Salvia sclarea Essential Oil Chemical Composition and Biological Activities
Abstract
1. Introduction
2. Results
2.1. Chemical Composition
2.2. Antioxidant Activity
2.3. Antimicrobial Activity In Vitro
2.3.1. Disc Diffusion Method
2.3.2. Minimal Inhibitory Concentration Assay
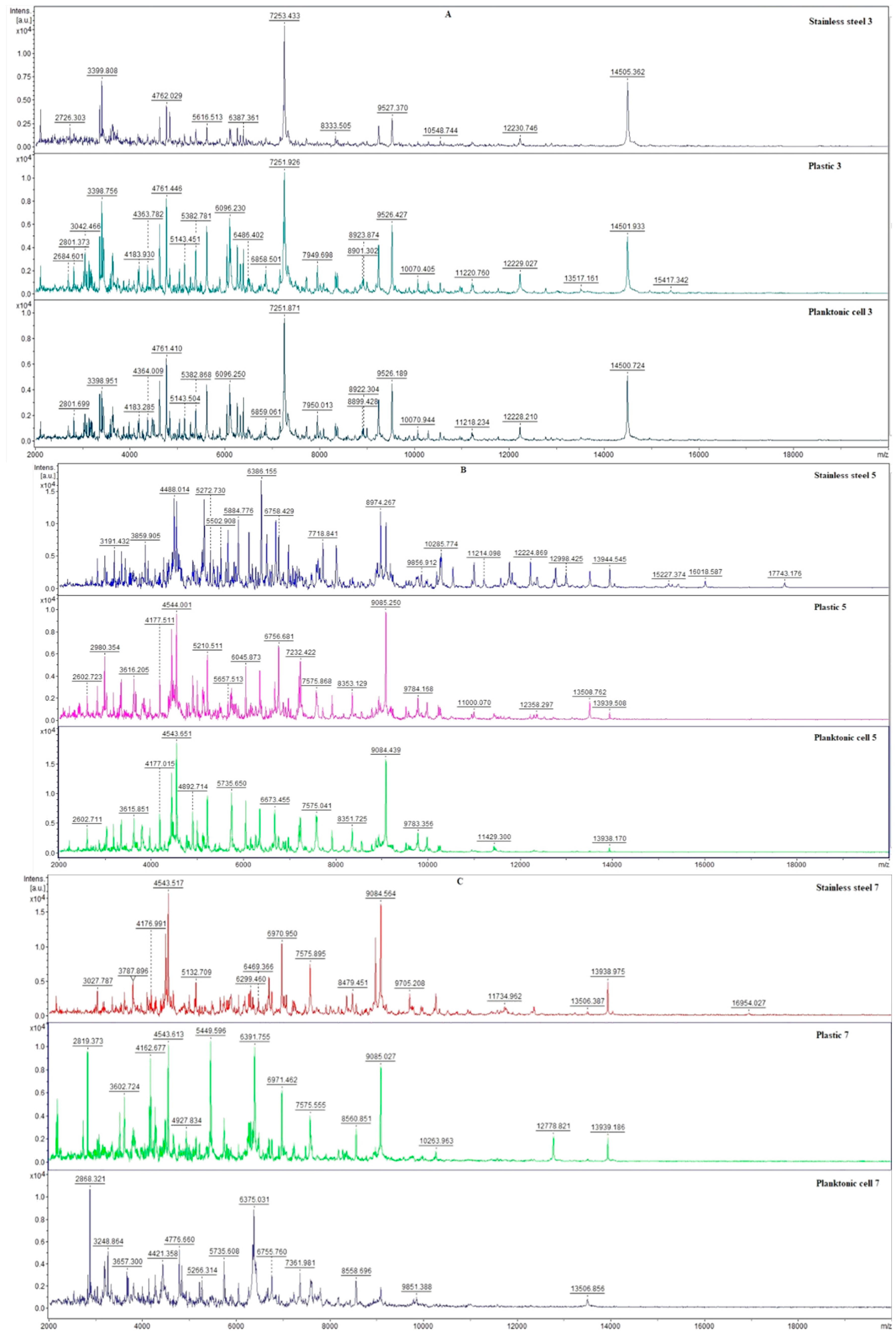
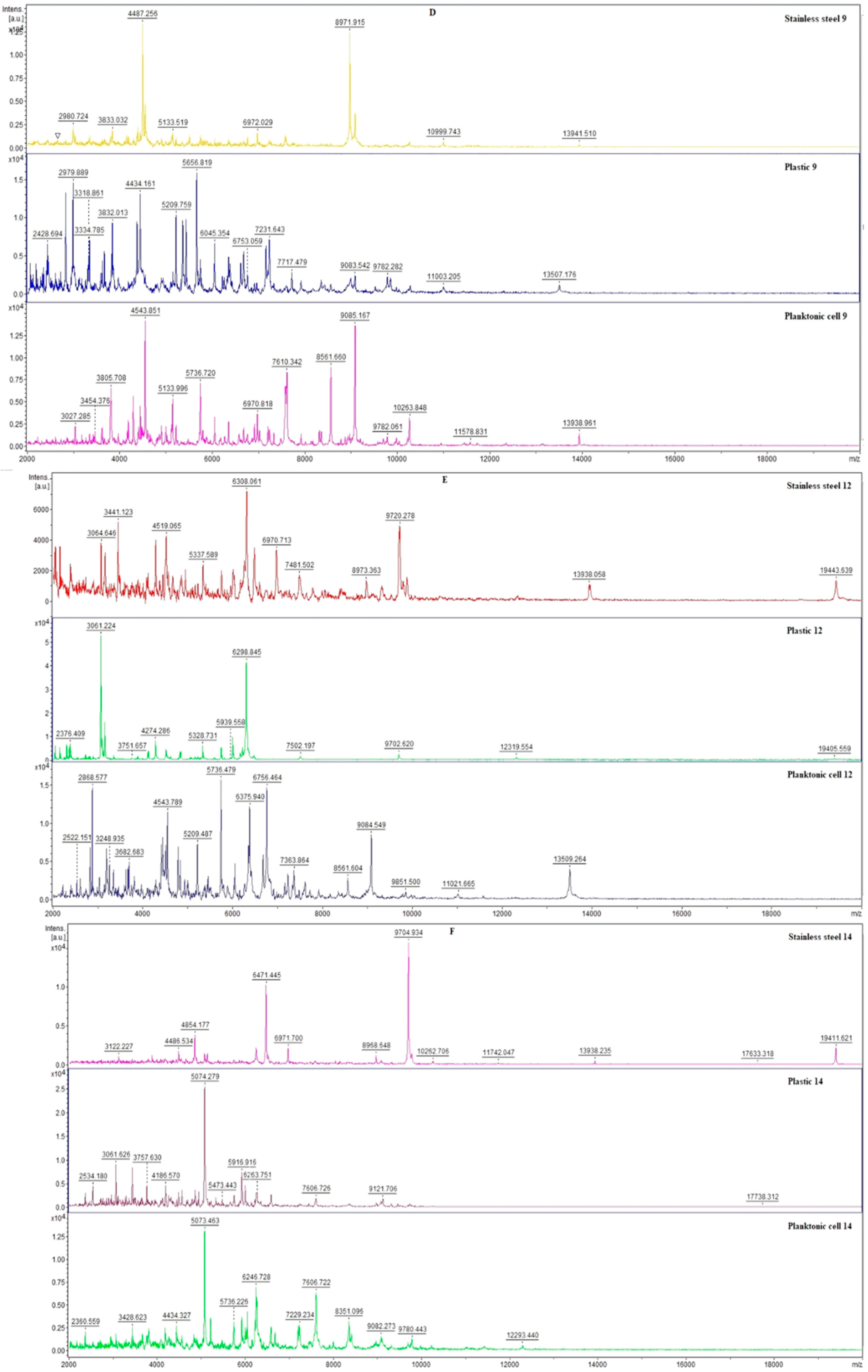
2.4. Antimicrobial Activity In Situ
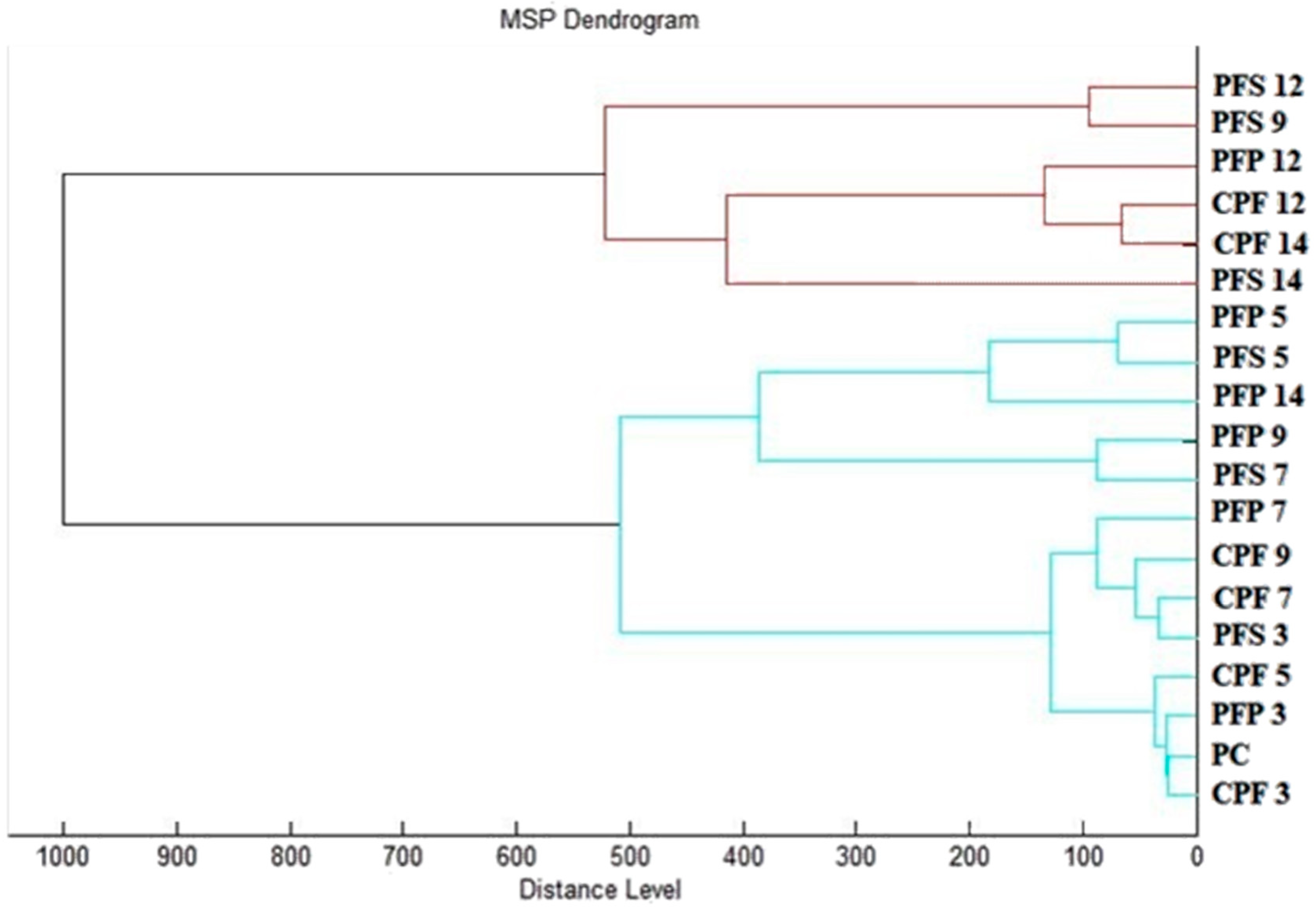
2.5. Antibiofilm Activity
2.6. Insecticidal Activity of SSEO
3. Discussion
4. Materials and Methods
4.1. Essential Oils and Standard
4.2. Gas Chromatography–Mass Spectrometry and Gas Chromatography Analyses
4.3. Antioxidant Activity
4.4. Microorganisms
4.5. Disc Diffusion Method
4.6. Broth Microdilution Method
4.7. Analysis of Biofilm Degradation
4.8. Insecticidal Activity
4.9. Statistical Data Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stefanakis, M.K.; Touloupakis, E.; Anastasopoulos, E.; Ghanotakis, D.; Katerinopoulos, H.E.; Makridis, P. Antibacterial Activity of Essential Oils from Plants of the Genus Origanum. Food Control 2013, 34, 539–546. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.; Zhou, H.; Zhao, C.; Lin, L. Antimicrobial Activity and Mechanisms of Salvia sclarea Essential Oil. Bot. Stud. 2015, 56, 16. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Mohan, M.; Singh, P.; Palni, U.T.; Tripathi, N.N. Chemical Composition, Antibacterial and Antioxidant Activity of Essential Oil of Eupatorium adenophorum Spreng. from Eastern Uttar Pradesh, India. Food Biosci. 2014, 7, 80–87. [Google Scholar] [CrossRef]
- Tian, J.; Zeng, X.; Zhang, S.; Wang, Y.; Zhang, P.; Lü, A.; Peng, X. Regional Variation in Components and Antioxidant and Antifungal Activities of Perilla frutescens Essential Oils in China. Ind. Crops. Prod. 2014, 59, 69–79. [Google Scholar] [CrossRef]
- Falleh, H.; ben Jemaa, M.; Saada, M.; Ksouri, R. Essential Oils: A Promising Eco-Friendly Food Preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G.; Pavoni, L.; Bonacucina, G.; Cespi, M.; Cianfaglione, K.; Bajalan, I.; Morshedloo, M.R.; Lupidi, G.; Romano, D.; et al. Microemulsions for Delivery of Apiaceae Essential Oils—Towards Highly Effective and Eco-Friendly Mosquito Larvicides? Ind. Crops. Prod. 2019, 129, 631–640. [Google Scholar] [CrossRef]
- El-Gohary, A.E.; Amer, H.M.; Salama, A.B.; Wahba, H.E.; Khalid, K.A. Characterization of the Essential Oil Components of Adapted Salvia sclarea L. (Clary sage) Plant Under Egyptian Environmental Conditions. J. Essent. Oil Bear. Plants 2020, 23, 788–794. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Cvetković, M.T.; Stanković Jeremić, J.M.; Pezo, L.L.; Varga, A.O.; Čabarkapa, I.S.; Kiprovski, B. Biological Activity and Profiling of Salvia sclarea Essential Oil Obtained by Steam and Hydrodistillation Extraction Methods via Chemometrics Tools. Flavour. Fragr. J. 2022, 37, 20–32. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Iapichino, G.; Licata, M.; Virga, G.; Leto, C.; la Bella, S. Agronomic Evaluation and Chemical Characterization of Sicilian Salvia sclarea L. Accessions. Agronomy 2020, 10, 1114. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Trikka, F.A.; Tsoktouridis, G.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Maloupa, E.; Makris, A.M. Micropropagation and Cultivation of Salvia sclarea for Essential Oil and Sclareol Production in Northern Greece. Vitr. Cell. Dev. Biol.-Plant 2020, 56, 51–59. [Google Scholar] [CrossRef]
