Cognitive Normal Older Adults with APOE-2 Allele Show a Distinctive Functional Connectivity Pattern in Response to Cerebral Aβ Deposition
Abstract
1. Introduction
2. Results
2.1. Baseline Demographic and Clinical Data
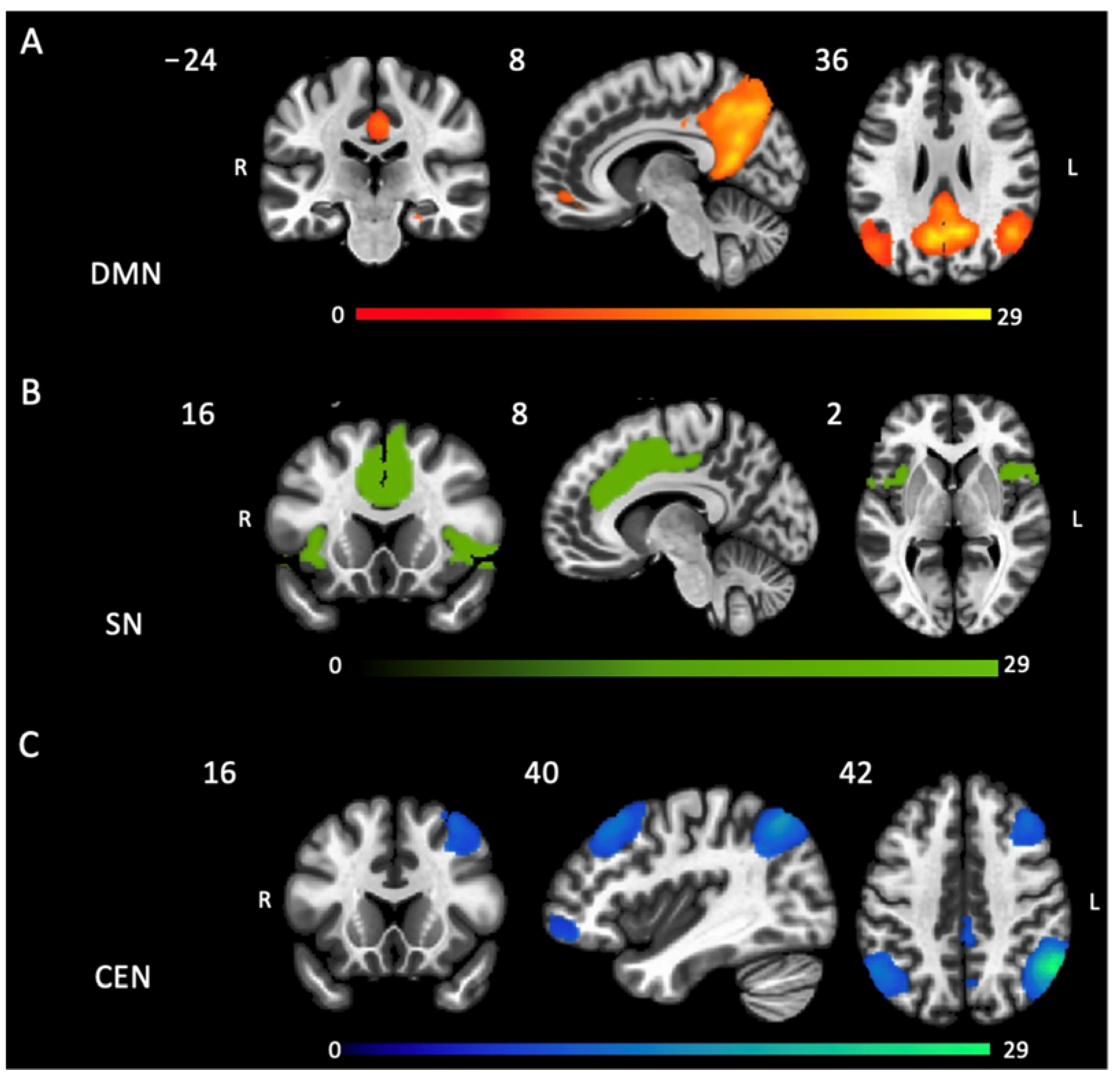
2.2. Group Difference in Intrinsic Functional Connectivity
2.3. Cerebral Aβ Deposition and Functional Connectivity
2.4. Graph Theory Measures
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Acquisition of MRI
4.3. [18F]-Flutemetamol PET Image Acquisition and Processing
4.4. Data Analysis
4.4.1. fMRI Data Preprocessing
4.4.2. Seed-to-Voxel Analysis
4.4.3. Graph Theory Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mattsson-Carlgren, N.; Salvado, G.; Ashton, N.J.; Tideman, P.; Stomrud, E.; Zetterberg, H.; Ossenkoppele, R.; Betthauser, T.J.; Cody, K.A.; Jonaitis, E.M.; et al. Prediction of Longitudinal Cognitive Decline in Preclinical Alzheimer Disease Using Plasma Biomarkers. JAMA Neurol. 2023, 80, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Tu, K.J.; Wei, A.; Lau, A.J.; Gonzalez-Gil, A.; Cao, T.; Braunstein, K.; Ling, J.P.; Troncoso, J.C.; Wong, P.C.; et al. Amyloid-beta and tau pathologies act synergistically to induce novel disease stage-specific microglia subtypes. Mol. Neurodegener. 2022, 17, 83. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s disease: Inhibition of amyloid beta and tau tangle formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Sehar, U.; Rawat, P.; Reddy, A.P.; Kopel, J.; Reddy, P.H. Amyloid Beta in Aging and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12924. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, R.; van der Kant, R.; Hansson, O. Tau biomarkers in Alzheimer’s disease: Towards implementation in clinical practice and trials. Lancet Neurol. 2022, 21, 726–734. [Google Scholar] [CrossRef]
- Bok, J.; Ha, J.; Ahn, B.J.; Jang, Y. Disease-Modifying Effects of Non-Invasive Electroceuticals on beta-Amyloid Plaques and Tau Tangles for Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 24, 679. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef]
- Buckner, R.L.; Snyder, A.Z.; Shannon, B.J.; LaRossa, G.; Sachs, R.; Fotenos, A.F.; Sheline, Y.I.; Klunk, W.E.; Mathis, C.A.; Morris, J.C.; et al. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 2005, 25, 7709–7717. [Google Scholar] [CrossRef]
