Abstract
Sustained tissue hypoxia is associated with many pathophysiological conditions, including chronic inflammation, chronic wounds, slow-healing fractures, microvascular complications of diabetes, and metastatic spread of tumors. This extended deficiency of oxygen (O2) in the tissue sets creates a microenvironment that supports inflammation and initiates cell survival paradigms. Elevating tissue carbon dioxide levels (CO2) pushes the tissue environment toward “thrive mode,” bringing increased blood flow, added O2, reduced inflammation, and enhanced angiogenesis. This review presents the science supporting the clinical benefits observed with the administration of therapeutic CO2. It also presents the current knowledge regarding the cellular and molecular mechanisms responsible for the biological effects of CO2 therapy. The most notable findings of the review include (a) CO2 activates angiogenesis not mediated by hypoxia-inducible factor 1a, (b) CO2 is strongly anti-inflammatory, (c) CO2 inhibits tumor growth and metastasis, and (d) CO2 can stimulate the same pathways as exercise and thereby, acts as a critical mediator in the biological response of skeletal muscle to tissue hypoxia.
1. Introduction
Oxygen (O2) is carried in the blood and delivered to cells in tissues. O2 is necessary for normal cell respiration, serving as an electron acceptor in the electron transport system. Carbon dioxide (CO2) is produced as a byproduct of cellular respiration. Normal tissue function requires a balance between O2 delivery and CO2 removal. When blood flow is too low or when tissue O2 demand exceeds delivery (such as during exercise), tissue hypoxia ensues, and metabolic byproducts, including CO2, build up in the tissues [1,2]. CO2 causes dilation of blood vessels, bringing about greater blood flow, increased O2 delivery, and removal of vasodilatory metabolites. The resulting reduction in vasodilatory signals brings blood flow back to homeostatic levels. This metabolic regulation of blood flow guarantees an adequate supply of blood to the tissues and microvascular stability. In this way, the local supply of O2 by the circulation is matched to the local demand by the tissues.
Cardiovascular and microvascular diseases can be caused by the chronic disruption of normal blood flow that makes previously healthy tissues cascade into a severe and, at times, permanent pathological condition. The condition caused by sustained hypoxia results in a slowed metabolism that is dependent on glycolytic pathways and a chronic inflammatory state that is perpetuated by an increase in hypoxia-inducible factor 1α (HIF-1α) production. Multiple transcriptional responses to HIF-1α promote a survival response in cells, preventing cell death in response to continued hypoxia [3]. Beyond cell survival, a major function of HIF-1α is the transcriptional activation of genes involved in new blood vessel growth. This neovascularization improves blood flow to supply more O2 to the tissue and reverse the hypoxia.
In contrast with the survival mode induced by severe or chronic hypoxia, the limited or transient hypoxia brought about by increased metabolic demand in tissues results in an acute decrease in ATP stores that is sensed by cells via the action of adenosine monophosphate-activated protein kinase (AMPK) [4]. AMPK senses cellular energy status by monitoring the ATP:AMP ratio. When this ratio is decreased, AMPK restores energy balance by inhibiting anabolic reactions that consume ATP and promoting catabolic processes that generate ATP. AMPK activation in vascular endothelial cells results in vasodilation and new blood vessel growth to increase blood flow and oxygen delivery to the tissue, thus restoring normal tissue function [4].
CO2 levels in the tissue rise immediately in response to increased metabolic demand. Elevated tissue CO2 acts as a sensitive trigger for cellular responses to hypoxia, modulating biological signaling pathways (e.g., HIF-1α and AMPK) to restore normal tissue function and homeostasis. This review presents data highlighting the potential use of CO2 as a therapeutic approach to tissue hypoxia to promote tissue repair, reduce inflammation, reverse the pathophysiological effects of hyperglycemia, and enhance the efficacy of cancer therapy. Moreover, it summarizes the status of knowledge of the cellular and molecular mechanisms underlying these beneficial effects of therapeutic CO2 delivery.
2. Therapeutic Effects of Carbon Dioxide
The use of CO2 as a therapeutic treatment for various ailments dates back hundreds of years when people visited and built cities around the hot springs of Europe. The medicinal effects of the waters found in hot springs have been attributed to dissolved CO2 in the form of carbonic acid [5]. There are now many more methods for using therapeutic CO2. Such treatment regimens used today include carbonated water (i.e., baths) (CB) [6], CO2 gas applied to the skin (transdermal [TD]) [7,8], intra-arterial infusions of carbonated solutions into tumors (IA) [9], intraperitoneal injection of CO2 (IP) [10], inhaled CO2 or hypercarbia (IH) [11], carbonated paste or gel (CG) [12], and subcutaneous microinjections of CO2 (SQ) [13]. In all these therapeutic applications, except for SQ, CO2 is applied for 10–20 min. Treatment schedules vary from 1–3 days apart, and the number of treatments varies from one (SQ, IA, and IP) to 4–20 for the other therapies. Dosing studies are rare, but one study showed that treatments must be applied every 2–3 days to be effective in healing fractures [14]. No studies have noted any adverse side effects or complications from CO2 therapy except for SQ, where bleeding or bruising can occur. It should be noted, however, that CO2 is anti-inflammatory, so treating an infected wound should be done with caution.
Modern studies have shown that therapeutic CO2 applied to wounds, tumors, and through the skin to underlying tissues has dramatic and long-lasting effects on inflammation, oxygenation, angiogenesis, and healing. Clinical and experimental findings using therapeutic CO2 are summarized here.
2.1. Accelerated Wound and Fracture Healing
There are now several studies investigating the effects of therapeutic CO2 on wounds and fractures. Studies in rats treated with TD CO2 demonstrated increased survival of skin flaps due to increased blood flow and greater capillary density [15]. This correlated with increased gene expression of basic fibroblast growth factor and vascular endothelial growth factor (VEGF) but a paradoxical decrease in HIF-1α. In a retrospective study of topical TD CO2 treatment of 86 human patients with chronic wounds and 17 patients with acute wounds, all patients had resolution of their wounds [16]. The CO2 gas was able to penetrate the granulation tissue of the wound bed to support/accelerate healing while improving the microcirculation and reducing the incidence of infection. Humans with diabetes often have chronic wounds that do not heal and eventually lead to the need for amputation. A double-blind study showed that TD CO2 therapy would heal these long-standing wounds [17]. By the end of the study, 66% of the CO2-treated wounds had healed completely, and wound volume decreased by 99%. In the control group, none of the wounds completely healed.
Clinicians have very limited options to biologically improve bone fracture repair. Blood flow and angiogenesis at the site of a fracture are critical components of fracture repair. When rats with long bone fractures were treated with TD CO2, fracture union was accelerated [14,18]. For example, fracture union was evident at week 3 in 86% of animals in the treated group compared with 36% in the control group [18]. Increased blood flow and greater capillary density at the fracture site were accompanied by elevated expression of chondrogenic and osteogenic genes resulting in early production of collagen and cartilage. Increased angiogenesis and blood flow induced by the TD CO2 treatment likely promoted blood vessel invasion and transformation of the avascular cartilaginous matrix into vascularized osseous tissue.
In the Phase I clinical trial involving 19 human patients with fractures of the lower extremities, CO2 was applied to the affected limb, and blood flow was measured in both limbs [19]. This TD CO2 therapy promoted an approximately two-fold increase in blood flow in the fractured limbs. As a result of the increase in blood flow, enhanced local oxygenation likely contributed to tissue healing. Importantly, no adverse events, including hypercapnia, were noted in these patients, supporting the validity of continuing assessments of CO2 therapy in additional clinical trials. Increased blood flow and greater O2 delivery suggest that CO2 therapy will be beneficial for the treatment of fractures in humans, including fractures in patients with the ischemic disease or diabetes mellitus, in smokers, and in those individuals with avascular nonunion [19].
2.2. Increased Blood Flow to Ischemic Limbs
Treatment of ischemia in limbs due to peripheral vascular disease is very challenging and commonly includes multiple surgeries and loss of toes and/or limbs. Experiments in mice and rats with unilateral hindlimb ischemia showed that CB and TD CO2 therapy induced elevated levels of VEGF in addition to activating the nitric oxide-cyclic guanosine-monophosphate (NO-cGMP) system to increase blood flow to the limb [20,21]. Treatment increased capillary density and collateral vessel formation in the ischemic limb as well as the number of endothelial cell progenitors in the circulation [21].
TD CO2 improved blood flow and preserved tissue in humans with peripheral vascular disease [22,23,24]. In a study of patients with ischemic ulceration due to severe stenosis or vascular occlusion, 83% of limbs were salvaged with CO2 therapy, thus reducing the need for amputation in patients with critical limb ischemia (CB) [22]. Transcutaneous CO2 acts as a vasodilator, opening nonfunctioning capillaries to enhance the O2 diffusion area (CB) [23]. Increases in tissue CO2 concentration affect both blood flow (tissue perfusion) and the capacity of hemoglobin to release O2 (the shift in the O2 dissociation curve known as the Bohr effect). Therapeutic TD CO2 reduced claudication (pain caused by reduced blood flow to the legs) and increased pain-free and total walking distance by 66% and 73%, respectively, in patients with critical limb ischemia [24]. Importantly, these effects were maintained for 12 months after cessation of therapy.
2.3. Greater Blood Flow and Vascularization in Diabetes
Diabetes is a major risk factor for the development of peripheral vascular disease. In a rat model of diabetes mellitus with hindlimb ischemia, TD therapy resulted in a two-fold increase in peak and mean blood flow in hindlimb skeletal muscle as well as a two-fold increase in the number of small blood vessels in skeletal muscle sections (CB) [6]. Both the structure and function of the microvasculature of skeletal muscle are known to be altered in diabetes. Hyperglycemia, resulting from poorly controlled diabetes, reduces skeletal muscle capillarization via an imbalance in pro- and anti-angiogenic factors [25]. TD CO2 therapy in rats with streptozotocin-(STZ-)-induced diabetes, a model of type I diabetes, increased blood flow in the soleus muscle, attenuated capillary rarefaction and restored the balance of angiogenic growth factors [26].
