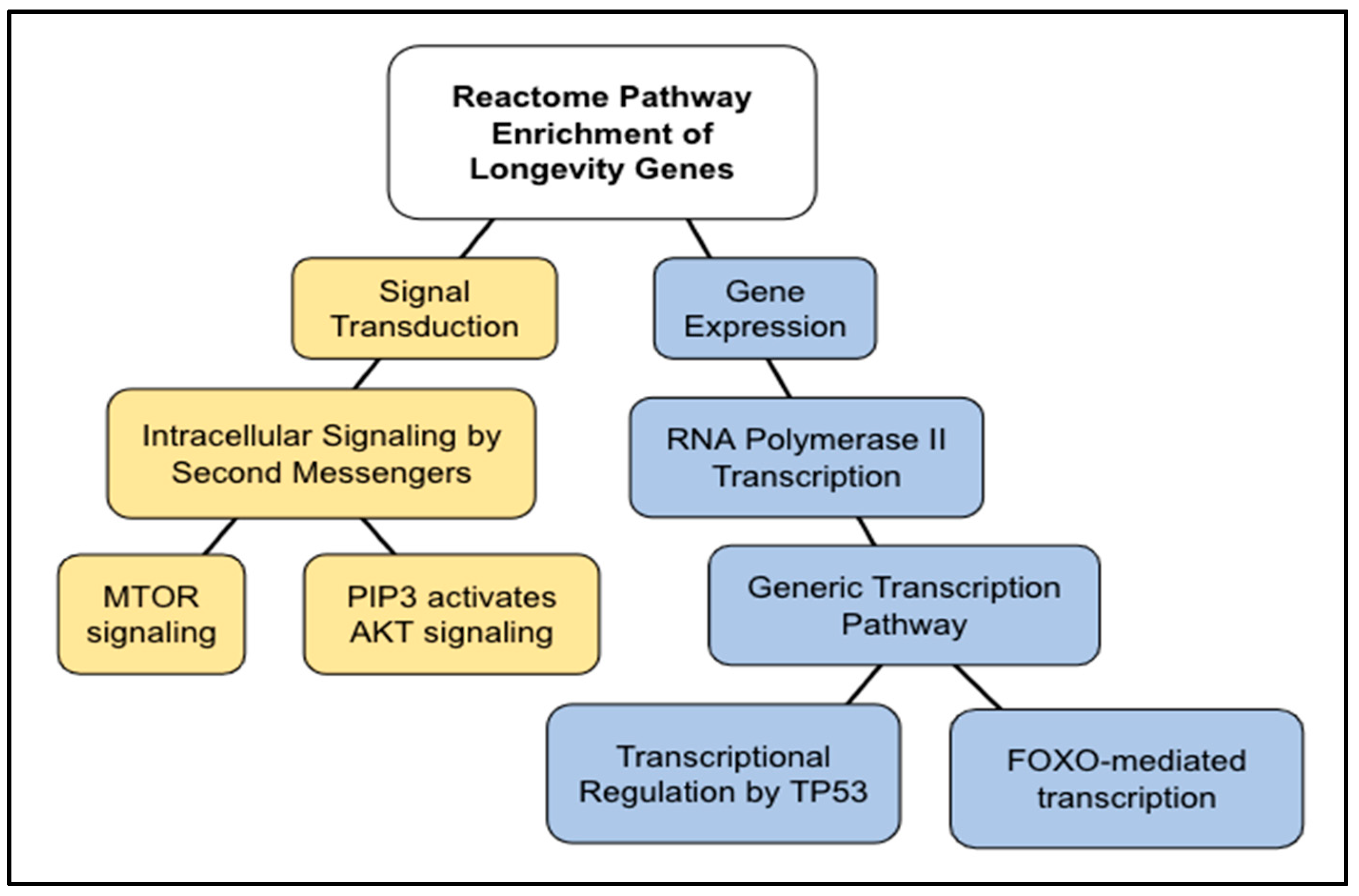
3.3. Longevity Genes
3.4. Comparing AR Pathways with Longevity Pathways
3.5. AD–AR Overlap Genes
3.6. AD–longevity Overlap Genes
3.7. Technical Advantages and Limitations
4. Materials and Methods
4.1. Datasets
4.2. Gene Ontology: Reactome Analysis
5. Conclusions and Future Direction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthews, K.A.; Xu, W.; Gaglioti, A.H.; Holt, J.B.; Croft, J.B.; Mack, D.; McGuire, L.C. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimer’s Dement. 2019, 15, 17–24. [Google Scholar] [CrossRef]
- Goate, A.; Chartier-Harlin, M.-C.; Mullan, M.; Brown, J.; Crawford, F.; Fidani, L.; Giuffra, L.; Haynes, A.; Irving, N.; James, L.; et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 1991, 349, 704–706. [Google Scholar] [CrossRef]
- Sherrington, R.; Rogaev, E.I.; Liang, Y.; Rogaeva, E.A.; Levesque, G.; Ikeda, M.; Chi, H.; Lin, C.; Li, G.; Holman, K.; et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 1995, 375, 754–760. [Google Scholar] [CrossRef]
- Rogaev, E.I.; Sherrington, R.; Rogaeva, E.A.; Levesque, G.; Ikeda, M.; Liang, Y.; Chi, H.; Lin, C.; Holman, K.; Tsuda, T.; et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature 1995, 376, 775–778. [Google Scholar] [CrossRef]
- Sherva, S.; Kowall, N.W. Genetics of Alzheimer Disease. 2022. Available online: https://www-uptodate-com.eu1.proxy.openathens.net (accessed on 6 January 2023).
- Harold, D.; Abraham, R.; Hollingworth, P.; Sims, R.; Gerrish, A.; Hamshere, M.L.; Pahwa, J.S.; Moskvina, V.; Dowzell, K.; Williams, A.; et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 2009, 41, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Vahdati Nia, B.; Kang, C.; Tran, M.G.; Lee, D.; Murakami, S. Meta Analysis of Human AlzGene Database: Benefits and Limitations of Using C. elegans for the Study of Alzheimer’s Disease and Co-morbid Conditions. Front. Genet. 2017, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Lacayo, P. Biological and disease hallmarks of Alzheimer’s disease defined by Alzheimer’s disease genes. Front Aging Neurosci. 2022, 14, 996030. [Google Scholar] [CrossRef]
- Mooser, V.; Helbecque, N.; Miklossy, J.; Marcovina, S.M.; Nicod, P.; Amouyel, P. Interactions between Apolipoprotein E and Apolipoprotein(a) in Patients with Late-Onset Alzheimer Disease. Ann. Intern. Med. 2000, 132, 533–537. [Google Scholar] [CrossRef]
- Sebastiani, P.; Gurinovich, A.; Nygaard, M.; Sasaki, T.; Sweigart, B.; Bae, H.; Andersen, S.L.; Villa, F.; Atzmon, G.; Christensen, K.; et al. APOE Alleles and Extreme Human Longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Johnson, T.E.; Vaupel, J.W. The quest for genetic determinants of human longevity: Challenges and insights. Nat. Rev. Genet. 2006, 7, 436–448. [Google Scholar] [CrossRef]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R.; et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef]
- Milacic, M.; Haw, R.; Rothfels, K.; Wu, G.; Croft, D.; Hermjakob, H.; D’Eustachio, P.; Stein, L. Annotating Cancer Variants and Anti-Cancer Therapeutics in Reactome. Cancers 2012, 4, 1180–1211. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018, 46, D649–D655. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Bertram, L.; McQueen, M.B.; Mullin, K.; Blacker, D.; E Tanzi, R. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat. Genet. 2007, 39, 17–23. [Google Scholar] [CrossRef] [PubMed]
- de Magalhães, J.P.; Curado, J.; Church, G.M. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics 2009, 25, 875–881. [Google Scholar] [CrossRef]
- de Magalhães, J.P.; Toussaint, O. GenAge: A genomic and proteomic network map of human ageing. FEBS Lett. 2004, 571, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Budovsky, A.; Craig, T.; Wang, J.; Tacutu, R.; Csordas, A.; Lourenço, J.; Fraifeld, V.E.; de Magalhães, J.P. LongevityMap: A database of human genetic variants associated with longevity. Trends Genet. 2013, 29, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Willcox, B.J.; Donlon, T.A.; He, Q.; Chen, R.; Grove, J.S.; Yano, K.; Masaki, K.H.; Willcox, D.C.; Rodriguez, B.; Curb, J.D. FOXO3A genotype is strongly associated with human longevity. Proc. Natl. Acad. Sci. USA 2008, 105, 13987–13992. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Giegé, T.; Giegé, R.; Giegé, P. tRNA Biology in Mitochondria. Int. J. Mol. Sci. 2015, 16, 4518–4559. [Google Scholar] [CrossRef] [PubMed]