- Barnett, A.J.; Reilly, W.; Dimsdale-Zucker, H.R.; Mizrak, E.; Reagh, Z.; Ranganath, C. Intrinsic connectivity reveals functionally distinct cortico-hippocampal networks in the human brain. PLoS Biol. 2021, 19, e3001275. [Google Scholar] [CrossRef]
- Mancuso, L.; Cavuoti-Cabanillas, S.; Liloia, D.; Manuello, J.; Buzi, G.; Cauda, F.; Costa, T. Tasks activating the default mode network map multiple functional systems. Brain Struct. Funct. 2022, 227, 1711–1734. [Google Scholar] [CrossRef]
- Hedden, T.; Van Dijk, K.R.; Becker, J.A.; Mehta, A.; Sperling, R.A.; Johnson, K.A.; Buckner, R.L. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J. Neurosci. 2009, 29, 12686–12694. [Google Scholar] [CrossRef]
- Sheline, Y.I.; Raichle, M.E.; Snyder, A.Z.; Morris, J.C.; Head, D.; Wang, S.; Mintun, M.A. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol. Psychiatry 2010, 67, 584–587. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Reidler, J.S.; Sepulcre, J.; Poulin, R.; Buckner, R.L. Functional-anatomic fractionation of the brain’s default network. Neuron 2010, 65, 550–562. [Google Scholar] [CrossRef]
- Lim, H.K.; Nebes, R.; Snitz, B.; Cohen, A.; Mathis, C.; Price, J.; Weissfeld, L.; Klunk, W.; Aizenstein, H.J. Regional amyloid burden and intrinsic connectivity networks in cognitively normal elderly subjects. Brain 2014, 137 Pt 12, 3327–3338. [Google Scholar] [CrossRef]
- Agosta, F.; Pievani, M.; Geroldi, C.; Copetti, M.; Frisoni, G.B.; Filippi, M. Resting state fMRI in Alzheimer’s disease: Beyond the default mode network. Neurobiol. Aging 2012, 33, 1564–1578. [Google Scholar] [CrossRef]
- Brier, M.R.; Thomas, J.B.; Snyder, A.Z.; Benzinger, T.L.; Zhang, D.; Raichle, M.E.; Holtzman, D.M.; Morris, J.C.; Ances, B.M. Loss of intranetwork and internetwork resting state functional connections with Alzheimer’s disease progression. J. Neurosci. 2012, 32, 8890–8899. [Google Scholar] [CrossRef]
- Cheung, E.Y.W.; Chau, A.C.M.; Shea, Y.F.; Chiu, P.K.C.; Kwan, J.S.K.; Mak, H.K.F. Level of Amyloid-beta (Abeta) Binding Leading to Differential Effects on Resting State Functional Connectivity in Major Brain Networks. Biomedicines 2022, 10, 2321. [Google Scholar] [CrossRef]