In addition to causing changes in vascular architecture and density in skeletal muscle, hyperglycemia can reduce oxidative capacity, the measure of a muscle’s maximal capacity to use O2, by reducing mitochondrial enzymatic activity and biogenesis [27]. The activity of citrate synthase (CS), a key mitochondrial enzyme in the tricarboxylic acid cycle, is used as an indicator of the oxidative capacity of skeletal muscle. CS activity in the soleus muscle was reduced in STZ-induced diabetic rats compared to nondiabetic rats, but cutaneous CO2 treatment increased CS activity in diabetic rats compared to diabetic rats without treatment and brought CS activity up to levels measured in nondiabetic control rats [27]. The cytochrome c oxidase (COX) complex catalyzes the final step in the mitochondrial electron transfer chain and is regarded as one of the major regulation sites for mitochondrial function and, thus, oxidative phosphorylation. Hyperglycemia reduced levels of COX subunit 4, essential for the assembly and respiratory function of the COX complex, while TD CO2 attenuated this effect [27].
Oxidative capacity is regulated by peroxisome proliferator-activated receptor-gamma co-activator-1 alpha (PGC-1α), and low oxidative capacity in diabetes correlates with decreased PGC-1α expression. CO2 therapy increases NO production in skeletal muscle via an increase in blood flow. NO can increase PGC-1α protein expression via activation of cGMP and promotes mitochondrial biogenesis and function. Of note, the application of TD CO2 not only increased oxidative capacity in skeletal muscle, but CO2 also improved hyperglycemia via an increase in glucose metabolism mediated by increased PGC-1α expression [27].
2.4. Improved Skeletal Muscle Function and Healing
Endurance (aerobic) exercise increases skeletal muscle oxidative capacity, mitochondrial biogenesis, and muscle fiber-type switching (via an increase in PGC-1α expression) while also stimulating angiogenesis (via upregulation of VEGF). This links the regulation of the consumption of O2 by mitochondria to the delivery of O2 and nutrients by the vasculature. Elevating tissue CO2 can cause a skeletal muscle to respond as though it has been exercising. For example, compared to nontreated rats, skeletal muscle in rats treated with CO2 displays an increase in mitochondria number as well as elevations in the expression of PGC-1α, VEGF, and sirtuin 1 (SIRT1) (TD) [28]. SIRT1 supports mitochondrial biogenesis and muscle fiber-type changes to increase ATP production and improve muscle performance. These data are consistent with the greater endurance [29] and more efficient muscle activity [30] noted in humans (CB) and rats (TD), respectively, following CO2 therapy.
TD application of CO2 may have therapeutic potential for muscle regeneration and strength recovery following injury. In a study of rats with a skeletal muscle injury, injured muscle fibers were completely repaired at week six in the CO2-treated group but only partially repaired in the untreated group [31]. Expression levels of the muscle-specific transcription factors MyoD and myogenin (involved in differentiation of myoblasts into myotubes) were increased at week two after injury in the CO2-treated group (signaling an acceleration of the regeneration process and differentiation of muscle cells), and significantly more capillaries were seen four weeks after injury. Angiogenesis is a prerequisite for morphological and functional healing, leading to the rebuilding of damaged vessels, re-establishment of blood flow, and restoration of the O2 supply to tissues. TD CO2 accelerates muscle injury repair by increasing O2 delivery as well as mitochondrial biogenesis, promoting VEGF upregulation to stimulate neovascularization and activating myogenesis to build muscle. TD CO2 therapy also ameliorated the atrophy of skeletal muscle caused by nerve injury or following fracture [32,33].
2.5. Reduced Inflammation
In addition to its regenerative effects, CO2 has anti-inflammatory effects. In laparoscopic rat models of sepsis, IP injection of CO2, but not helium or air, significantly increased survival in rats with lipopolysaccharide-induced sepsis [10]. This was accompanied by CO2-specific increases in plasma interleukin-10 (IL-10) levels and decreases in tumor necrosis factor-alpha (TNF-α) levels. The reduction in TNF-α levels correlated with the increase in survival and is consistent with previous demonstrations of IL-10-induced inhibition of macrophage-derived TNF-α production and suppression of nuclear factor kappa B (NF-kB) activation (responsible for the upregulation of many proinflammatory genes) as well as with studies showing that administration of recombinant IL-10 increases survival in septic animals and reduces inflammation [34,35,36,37].
The attenuation of the inflammatory response brought about by IP injection of CO2, including a reduction in the acute inflammatory response, was independent of peritoneal absorption of CO2 and subsequent systemic acidosis (IP) [38]. Similar effects were noted in a pig study investigating the use of IP CO2 gas vs. air during laparoscopic surgery [39]. Inflammatory responses were reduced by CO2, including a reduction in IL-6 release and peritoneal macrophage production of reactive O2 species (ROS).
Another key anti-inflammatory response to elevated CO2 was discovered in patients exhibiting hypercapnia (excessive CO2 in the bloodstream) due to inadequate respiration caused by extensive lung disease. These patients must be ventilated with minimal volumes to protect the lung from mechanical damage caused by lung stretching (ventilator-induced lung injury) so CO2 levels remain elevated in the blood. Interestingly, controlling the rise in CO2, the so-called “permissive hypercapnia”, was found to improve lung inflammation and reduce morbidity and mortality in patients with acute respiratory distress syndrome (ARDS) (IH) [11]. While hypercapnia-induced acidosis boosts cardiac output, increases peripheral perfusion, and enhances tissue hemoglobin O2 unloading, CO2 can penetrate and act directly on cells to modulate intracellular pathways leading to inflammation and oxidative stress as well as pathways linked to cell survival, proliferation, and apoptosis [11]. This has relevance for patients with coronavirus disease 2019 (COVID-19), in which the viral spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) binds and disrupts the functioning of angiotensin-converting enzyme 2 (ACE2) on cell surfaces, leading to the activation of mitogen-activated protein kinases (MAPKs) and production of proinflammatory cytokines (e.g., interferon-gamma, IL-1β, IL-6, and TNF-α) that cause pneumonia or ARDS [40]. The anti-inflammatory and antioxidant effects of increased tissue CO2 could be beneficial for these patients.
2.6. Decreased Tumor Growth and Metastasis
Tumor hypoxia is a common feature of malignant tumors and contributes to their growth, invasiveness, and metastatic potential [41]. HIF-1α, a key transcription factor induced by tissue hypoxia, regulates the transcription of genes involved in angiogenesis, cell survival, and tumor cell invasion. A hypoxic microenvironment also induces various molecular pathways that allow tumors to become resistant to chemotherapy, such as the induction of multidrug resistance (MDR) genes and radiation therapy. Multiple studies have shown that TD CO2 therapy can reduce tumor growth and metastasis [42,43,44,45,46], while one in vivo study showed that IA infusion of a carbonated solution reduced tumor growth in a liver tumor model [9]. Importantly, several of these studies demonstrated that CO2 therapy enhances the effects of chemotherapy [9,45] and radiation therapy [44,46]. Thus, while CO2 treatment alone can decrease the potential size of tumors, when CO2 therapy is combined with chemotherapy or radiation, the response is even more effective. CO2 therapy increases tumor cell apoptosis [9,42,43], which reduces tumor growth. The ability of CO2 to increase blood flow could increase oxygenation of the tumor core, decreasing HIF-1α levels and reducing inflammation.
3. Mechanisms of Action of Carbon Dioxide Therapy
CO2 has been seen simply as a by-product of cellular respiration, although it has roles in the control of breathing, acid–base balance, and cerebral blood flow [47]. The therapeutic effects of CO2 noted above cannot be explained by our current dogma of CO2 being carried in the blood away from tissues. Many new functions for tissue CO2 have been revealed, emphasizing the importance of controlling CO2 levels in tissues. The cellular and molecular mechanisms by which CO2 therapy has its effects are only beginning to be elucidated.
3.1. CO2/H+ Concentrations and Carbonic Anhydrase Govern the Sensing of CO2 in the Regulation of Cellular Function
This review describes the use of therapeutic CO2. It should be noted, however, that many of the findings described herein rely on the hydration of CO2 and the production of protons to activate sensors and initiate a response. While hydration happens spontaneously under physiological conditions, it happens as much as six orders of magnitude faster in the presence of the enzyme carbonic anhydrase (CA) [48]. CO2 hydration via the action of CA is an important pathway for many responses. While the relative importance of protons and CA is discussed in the context of the effect of CO2 on O2 release from hemoglobin in red blood cells, there are many additional responses where hydration is noted. If not discussed, it does not mean that protons are not important. The role of protons is complicated by the fact that CO2 can affect protons extracellularly, independent of how it affects protons intracellularly, indicating the importance of the location of the CA. As more is discovered about CO2 biology, the relative significance of protons, and CA, in sensing the levels of CO2 will come to light.
3.2. Increasing Blood Flow and Tissue Oxygenation
Increasing the tissue concentration of CO2 increases blood flow to that tissue (CB, TD) [6,26], and increased blood flow stimulates flow-mediated dilation of blood vessels [49,50]. This vasodilator action is partly due to the CO2-induced production of NO by the endothelium (which acts via activation of cyclic GMP to relax the underlying smooth muscle) as it can be attenuated by inhibition of the enzyme endothelial NO synthase [21,51]. NO can also reduce arterial stiffness. Indeed, a single TD CO2 treatment reduced arterial stiffness and, thus, peripheral vascular resistance in hypertensive patients [8]. However, this reduction in arterial stiffness by CO2 is controlled by both endothelium-derived NO and endothelium-derived hyperpolarizing factor (EDHF) [52]. EDHF plays an important role in CO2-mediated shear stress-induced endothelium-dependent relaxation, and potassium channels, especially calcium-activated potassium (KCa) channels, appear to be involved. EDHF-mediated hyperpolarization of the smooth muscle is evoked through myo-endothelial junctions and/or the accumulation of K+ in the intercellular space.
CO2-induced vasodilation can also be endothelium-independent. CO2 reacts with water to form carbonic acid, which is in equilibrium with HCO3− and H+ ions. Elevating CO2 pushes the equilibrium toward more H+ ions. The resulting reduction in tissue pH inhibits contractility of the underlying smooth muscle in the blood vessel wall, leading to vasodilatation (CB) [50]. CO2 in exercising skeletal muscle also activates perivascular sensory nerves, which release calcitonin gene-related peptide (CGRP) (IA) [53]. CGRP causes long-lasting local vasodilatation. Thus, during prolonged exhaustive exercise, CO2 liberated from exercising muscle can directly stimulate sensory nerves to release CGRP, while the local drop in pericellular pH may act together with CO2 to further activate sensory nerves, releasing more CGRP to dilate vessels and further increase blood flow [53]. This mechanism allows the vasodilatory response to occur locally, not systemically.