- Van Haute, L.; Pearce, S.F.; Powell, C.A.; D’Souza, A.R.; Nicholls, T.J.; Minczuk, M. Mitochondrial transcript maturation and its disorders. J. Inherit. Metab. Dis. 2015, 38, 655–680. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 62, 1403–1416. [Google Scholar] [CrossRef]
- Del Giudice, L.; Alifano, P.; Calcagnile, M.; Di Schiavi, E.; Bertapelle, C.; Aletta, M.; Pontieri, P. Mitochondrial ribosomal protein genes connected with Alzheimer’s and tellurite toxicity. Mitochondrion 2022, 64, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Piras, I.S.; Krate, J.; Delvaux, E.; Nolz, J.; Mastroeni, D.F.; Persico, A.M.; Jepsen, W.M.; Beach, T.G.; Huentelman, M.J.; Coleman, P.D. Transcriptome Changes in the Alzheimer’s Disease Middle Temporal Gyrus: Importance of RNA Metabolism and Mitochondria-Associated Membrane Genes. J. Alzheimer’s Dis. 2019, 70, 691–713. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Meng, Q.; De Acha, O.P.; Mustapic, M.; Cheng, A.; Eren, E.; Kundu, G.; Piao, Y.; Munk, R.; Wood, W.H., 3rd; et al. Mitochondrial RNA in Alzheimer’s Disease Circulating Extracellular Vesicles. Front. Cell Dev. Biol. 2020, 8, 581882. [Google Scholar] [CrossRef]
- Nagao, A.; Suzuki, T. Human Mitochondrial tRNAs: Biogenesis, Function, Structural Aspects, and Diseases. Annu. Rev. Genet. 2011, 45, 299–329. [Google Scholar] [CrossRef]
- Sarin, L.P.; A Leidel, S. Modify or die?—RNA modification defects in metazoans. RNA Biol. 2014, 11, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C.; Lott, M.T.; Shoffner, J.M.; Brown, M.D. Diseases resulting from mitochondrial DNA point mutations. J. Inherit. Metab. Dis. 1992, 15, 472–479. [Google Scholar] [CrossRef]
- Kirby, D.M.; McFarland, R.; Ohtake, A.; Dunning, C.; Ryan, M.T.; Wilson, C.; Ketteridge, D.; Turnbull, D.M.; Thorburn, D.R.; Taylor, R.W. Mutations of the mitochondrial ND1 gene as a cause of MELAS. J. Med. Genet. 2004, 41, 784–789. [Google Scholar] [CrossRef]
- Millan, M.J. Linking deregulation of non-coding RNA to the core pathophysiology of Alzheimer’s disease: An integrative review. Prog. Neurobiol. 2017, 156, 1–68. [Google Scholar] [CrossRef]
- Mathew, B.A.; Katta, M.; Ludhiadch, A.; Singh, P.; Munshi, A. Role of tRNA-Derived Fragments in Neurological Disorders: A Review. Mol. Neurobiol. 2023, 60, 655–671. [Google Scholar] [CrossRef]
- Gorbunova, V.; Seluanov, A. DNA double strand break repair, aging and the chromatin connection. Mutat. Res. 2016, 788, 2–6. [Google Scholar] [CrossRef]
- Golmard, L.; Delnatte, C.; Laugé, A.; Moncoutier, V.; Lefol, C.; Abidallah, K.; Tenreiro, H.; Copigny, F.; Giraudeau, M.; Guy, C.; et al. Breast and ovarian cancer predisposition due to de novo BRCA1 and BRCA2 mutations. Oncogene 2016, 35, 1324–1327. [Google Scholar] [CrossRef]
- Masutani, C.; Kusumoto, R.; Yamada, A.; Dohmae, N.; Yokoi, M.; Yuasa, M.; Araki, M.; Iwai, S.; Takio, K.; Hanaoka, F. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase η. Nature 1999, 399, 700–704. [Google Scholar] [CrossRef]
- Martens, M.C.; Emmert, S.; Boeckmann, L. Sunlight, Vitamin D, and Xeroderma Pigmentosum. Adv. Exp. Med. Biol. 2020, 1268, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Yokote, K.; Chanprasert, S.; Lee, L.; Eirich, K.; Takemoto, M.; Watanabe, A.; Koizumi, N.; Lessel, D.; Mori, T.; Hisama, F.M.; et al. WRNMutation Update: Mutation Spectrum, Patient Registries, and Translational Prospects. Hum. Mutat. 2017, 38, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Alter, B.P.; Rosenberg, P.S.; Brody, L.C. Clinical and molecular features associated with biallelic mutations in FANCD1/BRCA2. J. Med Genet. 2007, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.R.; Rothblum-Oviatt, C.; Ellis, N.A.; Hickson, I.D.; Meyer, S.; Crawford, T.O.; Smogorzewska, A.; Pietrucha, B.; Weemaes, C.; Stewart, G.S. Chromosome instability syndromes. Nat. Rev. Dis. Prim. 2019, 5, 64. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Calcinotto, A.; Kohli, J.; Zagato, E.; Pellegrini, L.; Demaria, M.; Alimonti, A. Cellular Senescence: Aging, Cancer, and Injury. Physiol. Rev. 2019, 99, 1047–1078. [Google Scholar] [CrossRef]
- Ou, H.; Hoffmann, R.; González-López, C.; Doherty, G.J.; Korkola, J.E.; Muñoz-Espín, D. Cellular senescence in cancer: From mechanisms to detection. Mol. Oncol. 2021, 15, 2634–2671. [Google Scholar] [CrossRef]
- Murakami, S.; Johnson, T.E. A Genetic Pathway Conferring Life Extension and Resistance to UV Stress in Caenorhabditis elegans. Genetics 1996, 143, 1207–1218. [Google Scholar] [CrossRef]