- Chow, T.E.; Veziris, C.R.; La Joie, R.; Lee, A.J.; Brown, J.A.; Yokoyama, J.S.; Rankin, K.P.; Kramer, J.H.; Miller, B.L.; Rabinovici, G.D.; et al. Increasing empathic concern relates to salience network hyperconnectivity in cognitively healthy older adults with elevated amyloid-beta burden. Neuroimage Clin. 2022, 37, 103282. [Google Scholar] [CrossRef]
- Zhao, S.; Rangaprakash, D.; Liang, P.; Deshpande, G. Deterioration from healthy to mild cognitive impairment and Alzheimer’s disease mirrored in corresponding loss of centrality in directed brain networks. Brain Inform. 2019, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zhang, Z. Global robustness and identifiability of random, scale-free, and small-world networks. Ann. N. Y. Acad. Sci. 2009, 1158, 82–92. [Google Scholar] [CrossRef]
- Behfar, Q.; Behfar, S.K.; von Reutern, B.; Richter, N.; Dronse, J.; Fassbender, R.; Fink, G.R.; Onur, O.A. Graph Theory Analysis Reveals Resting-State Compensatory Mechanisms in Healthy Aging and Prodromal Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 576627. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cao, W.; Wang, J.; Zhang, Y.; Yang, W.; Liu, Y. A spatial interaction incorporated betweenness centrality measure. PLoS ONE 2022, 17, e0268203. [Google Scholar] [CrossRef]
- Seo, E.H.; Lee, D.Y.; Lee, J.M.; Park, J.S.; Sohn, B.K.; Lee, D.S.; Choe, Y.M.; Woo, J.I. Whole-brain functional networks in cognitively normal, mild cognitive impairment, and Alzheimer’s disease. PLoS ONE 2013, 8, e53922. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010, 9, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Wink, A.M.; Tijms, B.M.; Ten Kate, M.; Raspor, E.; de Munck, J.C.; Altena, E.; Ecay-Torres, M.; Clerigue, M.; Estanga, A.; Garcia-Sebastian, M.; et al. Functional brain network centrality is related to APOE genotype in cognitively normal elderly. Brain Behav. 2018, 8, e01080. [Google Scholar] [CrossRef]
- Sheline, Y.I.; Morris, J.C.; Snyder, A.Z.; Price, J.L.; Yan, Z.; D’Angelo, G.; Liu, C.; Dixit, S.; Benzinger, T.; Fagan, A.; et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. J. Neurosci. 2010, 30, 17035–17040. [Google Scholar] [CrossRef]
- Li, Z.; Shue, F.; Zhao, N.; Shinohara, M.; Bu, G. APOE2: Protective mechanism and therapeutic implications for Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 63. [Google Scholar] [CrossRef]