CO2 and lactic acid are produced locally by metabolically active cells. The resultant decrease in intracellular pH provides a mechanism to balance vascular supply and metabolic activity by decreasing vascular resistance and increasing blood flow. While both CO2 and H+ individually can cause vasodilation via direct action on arterioles in skeletal muscle, they cannot conduct the spread of vasodilation throughout the microvascular network [54]. Instead, they likely work in concert to modify the effectiveness of other vasodilators. A physiological role for CO2 and pH in the regulation of coronary blood flow was initially proposed by Case and colleagues [55]. In an isolated heart preparation, CO2 evoked large increases in blood flow, presumably by generating adenosine (ADO) in the myocardium. The activation of ADO receptors on blood vessels by endogenously generated ADO contributes to the coronary vasodilation that occurs in response to an increase in IA CO2 and the resultant metabolic acidosis [56]. CO2 has also been reported to cause the release of ADO from cultured vascular endothelial cells [57].
An alternative mechanism for CO2-mediated increases in blood flow involves connexins and the release of ATP, which can act on ATP-sensitive potassium (KATP) channels in vascular smooth muscle to cause vasodilation, contributing both to resting blood flow and vasodilator-induced increases in flow. Connexins form large-pore channels that function either as dodecameric gap junctions or hexameric hemichannels to allow the regulated movement of small molecules and ions across cell membranes. Hemichannels are a particularly important mechanism for the release of ATP into the extracellular space [58]. CO2 binds to a carbamylation motif in connexins Cx26, Cx30, and Cx32 and causes their hemichannels to open. Fluctuations in CO2 levels within the tissue alter their open probability [59]. While it has been shown that direct CO2-gated Cx26 hemichannel opening and subsequent release of ATP mediate an important part of neurovascular coupling [47], the physiological significance of the CO2 sensitivity of Cx30 and Cx32 has not yet been elucidated during exercise. Endothelial cells express Cx32, which participates in gap junctional communication [60], and endothelial cells have been shown to release ATP via connexin hemichannels [61]. It is possible that CO2 binding to endothelial Cx32 could explain the release of ATP into the blood in skeletal muscle during exercise, as the source of ATP remains to be defined [62]. CO2-mediated CGRP release and the CA-dependent production of protons could also activate KATP channels in vascular smooth muscle [63,64].
It should be noted that all the aforementioned mechanisms for CO2-dependent increases in blood flow are not relevant to pulmonary circulation. Pulmonary circulation is unique. The role of CO2 in controlling the distribution of pulmonary blood flow is small compared to the powerful effects of hypoxia and pulmonary vasoconstriction [65]. Data shows that CO2 can induce both vasodilation and vasoconstriction, and it appears to depend on the level of resting vascular tone [66,67]. In sharp contrast to the peripheral vasculature, where protons cause vasodilation, CO2 and the CA-dependent production of carbonic acid cause vasoconstriction in the pulmonary vascular network [68].
In the in vivo hamster cheek pouch, CO2 alters both microvessel diameter and the perivascular partial pressure of O2 (pO2), as demonstrated by using microelectrodes to measure O2 [69]. When the CO2 concentration of the superfusion solution was increased, the tissue O2 concentration also increased [69]. Since increased O2 tensions cause vasoconstriction, there may be a limit to how much blood flow can increase as the CO2 rises. This may explain the limited increases seen in cremaster blood flow when CO2 in the superfusion solution was elevated above 10% [54].
Finally, it has been shown in the human forearm that there is enhanced O2 release from hemoglobin when CO2 is applied to the skin (TD) [70]. This CO2-based elevation in tissue oxygenation can be explained by the Bohr effect, a rightward shift of the O2–hemoglobin dissociation curve with an increase in pCO2 or a decrease in pH [71]. This relationship was discovered in 1904 by Christian Bohr [71], who showed that increased CO2 tensions decreased the O2 affinity of whole blood in dogs. It should be noted, however, that Malte et al. showed that the protons generated by CA have a larger effect on the binding of O2 to hemoglobin than does CO2 itself (Figure 1) [72].
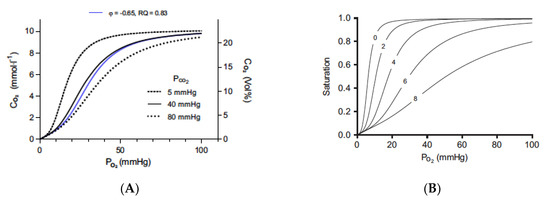
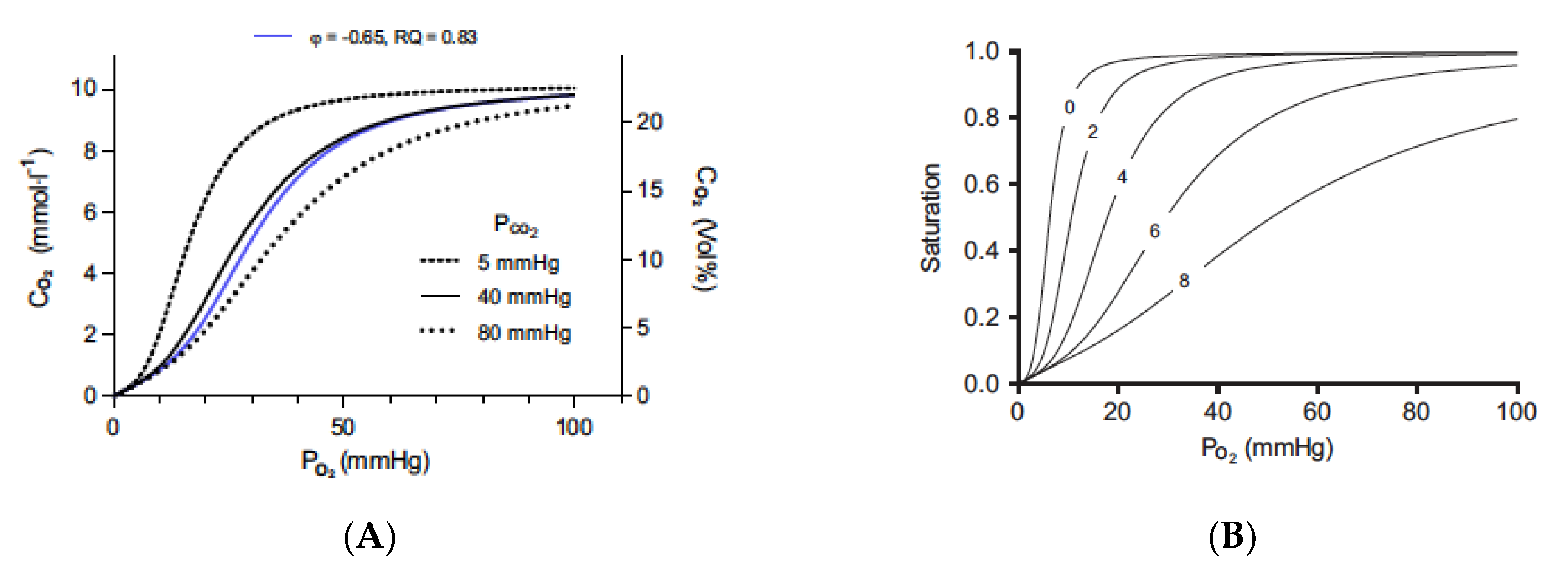
Figure 1.
The Bohr effect, as defined by CO2 and protons. The Bohr and Haldane effects have been modeled to describe the interactions between O2 and CO2 binding to hemoglobin [72]. The Bohr effect describes how O2 binding is decreased in the presence of CO2 and protons. Graph (A) shows the traditional oxygen (A) equilibrium curves modeled under different levels of CO2. The three black curves are the oxygen equilibrium curves obtained when the partial pressure of CO2 is held constant at the indicated values. The blue curve is obtained when CO2 is added at the same time as oxygen is removed at a gas exchange ratio, respiratory quotient (RQ), of 0.83. Graph (B) shows the effect of protons (B) on binding with a constant PCO2 value of 40 mmHg and pH 7.2 at a PO2 of 100 mmHg. Protons bind to the Bohr groups on the hemoglobin. The five oxygen equilibrium curves are obtained by increasing the number of bound Bohr groups (i.e., protons bound), as indicated by the numbers on the curves. The pH is not displayed because it is a poor indicator of proton binding. As protons are bound to hemoglobin, the pH increases. Figures are reprinted/adapted with permission from Ref. [72]. Copyright year 2018, Journal of Applied Physiology.
There are two Bohr groups on each hemoglobin moiety, allowing a total of eight protons to interact with the hemoglobin molecule. In the red blood cell, CO2 is rapidly interconverted to HCO3− and H+ ions via CA to generate the protons that can cause this effect. In the absence of CO2 and protons, hemoglobin will bind O2 until it reaches tissue that is essentially anoxic. Thus, tissues with low metabolism or which may be exposed to the body surface will have low CO2 levels and, therefore, will receive little O2 from the blood that may be flowing through the tissue. Elevating CO2 to generate protons will force the rightward shift in curves so that O2 will be released from hemoglobin and delivered to the tissue [Graph A]. The rightward shift caused by CO2 alone is relatively limited.
When external CO2 is applied, the CO2 is hydrated in the presence of CA, and higher concentrations of protons are produced. The rapid hydration of CO2 maintains the driving force for the diffusion of CO2 into the red blood cell and prevents the loss of CO2 by conversion to HCO3−. The effect of protons on oxygen binding is dramatic and decreases the binding of the oxygen, as defined in Graph B. When hemoglobin binds protons during the release of oxygen, it acts as a buffer. pH will actually increase, so red cell pH is not a good indicator of proton binding to hemoglobin. According to Malte and Lykkeboe, “The Bohr effect is exerted by protons preloaded on the Bohr groups at the given pH, as well as the protons taken up by the Bohr groups during gas exchange. The proton preload sets the oxygen affinity (i.e., the P50) at the beginning of the gas exchange. The protons taken up during gas exchange lead to a further progressive decrease in oxygen affinity. The concerted effect of these two processes is very large, and the size of the Bohr effect has a direct influence on the oxygen affinity of hemoglobin.” [72].