- Lattoo, S.K.; Dhar, R.S.; Dhar, A.K.; Sharma, P.R.; Agarwal, S.G. Dynamics of Essential Oil Biosynthesis in Relation to Inflorescence and Glandular Ontogeny InSalvia Sclarea. Flavour. Fragr. J. 2006, 21, 817–821. [Google Scholar] [CrossRef]
- Pešić, P.Ž.; Banković, V.M. Investigation on the Essential Oil of Cultivated Salvia sclarea L. Flavour. Fragr. J. 2003, 18, 228–230. [Google Scholar] [CrossRef]
- Karayel, H.B. Effect of Natural Boron Mineral Use on the Essential Oil Ratio and Components of Musk Sage (Salvia sclarea L.). Open Chem. 2020, 18, 732–739. [Google Scholar] [CrossRef]
- Randjelović, M.; Branković, S.; Miladinović, B.; Milutinović, M.; Živanović, S.; Mihajilov-Krstev, T.; Kitić, D. The Benefits of Salvia sclarea L. Ethanolic Extracts on Gastrointestinal and Respiratory Spasms. S. Afr. J. Bot. 2022, 150, 621–632. [Google Scholar] [CrossRef]
- Kumar Singh, V.; Das, S.; Kumar Dwivedy, A.; Kumar Chaudhari, A.; Upadhyay, N.; Dubey, N.K. Assessment of Chemically Characterized Salvia sclarea L. Essential Oil and Its Combination with Linalyl Acetate as Novel Plant Based Antifungal, Antiaflatoxigenic and Antioxidant Agent against Herbal Drugs Contamination and Probable Mode of Action. Nat. Prod. Res. 2021, 35, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Acimovic, M.G.; Loncar, B.L.; Jeliazkov, V.D.; Pezo, L.L.; Ljujic, J.P.; Miljkovic, A.R.; Vujisic, L.V. Comparison of Volatile Compounds from Clary Sage (Salvia sclarea L.) Verticillasters Essential Oil and Hydrolate. J. Essent. Oil Bear. Plants 2022, 25, 555–570. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; la Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Gyrdymova, Y.V.; Rubtsova, S.A. Caryophyllene and Caryophyllene Oxide: A Variety of Chemical Transformations and Biological Activities. Chemical. Pap. 2022, 76, 1–39. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-Caryophyllene and β-Caryophyllene Oxide-Natural Compounds of Anticancer and Analgesic Properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Myslivečková, Z.; Szotáková, B.; Špičáková, A.; Lněničková, K.; Ambrož, M.; Kubíček, V.; Krasulová, K.; Anzenbacher, P.; Skálová, L. The Inhibitory Effects of β-Caryophyllene, β-Caryophyllene Oxide and α-Humulene on the Activities of the Main Drug-Metabolizing Enzymes in Rat and Human Liver in Vitro. Chem. Biol. Interact. 2017, 278, 123–128. [Google Scholar] [CrossRef]
- Legault, J.; Pichette, A. Potentiating Effect of β-Caryophyllene on Anticancer Activity of α-Humulene, Isocaryophyllene and Paclitaxel. J. Pharm. Pharmacol. 2010, 59, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. The Pharmacology of Meropenem, a New Carbapenem Antibiotic. Clin. Infect. Dis. 1997, 24, S266–S275. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.R. Meropenem: A Microbiological Overview. J. Antimicrob. Chemother. 1995, 36, 1–17. [Google Scholar] [CrossRef]
- Raza, A.; Ngieng, S.C.; Sime, F.B.; Cabot, P.J.; Roberts, J.A.; Popat, A.; Kumeria, T.; Falconer, J.R. Oral Meropenem for Superbugs: Challenges and Opportunities. Drug Discov. Today 2021, 26, 551–560. [Google Scholar] [CrossRef]
