Respiratory Health Effects of In Vivo Sub-Chronic Diesel and Biodiesel Exhaust Exposure
Abstract
1. Introduction
2. Results
2.1. Exhaust Gas Characteristics
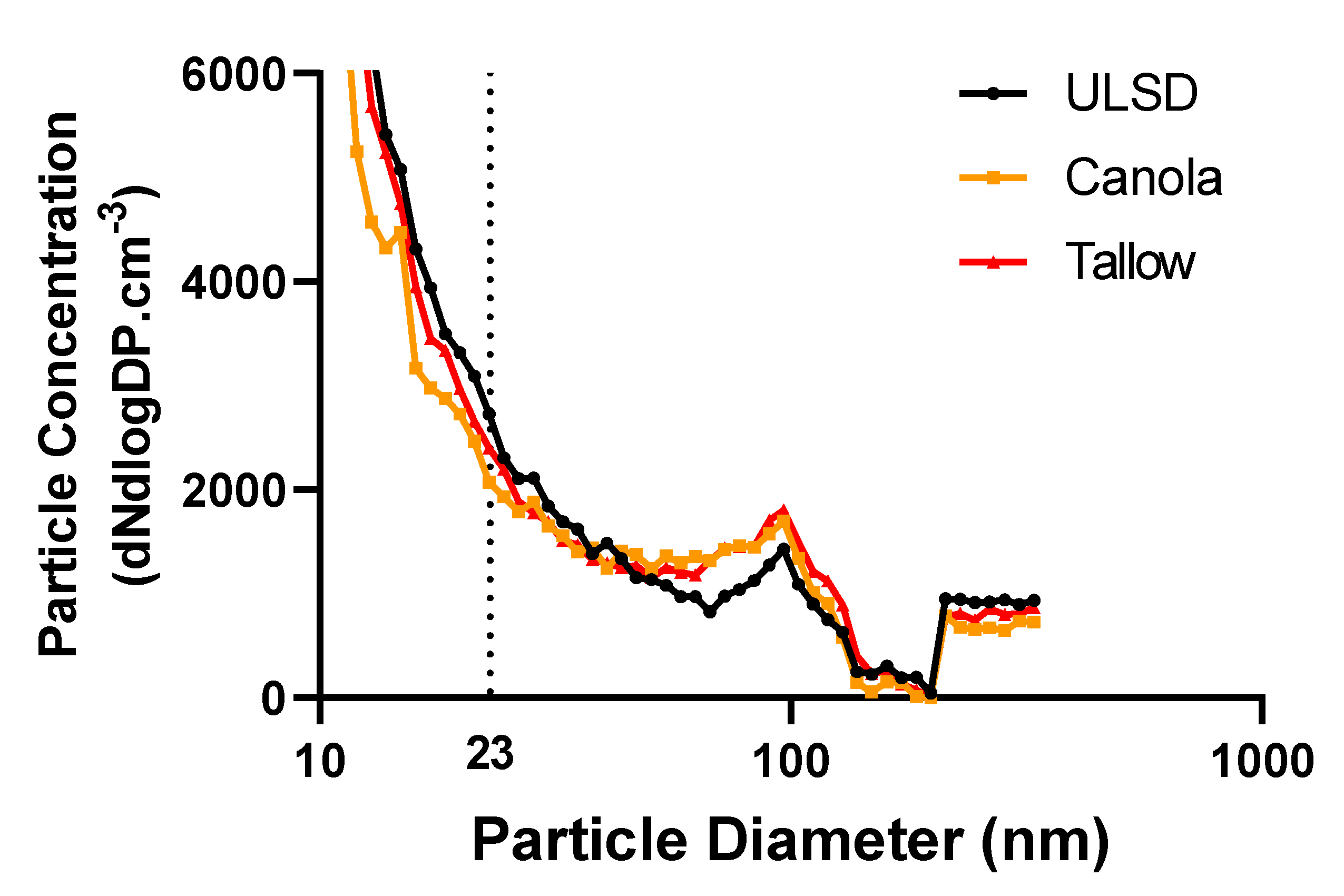
2.2. Exhaust Particle Characteristics
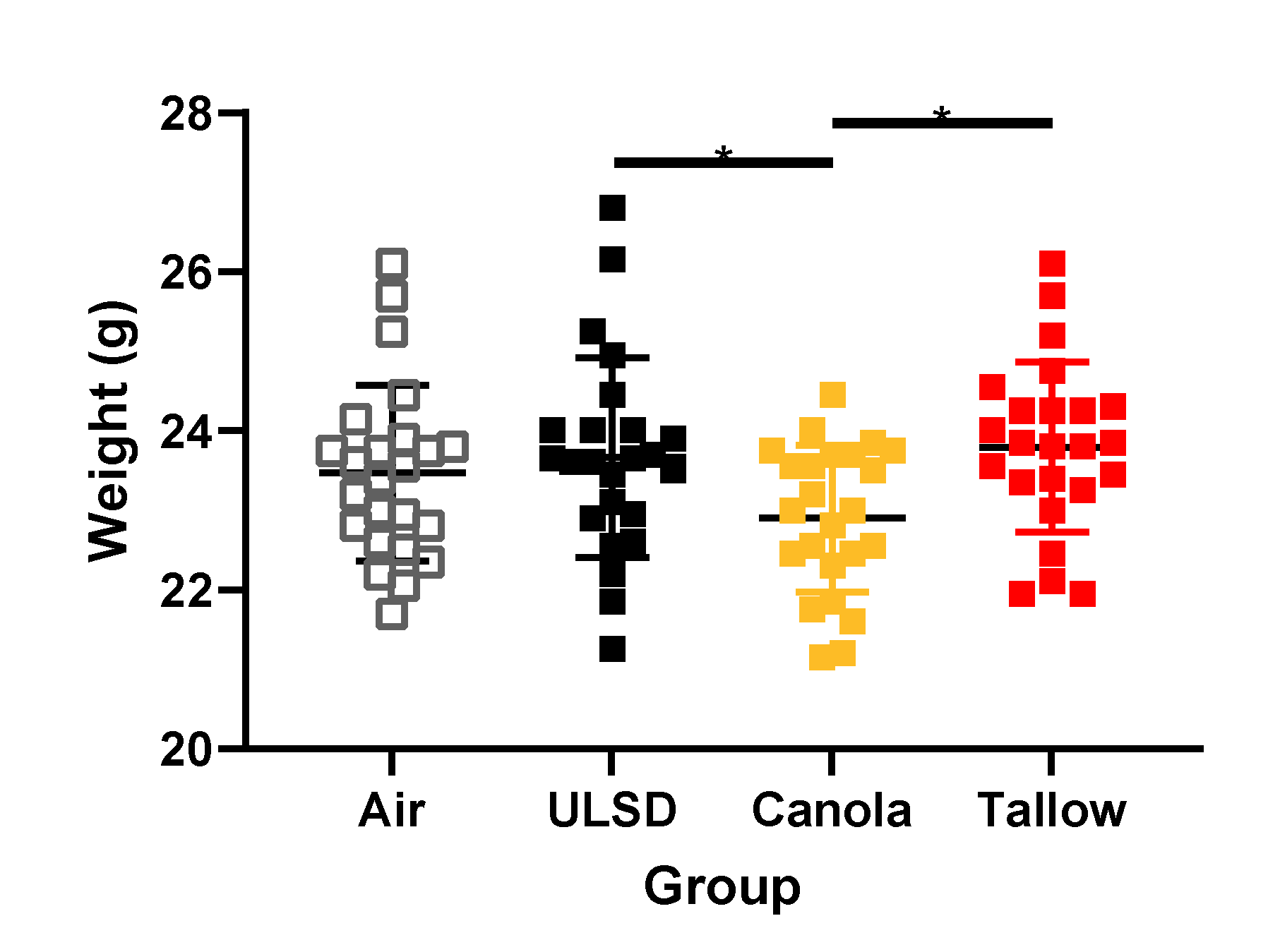
2.3. Mouse Weights
2.4. Lung Function at Functional Residual Capacity
2.5. Volume Dependence of Lung Function
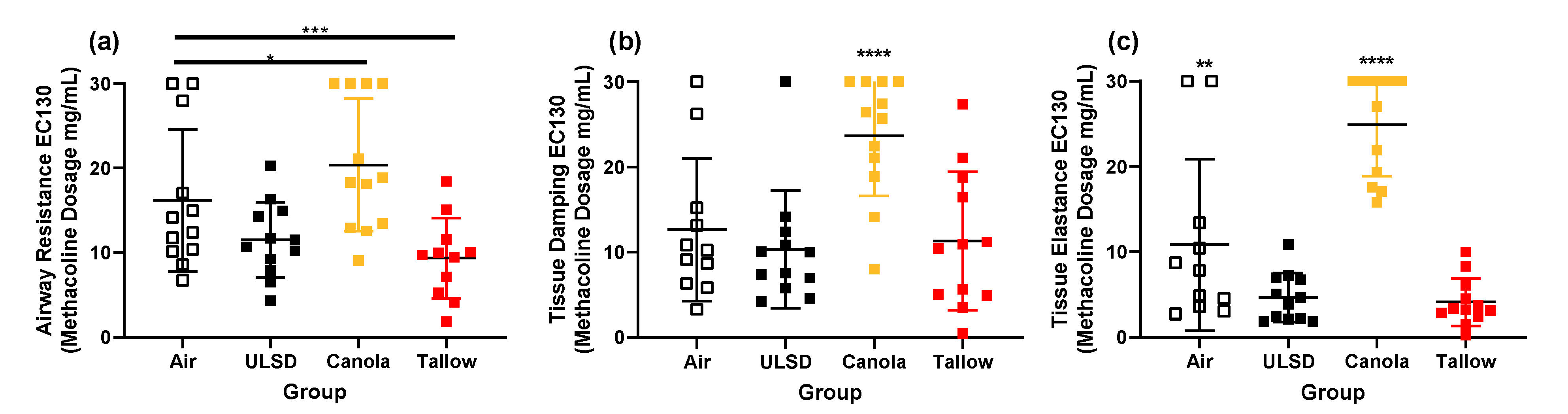
2.6. Responsiveness to Methacholine
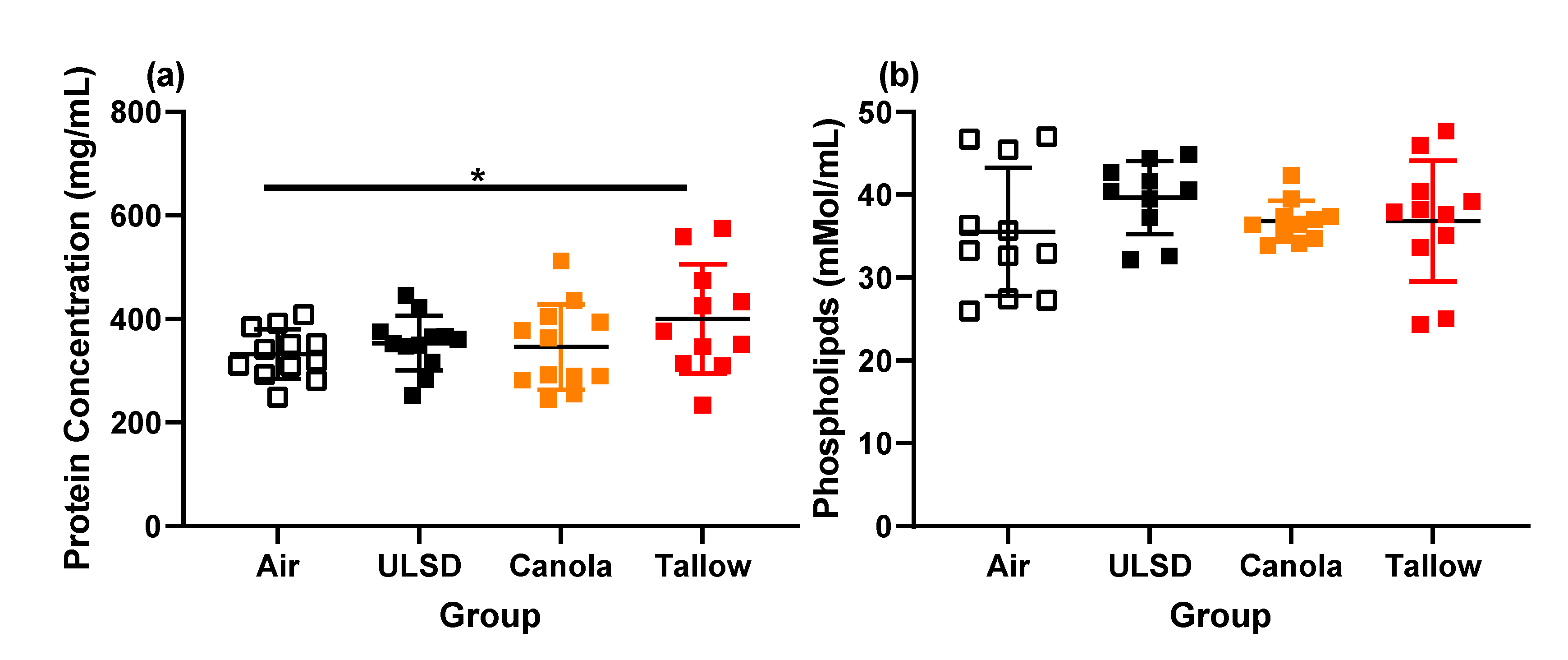
2.7. Bronchoalveolar Lavage Cells, Mediators, Protein, and Phospholipid Concentrations
2.8. Multivariate Analysis
2.9. Airway Morphometry
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Engine Configuration and Fuel Information
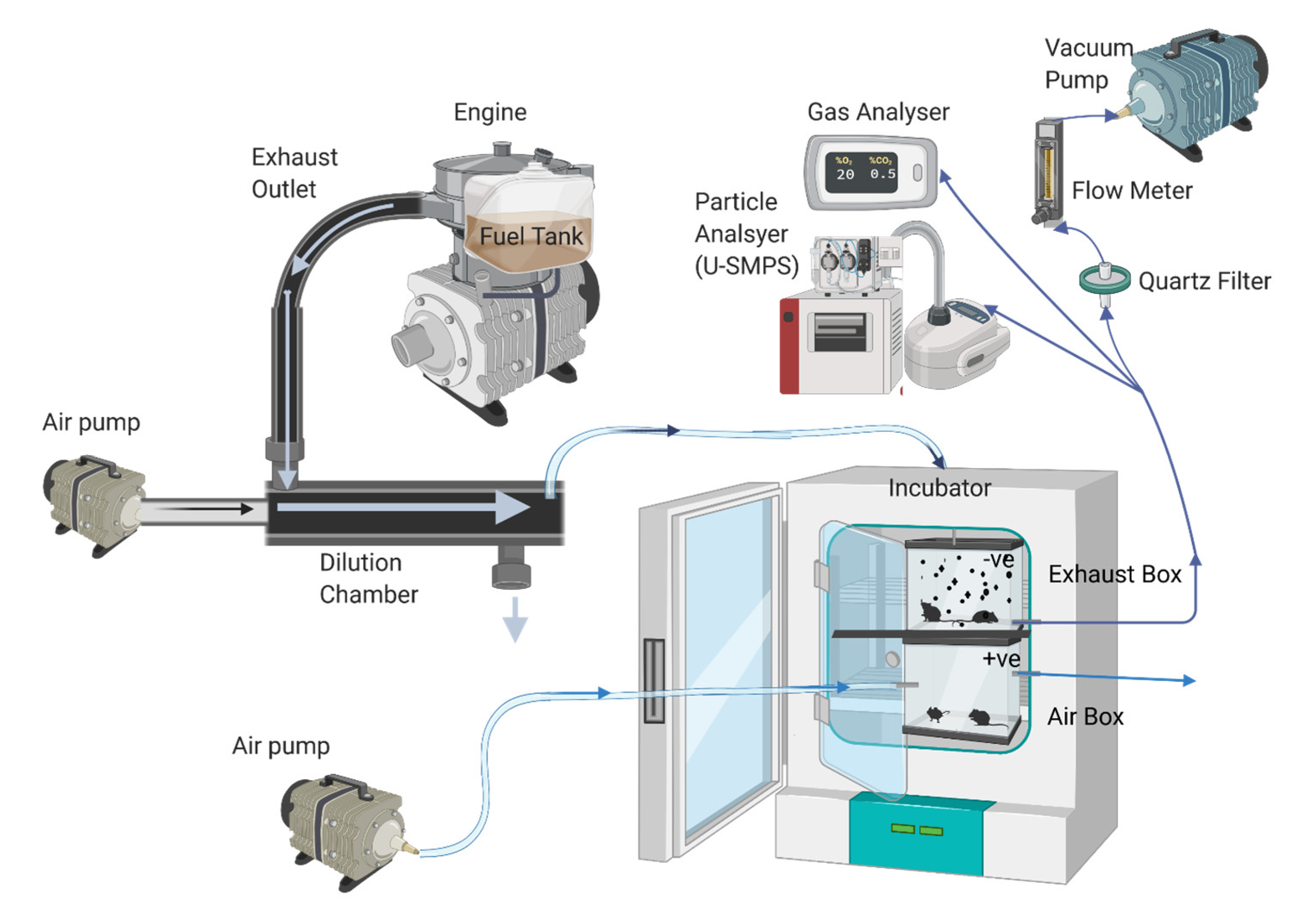
4.3. Exposure Protocol
4.4. Gas and Particle Analysis
4.5. Lung Function Measurements
4.6. Methacholine Challenge
4.7. Bronchoalveolar Lavage (BAL) Collection and Cell Measurement
4.8. Lung Fixation, Airway Morphometry, and Histology