Hartmann et al. [23] proposed that an increase in tissue O2 pressure caused by bathing with CO2-enriched water was due to the Bohr effect, but they were unable to show this directly. Sakai et al. [70] utilized near-infrared spectroscopy to track dynamic changes in tissue oxy- and deoxyhemoglobin levels in real-time, directly demonstrating that TD CO2 application causes O2 dissociation from oxy-hemoglobin, i.e., the Bohr effect, in humans.
3.3. Enhancing Angiogenesis
While a single treatment with CO2 can stimulate vasodilation and enhance O2 release from hemoglobin due to the Bohr effect, repeated CO2 applications maintain the tissue O2 supply and induce angiogenesis [6,15,18,26,28,32,33,73]. Responses to CO2 include increases in basic fibroblast growth factor [2,8], VEGF [6,15,18,26,28,32,33,73], SIRT1 [28], PGC-1α [16,17,20,22], and NO [26,73], all linked to neovascularization. Angiogenesis is a critical part of the healing process and is known to occur in response to tissue hypoxia. Hypoxia induces the transcription of angiogenic genes, such as VEGF, in part by the stabilization of hypoxia-inducible transcription factors [74]. A deficiency in blood flow leads to hypoxia, and elevated levels of CO2 accumulate in the tissue.
Interestingly, while the therapeutic introduction of CO2 causes angiogenesis, it decreases or prevents the production of HIF-1α [9,12,15,42,43,44,45,46,75,76]. One mechanism for CO2-mediated suppression of HIF-1α is via a pH-dependent, O2-independent degradation of HIF-1α [75]. Selfridge et al. proposed that elevated CO2 promotes lysosomal degradation of HIF-α subunits in an environment of decreasing intracellular pH rather than induction of proteasomal degradation [75]. Galganska et al. showed that a single treatment with CO2 efficiently inhibited HIF-1α expression in cultured human endothelial cells [40]. Alternatively, the decrease in HIF-1α could simply be the result of the increased oxygenation generated by the increased blood flow and increased release of oxygen because of the Bohr effect, as described above. Thus, while CO2 is a potent trigger for angiogenesis linked to hypoxia, it appears to raise VEGF levels via a non-HIF1 pathway.
Similarly, a non-HIF-mediated process may occur in skeletal muscle in response to exercise. Exercise generates high concentrations of CO2 in the skeletal muscle, and CO2 stimulates PGC-1α [77,78]. PGC-1α is a metabolic sensor induced by hypoxia that regulates VEGF expression and angiogenesis in cultured muscle cells and skeletal muscle in vivo [79]. PGC-1α promotes angiogenesis through interaction with estrogen-related receptor-alpha (ERRα), an orphan nuclear receptor that binds to the promoter region and a novel enhancer in the first intron of the VEGF gene to initiate robust VEGF gene activation in a HIF-independent manner [79,80,81]. A reduced increase in VEGF expression in PGC-1α knockout mice following exercise demonstrates the functional relevance of this non-HIF-1α pathway in coordinating the angiogenic response to exercise [80].
In contrast, Sopariwala et al. demonstrated that ERRα is a hypoxia-stimulated factor that activates a paracrine angiogenic gene program to promote normal as well as ischemic angiogenesis in skeletal muscle in a HIF-1-dependent manner [82]. In endothelial cells, ERRα receptors were positively linked to angiogenesis, as downregulation of ERRα receptors decreased angiogenesis [83]. This reduction in angiogenesis was mediated by inhibition of HIF-1α expression as well as inhibition of the phosphatidyl inositol-3-kinase/protein kinase B (Akt)/signal transducer and activator of transcription (PI3K/Akt/STAT3) pathway. Likhite et al., however, reported that ERRα receptors act as negative regulators of angiogenesis by transcriptionally repressing angiogenic genes [84]. The link between angiogenesis and exercise/ischemia is indeed complex, and further experimentation will be needed to elucidate the role of ERRα receptors.
The CO2 sensor triggering the angiogenic process has yet to be defined. Connexins have recently been shown to bind CO2 [58]. Cx32 is expressed on endothelial cells and regulates angiogenesis by enhancing tube formation and cell migration [85]. It is conceivable that CO2 binding to Cx32 initiates neovascularization in response to chronic tissue hypoxia. An alternate candidate for a tissue CO2 sensor is ubiquitin. While much of the control of angiogenic events is instigated through hypoxia-induced VEGF expression, the ubiquitin–proteasome system plays a central role in fine-tuning the functions of core proangiogenic proteins, including VEGF, VEGF receptors, angiogenic signaling proteins, and other non-VEGF pathways [86]. Ubiquitin is a highly conserved protein that regulates both protein activity and protein degradation through conjugation to target proteins [86,87]. CO2 binds to lysine residues in ubiquitin, forming carbamate, and this reversible carbamylation reaction may be involved in the diverse physiological responses to fluctuating pCO2 levels. Linthwaite et al. described multiple locations where CO2 could bind to ubiquitin and alter its properties [87]. Deng et al. described how the deubiquitination of AMPK is critical for its activation [88]. AMPK is a crucial sensor of cellular energy, and it is activated when intracellular ATP concentrations decrease, and AMP or ADP concentrations increase in response to energy or pathological stresses [89]. AMPK activation plays a critical role in the activation of PGC-1α and, thereby, could be the link between CO2 and exercise to initiate non-HIF-mediated angiogenesis [81].
3.4. Stimulating Skeletal Muscle Mitochondrial Biogenesis
Mitochondria are critical for aerobic ATP synthesis and proper cell function. Mitochondrial quantity and quality in skeletal muscle are not only important for performance but also relevant to health, as mitochondrial dysfunction is associated with muscle atrophy, diabetes, and aging. Mitochondrial biogenesis, an increase in mitochondrial number and function, is instrumental in exercise training-induced improvement of muscle function but is also important for conferring cytoprotection against several damaging stimuli and for maintaining whole-body metabolic homeostasis. As noted above, for angiogenesis, the molecular mechanisms triggered by therapeutic CO2 appear to mimic the mechanisms induced by exercise. Consistent with the known effects of PGC-1α, TD CO2, similar to exercise, increases mitochondrial biogenesis [28,30]. Oxidative and metabolic stresses induced by contractile activity in skeletal muscle stimulate PGC-1α expression and activity, which in turn promotes mitochondrial biogenesis through interactions with nuclear transcription factors nuclear respiratory factor 1 (NRF1) and NRF2 [81]. The coactivation of NRF1 and NRF2 by PGC-1α also induces mitochondrial transcription factor A (TFAM), which regulates mitochondrial DNA transcription [81]. PGC-1α thus coordinates the expression of both nuclear- and mitochondrial-encoded genes. As noted above, the exact mechanism(s) by which CO2, exercise, and contractile activity bring about activation of PGC-1α are not known, although p38γ3 MAPK, an upstream stress-activated kinase for PGC-1α, may have a role in activating mitochondrial biogenesis [81]. AMPK phosphorylates PCG-1a on several sites, leading to increased activation, while SIRT1 activates PCG-1a directly by removing acetylation groups on 13 lysines [90].
3.5. Reducing Inflammation
Some of the beneficial effects of therapeutic CO2 may be related to its ability to suppress inflammatory signaling and elevated ROS. Hypoxia, HIF-1α, and increased expression of glycolytic enzymes are linked to inflammation, and NF-κB can drive increased HIF-1α expression. Loss of oxygen supply, depletion of energy, and increased oxidative stress in ischemia/hypoxia result in the reduction of ATP synthesis and initiate a cascade of pathways that lead to cell death if not reversed. SIRT1 plays a major role in protecting against cellular stress and in controlling metabolic pathways during ischemia/hypoxia.
CO2 can elicit a specific repertoire of transcriptional events in a dose-dependent fashion [91]. NF-κB is the master regulator of the sensing and signaling pathways that induce the transcription of multiple proinflammatory genes. As described in the studies noted above and others [10,91], CO2 can counteract inflammation to restore homeostasis. CO2 disrupts inflammation by binding to ubiquitin. Once CO2 is bound, ubiquitin will remodel and transform into a proteasome that will directly diminish the NF-κB response [87,92]. During deubiquitination, ubiquitin is removed from a substrate to release its activity. This has been described as CO2-induced de-activation of NF-κB [92].
Alternatively, studies in human cells demonstrated that CO2 directly inhibited the activation of the MAPKs extracellular signal-regulated kinases 1 and 2 (ERK1/2) by the receptor-binding domain of the SARS-CoV-2 spike protein, independent of pH and independent of upstream activators, blocking the rise in proinflammatory cytokine production. Thus, ERK1/2 act as a direct CO2 sensor, mediating the anti-inflammatory response of elevated CO2 [40].
3.6. Antioxidant Effects
The presence of CO2 is essential for life due to its antioxidant effects. While CB CO2 can decrease inflammation, it will also reduce the ROS generated during the various phases of inflammation [93,94,95]. As Veselá and Wilhelm noted [95], CO2 prevents nitration and oxidative damage by scavenging peroxynitrite. Moreover, CO2 can protect superoxide dismutase from hydrogen-peroxide-induced damage, although this does result in the formation of carbonate radicals that can propagate oxidative damage. The most significant antioxidant role for CO2 in vivo is its ability to stabilize the iron–transferrin complex and thereby prevent the involvement of this complex in the initiation of free radical reactions [95].
While the evidence of the relationship between CO2 therapy and oxidative stress is limited, Veselá and Wilhelm found that CO2 plays a protective role in scavenging free radicals and suppressing oxidative metabolism [95]. A recent study demonstrated that CO2 treatment could reduce the level of asymmetric dimethylarginine, which is a marker of oxidative stress [96].
3.7. Benefits of Diabetes Mellitus
Microvascular disease caused by diabetes is a potential target for CO2 therapy. Studies have shown that the basic status of the microvasculature is one of low O2 and low metabolism, although the role of HIF-1α in the tissue response to hyperglycemia and hypoxia appears to be rather variable [97]. HIF-1α plays an unpredictable role in the response of the tissue to hyperglycemia, and current data suggest that it is probably better to activate the CO2 hypoxemic response than the HIF-1α hypoxic pathway. Hyperglycemia triggers the glycolytic pathway for ATP production. This pathway generates less CO2 compared to aerobic respiration. Therefore, the concentrations of CO2 in the tissue are lower than normal. This sets up an environment with low oxygenation, increased inflammation, and reduced capillary density. Notably, exercise can reduce these complications of diabetes [98], and it appears that CO2 therapy can mimic these same effects [28] as well as re-balance the pro- and anti-factors controlling angiogenesis, such as VEGF, endothelial NO synthase, and thrombospondin-1 [26].