- Chen, J.; Shu, H.; Wang, Z.; Liu, D.; Shi, Y.; Xu, L.; Zhang, Z. Protective effect of APOE epsilon 2 on intrinsic functional connectivity of the entorhinal cortex is associated with better episodic memory in elderly individuals with risk factors for Alzheimer’s disease. Oncotarget 2016, 7, 58789–58801. [Google Scholar] [CrossRef]
- Shu, H.; Shi, Y.; Chen, G.; Wang, Z.; Liu, D.; Yue, C.; Ward, B.D.; Li, W.; Xu, Z.; Chen, G.; et al. Opposite Neural Trajectories of Apolipoprotein E ϵ4 and ϵ2 Alleles with Aging Associated with Different Risks of Alzheimer’s Disease. Cereb. Cortex 2016, 26, 1421–1429. [Google Scholar] [CrossRef]
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.; Visser, P.J.; Amyloid Biomarker Study Group; Aalten, P.; Aarsland, D.; et al. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 2015, 313, 1924–1938. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, Q.; Luo, X.; Li, K.; Hong, H.; Wang, S.; Guan, X.; Wu, J.; Zhang, R.; Zhang, T.; et al. Effects of APOE epsilon2 on the Fractional Amplitude of Low-Frequency Fluctuation in Mild Cognitive Impairment: A Study Based on the Resting-State Functional MRI. Front. Aging Neurosci. 2021, 13, 591347. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, Q.; Luo, X.; Li, K.; Xu, X.; Hong, L.; Li, J.; Guan, X.; Xu, X.; Huang, P.; et al. Effects of APOE epsilon2 allele on basal forebrain functional connectivity in mild cognitive impairment. CNS Neurosci. Ther. 2023, 29, 597–608. [Google Scholar] [CrossRef]
- Ribaric, S. Detecting Early Cognitive Decline in Alzheimer’s Disease with Brain Synaptic Structural and Functional Evaluation. Biomedicines 2023, 11, 355. [Google Scholar] [CrossRef]
- Wang, S.M.; Kim, N.Y.; Um, Y.H.; Kang, D.W.; Na, H.R.; Lee, C.U.; Lim, H.K. Default mode network dissociation linking cerebral beta amyloid retention and depression in cognitively normal older adults. Neuropsychopharmacology 2021, 46, 2180–2187. [Google Scholar] [CrossRef]
- Insel, P.S.; Hansson, O.; Mattsson-Carlgren, N. Association Between Apolipoprotein E epsilon2 vs epsilon4, Age, and beta-Amyloid in Adults Without Cognitive Impairment. JAMA Neurol. 2021, 78, 229–235. [Google Scholar] [CrossRef]