- Johnson, T.E.; Henderson, S.; Murakami, S.; de Castro, E.; de Castro, S.H.; Cypser, J.; Rikke, B.; Tedesco, P.; Link, C. Longevity genes in the nematode Caenorhabditis elegans also mediate increased resistance to stress and prevent disease. J. Inherit. Metab. Dis. 2002, 25, 197–206. [Google Scholar] [CrossRef]
- Murakami, S.; Salmon, A.; Miller, R.A. Multiplex stress resistance in cells from long-lived dwarf mice. FASEB J. 2003, 17, 1565–1576. [Google Scholar] [CrossRef]
- Salmon, A.B.; Murakami, S.; Bartke, A.; Kopchick, J.; Yasumura, K.; Miller, R.A. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am. J. Physiol. Metab. 2005, 289, E23–E29. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S. Stress resistance in long-lived mouse models. Exp. Gerontol. 2006, 41, 1014–1019. [Google Scholar] [CrossRef]
- Min, K.-J.; Tatar, M. Unraveling the Molecular Mechanism of Immunosenescence in Drosophila. Int. J. Mol. Sci. 2018, 19, 2472. [Google Scholar] [CrossRef]
- Vitale, G.; Pellegrino, G.; Vollery, M.; Hofland, L.J. ROLE of IGF-1 System in the Modulation of Longevity: Controversies and New Insights from a Centenarians’ Perspective. Front. Endocrinol. 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Finkel, T. Key proteins and pathways that regulate lifespan. J. Biol. Chem. 2017, 292, 6452–6460. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Lithgow, G.J.; Link, W. Long live FOXO: Unraveling the role of FOXO proteins in aging and longevity. Aging Cell 2016, 15, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Lin, M.; Wu, R. The Regulation of Aging and Longevity: A New and Complex Role of p53. Genes Cancer 2011, 2, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Farmer, K.M.; Ghag, G.; Puangmalai, N.; Montalbano, M.; Bhatt, N.; Kayed, R. P53 aggregation, interactions with tau, and impaired DNA damage response in Alzheimer’s disease. Acta Neuropathol. Commun. 2020, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Abate, G.; Frisoni, G.B.; Bourdon, J.-C.; Piccirella, S.; Memo, M.; Uberti, D. The pleiotropic role of p53 in functional/dysfunctional neurons: Focus on pathogenesis and diagnosis of Alzheimer’s disease. Alzheimer’s Res. Ther. 2020, 12, 160. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Slade, N.; Hof, P.R.; Šimić, G. The interactions of p53 with tau and Aß as potential therapeutic targets for Alzheimer’s disease. Prog. Neurobiol. 2018, 168, 104–127. [Google Scholar] [CrossRef]
- Clark, J.S.; Kayed, R.; Abate, G.; Uberti, D.; Kinnon, P.; Piccirella, S. Post-translational Modifications of the p53 Protein and the Impact in Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci. 2022, 14, 835288. [Google Scholar] [CrossRef]
- Sola, M.; Magrin, C.; Pedrioli, G.; Pinton, S.; Salvadè, A.; Papin, S.; Paganetti, P. Tau affects P53 function and cell fate during the DNA damage response. Commun. Biol. 2020, 3, 245. [Google Scholar] [CrossRef]
- Cozachenco, D.; Ribeiro, F.C.; Ferreira, S.T. Defective proteostasis in Alzheimer’s disease. Ageing Res. Rev. 2023, 85, 101862. [Google Scholar] [CrossRef]
- Singh, P.P.; Demmitt, B.A.; Nath, R.D.; Brunet, A. The Genetics of Aging: A Vertebrate Perspective. Cell 2019, 177, 200–220. [Google Scholar] [CrossRef]
- Du, S.; Zheng, H. Role of FoxO transcription factors in aging and age-related metabolic and neurodegenerative diseases. Cell Biosci. 2021, 11, 188. [Google Scholar] [CrossRef]
- Du, S.; Maneix, L.; Zhang, Q.; Wan, Y.-W.; Zheng, H. The role of FOXO3 transcription factor in Alzheimer’s disease pathology. Innov. Aging 2019, 3, S842–S843. [Google Scholar] [CrossRef]
- Adlakha, Y.K.; Saini, N. miR-128 exerts pro-apoptotic effect in a p53 transcription-dependent and -independent manner via PUMA-Bak axis. Cell Death Dis. 2013, 4, e542. [Google Scholar] [CrossRef]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A Review: Inflammatory Process in Alzheimer’s Disease, Role of Cytokines. Sci. World J. 2012, 2012, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.P.; Krishnan, R.K.; Weaver, J.A.; Del Aguila, L.F.; Evans, W.J. Human aging is associated with altered TNF-α production during hyperglycemia and hyperinsulinemia. Am. J. Physiol. Metab. 2001, 281, E1137–E1143. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Ki, W.C.; Lee, E.K.; Chung, K.W.; Chung, S.; Lee, B.; Seo, A.Y.; Chung, J.H.; Jung, Y.S.; Im, E.; et al. Redefining Chronic Inflammation in Aging and Age-Related Diseases: Proposal of the Senoinflammation Concept. Aging Dis. 2019, 10, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Newman, A.B. Inflammatory markers in population studies of aging. Ageing Res. Rev. 2011, 10, 319–329. [Google Scholar] [CrossRef]