4.9. Mediator, Phospholipid, and Protein Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landwehr, K.R.; Larcombe, A.N.; Reid, A.; Mullins, B.J. Critical review of diesel exhaust exposure health impact research relevant to occupational settings: Are we controlling the wrong pollutants? Expo. Health 2021, 13, 141–171. [Google Scholar] [CrossRef]
- Long, E.; Carlsten, C. Controlled human exposure to diesel exhaust: Results illuminate health effects of traffic-related air pollution and inform future directions. Part. Fibre Toxicol. 2022, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.T.; Samanic, C.M.; Lubin, J.H.; Blair, A.E.; Stewart, P.A.; Vermeulen, R.; Coble, J.B.; Rothman, N.; Schleiff, P.L.; Travis, W.D.; et al. The Diesel Exhaust in Miners study: A nested case-control study of lung cancer and diesel exhaust. J. Natl. Cancer Inst. 2012, 104, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Attfield, M.D.; Schleiff, P.L.; Lubin, J.H.; Blair, A.; Stewart, P.A.; Vermeulen, R.; Coble, J.B.; Silverman, D.T. The Diesel Exhaust in Miners study: A cohort mortality study with emphasis on lung cancer. J. Natl. Cancer Inst. 2012, 104, 869–883. [Google Scholar] [CrossRef]