3.8. Reducing Tumor Hypoxia
Tumor growth is limited by the need for O2 and the limited diffusion of nutrients. Solid tumors display a hypoxic core that stimulates pathways triggered by HIF-1α [43,45,99]. As noted above, numerous studies have consistently confirmed that CO2 therapy will decrease the size of tumors by reducing the hypoxic core of the tumor, reversing the stabilization of HIF-1α to modulate survival pathways, and triggering non-HIF-related pathways [43,45,99]. CO2 therapy results in an oxygenated core in the tumor that increases tumor cell apoptosis and enhances sensitivity to chemotherapy and radiation therapy. A collection of studies describes multiple mechanisms that could come together, leading to apoptosis due to the presence of CO2 and improved oxygenation. These pathways involve increases in intracellular calcium, AMPK, PGC-1α, and caspases 3 and 9 [28,76,100,101,102,103,104,105].
4. Summary
Therapeutic CO2 gas has been found to be effective for many microvascular disorders. Figure 2 summarizes some of the tissue responses to hypoxia and elevated CO2 and demonstrates how CO2 protects the tissue from progressing toward survival mode (e.g., by reducing HIF-1α and activating AMPK) when hypoxia is severe and/or sustained. Elevated CO2 activates mechanisms that pull the microenvironment toward a “thrive mode” that maintains homeostasis. Physiological properties triggered by CO2 include elevated oxygenation, increased angiogenesis, increased mitochondrial biogenesis, anti-inflammation, reduced oxidative stress, and increased tissue blood flow. The balance between pro-inflammatory and anti-inflammatory phases is also pushed toward homeostasis when tissue CO2 is elevated. This is critical for healing wounds and fractures. Hyperglycemia in diabetes leads to poor oxygenation, loss of capillaries, and inflammation. Diabetes is a risk factor for peripheral arterial disease and reduced blood flow, often leading to chronic wounds. Therapeutic CO2 corrects this and restores homeostasis. Tumors have a hypoxic core that keeps metastatic cells alive due to pathways that are triggered by HIF-1α. Therapeutic CO2 can help oxygenate the hypoxic core and reduce HIF-1α. Finally, evidence supports the thesis that CO2 is a critical metabolic factor during exercise that triggers angiogenesis, mitochondrial biogenesis, and myocyte fiber switching. Thus, CO2 is not only a metabolic waste product but may be a gaseous signaling molecule, similar to NO, carbon monoxide, and hydrogen sulfide. Modulating its levels via therapeutic application may provide a path to multiple important health solutions.
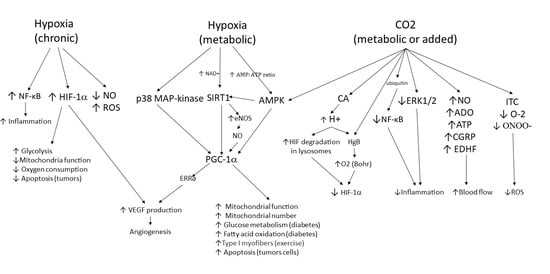
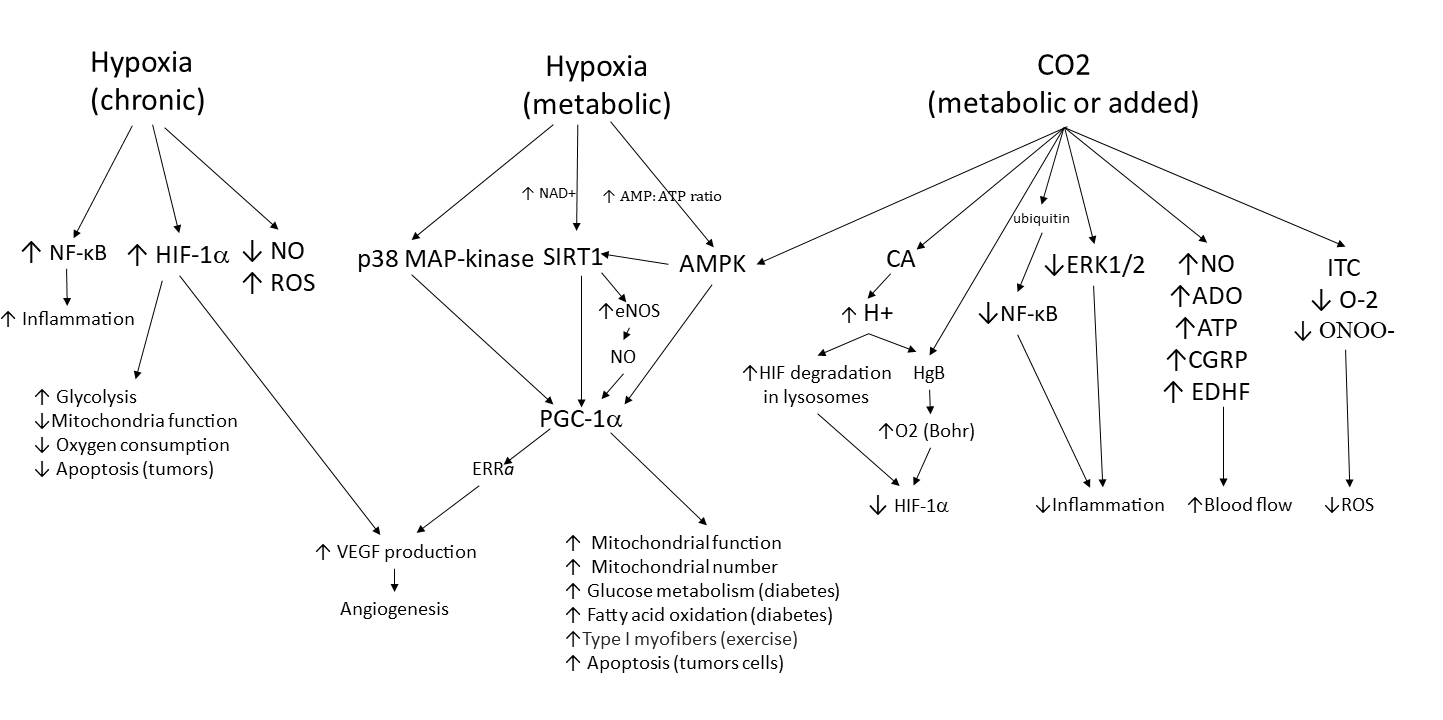
Figure 2.
Tissue response to hypoxia and carbon dioxide. This chart shows the molecular pathways that are involved in the tissue response to hypoxia and elevated CO2 levels. Three pathways are shown. The first two are tissue responses to hypoxia. Normal metabolic control of hypoxia initially involves the moment-to-moment response to O2 demands by alterations in blood flow. If demand persists, pathways for improving O2 supply are activated. Multiple pathways lead to the activation of PGC-1α, which induces metabolic changes and new blood vessel formation to allow the tissue to continue to thrive. If hypoxia is persistent and/or severe, as seen in pathological conditions, the left pathway is also activated. Cells experience increased inflammation and oxidative stress and activate HIF-1α-induced production of VEGF to further stimulate neovascularization and increase blood flow while also altering cell metabolism to reduce cell death (i.e., stimulate survival mode). The right pathway shows the responses activated by increases in tissue CO2. When blood flow is limited, CO2 levels rise and trigger activation of AMPK, stimulating the tissue to respond with changes in cell metabolism and neovascularization that allow the tissue to thrive. Multiple additional pathways are stimulated by elevated CO2, resulting in increased blood flow, decreased inflammation, decreased oxidative stress, as well as a reduction in survival pathways stimulated by HIF-1α. Thus, elevated CO2 levels (either metabolically generated or brought about by therapeutic CO2 delivery) will produce a more homeostatic state in the tissue.
Author Contributions
Both authors contributed to the conceptualization, analysis, writing, reviewing, and editing of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data reported is publicly available.
Conflicts of Interest
R.J. Rivers is a scientific advisor to Serendi Medical, LL. C.J. Meininger has no conflicts of interest.