- Di Bona, D.; Vasto, S.; Capurso, C.; Christiansen, L.; Deiana, L.; Franceschi, C.; Hurme, M.; Mocchegiani, E.; Rea, M.; Lio, D.; et al. Effect of interleukin-6 polymorphisms on human longevity: A systematic review and meta-analysis. Ageing Res. Rev. 2009, 8, 36–42. [Google Scholar] [CrossRef]
- Wei, G.-Z.; Wang, F.; Zhao, Y.-G.; Li, S.-S.; Shi, M.-L.; Gao, K.; Luo, Y.; Tang, W.-R. Association of longevity with TNF-α G308A and IL-6 G174C polymorphic inflammatory biomarkers in Caucasians: A meta-analysis. Z. Gerontol. Geriatr. 2016, 49, 706–713. [Google Scholar] [CrossRef]
- Giuliani, C.; Garagnani, P.; Franceschi, C. Genetics of Human Longevity Within an Eco-Evolutionary Nature-Nurture Framework. Circ. Res. 2018, 123, 745–772. [Google Scholar] [CrossRef]
- Gadani, S.P.; Cronk, J.C.; Norris, G.T.; Kipnis, J. IL-4 in the Brain: A Cytokine to Remember. J. Immunol. 2012, 189, 4213–4219. [Google Scholar] [CrossRef] [PubMed]
- Dugan, B.; Conway, J.; A Duggal, N. Inflammaging as a target for healthy ageing. Age Ageing 2023, 52, afac328. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.S.; Sampson, E.L. Lipid composition of the normal human brain: Gray matter, white matter, and myelin. J. Lipid Res. 1965, 6, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Ke, D.-S.; Chen, J.-Y. Essential fatty acids and human brain. Acta Neurol. Taiwanica 2009, 18, 231–241. [Google Scholar]
- Herbst-Robinson, K.J.; Liu, L.; James, M.; Yao, Y.; Xie, S.X.; Brunden, K.R. Inflammatory Eicosanoids Increase Amyloid Precursor Protein Expression via Activation of Multiple Neuronal Receptors. Sci. Rep. 2015, 5, 18286. [Google Scholar] [CrossRef]
- Biringer, R.G. The Role of Eicosanoids in Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2019, 16, 2560. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.-C.; Bu, G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef]
- Arias-Vásquez, A.; Isaacs, A.; Aulchenko, Y.S.; Hofman, A.; Oostra, B.A.; Breteler, M.; Van Duijn, C.M. The cholesteryl ester transfer protein (CETP) gene and the risk of Alzheimer’s disease. Neurogenetics 2007, 8, 189–193. [Google Scholar] [CrossRef]
- Tuminello, E.R.; Han, S.D. The Apolipoprotein E Antagonistic Pleiotropy Hypothesis: Review and Recommendations. Int. J. Alzheimer’s Dis. 2011, 2011, 726197. [Google Scholar] [CrossRef]
- Matsuura, E.; Hughes, G.R.; A Khamashta, M. Oxidation of LDL and its clinical implication. Autoimmun. Rev. 2008, 7, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, S.; Quintin, J.; Joosten, L.A.; van der Meer, J.W.; Netea, M.G.; Riksen, N.P. Oxidized Low-Density Lipoprotein Induces Long-Term Proinflammatory Cytokine Production and Foam Cell Formation via Epigenetic Reprogramming of Monocytes. Arter. Thromb. Vasc. Biol. 2014, 34, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, J.P.; Major, A.S. How Oxidized Low-Density Lipoprotein Activates Inflammatory Responses. Crit. Rev. Immunol. 2018, 38, 333–342. [Google Scholar] [CrossRef]
- Basseri, S.; Austin, R.C. Endoplasmic Reticulum Stress and Lipid Metabolism: Mechanisms and Therapeutic Potential. Biochem. Res. Int. 2012, 2012, 841362. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kaufman, R.J. The role of ER stress in lipid metabolism and lipotoxicity. J. Lipid Res. 2016, 57, 1329–1338. [Google Scholar] [CrossRef]
- Moncan, M.; Mnich, K.; Blomme, A.; Almanza, A.; Samali, A.; Gorman, A.M. Regulation of lipid metabolism by the unfolded protein response. J. Cell. Mol. Med. 2021, 25, 1359–1370. [Google Scholar] [CrossRef]
- Merighi, A.; Lossi, L. Endoplasmic Reticulum Stress Signaling and Neuronal Cell Death. Int. J. Mol. Sci. 2022, 23, 15186. [Google Scholar] [CrossRef]
- de Magalhães, J.P.; Passos, J.F. Stress, cell senescence and organismal ageing. Mech. Ageing Dev. 2018, 170, 2–9. [Google Scholar] [CrossRef]
- Young, A.R.; Narita, M. SASP reflects senescence. EMBO Rep. 2009, 10, 228–230. [Google Scholar] [CrossRef]
- Pezone, A.; Olivieri, F.; Napoli, M.V.; Procopio, A.; Avvedimento, E.V.; Gabrielli, A. Inflammation and DNA damage: Cause, effect or both. Nat. Rev. Rheumatol. 2023. [Google Scholar] [CrossRef]