- Liebchen, U.; Rakete, S.; Vogeser, M.; Arend, F.M.; Kinast, C.; Scharf, C.; Zoller, M.; Schönermarck, U.; Paal, M. The Role of Non-Enzymatic Degradation of Meropenem—Insights from the Bottle to the Body. Antibiotics 2021, 10, 715. [Google Scholar] [CrossRef] [PubMed]
- Lutsar, I.; Chazallon, C.; Trafojer, U.; de Cabre, V.M.; Auriti, C.; Bertaina, C.; Calo Carducci, F.I.; Canpolat, F.E.; Esposito, S.; Fournier, I.; et al. Meropenem vs Standard of Care for Treatment of Neonatal Late Onset Sepsis (NeoMero1): A Randomised Controlled Trial. PLoS ONE 2020, 15, e0229380. [Google Scholar] [CrossRef]
- Pop-Vicas, A.; Opal, S.M. The Clinical Impact of Multidrug-Resistant Gram-Negative Bacilli in the Management of Septic Shock. Virulence 2014, 5, 206–212. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Kalemba, D.; Różalski, M.; Różalska, B.; Więckowska-Szakiel, M.; Krajewska, U.; Wysokińska, H. Chemical Composition and Biological Activities of Essential Oil from Salvia sclarea Plants Regenerated in Vitro. Molecules 2009, 14, 1438–1447. [Google Scholar] [CrossRef]
- Dzamic, A.; Sokovic, M.; Ristic, M.; Grujic-Jovanovic, S.; Vukojevic, J.; Marin, P.D. Chemical Composition and Antifungal Activity of Salvia sclarea (Lamiaceae) Essential Oil. Arch. Biol. Sci. 2008, 60, 233–237. [Google Scholar] [CrossRef]
- Souleles, C.; Argyriadou, N. Constituents of the Essential Oil of Salvia sclarea Growing Wild in Greece. Int. J. Pharmacogn. 1997, 35, 218–220. [Google Scholar] [CrossRef]
- Pitarokili, D.; Couladis, M.; Petsikos-Panayotarou, N.; Tzakou, O. Composition and Antifungal Activity on Soil-Borne Pathogens of the Essential Oil of Salvia sclarea from Greece. J. Agric. Food Chem. 2002, 50, 6688–6691. [Google Scholar] [CrossRef] [PubMed]
- Ovidi, E.; Laghezza Masci, V.; Zambelli, M.; Tiezzi, A.; Vitalini, S.; Garzoli, S. Laurus Nobilis, Salvia Sclarea and Salvia Officinalis Essential Oils and Hydrolates: Evaluation of Liquid and Vapor Phase Chemical Composition and Biological Activities. Plants 2021, 10, 707. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH Assays to Measure Antioxidant Capacity in Popular Antioxidant-Rich US Foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Pierozan, M.K.; Pauletti, G.F.; Rota, L.; dos Santos, A.C.A.; Lerin, L.A.; di Luccio, M.; Mossi, A.J.; Atti-Serafini, L.; Cansian, R.L.; Oliveira, J.V. Chemical Characterization and Antimicrobial Activity of Essential Oils of Salvia L. Species. Ciência Tecnol. Aliment. 2009, 29, 764–770. [Google Scholar] [CrossRef]
- Aćimović, M.; Kiprovski, B.; Rat, M.; Sikora, V.; Popović, V.; Koren, A.; Brdar-Jokanović, M. Salvia sclarea: Chemical Composition and Biological Activity. J. Agron. Technol. Eng. Manag. 2018, 1, 18–28. [Google Scholar]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Schmidt, E.; Bail, S.; Friedl, S.M.; Jirovetz, L.; Buchbauer, G.; Wanner, J.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Geissler, M. Antimicrobial Activities of Single Aroma Compounds. Nat. Prod. Commun. 2010, 5, 1365–1368. [Google Scholar] [CrossRef]
- Yoo, H.-J.; Jwa, S.-K. Efficacy of β-Caryophyllene for Periodontal Disease Related Factors. Arch. Oral Biol. 2019, 100, 113–118. [Google Scholar] [CrossRef]