Abbreviations
| ACE2 | angiotensin-converting enzyme 2 |
| ADO | adenosine |
| AMP | adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| ARDS | acute respiratory distress syndrome |
| ATP | adenosine triphosphate |
| CA | carbonic anhydrase |
| CB | carbonated water bath |
| CG | carbonated paste or gel |
| cGMP | cyclic guanosine monophosphate |
| CGRP | calcitonin gene-related peptide |
| CO2 | carbon dioxide |
| COX | cytochrome c oxidase |
| COVID-19 | coronavirus disease 2019 |
| CS | citrate synthase |
| EDHF | endothelium-derived hyperpolarizing factor |
| eNOS | endothelial nitric oxide synthase |
| ERK1/2 | extracellular signal-regulated kinase 1/2 |
| ERRα | orphan nuclear receptor estrogen-related receptor-alpha |
| H+ | hydrogen proton |
| HIF-1α | hypoxia-inducible factor 1-alpha |
| IA | intra-arterial infusion of a carbonated solution |
| IH | inhaled CO2 or hypercarbia due to hypoventilation |
| IL-10 | interleukin-10 |
| IL-1β | interleukin 1 beta |
| IL-6 | interleukin 6 |
| IP | intraperitoneal insufflation of CO2 |
| ITC | iron–transferrin complex |
| KCa | calcium-activated potassium |
| KATP | ATP-sensitive potassium |
| MAPKs | mitogen-activated protein kinases |
| MDM-2 | mouse double minute 2 |
| NAD+ | nicotinamide adenine dinucleotide |
| NF-κB | nuclear factor-κB |
| NO | nitric oxide |
| NRF1, NRF2 | nuclear transcription factors nuclear respiratory factor 1, 2 |
| O-2 | superoxide |
| O2 | molecular oxygen |
| p38 MAP-kinase | mitogen-activated protein (MAP) kinase |
| PGC-1α | peroxisome proliferator-activated receptor-gamma coactivator—1 alpha |
| ROS | reactive oxygen species |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SIRT1 | sirtuin 1 |
| SQ | subcutaneous injections of CO2 |
| STZ | streptozotocin |
| TD | transdermal application of CO2 |
| TFAM | mitochondrial transcription factor A |
| TNF-α | tumor necrosis factor-alpha |
| TSP-1t | hrombospondin 1 |
| VEGF | vascular endothelial growth factor |
References
- Crystal, G.J. Carbon dioxide and the heart: Physiology and clinical implications. Anesth. Analg. 2015, 121, 610–623. [Google Scholar] [CrossRef]
- Reglin, B.; Pries, A.R. Metabolic control of microvascular networks: Oxygen sensing and beyond. J. Vasc. Res. 2014, 51, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Kierans, S.J.; Taylor, C.T. Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J. Physiol. 2021, 599, 23–37. [Google Scholar] [CrossRef]
- Rodríguez, C.; Muñoz, M.; Contreras, C.; Prieto, D. AMPK, metabolism, and vascular function. FEBS J. 2021, 288, 3746–3771. [Google Scholar] [CrossRef]
- Bottoni, P.; Óvári, M.; Záray, G.; Caroli, S. Characteristics of spring waters in budapest: A short review. Microchem. J. 2013, 110, 770–774. [Google Scholar] [CrossRef]
- Xu, Y.-J.; Elimban, V.; Bhullar, S.K.; Dhalla, N.S. Effects of CO2 water-bath treatment on blood flow and angiogenesis in ischemic hind limb of diabetic rat. Can. J. Physiol. Pharmacol. 2018, 96, 1017–1021. [Google Scholar] [CrossRef]
- Yatagai, N.; Hasegawa, T.; Amano, R.; Saito, I.; Arimoto, S.; Takeda, D.; Kakei, Y.; Akashi, M. Transcutaneous carbon dioxide decreases immunosuppressive factors in squamous cell carcinoma. In Vivo. BioMed Res. Int. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Németh, B.; Kiss, I.; Ajtay, B.; Péter, I.; Kreska, Z.; Cziráki, A.; Horváth, I.G.; Ajtay, Z. Transcutaneous carbon dioxide treatment is capable of reducing peripheral vascular resistance in hypertensive patients. Vivo 2018, 32, 1555–1559. [Google Scholar] [CrossRef]
- Katayama, N.; Sugimoto, K.; Okada, T.; Ueha, T.; Sakai, Y.; Akiyoshi, H.; Mie, K.; Ueshima, E.; Sofue, K.; Koide, Y.; et al. Intra-arterially infused carbon dioxide-saturated solution for sensitizing the anticancer effect of cisplatin in a rabbit VX2 liver tumor model. Int. J. Oncol. 2017, 51, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Hanly, E.J.; Fuentes, J.M.; Aurora, A.R.; Bachman, S.L.; De Maio, A.; Marohn, M.R.; Talamini, M.A. Carbon dioxide pneumoperitoneum prevents mortality from sepsis. Surg. Endosc. 2006, 20, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Masterson, C.; Laffey, J.G. Permissive hypercapnia: What to remember. Curr. Opin. Anaesthesiol. 2015, 28, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Amano-Iga, R.; Hasegawa, T.; Takeda, D.; Murakami, A.; Yatagai, N.; Saito, I.; Arimoto, S.; Kakei, Y.; Sakakibara, A.; Akashi, M. Local application of transcutaneous carbon dioxide paste decreases inflammation and accelerates wound healing. Cureus 2021, 13, e19518. [Google Scholar] [CrossRef]
- Ahramiyanpour, N.; Shafie’ei, M.; Sarvipour, N.; Amiri, R.; Akbari, Z. Carboxytherapy in dermatology: A systematic review. J. Cosmet. Dermatol. 2022, 21, 1874–1894. [Google Scholar] [CrossRef]
- Oda, T.; Iwakura, T.; Fukui, T.; Oe, K.; Mifune, Y.; Hayashi, S.; Matsumoto, T.; Matsushita, T.; Kawamoto, T.; Sakai, Y.; et al. Effects of the duration of transcutaneous CO2 application on the facilitatory effect in rat fracture repair. J. Orthop. Sci. 2020, 25, 886–891. [Google Scholar] [CrossRef]
- Saito, I.; Hasegawa, T.; Ueha, T.; Takeda, D.; Iwata, E.; Arimoto, S.; Sakakibara, A.; Sakakibara, S.; Akashi, M.; Sakai, Y.; et al. Effect of local application of transcutaneous carbon dioxide on survival of random-pattern skin flaps. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Heinig, B.; Uhlemann, C. Transdermal CO2 application in chronic wounds. Int. J. Low. Extrem Wounds 2004, 3, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Macura, M.; Ban Frangez, H.; Cankar, K.; Finžgar, M.; Frangez, I. The effect of transcutaneous application of gaseous CO2 on diabetic chronic wound healing—A double-blind randomized clinical trial. Int. Wound J. 2020, 17, 1607–1614. [Google Scholar] [CrossRef]
- Koga, T.; Niikura, T.; Lee, S.Y.; Okumachi, E.; Ueha, T.; Iwakura, T.; Sakai, Y.; Miwa, M.; Kuroda, R.; Kurosaka, M. Topical cutaneous CO2 application by means of a novel hydrogel accelerates fracture repair in rats. J. Bone Jt. Surg. 2014, 96, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Niikura, T.; Iwakura, T.; Omori, T.; Lee, S.Y.; Sakai, Y.; Akisue, T.; Oe, K.; Fukui, T.; Matsushita, T.; Matsumoto, T.; et al. Topical cutaneous application of carbon dioxide via a hydrogel for improved fracture repair: Results of phase I clinical safety trial. BMC Musculoskelet. Disord. 2019, 20, 563. [Google Scholar] [CrossRef]
- Izumi, Y.; Yamaguchi, T.; Yamazaki, T.; Yamashita, N.; Nakamura, Y.; Shiota, M.; Tanaka, M.; Sano, S.; Osada-Oka, M.; Shimada, K.; et al. Percutaneous carbon dioxide treatment using a gas mist generator enhances the collateral blood flow in the ischemic hindlimb. J. Atheroscl. Thromb. 2015, 22, 38–51. [Google Scholar] [CrossRef]
- Irie, H.; Tatsumi, T.; Takamiya, M.; Zen, K.; Takahashi, T.; Azuma, A.; Tateishi, K.; Nomura, T.; Hayashi, H.; Nakajima, N.; et al. Carbon dioxide-rich water bathing enhances collateral blood flow in ischemic hindlimb via mobilization of endothelial progenitor cells and activation of NO-cGMP system. Circulation 2005, 111, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Toriyama, T.; Kumada, Y.; Matsubara, T.; Murata, A.; Ogino, A.; Hayashi, H.; Nakashima, H.; Takahashi, H.; Matsuo, H.; Kawahara, H. Effect of artificial carbon dioxide foot bathing on critical limb ischemia (fontaine IV) in peripheral arterial disease patients. Int. Angiol. 2002, 21, 367–373. [Google Scholar] [PubMed]
- Hartmann, B.R.; Bassenge, E.; Hartmann, M. Effects of serial percutaneous application of carbon dioxide in intermittent claudication: Results of a controlled trial. Angiology 1997, 48, 957–963. [Google Scholar] [CrossRef]
- Fabry; Monnet; Schmidt; Lusson; Carpentier; Baguet; Dubray. Clinical and microcirculatory effects of transcutaneous CO2 therapy in intermittent claudication. randomized double-blind clinical trial with a parallel design. Vasa 2009, 38, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, R.; Silvennoinen, M.; Touvra, A.; Lehti, T.M.; Kainulainen, H.; Vihko, V. Effects of experimental type 1 diabetes and exercise training on angiogenic gene expression and capillarization in skeletal muscle. FASEB J. 2006, 20, 1570–1572. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tanaka, M.; Ikeji, T.; Maeshige, N.; Sakai, Y.; Akisue, T.; Kondo, H.; Ishihara, A.; Fujino, H. Application of transcutaneous carbon dioxide improves capillary regression of skeletal muscle in hyperglycemia. J. Physiol. Sci. 2019, 69, 317–326. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tanaka, M.; Nakanish, R.; Takuwa, M.; Hirabayashi, T.; Ono, K.; Ikeji, T.; Maeshige, N.; Sakai, Y.; Akisue, T.; et al. Transcutaneous carbon dioxide attenuates impaired oxidative capacity in skeletal muscle in hyperglycemia model. Gen. Physiol. Biophys. 2019, 38, 237–244. [Google Scholar] [CrossRef]
- Oe, K.; Ueha, T.; Sakai, Y.; Nikura, T.; Lee, S.Y.; Koh, A.; Hasegawa, T.; Tanaka, M.; Miwa, M.; Kurosaka, M. The effect of transcutaneous application of carbon dioxide (CO2) on skeletal muscle. Biochem. Biophys. Res. Commun. 2011, 407, 148–152. [Google Scholar] [CrossRef]