- Andersen, Z.J.; Pedersen, M.; Weinmayr, G.; Stafoggia, M.; Galassi, C.; Jørgensen, J.T.; Sommar, J.N.; Forsberg, B.; Olsson, D.; Oftedal, B.; et al. Long-term exposure to ambient air pollution and incidence of brain tumor: The European Study of Cohorts for Air Pollution Effects (ESCAPE). Neuro Oncol. 2018, 20, 420–432. [Google Scholar] [CrossRef]
- Latifovic, L.; Villeneuve, P.J.; Parent, M.-É.; Johnson, K.C.; Kachuri, L.; Canadian Cancer Registries Epidemiology Group; Harris, S.A. Bladder cancer and occupational exposure to diesel and gasoline engine emissions among Canadian men. Cancer Med. 2015, 4, 1948–1962. [Google Scholar] [CrossRef]
- Cosselman, K.E.; Krishnan, R.; Oron, A.P.; Jansen, K.; Peretz, A.; Sullivan, J.H.; Larson, T.V.; Kaufman, J.D. Blood pressure response to controlled diesel exhaust exposure in human subjects. Hypertension 2012, 59, 943–948. [Google Scholar] [CrossRef]
- Crüts, B.; van Etten, L.; Törnqvist, H.; Blomberg, A.; Sandström, T.; Mills, N.L.; Borm, P.J. Exposure to diesel exhaust induces changes in EEG in human volunteers. Part. Fibre Toxicol. 2008, 5, 4. [Google Scholar] [CrossRef]