- Papp, K.V.; Buckley, R.; Mormino, E.; Maruff, P.; Villemagne, V.L.; Masters, C.L.; Johnson, K.A.; Rentz, D.M.; Sperling, R.A.; Amariglio, R.E.; et al. Clinical meaningfulness of subtle cognitive decline on longitudinal testing in preclinical AD. Alzheimers Dement 2020, 16, 552–560. [Google Scholar] [CrossRef]
- Bubb, E.J.; Metzler-Baddeley, C.; Aggleton, J.P. The cingulum bundle: Anatomy, function, and dysfunction. Neurosci. Biobehav. Rev. 2018, 92, 104–127. [Google Scholar] [CrossRef]
- Rolls, E.T. The cingulate cortex and limbic systems for action, emotion, and memory. Handb. Clin. Neurol. 2019, 166, 23–37. [Google Scholar]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Thal, D.R.; Beach, T.G.; Zanette, M.; Heurling, K.; Chakrabarty, A.; Ismail, A.; Smith, A.P.; Buckley, C. [(18)F]flutemetamol amyloid positron emission tomography in preclinical and symptomatic Alzheimer’s disease: Specific detection of advanced phases of amyloid-beta pathology. Alzheimers Dement. 2015, 11, 975–985. [Google Scholar] [CrossRef]
- Raulin, A.-C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.-C. ApoE in Alzheimer’s disease: Pathophysiology and therapeutic strategies. Mol. Neurodegener. 2022, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Mormino, E.C.; Smiljic, A.; Hayenga, A.O.; Onami, S.H.; Greicius, M.D.; Rabinovici, G.D.; Janabi, M.; Baker, S.L.; Yen, I.V.; Madison, C.M.; et al. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cereb. Cortex 2011, 21, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.D.; Price, J.C.; Weissfeld, L.A.; James, J.; Rosario, B.L.; Bi, W.; Nebes, R.D.; Saxton, J.A.; Snitz, B.E.; Aizenstein, H.A.; et al. Basal cerebral metabolism may modulate the cognitive effects of Abeta in mild cognitive impairment: An example of brain reserve. J. Neurosci. 2009, 29, 14770–14778. [Google Scholar] [CrossRef]
- Johnson, S.C.; Christian, B.T.; Okonkwo, O.C.; Oh, J.M.; Harding, S.; Xu, G.; Hillmer, A.T.; Wooten, D.W.; Murali, D.; Barnhart, T.E.; et al. Amyloid burden and neural function in people at risk for Alzheimer’s Disease. Neurobiol. Aging 2014, 35, 576–584. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Kelly, A.M.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum. Brain Mapp. 2009, 30, 625–637. [Google Scholar] [CrossRef]
- Fang, K.; Han, S.; Li, Y.; Ding, J.; Wu, J.; Zhang, W. The Vital Role of Central Executive Network in Brain Age: Evidence From Machine Learning and Transcriptional Signatures. Front. Neurosci. 2021, 15, 733316. [Google Scholar] [CrossRef]