- Pieri, F.A.; de Castro Souza, M.C.; Vermelho, L.L.R.; Vermelho, M.L.R.; Perciano, P.G.; Vargas, F.S.; Borges, A.P.B.; da Veiga-Junior, V.F.; Moreira, M.A.S. Use of β-Caryophyllene to Combat Bacterial Dental Plaque Formation in Dogs. BMC Vet. Res. 2016, 12, 216. [Google Scholar] [CrossRef]
- Jones, R.N.; Rhomberg, P.R.; Varnam, D.J.; Mathai, D. A Comparison of the Antimicrobial Activity of Meropenem and Selected Broad-Spectrum Antimicrobials Tested against Multi-Drug Resistant Gram-Negative Bacilli Including Bacteraemic Salmonella spp. Int. J. Antimicrob. Agents 2002, 20, 426–431. [Google Scholar] [CrossRef]
- Joly-Guillou, M.-L.; Kempf, M.; Cavallo, J.-D.; Chomarat, M.; Dubreuil, L.; Maugein, J.; Muller-Serieys, C.; Roussel-Delvallez, M. Comparative in Vitro Activity of Meropenem, Imipenem and Piperacillin/Tazobactam against 1071 Clinical Isolates Using 2 Different Methods: A French Multicentre Study. BMC Infect. Dis. 2010, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Sumitani, Y.; Inose, R.; Katohno, Y. Antimicrobial Activity of Meropenem against Main Bacterial Species Isolated from Patient Blood in 2010. Jpn. J. Antibiot. 2011, 64, 355–365. [Google Scholar] [PubMed]
- Yang, H.S.; Lee, E.J.; Moon, S.; Paik, H.D.; Ahn, D.U. Effect of Garlic, Onion, and Their Combination on the Quality and Sensory Characteristics of Irradiated Raw Ground Beef. Meat Sci. 2013, 89, 202–208. [Google Scholar] [CrossRef] [PubMed]
- van Haute, S.; Raes, K.; van der Meeren, P.; Sampers, I. The Effect of Cinnamon, Oregano and Thyme Essential Oils in Marinade on the Microbial Shelf Life of Fish and Meat Products. Food Control 2016, 68, 30–39. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Antimicrobial Potential and Chemical Composition of Eucalyptus globulus Oil in Liquid and Vapour Phase against Food Spoilage Microorganisms. Food Chem. 2011, 126, 228–235. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J. Constituents of the Essential Oils of Garlic and Citronella and Their Vapor-Phase Inhibition Mechanism against S. aureus. Food Sci. Technol. Res. 2019, 25, 65–74. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cran, M.J.; Bigger, S.W.; Hernández-Muñoz, P.; Gavara, R. Antioxidant and Antimicrobial Properties of Ethylene Vinyl Alcohol Copolymer Films Based on the Release of Oregano Essential Oil and Green Tea Extract Components. J. Food Eng. 2015, 149, 9–16. [Google Scholar] [CrossRef]
- Kwon, S.-J.; Chang, Y.; Han, J. Oregano Essential Oil-Based Natural Antimicrobial Packaging Film to Inactivate Salmonella enterica and Yeasts/Molds in the Atmosphere Surrounding Cherry Tomatoes. Food Microbiol. 2017, 65, 114–121. [Google Scholar] [CrossRef]
- dos Santos Rodrigues, J.B.; de Carvalho, R.J.; de Souza, N.T.; de Sousa Oliveira, K.; Franco, O.L.; Schaffner, D.; de Souza, E.L.; Magnani, M. Effects of Oregano Essential Oil and Carvacrol on Biofilms of Staphylococcus aureus from Food-Contact Surfaces. Food Control 2017, 73, 1237–1246. [Google Scholar] [CrossRef]