- Mills, N.L.; Törnqvist, H.; Gonzalez, M.C.; Vink, E.; Robinson, S.D.; Söderberg, S.; Boon, N.A.; Donaldson, K.; Sandström, T.; Blomberg, A.; et al. Ischemic and Thrombotic Effects of Dilute Diesel-Exhaust Inhalation in Men with Coronary Heart Disease. N. Engl. J. Med. 2007, 357, 1075–1082. [Google Scholar] [CrossRef]
- Zhu, N.; Li, H.; Han, M.; Guo, L.; Chen, L.; Yun, Y.; Guo, Z.; Li, G.; Sang, N. Environmental nitrogen dioxide (NO2) exposure influences development and progression of ischemic stroke. Toxicol. Lett. 2012, 214, 120–130. [Google Scholar] [CrossRef]
- Balti, E.V.; Echouffo-Tcheugui, J.B.; Yako, Y.Y.; Kengne, A.P. Air pollution and risk of type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2014, 106, 161–172. [Google Scholar] [CrossRef]
- Eze, I.C.; Hemkens, L.G.; Bucher, H.C.; Hoffmann, B.; Schindler, C.; Künzli, N.; Schikowski, T.; Probst-Hensch, N.M. Association between ambient air pollution and diabetes mellitus in Europe and North America: Systematic review and meta-analysis. Environ. Health Perspect. 2015, 123, 381–389. [Google Scholar] [CrossRef]
- Fleisch, A.F.; Kloog, I.; Luttmann-Gibson, H.; Gold, D.R.; Oken, E.; Schwartz, J.D. Air pollution exposure and gestational diabetes mellitus among pregnant women in Massachusetts: A cohort study. Environ. Health A Glob. Access Sci. Source 2016, 15, 40. [Google Scholar] [CrossRef]