- Wiley, C.D.; Campisi, J. The metabolic roots of senescence: Mechanisms and opportunities for intervention. Nat. Metab. 2021, 3, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Hamsanathan, S.; Gurkar, A.U. Lipids as Regulators of Cellular Senescence. Front. Physiol. 2022, 13, 796850. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.; Del Pilar Sosa Idelchik, M.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef]
- Miller, B.F.; Seals, D.R.; Hamilton, K.L. A viewpoint on considering physiological principles to study stress resistance and resilience with aging. Ageing Res. Rev. 2017, 38, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dato, S.; Crocco, P.; De Rango, F.; Iannone, F.; Maletta, R.; Bruni, A.C.; Saiardi, A.; Rose, G.; Passarino, G. IP6K3 and IPMK variations in LOAD and longevity: Evidence for a multifaceted signaling network at the crossroad between neurodegeneration and survival. Mech. Ageing Dev. 2021, 195, 111439. [Google Scholar] [CrossRef]
- Mackay, T.F.C. Epistasis and quantitative traits: Using model organisms to study gene–gene interactions. Nat. Rev. Genet. 2014, 15, 22–33. [Google Scholar] [CrossRef]
- Mackay, T.F.; Moore, J.H. Why epistasis is important for tackling complex human disease genetics. Genome Med. 2014, 6, 124, Erratum in Genome Med. 2015, 7, 85. [Google Scholar] [CrossRef]
- Wolfson, M.; Budovsky, A.; Tacutu, R.; Fraifeld, V. The signaling hubs at the crossroad of longevity and age-related disease networks. Int. J. Biochem. Cell Biol. 2009, 41, 516–520. [Google Scholar] [CrossRef]
- Li, L.; Murakami, S. Artificial Intelligence-Assisted Meta-Analysis of the Frequency of ACE I/D Polymorphisms in Centenarians and Other Long-Lived Individuals. Int. J. Mol. Sci. 2023, 24, 3411. [Google Scholar] [CrossRef]
- Olgiati, P.; Politis, A.M.; Papadimitriou, G.N.; De Ronchi, D.; Serretti, A. Genetics of Late-Onset Alzheimer’s Disease: Update from the Alzgene Database and Analysis of Shared Pathways. Int. J. Alzheimer’s Dis. 2011, 2011, 832379. [Google Scholar] [CrossRef]
- Tacutu, R.; Thornton, D.; Johnson, E.; Budovsky, A.; Barardo, D.; Craig, T.; Diana, E.; Lehmann, G.; Toren, D.; Wang, J.; et al. Human Ageing Genomic Resources: New and updated databases. Nucleic Acids Res. 2018, 46, D1083–D1090. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Haw, R. Functional Interaction Network Construction and Analysis for Disease Discovery. In Protein Bioinformatics; Wu, C., Arighi, C., Ross, K., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1558, pp. 235–253. [Google Scholar] [CrossRef]
- Le, D.; Brown, L.; Malik, K.; Murakami, S. Two Opposing Functions of Angiotensin-Converting Enzyme (ACE) That Links Hypertension, Dementia, and Aging. Int. J. Mol. Sci. 2021, 22, 13178. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.M.; Holly, J.M.P.; Biernacka, K.M.; Allen, S.; Perks, C. Mini Review: Opposing Pathologies in Cancer and Alzheimer’s Disease: Does the PI3K/Akt Pathway Provide Clues? Front. Endocrinol. 2020, 11, 403. [Google Scholar] [CrossRef]
- Murakami, S. Age-dependent modulation of learning and memory in C. elegans. In Invertebrate Learning and Memory; Handbook of Behavioral Neuroscience; Menzel, R., Benjamin, P.R., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 140–150. [Google Scholar] [CrossRef]
- Murakami, S.; Cabana, K.; Anderson, D. Current advances in the study of oxidative stress and age-related memory impairment in C. elegans. In Molecular Aspects of Oxidative Stress on Cell Signaling in Vertebrates and Invertebrates; Farooqui, T., Farooqui, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 347–360. [Google Scholar] [CrossRef]
- Murakami, S.; Halperin, A. Alzheimer’s patient feedback to complement research using model systems for cognitive aging and dementia. Front. Genet. 2014, 5, 269. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S. Editorial: Biology of Cognitive Aging: Model Systems, Technologies, and Beyond. Front. Genet. 2016, 6, 366. [Google Scholar] [CrossRef]

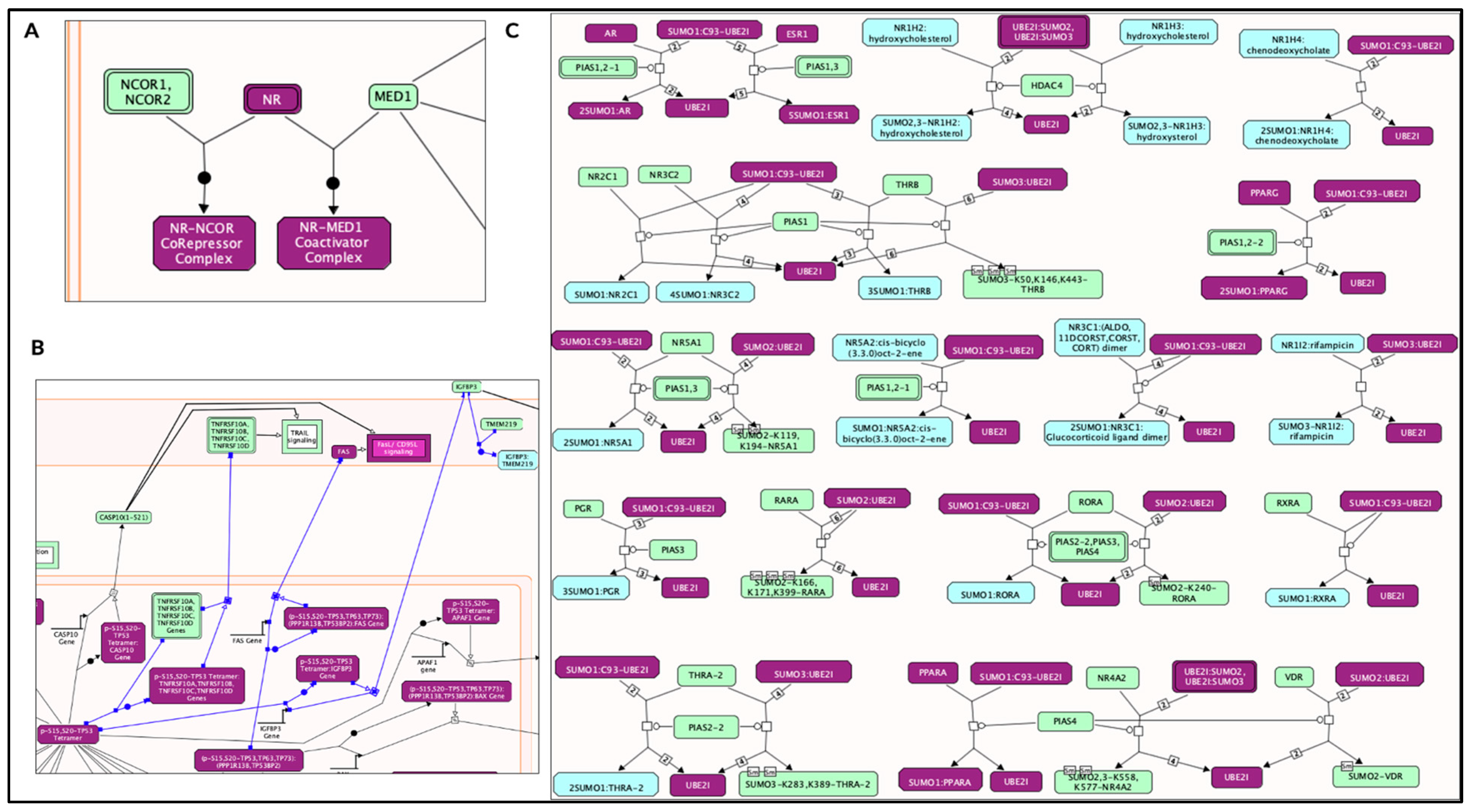
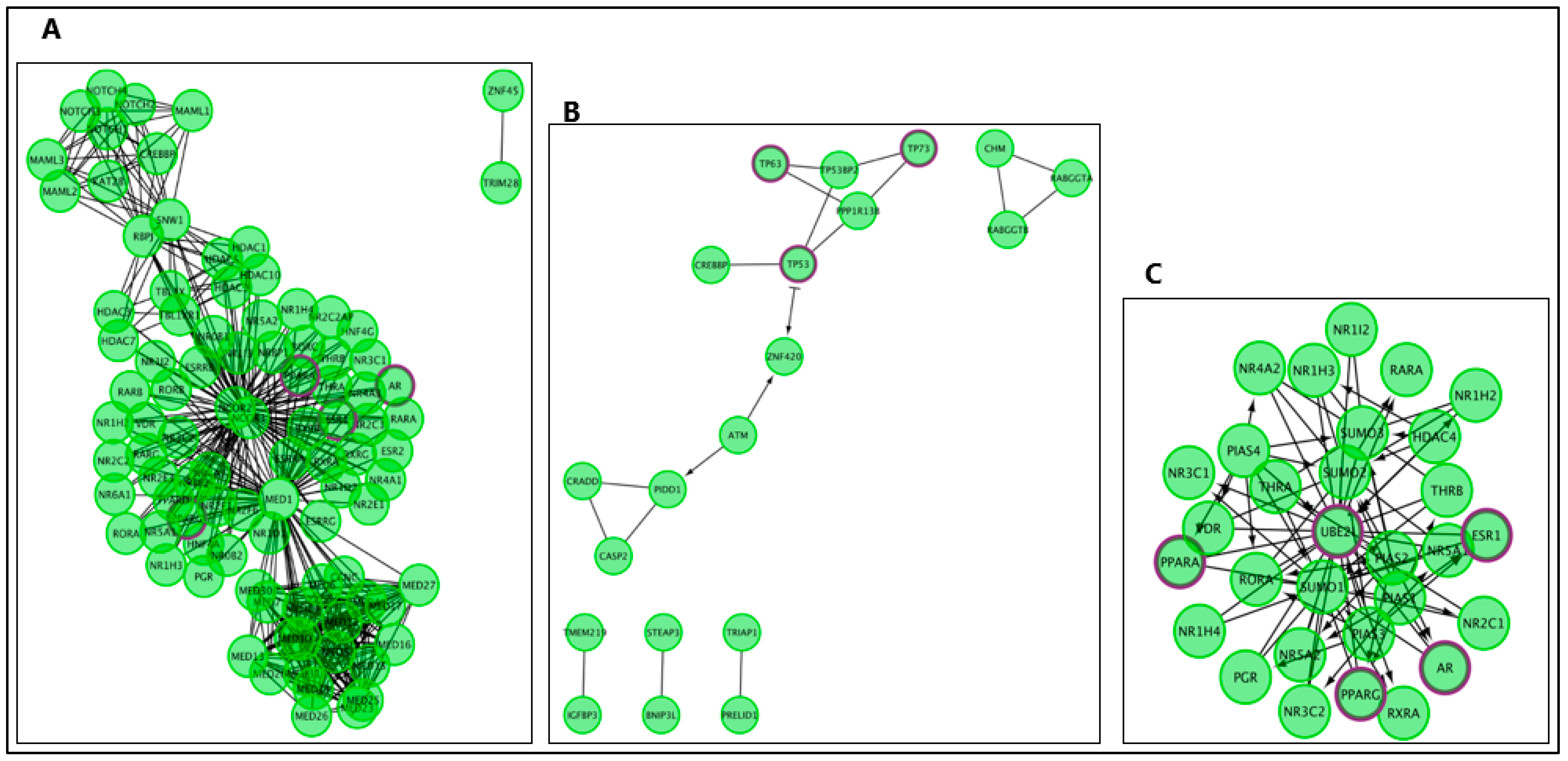
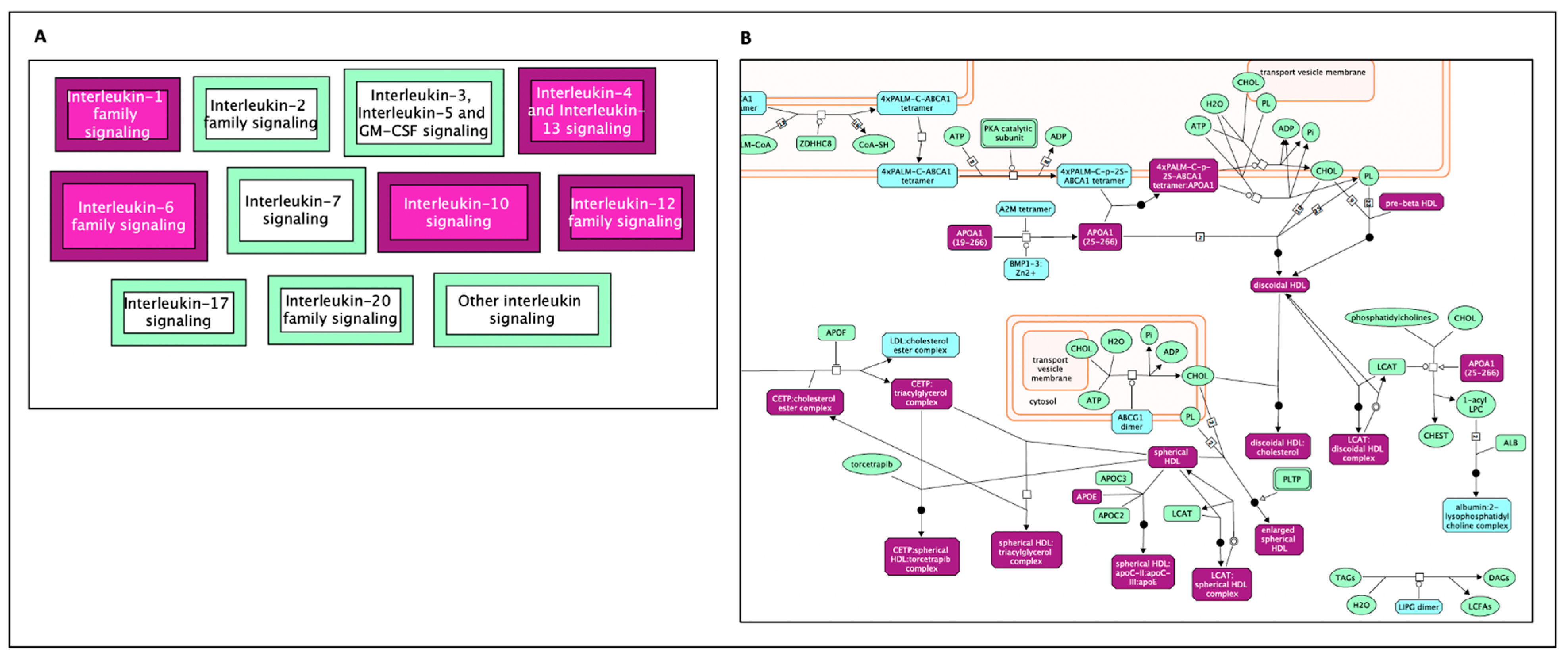
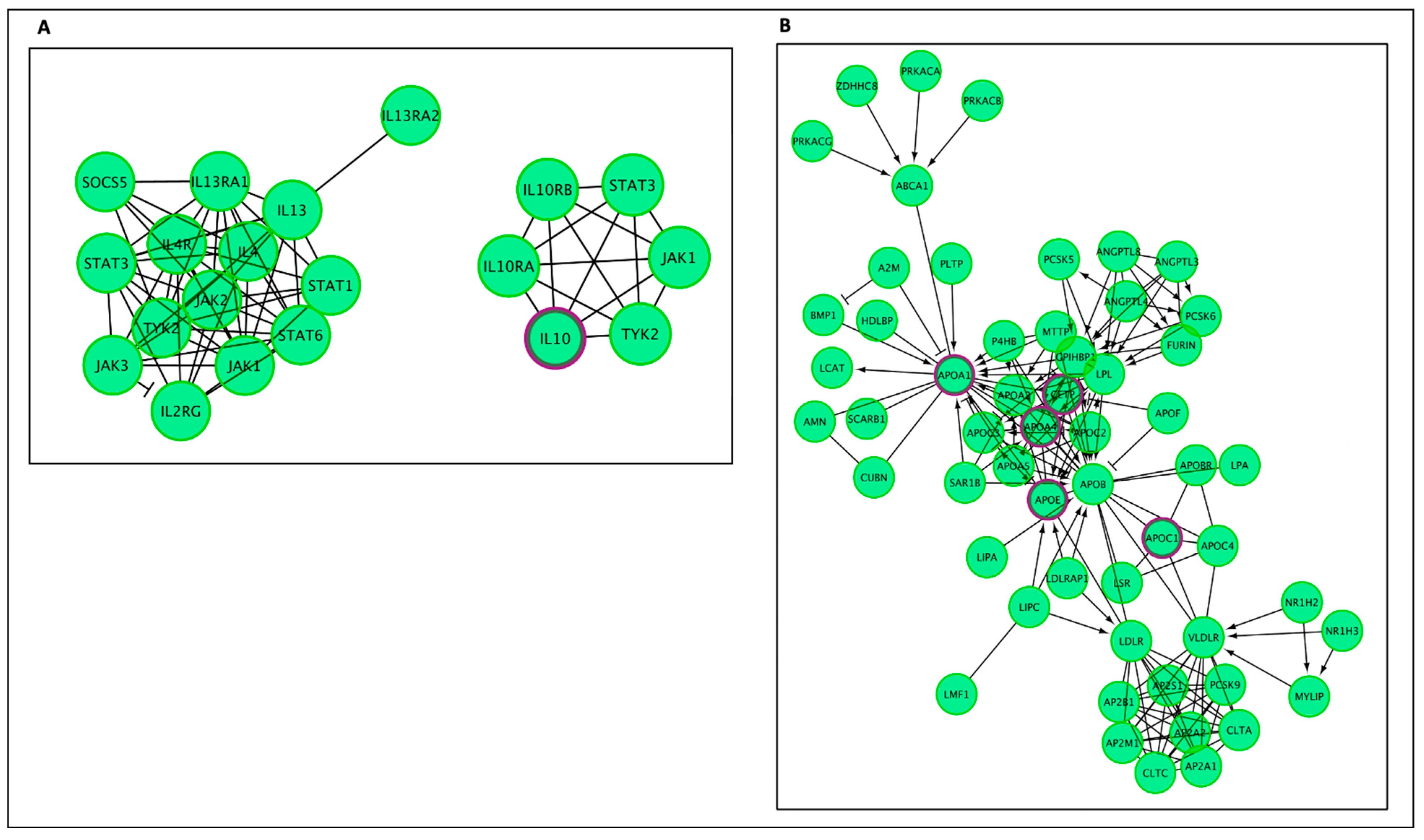

| Reactome Pathway | No. of Total Proteins in Pathway | No. of Hits in Pathway | p-Value | FDR Value | Hit Gene |
|---|---|---|---|---|---|
| tRNA processing in the mitochondrion | 42 | 19 | 1.11 × 10−16 | 1.01 × 10−13 | MT-ND6, MT-TQ, MT-ND4L, MT-ND4, MT-CO1, MT-TT, MT-TR, MT-ND2, MT-ND3, MT-ND1, MT-TH, MT-CO2, MT-TG, MT-CO3, MT-TS2, MT-ATP6, MT-ATP8, MT-RNR1, MT-CYB |