- Camo, J.; Lorés, A.; Djenane, D.; Beltrán, J.A.; Roncalés, P. Display Life of Beef Packaged with an Antioxidant Active Film as a Function of the Concentration of Oregano Extract. Meat Sci. 2011, 88, 174–178. [Google Scholar] [CrossRef]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The Antibacterial and Antifungal Activity of Six Essential Oils and Their Cyto/Genotoxicity to Human HEL 12469 Cells. Sci. Rep. 2017, 7, 8211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, X.; Yu, C.; Wang, Y. Promising Therapeutic Strategies against Microbial Biofilm Challenges. Front. Cell Infect. Microbiol. 2020, 10, 359. [Google Scholar] [CrossRef]
- Vetrivel, A.; Ramasamy, M.; Vetrivel, P.; Natchimuthu, S.; Arunachalam, S.; Kim, G.-S.; Murugesan, R. Pseudomonas Aeruginosa Biofilm Formation and Its Control. Biologics 2021, 1, 312–336. [Google Scholar] [CrossRef]
- de Jesus Pimentel-Filho, N.; Martins, M.C.d.F.; Nogueira, G.B.; Mantovani, H.C.; Vanetti, M.C.D. Bovicin HC5 and Nisin Reduce Staphylococcus aureus Adhesion to Polystyrene and Change the Hydrophobicity Profile and Gibbs Free Energy of Adhesion. Int. J. Food Microbiol. 2014, 190, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fastenberg, J.H.; Hsueh, W.D.; Mustafa, A.; Akbar, N.A.; Abuzeid, W.M. Biofilms in Chronic Rhinosinusitis: Pathophysiology and Therapeutic Strategies. World J. Otorhinolaryngol. Head Neck Surg. 2016, 2, 219–229. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus Biofilm: An Emerging Battleground in Microbial Communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; de Martino, L.; Coppola, R.; de Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; El-Saadony, M.T.; Alagawany, M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Elwan, H.A.M.; Elnesr, S.S.; El-Hack, M.E.A. Using Essential Oils to Overcome Bacterial Biofilm Formation and Their Antimicrobial Resistance. Saudi. J. Biol. Sci. 2021, 28, 5145–5156. [Google Scholar] [CrossRef]
- Szczepanski, S.; Lipski, A. Essential Oils Show Specific Inhibiting Effects on Bacterial Biofilm Formation. Food Control 2014, 36, 224–229. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Pejčić, M.; Stojanović, N.; Sharifi-Rad, J.; Stanković, N. Potential of Ocimum basilicum L. and Salvia officinalis L. Essential Oils against Biofilms of P. aeruginosa Clinical Isolates. Cell Mol. Biol. 2016, 62, 27–33. [Google Scholar]
- Isman, M.B. Plant Essential Oils for Pest and Disease Management. Crop. Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Ayvaz, A.; Sagdic, O.; Karaborklu, S.; Ozturk, I. Insecticidal Activity of the Essential Oils from Different Plants against Three Stored-Product Insects. J. Insect Sci. 2010, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Chantraine, J.-M.; Laurent, D.; Ballivian, C.; Saavedra, G.; Ibañez, R.; Vilaseca, L.A. Insecticidal Activity of Essential Oils On Aedes aegypti Larvae. Phytother. Res. 1998, 12, 350–354. [Google Scholar] [CrossRef]
- Enan, E. Insecticidal Activity of Essential Oils: Octopaminergic Sites of Action. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Pavela, R. Insecticidal Activity of Some Essential Oils against Larvae of Spodoptera Littoralis. Fitoterapia 2005, 76, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Neem and Other Botanical Insecticides: Barriers to Commercialization. Phytoparasitica 1997, 25, 339–344. [Google Scholar] [CrossRef]