- Lanfranco, M.F.; Sepulveda, J.; Kopetsky, G.; Rebeck, G.W. Expression and secretion of apoE isoforms in astrocytes and microglia during inflammation. Glia 2021, 69, 1478–1493. [Google Scholar] [CrossRef]
- Wang, N.; Wang, M.; Jeevaratnam, S.; Rosenberg, C.; Ikezu, T.C.; Shue, F.; Doss, S.V.; Alnobani, A.; Martens, Y.A.; Wren, M.; et al. Opposing effects of apoE2 and apoE4 on microglial activation and lipid metabolism in response to demyelination. Mol. Neurodegener. 2022, 17, 75. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, K.U.; Lee, D.Y.; Kim, K.W.; Jhoo, J.H.; Kim, J.H.; Lee, K.H.; Kim, S.Y.; Han, S.H.; Woo, J.I. Development of the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet (CERAD-K): Clinical and neuropsychological assessment batteries. J. Gerontol. B Psychol. Sci. Soc. Sci. 2002, 57, P47–P53. [Google Scholar] [CrossRef]
- Thurfjell, L.; Lilja, J.; Lundqvist, R.; Buckley, C.; Smith, A.; Vandenberghe, R.; Sherwin, P. Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: Concordance with visual image reads. J. Nucl. Med. 2014, 55, 1623–1628. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef]
- Buckner, R.L.; Sepulcre, J.; Talukdar, T.; Krienen, F.M.; Liu, H.; Hedden, T.; Andrews-Hanna, J.R.; Sperling, R.A.; Johnson, K.A. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. J. Neurosci. 2009, 29, 1860–1873. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- van den Heuvel, M.P.; Sporns, O. Network hubs in the human brain. Trends Cogn. Sci. 2013, 17, 683–696. [Google Scholar] [CrossRef]
- Tong, C.; Niu, J.; Dai, B.; Xie, Z. A novel complex networks clustering algorithm based on the core influence of nodes. Sci. World, J. 2014, 2014, 801854. [Google Scholar] [CrossRef]
- delEtoile, J.; Adeli, H. Graph Theory and Brain Connectivity in Alzheimer’s Disease. Neuroscientist 2017, 23, 616–626. [Google Scholar] [CrossRef]
- The jamovi Project (2023). jamovi (Version 2.3.210) [Computer Software]. Available online: https://www.jamovi.org (accessed on 1 March 2023.).
- Kac, P.R.; Gonzalez-Ortiz, F.; Simren, J.; Dewit, N.; Vanmechelen, E.; Zetterberg, H.; Blennow, K.; Ashton, N.J.; Karikari, T.K. Diagnostic value of serum versus plasma phospho-tau for Alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 65. [Google Scholar] [CrossRef]