| Plasma lipoprotein assembly, remodeling, and clearance | 71 | 21 | 4.44 × 10−16 | 1.34 × 10−13 | LIPA, LIPC, SOAT1, CETP, APOE, A2M, ABCA1, VLDLR, LDLR, NR1H2, ABCG1, LPL, ALB, APOA1, APOA4, APOA5, NPC1, NPC2, APOC4, APOC2, APOC1 |
| rRNA processing in the mitochondrion | 38 | 17 | 4.44 × 10−16 | 1.34 × 10−13 | MT-ND4L, MT-ND4, MT-CO1, MT-TT, MT-TR, MT-ND2, MT-ND3, MT-ND1, MT-TH, MT-CO2, MT-TG, MT-CO3, MT-TS2, MT-ATP6, MT-ATP8, MT-RNR1, MT-CYB |
| Interleukin-4 and Interleukin-13 signaling | 112 | 21 | 2.57 × 10−12 | 5.80 × 10−10 | ICAM1, TP53, MAOA, PIK3R1, HMOX1, CD36, IL10, IL18, IL1A, IL1B, PTGS2, ALOX5, F13A1, TNF, TGFB1, POU2F1, IL6, IL8, MMP1, MMP3, CCL2 |
| Plasma lipoprotein clearance | 33 | 12 | 9.58 × 10−11 | 1.73 × 10−8 | LIPA, LIPC, SOAT1, APOE, VLDLR, LDLR, NR1H2, APOA1, NPC1, NPC2, APOC4, APOC1 |
| Interleukin-10 signaling | 47 | 13 | 4.28 × 10−10 | 6.46 × 10−8 | IL1RN, ICAM1, CCR2, IL10, IL18, IL1A, IL1B, PTGS2, TNF, IL6, IL8, CCL3, CCL2 |
| Retinoid metabolism and transport | 44 | 12 | 2.36 × 10−9 | 3.05 × 10−7 | LRAT, HSPG2, APOE, LDLR, LPL, APOA1, APOA4, LRP1, LRP2, LRP8, TTR, APOC2 |
| Metabolism of fat-soluble vitamins | 48 | 12 | 6.14 × 10−9 | 6.93 × 10−7 | LRAT, HSPG2, APOE, LDLR, LPL, APOA1, APOA4, LRP1, LRP2, LRP8, TTR, APOC2 |
| tRNA processing | 146 | 19 | 1.09 × 10−8 | 1.09 × 10−6 | MT-ND6, MT-TQ, MT-ND4L, MT-ND4, MT-CO1, MT-TT, MT-TR, MT-ND2, MT-ND3, MT-ND1, MT-TH, MT-CO2, MT-TG, MT-CO3, MT-TS2, MT-ATP6, MT-ATP8, MT-RNR1, MT-CYB |
| Plasma lipoprotein remodeling | 32 | 10 | 1.47 × 10−8 | 1.32 × 10−6 | LIPC, CETP, APOE, ABCG1, LPL, ALB, APOA1, APOA4, APOA5, APOC2 |
| Reactome Pathway | No. of Total Proteins in Pathway | No. of Hits in Pathway | p-Value | FDR Value | Hit Gene |
|---|---|---|---|---|---|
| Signaling by Interleukins | 452 | 59 | 1.11 × 10−16 | 8.66 × 10−15 | APP, ATF2, MYC, AKT1, MIF, TP53, PIK3R1, HIF1A, STAT5A, STAT5B, JUN, GRB2, CDKN1A, IKBKB, JAK2, FOS, CTF1, PTGS2, RELA, STAT3, VEGFA, IRS1, IRS2, TNF, SQSTM1, SHC1, FOXO3, FOXO1, SOCS2, UBB, HSPA9, HSPA8, TGFB1, IL2, NFKB1, NFKB2, NFKBIA, IL6, IL7, BCL2, IL7R, HMGB1, PIK3CB, LMNB1, HSP90AA1, YWHAZ, CREB1, PIK3CA, IL2RG, CDC42, MAPK9, MAPK8, PTK2B, MAPK3, PTPN11, MAPK14, S100B, SOD2, SOD1 |
| DNA Double-Strand Break Repair | 149 | 30 | 1.11 × 10−16 | 8.66 × 10−15 | CHEK2, TP53, PRKDC, TP53BP1, XRCC6, XRCC5, ATM, ATR, FEN1, BRCA1, BRCA2, PARP1, SIRT6, PCNA, WRN, RPA1, NBN, BLM, UBB, ABL1, UBE2I, SUMO1, H2AFX, CCNA2, MAPK8, POLD1, RAD52, RAD51, ERCC4, ERCC1 |
| PIP3 activates AKT signaling | 286 | 46 | 1.11 × 10−16 | 8.66 × 10−15 | ATF2, AKT1, TP53, PDGFB, PIK3R1, EGR1, JUN, INSR, GRB2, CDKN1A, BMI1, PPARG, EGFR, RICTOR, IRS1, IRS2, PDGFRB, PDGFRA, FOXO4, FOXO3, FOXO1, UBB, FGF23, KL, ESR1, MDM2, FGFR1, GSK3B, GSK3A, PTEN, PIK3CB, PDPK1, NRG1, CREB1, PIK3CA, HDAC2, HDAC3, HDAC1, INS, ERBB2, MAPK3, EGF, PTPN11, MTOR, PML, STUB1 |
| Intracellular signaling by second messengers | 326 | 49 | 1.11 × 10−16 | 8.66 × 10−15 | ATF2, AKT1, PRKCD, PRKCA, TP53, PDGFB, PIK3R1, EGR1, JUN, INSR, GRB2, CDKN1A, BMI1, PPARG, EGFR, ADCY5, RICTOR, IRS1, IRS2, PDGFRB, PDGFRA, FOXO4, FOXO3, FOXO1, UBB, FGF23, KL, ESR1, MDM2, FGFR1, GSK3B, GSK3A, PTEN, PIK3CB, PDPK1, NRG1, CREB1, PIK3CA, HDAC2, HDAC3, HDAC1, INS, ERBB2, MAPK3, EGF, PTPN11, MTOR, PML, STUB1 |
| Signaling by Receptor Tyrosine Kinases | 493 | 60 | 1.11 × 10−16 | 8.66 × 10−15 | ATF2, AKT1, PRKCD, PRKCA, PDGFB, PIK3R1, APOE, STAT5A, EGR1, STAT5B, JUND, INSR, IGF2, IGF1, PTK2, GRB2, JAK2, HRAS, FOS, NCOR1, EGFR, RICTOR, STAT3, VEGFA, CTNNB1, IRS1, IRS2, IGF1R, EP300, PDGFRB, PDGFRA, SHC1, PSEN1, UBB, FGF23, KL, BDNF, ESR1, FGFR1, FLT1, PIK3CB, HSP90AA1, PDPK1, NRG1, NGF, CREB1, PIK3CA, INS, CDC42, ERBB2, PTK2B, MAPK3, PTPN1, EGF, PTPN11, MAPK14, S100B, MTOR, BAX, STUB1 |
| FOXO-mediated transcription | 66 | 22 | 1.11 × 10−16 | 8.66 × 10−15 | AKT1, DDIT3, TXN, PCK1, PPARGC1A, CREBBP, CDKN1A, SIRT1, SIRT3, NR3C1, STK11, EP300, FOXO4, FOXO3, FOXO1, SIN3A, YWHAZ, CAT, HDAC2, HDAC1, INS, SOD2 |