- Rozman, V.; Kalinovic, I.; Korunic, Z. Toxicity of Naturally Occurring Compounds of Lamiaceae and Lauraceae to Three Stored-Product Insects. J. Stored Prod. Res. 2007, 43, 349–355. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- van den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Proestos, C.; Lytoudi, K.; Mavromelanidou, O.; Zoumpoulakis, P.; Sinanoglou, V. Antioxidant Capacity of Selected Plant Extracts and Their Essential Oils. Antioxidants 2013, 2, 11–22. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Galovičová, L.; Ivanišová, E.; Štefániková, J.; Valková, V.; Borotová, P.; Kowalczewski, P.Ł.; Kunová, S.; Felšöciová, S.; et al. Biological Activity and Antibiofilm Molecular Profile of Citrus aurantium Essential Oil and Its Application in a Food Model. Molecules 2020, 25, 3956. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Valková, V.; Ďuranová, H.; Borotová, P.; Štefániková, J.; Vukovic, N.L.; Vukic, M.; Kunová, S.; Felsöciová, S.; et al. Chemical Composition and Biological Activity of Salvia officinalis Essential Oil. Acta Hortic. Regiotect. 2021, 24, 81–88. [Google Scholar] [CrossRef]
- Vatľák, A.; Kolesárová, A.; Vukovič, N.; Rovná, K.; Petrová, J.; Vimmerová, V.; Hleba, L.; Mellen, M.; Kačániová, M. Antimicrobial activity of medicinal plants against different strains of bacteria. J. Microb. Biot. Food Sci. 2014, 3, 174–176. [Google Scholar]
| No | RI(calc) a | RI(lit) | Compound b | % |
|---|---|---|---|---|
| 1 | 858 | 859 | cis-3-hexenol | tr c |
| 2 | 938 | 939 | α-pinene | 2.4 |
| 3 | 948 | 954 | camphene | tr |
| 4 | 977 | 975 | sabinene | tr |
| 5 | 980 | 979 | β-pinene | 0.2 |
| 6 | 992 | 990 | β-myrcene | 0.6 |
| 7 | 1004 | 1002 | α-phellandrene | tr |
| 8 | 1009 | 1011 | δ-3-carene | tr |
| 9 | 1016 | 1010 | α-terpinene | tr |
| 10 | 1023 | 1024 | p-cymene | 4.9 |
| 11 | 1028 | 1029 | α-limonene | 2.2 |
| 12 | 1038 | 1037 | (Z)-β-ocimene | 0.3 |
| 13 | 1047 | 1050 | (E)-β-ocimene | 0.5 |
| 14 | 1074 | 1072 | cis-linalool oxide | tr |
| 15 | 1088 | 1088 | α-terpinolene | tr |
| 16 | 1089 | 1086 | trans-linalool oxide | tr |
| 17 | 1098 | 1096 | linalool | 20.6 |
| 18 | 1189 | 1188 | α-terpineol | 4.9 |
| 19 | 1227 | 1229 | nerol | 1.1 |
| 20 | 1238 | 1238 | neral | tr |
| 21 | 1255 | 1257 | linalool acetate | 49.1 |
| 22 | 1286 | 1285 | bornyl acetate | 0.6 |
| 26 | 1299 | 1298 | geranyl formate | tr |
| 27 | 1364 | 1361 | neryl acetate | 1.7 |
| 28 | 1379 | 1375 | α-copaene | 0.2 |
| 29 | 1380 | 1381 | geranyl acetate | 4.4 |
| 30 | 1385 | 1388 | β-bourbonene | tr |
| 31 | 1388 | 1390 | β-elemene | 0.2 |
| 32 | 1408 | 1409 | α-gurjunene | tr |
| 33 | 1422 | 1419 | (E)-caryophyllene | 5.1 |
| 34 | 1456 | 1454 | α-humulene | tr |
| 35 | 1483 | 1481 | germacrene D | 0.2 |
| 36 | 1498 | 1496 | ledene | tr |
| 37 | 1502 | 1500 | bicyclogermacrene | 0.2 |
| 38 | 1525 | 1523 | δ-cadinene | tr |
| 39 | 1583 | 1583 | caryophyllene oxide | 0.3 |
| total | 99.6 |
| Class of Compounds | % |
|---|---|
| Monoterpenes | 93.5 |
| Monoterpene hydrocarbons | 11.1 |
| Oxygenated monoterpenes | 82.4 |
| Monoterpene epoxide | tr a |
| Monoterpene alcohols | 26.6 |
| Monoterpene aldehydes | tr |
| Monoterpene esters | 55.8 |
| Sesquiterpenes | 6.1 |
| Sesquiterpene hydrocarbons | 5.9 |
| Oxygenated sesquiterpenes | 0.2 |
| Sesquiterpene alcohols | tr |
| Sesquiterpene epoxides | 0.3 |
| Non-terpenic | tr |
| Alcohols | tr |
| Total | 99.6 |
| Microorganism | Inhibition Zone | Activity of EO | Control |