- Evans, K.A.; Halterman, J.S.; Hopke, P.K.; Fagnano, M.; Rich, D.Q. Increased ultrafine particles and carbon monoxide concentrations are associated with asthma exacerbation among urban children. Environ. Res. 2014, 129, 11–19. [Google Scholar] [CrossRef]
- Kittelson, D.; Watts, W.; Johnson, J. Diesel Aerosol Sampling Methodology; CRC E-43 Final Report; Coordinating Research Council: Alpharetta, GA, USA, 19 August 2002. [Google Scholar]
- Ris, C.U.S. EPA Health Assessment for Diesel Engine Exhaust: A Review. Inhal. Toxicol. 2007, 19, 229–239. [Google Scholar] [CrossRef]
- Yusuf, A.A.; Yusuf, D.A.; Jie, Z.; Bello, T.Y.; Tambaya, M.; Abdullahi, B.; Muhammed-Dabo, I.A.; Yahuza, I.; Dandakouta, H. Influence of waste oil-biodiesel on toxic pollutants from marine engine coupled with emission reduction measures at various loads. Atmos. Pollut. Res. 2022, 13, 101258. [Google Scholar] [CrossRef]
- Fontaras, G.; Karavalakis, G.; Kousoulidou, M.; Tzamkiozis, T.; Ntziachristos, L.; Bakeas, E.; Stournas, S.; Samaras, Z. Effects of biodiesel on passenger car fuel consumption, regulated and non-regulated pollutant emissions over legislated and real-world driving cycles. Fuel 2009, 88, 1608–1617. [Google Scholar] [CrossRef]