| RNA Polymerase II Transcription | 1363 | 110 | 1.11 × 10−16 | 8.66 × 10−15 | RB1, ATF2, MT-CO1, SERPINE1, CHEK2, MYC, AKT1, TP63, MED1, AR, DDIT3, TP53, TXN, PRDX1, APOE, PCK1, PPARGC1A, CREBBP, JUN, ATM, ATR, TP73, CDKN1A, BRCA1, BMI1, PARP1, FOS, SIRT1, SIRT3, NCOR2, NCOR1, PPARG, PPARA, PCNA, HTT, EGFR, RELA, WRN, RICTOR, CDKN2B, CDKN2A, GSR, RPA1, GTF2H2, MLH1, VEGFA, CTNNB1, FAS, NR3C1, CTGF, STK11, EP300, NBN, IGFBP3, BLM, MAX, FOXO4, FOXO3, FOXO1, UBB, ABL1, TFAP2A, TGFB1, UBE2I, BDNF, ESR1, IL2, NFKB1, CDK7, IL6, SP1, CDK1, MDM2, PIN1, TCF3, GSK3B, PTEN, SUMO1, SIN3A, TBP, PDPK1, H2AFX, YWHAZ, CCNA2, CREB1, TFDP1, CAT, CEBPB, HDAC2, HDAC3, HDAC1, PPM1D, HSPD1, INS, ERBB2, E2F1, MAPK3, PTPN1, PTPN11, MAPK14, SOD2, MTOR, PML, RAD51, ERCC3, SST, ERCC2, BAX, STUB1, TAF1 |
| Generic Transcription Pathway | 1234 | 110 | 1.11 × 10−16 | 8.66 × 10−15 | RB1, ATF2, MT-CO1, SERPINE1, CHEK2, MYC, AKT1, TP63, MED1, AR, DDIT3, TP53, TXN, PRDX1, APOE, PCK1, PPARGC1A, CREBBP, JUN, ATM, ATR, TP73, CDKN1A, BRCA1, BMI1, PARP1, FOS, SIRT1, SIRT3, NCOR2, NCOR1, PPARG, PPARA, PCNA, HTT, EGFR, RELA, WRN, RICTOR, CDKN2B, CDKN2A, GSR, RPA1, GTF2H2, MLH1, VEGFA, CTNNB1, FAS, NR3C1, CTGF, STK11, EP300, NBN, IGFBP3, BLM, MAX, FOXO4, FOXO3, FOXO1, UBB, ABL1, TFAP2A, TGFB1, UBE2I, BDNF, ESR1, IL2, NFKB1, CDK7, IL6, SP1, CDK1, MDM2, PIN1, TCF3, GSK3B, PTEN, SUMO1, SIN3A, TBP, PDPK1, H2AFX, YWHAZ, CCNA2, CREB1, TFDP1, CAT, CEBPB, HDAC2, HDAC3, HDAC1, PPM1D, HSPD1, INS, ERBB2, E2F1, MAPK3, PTPN1, PTPN11, MAPK14, SOD2, MTOR, PML, RAD51, ERCC3, SST, ERCC2, BAX, STUB1, TAF1 |
| Cellular responses to stress | 554 | 61 | 1.11 × 10−16 | 8.66 × 10−15 | RB1, ATF2, AR, DDIT3, TP53, TXN, HIF1A, PRDX1, CREBBP, JUN, ATM, ATR, CDKN1A, BMI1, FOS, TERF1, SIRT1, TERF2, GSTP1, RELA, HSF1, RAE1, CDKN2B, CDKN2A, IFNB1, GSR, STAT3, RPA1, VEGFA, HSPA1B, HSPA1A, NR3C1, EP300, NBN, MAP3K5, EEF1A1, VCP, UBB, HSPA9, HSPA8, NFKB1, IL6, SP1, MDM2, GSK3B, LMNB1, HSP90AA1, GPX1, H2AFX, CCNA2, TFDP1, CAT, CEBPB, MAPK9, MAPK8, E2F1, MAPK3, MAPK14, SOD2, MTOR, SOD1 |
| SUMO E3 ligases SUMOylate target proteins | 169 | 36 | 1.11 × 10−16 | 8.66 × 10−15 | AR, TP53, TP53BP1, PPARGC1A, CREBBP, TOP2A, TOP2B, BRCA1, BMI1, PARP1, NCOR2, PPARG, PPARA, PCNA, RELA, WRN, RAE1, CDKN2A, RPA1, NR3C1, EP300, HIC1, BLM, TFAP2A, UBE2I, ESR1, NFKB2, NFKBIA, MDM2, SUMO1, SIN3A, TOP1, HDAC2, HDAC1, PML, RAD52 |
| Reactome Pathway | No. of Total Proteins in Pathway | No. of Hits in Pathway | p-Value | FDR | HitGenes |
|---|---|---|---|---|---|
| mTOR signalling | 41 | 19 | 1.11 × 10−16 | 5.15 × 10−14 | AKT1, RRAGA, RRAGC, RRAGB, RRAGD, RPTOR, LAMTOR2, LAMTOR3, RPS6, TSC2, TSC1, EIF4EBP1, RHEB, MLST8, AKT1S1, EIF4E, EIF4B, MTOR, RPS6KB1 |
| mTORC1-mediated signalling | 24 | 16 | 1.11 × 10−16 | 5.15 × 10−14 | RRAGA, RRAGC, RRAGB, RRAGD, RPTOR, LAMTOR2, LAMTOR3, RPS6, EIF4EBP1, RHEB, MLST8, AKT1S1, EIF4E, EIF4B, MTOR, RPS6KB1 |
| Intracellular signaling by second messengers | 326 | 34 | 1.74 × 10−14 | 5.39 × 10−12 | AKT1, PRKCA, RRAGA, RRAGC, RRAGB, RRAGD, TP53, MAPKAP1, TGFA, INSR, CDKN1A, RPTOR, PPARG, EGFR, RICTOR, CAMK4, LAMTOR2, LAMTOR3, IRS2, TSC2, FOXO4, FOXO3, FOXO1, KL, ESR1, NBEA, RHEB, FGFR1, PRR5, MLST8, PIK3CA, AKT1S1, INS, MTOR |
| PIP3 activates AKT signaling | 286 | 31 | 1.03 × 10−13 | 2.39 × 10−11 | AKT1, RRAGA, RRAGC, RRAGB, RRAGD, TP53, MAPKAP1, TGFA, INSR, CDKN1A, RPTOR, PPARG, EGFR, RICTOR, LAMTOR2, LAMTOR3, IRS2, TSC2, FOXO4, FOXO3, FOXO1, KL, ESR1, RHEB, FGFR1, PRR5, MLST8, PIK3CA, AKT1S1, INS, MTOR |
| Generic Transcription Pathway | 1234 | 66 | 3.56 × 10−13 | 6.59 × 10−11 | SERPINE1, AKT1, RUNX3, RRAGA, RRAGC, RRAGB, RRAGD, TP53, MAPKAP1, TGFA, GATA4, APOE, ATRIP, PPARGC1A, ATM, CDKN1A, MSTN, RPTOR, SREBF1, SIRT1, SIRT3, RAD51D, RARB, PPARG, SGK1, EGFR, WRN, RICTOR, WWOX, CDKN2B, GSR, MLH1, VEGFA, CAMK4, FAS, LAMTOR2, LAMTOR3, NR3C1, YY1, KCTD1, TSC2, TSC1, TBL1XR1, CSF1R, FOXO4, FOXO3, FOXO1, PLXNA4, TGFB1, ESRRG, ESR1, NFKB1, IL6, CDK6, RHEB, PRR5, EXO1, MLST8, YWHAG, H2AFX, IFNG, INS, TXNRD1, SOD2, MTOR, ERCC2 |
| Energy dependent regulation of mTOR by LKB1-AMPK | 29 | 12 | 2.20 × 10−12 | 3.39 × 10−10 | RRAGA, RRAGC, RRAGB, RRAGD, RPTOR, LAMTOR2, LAMTOR3, TSC2, TSC1, RHEB, MLST8, MTOR |
| RNA Polymerase II Transcription | 1363 | 67 | 1.04 × 10−11 | 1.29 × 10−9 | SERPINE1, AKT1, RUNX3, RRAGA, RRAGC, RRAGB, RRAGD, TP53, MAPKAP1, TGFA, GATA4, APOE, ATRIP, PPARGC1A, ATM, CDKN1A, MSTN, RPTOR, SREBF1, SIRT1, SIRT3, RAD51D, RARB, PPARG, SGK1, EGFR, WRN, RICTOR, WWOX, CDKN2B, GSR, MLH1, VEGFA, CAMK4, FAS, LAMTOR2, LAMTOR3, NR3C1, YY1, KCTD1, TSC2, TSC1, TBL1XR1, CSF1R, FOXO4, FOXO3, FOXO1, PLXNA4, TGFB1, ESRRG, ESR1, NFKB1, IL6, CDK6, RHEB, PRR5, EXO1, MLST8, YWHAG, H2AFX, IFNG, POLDIP3, INS, TXNRD1, SOD2, MTOR, ERCC2 |