|---|---|---|---|
| Gram-positive bacteria | |||
| Bacillus subtilis | 12.00 ± 1.00 | *** | 33 ± 1.0 |
| Enterococcus faecalis | 4.67 ± 0.58 | * | 29 ± 0.5 |
| Staphylococcus aureus | 6.67 ± 0.58 | ** | 32 ± 1.0 |
| Gram-negative bacteria | |||
| Pseudomonas aeruginosa | 8.00 ± 1.00 | ** | 25 ± 1.0 |
| Salmonella enterica | 3.67 ± 0.58 | * | 27 ± 2.0 |
| Yersinia enterocolitica | 3.67 ± 0.58 | * | 27 ± 1.5 |
| Pseudomonas fluorescens biofilm | 7.67 ± 0.58 | ** | 28 ± 1.0 |
| Yeasts | |||
| Candida albicans | 11.33 ± 0.58 | *** | 28 ± 2.0 |
| Candida glabrata | 8.33 ± 0.58 | ** | 33 ± 1.5 |
| Candida krusei | 7.67 ± 0.58 | ** | 33 ± 3.0 |
| Candida tropicalis | 7.67 ± 0.58 | ** | 33 ± 1.0 |
| Fungi | |||
| Aspergillus flavus | 10.33 ± 0.58 | ** | 32 ± 0.58 |
| Botrytis cinerae | 9.67 ± 0.58 | ** | 33 ± 1.0 |
| Penicillium citrinum | 8.67 ± 0.58 | ** | 31 ± 0.58 |
| Microorganism | Inhibition Zone | Activity | Control |
|---|---|---|---|
| Gram-positive bacteria | |||
| Bacillus subtilis | 3.67 ± 0.58 | * | 35 ± 0.5 |
| Enterococcus faecalis | 5.33 ± 0.58 | ** | 34 ± 0.5 |
| Staphylococcus aureus | 5.67 ± 0.58 | ** | 33 ± 1.0 |
| Gram-negative bacteria | |||
| Pseudomonas aeruginosa | 5.33 ± 1.00 | ** | 35 ± 1.0 |
| Salmonella enterica | 5.67 ± 0.58 | ** | 34 ± 2.0 |
| Yersinia enterocolitica | 6.67 ± 0.58 | ** | 34 ± 1.5 |
| Pseudomonas fluorescens biofilm | 5.67 ± 0.58 | ** | 33 ± 1.0 |
| Yeasts | |||
| Candida albicans | 5.33 ± 0.58 | ** | 34 ± 2.0 |
| Candida glabrata | 5.33 ± 0.58 | ** | 31 ± 1.5 |
| Candida krusei | 7.67 ± 0.58 | ** | 33 ± 2.0 |
| Candida tropicalis | 5.67 ± 0.58 | ** | 33 ± 1.0 |
| Fungi | |||
| Aspergillus flavus | 5.33 ± 0.58 | ** | 28 ± 1.0 |
| Botrytis cinerae | 5.67 ± 0.58 | ** | 31 ± 1.0 |
| Penicillium citrinum | 5.67 ± 0.58 | ** | 28 ± 1.5 |
| Microorganism | SSEO | (E)-Caryophyllene | Meropenem | |||
|---|---|---|---|---|---|---|
| MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | |
| µL/mL | µg/mL | µg/mL | ||||
| Gram-positive bacteria | ||||||
| Bacillus subtilis | 187.31 | 199.21 | 0.37 | 0.44 | 23.44 | 4.24 |
| Enterococcus faecalis | 46.89 | 50.07 | 0.22 | 0.39 | 11.72 | 13.74 |
| Staphylococcus aureus | 1.49 | 1.59 | 0.22 | 0.39 | 93.80 | 98.56 |
| Gram-negative bacteria | ||||||
| Pseudomonas aeruginosa | 46.89 | 50.07 | 0.37 | 0.44 | 187.31 | 199.87 |
| Salmonella enterica | 6.56 | 25.46 | 0.37 | 0.44 | 93.80 | 98.56 |
| Yersinia enterocolitica | 31.02 | 53.60 | 0.37 | 0.44 | 0.73 | 0.98 |
| Pseudomonas fluorescens biofilm | 2.93 | 3.17 | 0.22 | 0.39 | 93.80 | 98.56 |
| Yeasts | ||||||
| Candida albicans | 131.99 | 153.98 | 0.56 | 0.67 | 2.93 | 4.24 |
| Candida glabrata | 31.02 | 53.60 | 0.75 | 0.89 | 2.93 | 4.24 |
| Candida krusei | 11.72 | 12.58 | 0.75 | 0.89 | 5.86 | 7.89 |
| Candida tropicalis | 2.93 | 3.17 | 0.56 | 0.67 | 298.92 | 324.56 |
| Fungi | Concentration of SSEO | Inhibition Zone in mm |
|---|---|---|
| Aspergillus flavus | 500 µL/mL | 8.00 ± 3.00 a |
| 250 µL/mL | 3.33 ± 0.58 b,a | |
| 125 µL/mL | 1.67 ± 0.58 c,a | |
| 62.5 µL/mL | 2.33 ± 0.58 d,a | |
| Botrytis cinerea | 500 µL/mL | 8.67 ± 0.58 a |
| 250 µL/mL | 9.67 ± 1.53 b | |
| 125 µL/mL | 5.67 ± 0.58 c,a,b | |
| 62.5 µL/mL | 6.67 ± 0.58 d,b | |
| Penicillium citrinum | 500 µL/mL | 6.00 ± 1.00 a |