- Prokopowicz, A.; Zaciera, M.; Sobczak, A.; Bielaczyc, P.; Woodburn, J. The Effects of Neat Biodiesel and Biodiesel and HVO Blends in Diesel Fuel on Exhaust Emissions from a Light Duty Vehicle with a Diesel Engine. Environ. Sci. Technol. 2015, 49, 7473–7482. [Google Scholar] [CrossRef]
- Graver, B.M.; Frey, H.C.; Hu, J. Effect of Biodiesel Fuels on Real-World Emissions of Passenger Locomotives. Environ. Sci. Technol. 2016, 50, 12030–12039. [Google Scholar] [CrossRef]
- Giakoumis, E.G.; Rakopoulos, C.D.; Dimaratos, A.M.; Rakopoulos, D.C. Exhaust emissions of diesel engines operating under transient conditions with biodiesel fuel blends. Prog. Energy Combust. Sci. 2012, 38, 691–715. [Google Scholar] [CrossRef]
- Mullins, B.J.; Kicic, A.; Ling, K.-M.; Mead-Hunter, R.; Larcombe, A.N. Biodiesel Exhaust–Induced Cytotoxicity and Proinflammatory Mediator Production in Human Airway Epithelial Cells. Environ. Toxicol. 2016, 31, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Westphal, G.A.; Krahl, J.; Munack, A.; Rosenkranz, N.; Schröder, O.; Schaak, J.; Pabst, C.; Brüning, T.; Bünger, J. Combustion of Hydrotreated Vegetable Oil and Jatropha Methyl Ester in a Heavy Duty Engine: Emissions and Bacterial Mutagenicity. Environ. Sci. Technol. 2013, 47, 6038–6046. [Google Scholar] [CrossRef] [PubMed]
- Gioda, A.; Rodríguez-Cotto, R.I.; Amaral, B.S.; Encarnación-Medina, J.; Ortiz-Martínez, M.G.; Jiménez-Vélez, B.D. Biodiesel from Soybean Promotes Cell Proliferation in Vitro. Toxicol. Vitr. 2016, 34, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G.; Celein, R.M.; Ferin, J.; Weiss, B. Association of Particulate Air Pollution and Acute Mortality: Involvement of Ultrafine Particles? Inhal. Toxicol. 1995, 7, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Breitner, S.; Liu, L.; Cyrys, J.; Brüske, I.; Franck, U.; Schlink, U.; Leitte, A.M.; Herbarth, O.; Wiedensohler, A.; Wehner, B.; et al. Sub-micrometer particulate air pollution and cardiovascular mortality in Beijing, China. Sci. Total Environ. 2011, 409, 5196–5204. [Google Scholar] [CrossRef]
- EIA. International Energy Statistics. Available online: https://www.eia.gov/international/overview/world (accessed on 28 October 2022).