| TP53 Regulates Metabolic Genes | 89 | 17 | 1.11 × 10−11 | 1.29 × 10−9 | AKT1, RRAGA, RRAGC, RRAGB, RRAGD, TP53, RPTOR, GSR, LAMTOR2, LAMTOR3, TSC2, TSC1, RHEB, MLST8, YWHAG, TXNRD1, MTOR |
| FOXO-mediated transcription | 66 | 15 | 1.73 × 10−11 | 1.78 × 10−9 | AKT1, PPARGC1A, CDKN1A, MSTN, SREBF1, SIRT1, SIRT3, NR3C1, FOXO4, FOXO3, FOXO1, PLXNA4, YWHAG, INS, SOD2 |
| Transcriptional Regulation by TP53 | 368 | 30 | 2.68 × 10−10 | 2.25 × 10−8 | AKT1, RRAGA, RRAGC, RRAGB, RRAGD, TP53, MAPKAP1, ATRIP, ATM, CDKN1A, RPTOR, RAD51D, SGK1, WRN, RICTOR, GSR, MLH1, FAS, LAMTOR2, LAMTOR3, TSC2, TSC1, RHEB, PRR5, EXO1, MLST8, YWHAG, TXNRD1, MTOR, ERCC2 |
| GenAge (AR) Genes | AlzGene (AD) Genes | Overlap Genes | Overlapped in AR Genes (%) |
|---|---|---|---|
| 307 | 356 | 41 | 13% |
| Reactome Pathway | No. of Total Proteins in Pathway | No. of Hits in Pathway | p-Value | FDR Value | Hit Gene |
|---|---|---|---|---|---|
| Generic Transcription Pathway | 1234 | 22 | 4.05 × 10−11 | 1.85 × 10−8 | GSK3B, MT-CO1, SERPINE1, APOE, PCK1, TP63, TGFB1, UBE2I, PARP1, BDNF, CDKN2A, SOD2, ESR1, AR, IL6, SST, FAS, PIN1, PPARG, PPARA, TP53, TP73 |
| RNA Polymerase II Transcription | 1363 | 22 | 2.84 × 10−10 | 6.47 × 10−8 | GSK3B, MT-CO1, SERPINE1, APOE, PCK1, TP63, TGFB1, UBE2I, PARP1, BDNF, CDKN2A, SOD2, ESR1, AR, IL6, SST, FAS, PIN1, PPARG, PPARA, TP53, TP73 |
| SUMOylation of intracellular receptors | 30 | 5 | 1.08 × 10−7 | 1.62 × 10−5 | UBE2I, ESR1, AR, PPARG, PPARA |
| TP53 Regulates Transcription of Death Receptors and Ligands | 12 | 4 | 1.42 × 10−7 | 1.62 × 10−5 | TP63, FAS, TP53, TP73 |
| SUMO E3 ligases SUMOylate target proteins | 169 | 8 | 1.98 × 10−7 | 1.80 × 10−5 | UBE2I, PARP1, CDKN2A, ESR1, AR, PPARG, PPARA, TP53 |
| Signaling by ERBB4 | 58 | 5 | 2.69 × 10−6 | 1.75 × 10−4 | PIK3R1, PSEN1, APOE, S100B, ESR1 |
| Interleukin-4 and Interleukin-13 signaling | 112 | 6 | 3.83 × 10−6 | 2.18 × 10−4 | PIK3R1, PTGS2, TNF, TGFB1, IL6, TP53 |
| Apoptosis | 179 | 7 | 4.36 × 10−6 | 2.18 × 10−4 | LMNA, TP63, CDKN2A, FAS, MAPT, TP53, TP73 |
| Nuclear signaling by ERBB4 | 32 | 4 | 6.83 × 10−6 | 2.80 × 10−4 | PSEN1, APOE, S100B, ESR1 |
| Platelet degranulation | 128 | 6 | 8.16 × 10−6 | 3.10 × 10−4 | APP, SERPINE1, CLU, A2M, TGFB1, IGF1 |
| Longevity Genes | Positive AlzGene (AD) Genes | Overlap Genes | Overlapped in Longevity Genes % |
|---|---|---|---|
| 357 | 356 | 43 | 12% |
| Reactome Pathway | No. of Total Proteins in Pathway | No. of Hits in Pathway | p-Value | FDR Value | Hit Gene |
|---|---|---|---|---|---|
| Interleukin-4 and Interleukin-13 signaling | 112 | 7 | 7.64 × 10−8 | 2.32 × 10−5 | TNF, HMOX1, IL10, TGFB1, IL18, IL6, TP53 |
| Plasma lipoprotein assembly | 19 | 4 | 5.15 × 10−7 | 7.82 × 10−5 | APOE, APOA1, APOA4, APOC1 |
| Plasma lipoprotein assembly, remodeling, and clearance | 71 | 5 | 3.69 × 10−6 | 2.30 × 10−4 | CETP, APOE, APOA1, APOA4, APOC1 |
| Plasma lipoprotein remodeling | 32 | 4 | 4.02 × 10−6 | 2.30 × 10−4 | CETP, APOE, APOA1, APOA4 |
| Chylomicron remodeling | 10 | 3 | 5.35 × 10−6 | 2.30 × 10−4 | APOE, APOA1, APOA4 |
| Chylomicron assembly | 10 | 3 | 5.35 × 10−6 | 2.30 × 10−4 | APOE, APOA1, APOA4 |
| HDL remodeling | 10 | 3 | 5.35 × 10−6 | 2.30 × 10−4 | CETP, APOE, APOA1 |
| Signaling by Interleukins | 452 | 9 | 1.20 × 10−5 | 4.57 × 10−4 | AGER, TNF, HMOX1, IL10, TGFB1, IL18, SOD2, IL6, TP53 |
| Retinoid metabolism and transport | 44 | 4 | 1.40 × 10−5 | 4.61 × 10−4 | TTR, APOE, APOA1, APOA4 |
| Interleukin-10 signaling | 47 | 4 | 1.80 × 10−5 | 5.29 × 10−4 | TNF, IL10, IL18, IL6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balmorez, T.; Sakazaki, A.; Murakami, S. Genetic Networks of Alzheimer’s Disease, Aging, and Longevity in Humans. Int. J. Mol. Sci. 2023, 24, 5178. https://doi.org/10.3390/ijms24065178
Balmorez T, Sakazaki A, Murakami S. Genetic Networks of Alzheimer’s Disease, Aging, and Longevity in Humans. International Journal of Molecular Sciences. 2023; 24(6):5178. https://doi.org/10.3390/ijms24065178
Chicago/Turabian StyleBalmorez, Timothy, Amy Sakazaki, and Shin Murakami. 2023. "Genetic Networks of Alzheimer’s Disease, Aging, and Longevity in Humans" International Journal of Molecular Sciences 24, no. 6: 5178. https://doi.org/10.3390/ijms24065178
APA StyleBalmorez, T., Sakazaki, A., & Murakami, S. (2023). Genetic Networks of Alzheimer’s Disease, Aging, and Longevity in Humans. International Journal of Molecular Sciences, 24(6), 5178. https://doi.org/10.3390/ijms24065178