- Akamine, T.; Taguchi, N. Effects of an artificially carbonated bath on athletic warm-up. J. Hum. Ergol. 1998, 27, 22–29. [Google Scholar] [CrossRef]
- Ueha, T.; Oe, K.; Miwa, M.; Hasegawa, T.; Koh, A.; Nishimoto, H.; Lee, S.; Niikura, T.; Kurosaka1, M.; Kuroda1, R.; et al. Increase in carbon dioxide accelerates the performance of endurance exercise in rats. J. Physiol. Sci. 2018, 68, 463–470. [Google Scholar] [CrossRef]
- Akahane, S.; Sakai, Y.; Ueha, T.; Nishimoto, H.; Inoue, M.; Niikura, T.; Kuroda, R. Transcutaneous carbon dioxide application accelerates muscle injury repair in rat models. Int. Orthop. 2017, 41, 1007–1015. [Google Scholar] [CrossRef]
- Nishimoto, H.; Inui, A.; Ueha, T.; Inoue, M.; Akahane, S.; Harada, R.; Mifune, Y.; Kokubu, T.; Nishida, K.; Kuroda, R.; et al. Transcutaneous carbon dioxide application with hydrogel prevents muscle atrophy in a rat sciatic nerve crush model. J. Orthop. Res. 2018, 36, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Sakai, Y.; Oe, K.; Ueha, T.; Koga, T.; Nishimoto, H.; Akahane, S.; Harada, R.; Lee, S.Y.; Niikura, T.; et al. Transcutaneous carbon dioxide application inhibits muscle atrophy after fracture in rats. J. Orthop. Sci. 2020, 25, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.; Muchamuel, T.; Andrade, S.; Menon, S. Interleukin 10 protects mice from lethal endotoxemia. J. Exp. Med. 1993, 177, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Rongione, A.J.; Kusske, A.M.; Ashley, S.W.; Reber, H.A.; McFadden, D.W. Interleukin-10 prevents early cytokine release in severe intraabdominal infection and sepsis. J. Surg. Res. 1997, 70, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Rongione, A.J.; Kusske, A.M.; Kwan, K.; Ashley, S.W.; Reber, H.A.; McFadden, D.W. Interleukin-10 protects against lethality of intra-abdominal infection and sepsis. J. Gastrointest. Surg. 2000, 4, 70–76. [Google Scholar] [CrossRef]
- Yoshidome, H.; Kato, A.; Edwards, M.J.; Lentsch, A.B. Interleukin-10 suppresses hepatic ischemia/reperfusion injury in mice: Implications of a central role for nuclear factor κB. Hepatology 1999, 30, 203–208. [Google Scholar] [CrossRef]
- Hanly, E.J.; Bachman, S.L.; Marohn, M.R.; Boden, J.H.; Herring, A.E.; De Maio, A.; Talamini, M.A. Carbon dioxide pneumoperitoneum–mediated attenuation of the inflammatory response is independent of systemic acidosis. Surgery 2005, 137, 559–566. [Google Scholar] [CrossRef]
- Ure, B.M.; Niewold, T.A.; Bax, N.M.A.; Ham, M.; van de Zee, D.C.; Essen, G.J. Peritoneal, systemic, and distant organ inflammatory responses are reduced by a laparoscopic approach and carbon dioxide vs air. Surg. Endosc. 2002, 16, 836–842. [Google Scholar] [CrossRef]
- Galganska, H.; Jarmuszkiewicz, W.; Galganski, L. Carbon dioxide inhibits COVID-19-type proinflammatory responses through extracellular signal-regulated kinases 1 and 2, novel carbon dioxide sensors. Cell. Mol. Life Sci. 2021, 78, 8229–8242. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J. Clin. Investig. 2013, 123, 3664–3671. [Google Scholar] [CrossRef] [PubMed]
- Takeda, D.; Hasegawa, T.; Ueha, T.; Imai, Y.; Sakakibara, A.; Minoda, M.; Kawamoto, T.; Minamikawa, T.; Shibuya, Y.; Akisue, T.; et al. Transcutaneous carbon dioxide induces mitochondrial apoptosis and suppresses metastasis of oral squamous cell carcinoma in vivo. PLoS ONE 2014, 9, e100530. [Google Scholar] [CrossRef]
- Harada, R.; Kawamoto, T.; Ueha, T.; Minoda, M.; Toda, M.; Onishi, Y.; Fukase, N.; Hara, H.; Sakai, Y.; Miwa, M.; et al. Reoxygenation using a novel CO2 therapy decreases the metastatic potential of osteosarcoma cells. Exp. Cell Res. 2013, 319, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Akisue, T.; Kawamoto, T.; Ueha, T.; Hara, H.; Toda, M.; Harada, R.; Minoda, M.; Morishita, M.; Sasaki, R.; et al. Transcutaneous application of CO2 enhances the antitumor effect of radiation therapy in human malignant fibrous histiocytoma. Int. J. Oncol. 2014, 45, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Kawamoto, T. Transcutaneous application of carbon dioxide (CO2) enhances chemosensitivity by reducing hypoxic conditions in human malignant fibrous histiocytoma. J. Cancer Sci. Ther. 2012, 4, 74–181. [Google Scholar] [CrossRef]
- Iwata, E.; Hasegawa, T.; Ueha, T.; Takeda, D.; Saito, I.; Kawamoto, T.; Akisue, T.; Sakai, Y.; Sasaki, R.; Kuroda, R.; et al. Transcutaneous carbon dioxide enhances the antitumor effect of radiotherapy on oral squamous cell carcinoma. Oncol. Rep. 2018, 40, 434–442. [Google Scholar] [CrossRef]
- Hosford, P.S.; Wells, J.A.; Nizari, S.; Christie, I.N.; Theparambil, S.M.; Castro, P.A.; Hadjihambi, A.; Barros, L.F.; Ruminot, I.; Lythgoe, M.F.; et al. CO2 signaling mediates neurovascular coupling in the cerebral cortex. Nat. Commun. 2022, 13, 2125. [Google Scholar] [CrossRef]
- Lahiri, S.; Forster, R.E., 2nd. CO2/H+ sensing: Peripheral and central chemoreception. Int. J. Biochem. Cell Biol. 2003, 35, 1413–1435. [Google Scholar] [CrossRef]
- Ogoh, S.; Nagaoka, R.; Mizuno, T.; Kimura, S.; Shidahara, Y.; Ishii, T.; Kudoh, M.; Iwamoto, E. Acute vascular effects of carbonated warm water lower leg immersion in healthy young adults. Physiol. Rep. 2016, 4, e13046. [Google Scholar] [CrossRef]
- Minamiyama, M.; Yamamoto, A. Direct evidence of the vasodilator action of carbon dioxide on subcutaneous microvasculature in rats by use of intra-vital video-microscopy. J. Biorheol. 2010, 24, 42–46. [Google Scholar] [CrossRef]
- Carr, P.; Graves, J.E.; Poston, L. Carbon dioxide induced vasorelaxation in rat mesenteric small arteries precontracted with noradrenaline is endothelium dependent and mediated by nitric oxide. Pflügers Arch. 1993, 423, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Bellien, J.; Favre, J.; Iacob, M.; Gao, J.; Thuillez, C.; Richard, V.; Joannidès, R. Arterial stiffness is regulated by nitric oxide and endothelium-derived hyperpolarizing factor during changes in blood flow in humans. Hypertension 2010, 55, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Ishikawa, T.; Yamanaka, A.; Fujimori, A.; Goto, K. Local neurogenic regulation of rat hindlimb circulation: CO2-induced release of calcitonin gene-related peptide from sensory nerves. Br. J. Pharmacol. 1997, 122, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Charter, M.E.; Lamb, I.R.; Murrant, C.L. Arteriolar and capillary responses to CO2 and H+ in hamster skeletal muscle microvasculature: Implications for active hyperemia. Microcirculation 2018, 25, e12494. [Google Scholar] [CrossRef] [PubMed]
- Case, R.; Greenberg, H.; Moskowitz, R. Alterations in coronary sinus pO2 and O2 saturation resulting from pCO2 changes. Cardiovasc. Res. 1975, 9, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Phillis, J.W.; O’Regan, M.H.; Song, D. Further evidence for the role of adenosine in hypercapnia/acidosis-evoked coronary flow regulation. Gen. Pharmacol. 1999, 33, 431–437. [Google Scholar] [CrossRef]
- Nees, S.; Gerlach, E. Adenine Nucleotide and Adenosine Metabolism in Cultured Coronary Endothelial Cells: Formation and Release of Adenine Compounds and Possible Functional Implications. In Regulatory Function of Adenosine; Berne, R.M., Rall, T.W., Rubio, R., Eds.; Springer: Boston, MA, USA, 1983; pp. 347–360. [Google Scholar]
- Dospinescu, V.; Nijjar, S.; Spanos, F.; Cook, J.; de Wolf, E.; Biscotti, M.A.; Gerdol, M.; Dale, N. Structural determinants of CO2-sensitivity in the β connexin family suggested by evolutionary analysis. Commun. Biol. 2019, 2, 331. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.; Dale, N.; Wall, M.J. CO2-sensitive connexin hemichannels in neurons and glia: Three different modes of signalling? Int. J. Mol. Sci. 2021, 22, 7254. [Google Scholar] [CrossRef]
- Okamoto, T.; Akiyama, M.; Takeda, M.; Gabazza, E.C.; Hayashi, T.; Suzuki, K. Connexin32 is expressed in vascular endothelial cells and participates in gap-junction intercellular communication. Biochem. Biophys. Res. Commun. 2009, 382, 264–268. [Google Scholar] [CrossRef]
- Braet, K.; Aspeslagh, S.; Vandamme, W.; Willecke, K.; Martin, P.E.M.; Evans, W.H.; Leybaert, L. Pharmacological sensitivity of ATP release triggered by photoliberation of inositol-1,4,5-trisphosphate and zero extracellular calcium in brain endothelial cells. J. Cell. Physiol. 2003, 197, 205–213. [Google Scholar] [CrossRef]
- Kirby, B.S.; Crecelius, A.R.; Richards, J.C.; Dinenno, F.A. Sources of intravascular ATP during exercise in humans: Critical role for skeletal muscle perfusion. Exp. Physiol. 2013, 98, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Brayden, J.E. Functional roles of KATP channels in vascular smooth muscle. Clin. Exp. Pharmacol. Physiol. 2002, 29, 312–316. [Google Scholar] [CrossRef]
- Davies, N.W. Modulation of ATP-sensitive K+ channels in skeletal muscle by intracellular protons. Nature 1990, 343, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.K.; Sá, R.C.; Arai, T.J.; Theilmann, R.J.; Hopkins, S.R.; Buxton, R.B.; Prisk, G.K. Regional pulmonary perfusion patterns in humans are not significantly altered by inspiratory hypercapnia. J. Appl. Physiol. 2019, 127, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, S.V.; Evans, T.W. Action of carbon dioxide on hypoxic pulmonary vasoconstriction in the rat lung: Evidence against specific endothelium-derived relaxing factor-mediated vasodilation. Cri.t Care Med. 1993, 21, 740–746. [Google Scholar] [CrossRef]