| 250 µL/mL | 7.33 ± 0.58 b,a | |
| 125 µL/mL | 6.00 ± 1.00 c,b | |
| 62.5 µL/mL | 5.33 ± 0.58 d,b |
| Bacteria | Bacterial Growth Inhibition (%) | ||||
|---|---|---|---|---|---|
| The Concentration of SSEO | |||||
| 62.5 μL/L | 125 μL/L | 250 μL/L | 500 μL/L | ||
| Gram-positive | B. subtilis | 24.86 ± 2.56 b | 46.85 ± 1.52 d | 32.34 ± 2.08 c | −11.59 ± 0.90 a |
| E. faecalis | 64.71 ± 0.78 d | 42.22 ± 1.36 c | 24.85 ± 2.66 b | 15.27 ± 1.62 a | |
| S. aureus | 73.71 ± 2.06 d | 53.98 ± 2.66 c | 24.07 ± 2.53 b | 12.75 ± 0.96 a | |
| Gram-negative | P. flourescens biofilm | 56.46 ± 1.33 d | 46.85 ± 1.52 c | 24.92 ± 1.68 b | 15.04 ± 0.57 a |
| P. aeroginosa | 25.37 ± 1.58 b | 8.48 ± 1.51 a | 67.74 ± 1.89 c | 7.77 ± 1.94 a | |
| S. enterica | 74.79 ± 3.66 d | 31.87 ± 1.48 b | 54.66 ± 1.82 c | 7.56 ± 1.09 a | |
| Y. enterocolitica | 67.61 ± 1.84 c | 43.78 ± 1.89 b | 12.26 ± 1.51 a | 64.87 ± 2.60 c | |
| Yeasts | C. albicans | 34.09 ± 1.25 d | 8.71 ± 0.84 c | −7.96 ± 1.35 b | −12.37 ± 1.40 a |
| C. glabrata | 64.01 ± 1.68 d | 44.59 ± 1.20 c | 33.66 ± 1.98 b | 14.92 ± 1.10 a | |
| C. krusei | 43.71 ± 0.95 c | 67.61 ± 0.86 d | 16.94 ± 1.54 b | 7.89 ± 1.45 a | |
| C. tropicalis | −6.25 ± 0.65 b | 14.20 ± 1.56 c | 44.66 ± 1.20 d | −15.26 ± 2.62 a | |
| Microscopic fungi | A. flavus | 64.11 ± 1.15 d | 35.88 ± 1.06 c | 7.11 ± 0.47 a | 13.63 ± 0.99 b |
| B. cinerea | 73.00 ± 1.16 d | 53.66 ± 1.91 c | 34.81 ± 2.0 b | 16.63 ± 0.88 a | |
| P. citrinum | 87.34 ± 2.22 d | 53.66 ± 2.06 c | 35.11 ± 1.50 b | 23.70 ± 2.22 a | |
| Concentration (%) | Number of Living Individuals | Number of Dead Individuals | Insecticidal Activity (%) |
|---|---|---|---|
| 100 | 10 | 20 | 66.66 |
| 50 | 15 | 15 | 50.00 |
| 25 | 17 | 13 | 43.33 |
| 12.5 | 25 | 5 | 16.66 |
| 6.25 | 30 | 30 | 0.00 |
| 3.125 | 30 | 0 | 0.00 |
| Control group | 30 | 0 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kačániová, M.; Vukovic, N.L.; Čmiková, N.; Galovičová, L.; Schwarzová, M.; Šimora, V.; Kowalczewski, P.Ł.; Kluz, M.I.; Puchalski, C.; Bakay, L.; et al. Salvia sclarea Essential Oil Chemical Composition and Biological Activities. Int. J. Mol. Sci. 2023, 24, 5179. https://doi.org/10.3390/ijms24065179
Kačániová M, Vukovic NL, Čmiková N, Galovičová L, Schwarzová M, Šimora V, Kowalczewski PŁ, Kluz MI, Puchalski C, Bakay L, et al. Salvia sclarea Essential Oil Chemical Composition and Biological Activities. International Journal of Molecular Sciences. 2023; 24(6):5179. https://doi.org/10.3390/ijms24065179
Chicago/Turabian StyleKačániová, Miroslava, Nenad L. Vukovic, Natália Čmiková, Lucia Galovičová, Marianna Schwarzová, Veronika Šimora, Przemysław Łukasz Kowalczewski, Maciej Ireneusz Kluz, Czeslaw Puchalski, Ladislav Bakay, and et al. 2023. "Salvia sclarea Essential Oil Chemical Composition and Biological Activities" International Journal of Molecular Sciences 24, no. 6: 5179. https://doi.org/10.3390/ijms24065179
APA StyleKačániová, M., Vukovic, N. L., Čmiková, N., Galovičová, L., Schwarzová, M., Šimora, V., Kowalczewski, P. Ł., Kluz, M. I., Puchalski, C., Bakay, L., & Vukic, M. D. (2023). Salvia sclarea Essential Oil Chemical Composition and Biological Activities. International Journal of Molecular Sciences, 24(6), 5179. https://doi.org/10.3390/ijms24065179