- Gonzalez-Ortiz, F.; Kac, P.R.; Brum, W.S.; Zetterberg, H.; Blennow, K.; Karikari, T.K. Plasma phospho-tau in Alzheimer’s disease: Towards diagnostic and therapeutic trial applications. Mol. Neurodegener. 2023, 18, 18. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Kesari, K.K.; Rachamalla, M.; Mani, S.; Ashraf, G.M.; Jha, S.K.; Kumar, P.; Ambasta, R.K.; Dureja, H.; Devkota, H.P.; et al. CRISPR/Cas9 gene editing: New hope for Alzheimer’s disease therapeutics. J. Adv. Res. 2022, 40, 207–221. [Google Scholar] [CrossRef]
| Amyloid-PET Negative Group (N = 29) | Amyloid-PET Positive Group (N = 15) | p-Value | |
|---|---|---|---|
| Age (years ± SD) | 71.62 ± 8.13 | 71.67 ± 8.76 | NS |
| Education (years ± SD) | 11.69 ± 4.54 | 11.00 ± 6.43 | NS |
| Sex (M:F) | 9:20 | 4:11 | NS |
| CDR (SD) | 0 | 0 | NS |
| SUVR (mean ± SD) | 0.484 ± 0.080 | 0.695 ± 0.084 | <0.01 |
| APOE 2/2:2/3 (APOE 2/2%) | 1:28 (3.4%) | 0:14 (0%) | NS |
| CERAD-K Battery (SD) | |||
| VF | 14.97 ± 4.41 | 13.67 ± 5.19 | NS |
| BNT | 12.10 ± 2.24 | 11.50 ± 1.88 | NS |
| MMSE | 27.93 ± 1.39 | 27.53± 1.95 | NS |
| WLM | 18.17 ± 3.09 | 16.76± 4.17 | NS |
| CP | 10.38 ± 1.05 | 9.80 ± 1.90 | NS |
| WLR | 5.93 ± 1.41 | 5.47 ± 1.47 | NS |
| WLRc | 9.24 ± 1.02 | 8.80 ± 1.47 | NS |
| CR | 6.86 ± 2.63 | 6.64 ± 3.00 | NS |
| CERAD total score | 70.79 ± 9.45 | 67.14 ± 11.77 | NS |
| Region | L/R | Cluster | T Score | p-Value * | MNI (x, y, z) | ||
|---|---|---|---|---|---|---|---|
| Group Differences | |||||||
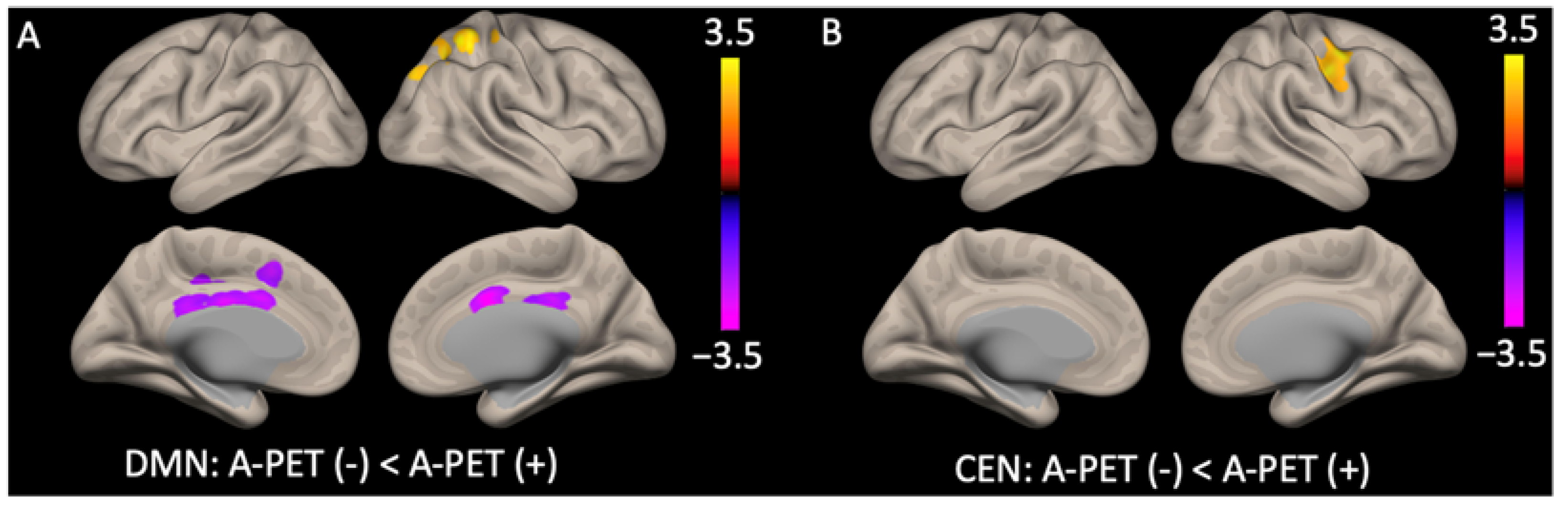
| Anterior DMN: A-PET-positive group < A-PET-negative group | |||||||
| Anterior cingulate and middle cingulate cortex | B | 867 | −3.14 | <0.05 | 6 | 6 | 28 |
| Posterior DMN: A-PET-positive group > A-PET-negative group | |||||||
| Superior parietal cortex and precuneus | R | 671 | 2.96 | <0.05 | 30 | −52 | 56 |
| CEN: A-PET-positive group > A-PET-negative group | |||||||
| Middle frontal gyrus | R | 558 | 2.70 | <0.05 | 52 | 00 | 50 |
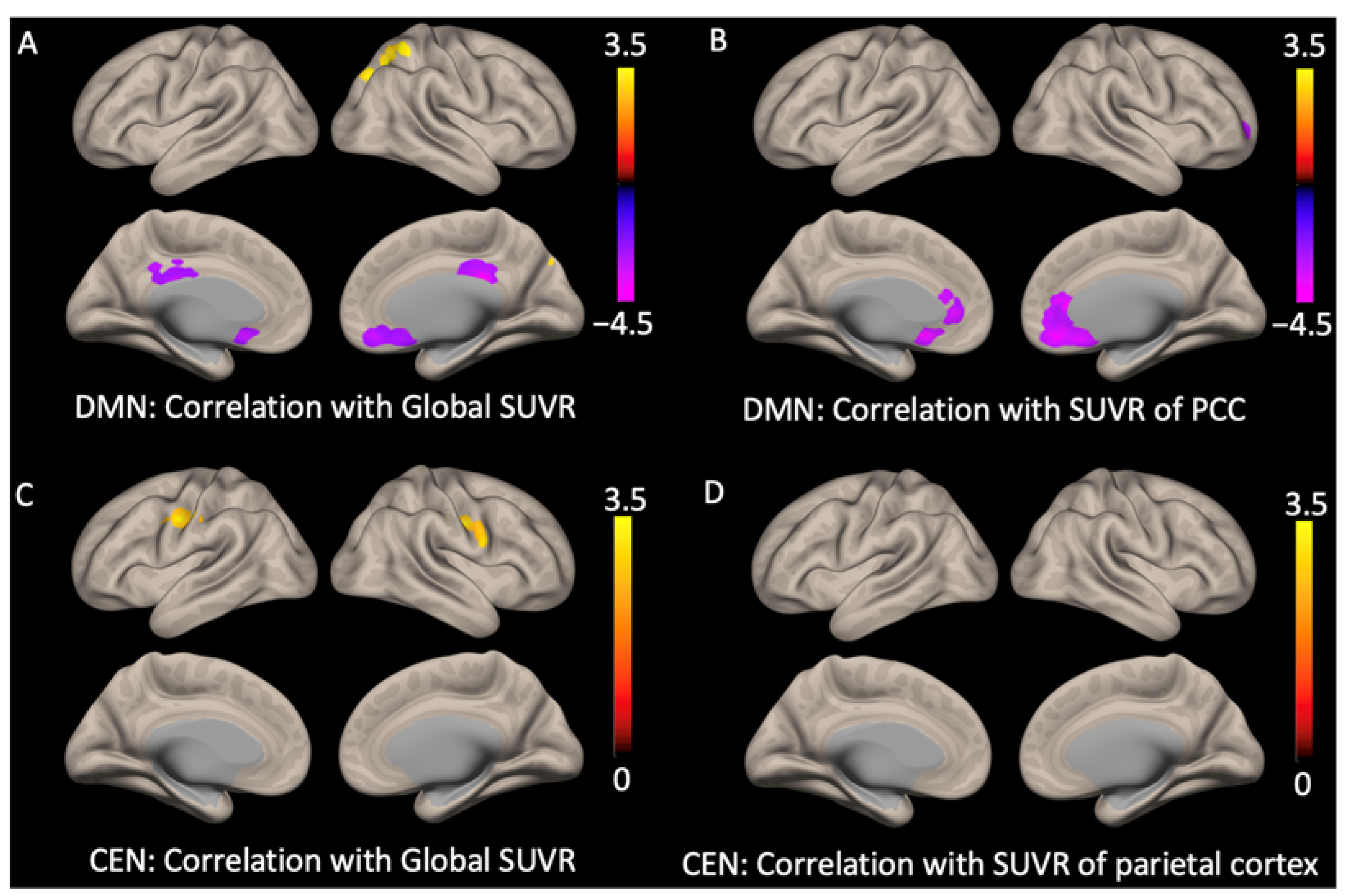
| Mean SUVR—functional connectivity relationship | |||||||
| DMN with Global SUVR | |||||||
| Anterior DMN: Subgenual anterior cingulate with global SUVR showed negative correlation | B | 242 | −3.36 | <0.05 | −4 | 20 | −10 |