- Dorrington, K.L.; Talbot, N.P. Human pulmonary vascular responses to hypoxia and hypercapnia. Pflugers Arch. 2004, 449, 1–15. [Google Scholar] [CrossRef]
- Barer, G.R.; Shaw, J.W. Pulmonary vasodilator and vasoconstrictor actions of carbon dioxide. J. Physiol. 1971, 213, 633–645. [Google Scholar] [CrossRef]
- Duling, B.R. Changes in microvascular diameter and oxygen tension induced by carbon dioxide. Circ. Res. 1973, 32, 370–376. [Google Scholar] [CrossRef]
- Sakai, Y.; Miwa, M.; Oe, K.; Ueha, T.; Koh, A.; Niikura, T.; Iwakura, T.; Lee, S.Y.; Tanaka, M.; Kurosaka, M. A novel system for transcutaneous application of carbon dioxide causing an “artificial bohr effect” in the human body. PLoS ONE 2011, 6, e24137. [Google Scholar] [CrossRef]
- Bohr, C.; Hasselbalch, K.; Krogh, A. Ueber einen in biologischer beziehung wichtigen einfluss, den die kohlensäurespannung des blutes auf dessen sauerstoffbindung übt1. Skandinavisches Archiv Für Physiologie 1904, 16, 402–412. [Google Scholar] [CrossRef]
- Malte, H.; Lykkeboe, G. The bohr/haldane effect: A model-based uncovering of the full extent of its impact on O2 delivery to and CO2 removal from tissues. J. Appl. Physiol. 2018, 125, 916–922. [Google Scholar] [CrossRef]
- Xu, Y.; Elimban, V.; Dhalla, N.S. Carbon dioxide water-bath treatment augments peripheral blood flow through the development of angiogenesis. Can. J. Physiol. Pharmacol. 2017, 95, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Selfridge, A.C.; Cavadas, M.A.S.; Scholz, C.C.; Campbell, E.L.; Welch, L.C.; Lecuona, E.; Colgan, S.P.; Barrett, K.E.; Sporn, P.H.S.; Sznajder, J.I.; et al. Hypercapnia suppresses the HIF-dependent adaptive response to hypoxia. J. Biol. Chem. 2016, 291, 11800–11808. [Google Scholar] [CrossRef]
- Takemori, T.; Kawamoto, T.; Ueha, T.; Toda, M.; Morishita, M.; Kamata, E.; Fukase, N.; Hara, H.; Fujiwara, S.; Niikura, T.; et al. Transcutaneous carbon dioxide application suppresses bone destruction caused by breast cancer metastasis. Oncol. Rep. 2018, 40, 2079–2087. [Google Scholar] [CrossRef]
- Coffey, V.G.; Jemiolo, B.; Edge, J.; Garnham, A.P.; Trappe, S.W.; Hawley, J.A. Effect of consecutive repeated sprint and resistance exercise bouts on acute adaptive responses in human skeletal muscle. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 297, R1441–R1451. [Google Scholar] [CrossRef]
- Coffey, V.G.; Hawley, J.A. Concurrent exercise training: Do opposites distract? J. Physiol. 2017, 595, 2883–2896. [Google Scholar] [CrossRef] [PubMed]
- Arany, Z.; Foo, S.; Ma, Y.; Ruas, J.L.; Bommi-Reddy, A.; Girnun, G.; Cooper, M.; Laznik, D.; Chinsomboon, J.; Rangwala, S.M.; et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature 2008, 451, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.L.; Lloyd, P.G.; Yang, H.; Terjung, R.L. Exercise Training and Peripheral Arterial Disease. Compr. Physiol. 2012, 2, 2933–3017. [Google Scholar] [CrossRef]
- Yan, Z.; Okutsu, M.; Akhtar, Y.N.; Lira, V.A. Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J. Appl. Physiol. 2011, 110, 264–274. [Google Scholar] [CrossRef]
- Sopariwala, D.H.; Likhite, N.; Pei, G.; Haroon, F.; Lin, L.; Yadav, V.; Zhao, Z.; Narkar, V.A. Estrogen-related receptor α is involved in angiogenesis and skeletal muscle revascularization in hindlimb ischemia. Faseb. J. 2021, 35, e21480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, L.; Zhang, M.; Qi, H.; Chen, L.; Chen, H.; Zhong, M.; Shi, X.; Li, Q. Downregulation of ERRα inhibits angiogenesis in human umbilical vein endothelial cells through regulating VEGF production and PI3K/akt/STAT3 signaling pathway. Eur. J. Pharmacol. 2015, 769, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Likhite, N.; Yadav, V.; Milliman, E.J.; Sopariwala, D.H.; Lorca, S.; Narayana, N.P.; Sheth, M.; Reineke, E.L.; Giguère, V.; Narkar, V. Loss of estrogen-related receptor alpha facilitates angiogenesis in endothelial cells. Mol. Cell Biol. 2019, 39, 411. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Akita, N.; Kawamoto, E.; Hayashi, T.; Suzuki, K.; Shimaoka, M. Endothelial connexin32 enhances angiogenesis by positively regulating tube formation and cell migration. Exp. Cell Res. 2014, 321, 133–141. [Google Scholar] [CrossRef]
- Rahimi, N. The ubiquitin-proteasome system meets angiogenesis. Mol. Cancer Res. 2012, 11, 538–548. [Google Scholar] [CrossRef]
- Linthwaite, V.L.; Pawloski, W.; Pegg, H.B.; Townsend, P.D.; Thomas, M.J.; So, V.K.H.; Brown, A.P.; Hodgson, D.R.W.; Lorimer, G.H.; Fushman, D.; et al. Ubiquitin is a carbon dioxide–binding protein. Sci. Adv. 2021, 7, eabi5507. [Google Scholar] [CrossRef]
- Deng, M.; Yang, X.; Qin, B.; Liu, T.; Zhang, H.; Guo, W.; Lee, S.; Kim, J.; Yuan, J.; Pei, H.; et al. Deubiquitination and activation of AMPK by USP10. Mol. Cell. 2016, 61, 614–624. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Shoag, J.; Arany, Z. Regulation of hypoxia-inducible genes by PGC-1α. Arterioscl. Throm. Vas. 2010, 30, 662–666. [Google Scholar] [CrossRef]
- Cummins, E.P.; Oliver, K.M.; Lenihan, C.R.; Fitzpatrick, S.F.; Bruning, U.; Scholz, C.C.; Slattery, C.; Leonard, M.O.; McLoughlin, P.; Taylor, C.T. NF-κB links CO2 sensing to innate immunity and inflammation in mammalian cells. J. Immunol. 2010, 185, 4439–4445. [Google Scholar] [CrossRef]
- Keogh, C.E.; Scholz, C.C.; Rodriguez, J.; Selfridge, A.C.; von Kriegsheim, A.; Cummins, E.P. Carbon dioxide-dependent regulation of NF-kappaB family members RelB and p100 gives molecular insight into CO2-dependent immune regulation. J. Biol. Chem. 2017, 292, 11561–11571. [Google Scholar] [CrossRef] [PubMed]
- Dogliotti, G.; Galliera, E.; Iorio, E.; De Bernardi Di Valserra, M.; Solimene, U.; Corsi, M.M. Effect of immersion in CO2-enriched water on free radical release and total antioxidant status in peripheral arterial occlusive disease. Int. Angiol. 2011, 30, 12–17. [Google Scholar]
- Bolevich, S.; Kogan, A.; Zivkovic, V.; Djuric, D.; Novikov, A.; Vorobyev, S.; Jakovljevic, V. Protective role of carbon dioxide (CO2) in generation of reactive oxygen species. Mol. Cell Biochem. 2016, 411, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Veselá, A.; Wilhelm, J. The role of carbon dioxide in free radical reactions of the organism. Physiol. Res. 2002, 51, 335–339. [Google Scholar]
- Nemeth, B.; Kiss, I.; Jencsik, T.; Peter, I.; Kreska, Z.; Koszegi, T.; Miseta, A.; Kustan, P.; Boncz, I.; Laczo, A.; et al. Angiotensin-converting enzyme inhibition improves the effectiveness of transcutaneous carbon dioxide treatment. vivo 2017, 31, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Botusan, I.R.; Sunkari, V.G.; Savu, O.; Catrina, A.I.; Grünler, J.; Lindberg, S.; Pereira, T.; Ylä-Herttuala, S.; Poellinger, L.; Brismar, K.; et al. Stabilization of HIF-1α is critical to improve wound healing in diabetic mice. Proc. Nat. Acad. Sci. USA 2008, 105, 19426–19431. [Google Scholar] [CrossRef]
- Stewart, K.J. Role of exercise training on cardiovascular disease in persons who have type 2 diabetes and hypertension. Cardiol. Clin. 2004, 22, 569–586. [Google Scholar] [CrossRef]
- Maruyama, K.; Okada, T.; Ueha, T.; Isohashi, K.; Ikeda, H.; Kanai, Y.; Sasaki, K.; Gentsu, T.; Ueshima, E.; Sofue, K.; et al. In vivo evaluation of percutaneous carbon dioxide treatment for improving intratumoral hypoxia using 18F-fluoromisonidazole PET-CT. Oncol. Lett. 2021, 21, 207. [Google Scholar] [CrossRef]
- Summers, B.A.; Overholt, J.L.; Prabhakar, N.R. CO2 and pH independently modulate L-type Ca2+ current in rabbit carotid body glomus cells. J. Neurophysiol. 2002, 88, 604–612. [Google Scholar] [CrossRef]
- Vadász, I.; Dada, L.A.; Briva, A.; Trejo, H.E.; Welch, L.C.; Chen, J.; Tóth, P.T.; Lecuona, E.; Witters, L.A.; Schumacker, P.T.; et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting na, K-ATPase endocytosis. J. Clin. Investig. 2008, 118, 752–762. [Google Scholar] [CrossRef]
- Irrcher, I.; Adhihetty, P.J.; Sheehan, T.; Joseph, A.; Hood, D.A. PPARgamma coactivator-1alpha expression during thyroid hormone- and contractile activity-induced mitochondrial adaptations. Am. J. Physiol: Cell Physiol. 2003, 284, C1669–C1677. [Google Scholar] [CrossRef] [PubMed]
- Ojuka, E.O.; Jones, T.E.; Han, D.; Chen, M.; Holloszy, J.O. Raising Ca2+ in L6 myotubes mimics effects of exercise on mitochondrial biogenesis in muscle. FASEB J. 2003, 17, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Montalto, A.S.; Currò, M.; Russo, T.; Visalli, G.; Impellizzeri, P.; Antonuccio, P.; Arena, S.; Borruto, F.A.; Scalfari, G.; Ientile, R.; et al. In Vitro CO2-induced ROS production impairs cell cycle in SH-SY5Y neuroblastoma cells. Pediatr. Surg. Int. 2013, 29, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; Yamaguchi, M.; Namiki, S.; Nakayama, R.; Tabata, M.; et al. Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1. Nature 2010, 464, 1313–1319. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).