| Posterior DMN: Posterior cingulate cortex with global SUVR showed negative correlation | B | 377 | −4.12 | <0.05 | 8 | −34 | 28 |
| Posterior DMN: Superior parietal cortex and precuneus with global SUVR showed positive correlation | R | 269 | 2.96 | <0.05 | 26 | −66 | 50 |
| DMN with regional SUVR of posterior cingulate cortex | |||||||
| Posterior DMN: Subgenual anterior cingulate with regional SUVR of posterior cingulate cortex showed negative correlation | B | 679 | −3.72 | <0.0001 | 8 | 32 | 8 |
| CEN with global SUVR | |||||||
| Precentral gyrus and middle frontal gyrus with global SUVR showed positive correlation | R | 393 | 3.37 | <0.01 | 14 | 32 | 4 |
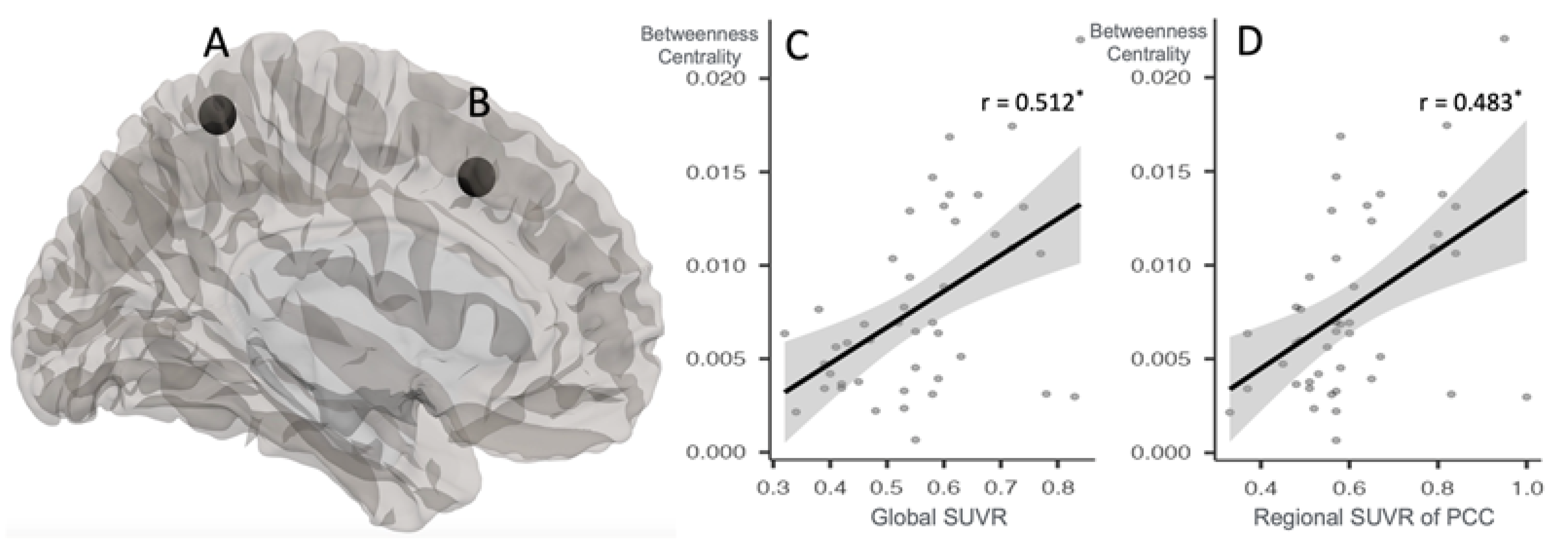
| Graph theory analysis | |||||||
| Betweenness centrality: A-PET-negative group > A-PET-positive group | |||||||
| Middle frontal gyrus | L | NA | 4.75 | <0.05 | −38 | 18 | 42 |
| Fronto-parietal regions | L | NA | 3.71 | <0.05 | −46 | −58 | 49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-M.; Kang, D.W.; Um, Y.H.; Kim, S.; Kim, R.E.Y.; Kim, D.; Lee, C.U.; Lim, H.K. Cognitive Normal Older Adults with APOE-2 Allele Show a Distinctive Functional Connectivity Pattern in Response to Cerebral Aβ Deposition. Int. J. Mol. Sci. 2023, 24, 11250. https://doi.org/10.3390/ijms241411250
Wang S-M, Kang DW, Um YH, Kim S, Kim REY, Kim D, Lee CU, Lim HK. Cognitive Normal Older Adults with APOE-2 Allele Show a Distinctive Functional Connectivity Pattern in Response to Cerebral Aβ Deposition. International Journal of Molecular Sciences. 2023; 24(14):11250. https://doi.org/10.3390/ijms241411250
Chicago/Turabian StyleWang, Sheng-Min, Dong Woo Kang, Yoo Hyun Um, Sunghwan Kim, Regina E. Y. Kim, Donghyeon Kim, Chang Uk Lee, and Hyun Kook Lim. 2023. "Cognitive Normal Older Adults with APOE-2 Allele Show a Distinctive Functional Connectivity Pattern in Response to Cerebral Aβ Deposition" International Journal of Molecular Sciences 24, no. 14: 11250. https://doi.org/10.3390/ijms241411250
APA StyleWang, S.-M., Kang, D. W., Um, Y. H., Kim, S., Kim, R. E. Y., Kim, D., Lee, C. U., & Lim, H. K. (2023). Cognitive Normal Older Adults with APOE-2 Allele Show a Distinctive Functional Connectivity Pattern in Response to Cerebral Aβ Deposition. International Journal of Molecular Sciences, 24(14), 11250. https://doi.org/10.3390/ijms241411250