- Larcombe, A.N.; Kicic, A.; Mullins, B.J.; Knothe, G. Biodiesel exhaust: The need for a systematic approach to health effects research. Respirology 2015, 20, 1034–1045. [Google Scholar] [CrossRef]
- André, V.; Barraud, C.; Capron, D.; Preterre, D.; Keravec, V.; Vendeville, C.; Cazier, F.; Pottier, D.; Morin, J.P.; Sichel, F. Comparative mutagenicity and genotoxicity of particles and aerosols emitted by the combustion of standard vs. rapeseed methyl ester supplemented bio-diesel fuels: Impact of after treatment devices: Oxidation catalyst and particulate filter. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2015, 777, 33–42. [Google Scholar] [CrossRef]
- Møller, P.; Scholten, R.H.; Roursgaard, M.; Krais, A.M. Inflammation, oxidative stress and genotoxicity responses to biodiesel emissions in cultured mammalian cells and animals. Crit. Rev. Toxicol. 2020, 50, 383–401. [Google Scholar] [CrossRef]
- Madden, M.C. A paler shade of green? The toxicology of biodiesel emissions: Recent findings from studies with this alternative fuel. Biochim. Biophys. Acta-Gen. Subj. 2016, 1860, 2856–2862. [Google Scholar] [CrossRef]
- Swanson, K.J.; Madden, M.l.C.; Ghio, A.J. Biodiesel Exhaust: The Need for Health Effects Research. Environ. Health Perspect. 2007, 115, 496–499. [Google Scholar] [CrossRef]
- Yanamala, N.; Hatfield, M.K.; Farcas, M.T.; Schwegler-Berry, D.; Hummer, J.A.; Shurin, M.R.; Birch, M.E.; Gutkin, D.W.; Kisin, E.; Kagan, V.E.; et al. Biodiesel versus diesel exposure: Enhanced pulmonary inflammation, oxidative stress, and differential morphological changes in the mouse lung. Toxicol. Appl. Pharmacol. 2013, 272, 373–383. [Google Scholar] [CrossRef]
- Chen, T.-M.; Kuschner, W.G.; Gokhale, J.; Shofer, S. Outdoor Air Pollution: Nitrogen Dioxide, Sulfur Dioxide, and Carbon Monoxide Health Effects. Am. J. Med. Sci. 2007, 333, 249–256. [Google Scholar] [CrossRef]
- Morin, J.-P.; Hasson, V.; Fall, M.; Papaioanou, E.; Preterre, D.; Gouriou, F.; Keravec, V.; Konstandopoulos, A.; Dionnet, F. Prevalidation of in vitro continuous flow exposure systems as alternatives to in vivo inhalation safety evaluation experimentations: Outcome from MAAPHRI-PCRD5 research program. Exp. Toxicol. Pathol. 2008, 60, 195–205. [Google Scholar] [CrossRef]
- Lichtveld, K.M.; Ebersviller, S.M.; Sexton, K.G.; Vizuete, W.; Jaspers, I.; Jeffries, H.E. In Vitro Exposures in Diesel Exhaust Atmospheres: Resuspension of PM from Filters versus Direct Deposition of PM from Air. Environ. Sci. Technol. 2012, 46, 9062–9070. [Google Scholar] [CrossRef]
- Bünger, J.; Krahl, J.; Baum, K.; Schröder, O.; Müller, M.; Westphal, G.; Ruhnau, P.; Schulz, T.G.; Hallier, E. Cytotoxic and mutagenic effects, particle size and concentration analysis of diesel engine emissions using biodiesel and petrol diesel as fuel. Arch. Toxicol. 2000, 74, 490–498. [Google Scholar] [CrossRef]
- Cervena, T.; Rossnerova, A.; Sikorova, J.; Beranek, V.; Vojtisek-Lom, M.; Ciganek, M.; Topinka, J.; Rossner, P. DNA Damage Potential of Engine Emissions Measured In Vitro by Micronucleus Test in Human Bronchial Epithelial Cells. Basic Clin. Pharmacol. Toxicol. 2017, 121, 102–108. [Google Scholar] [CrossRef]
- Jalava, P.I.; Aakko-Saksa, P.; Murtonen, T.; Happo, M.S.; Markkanen, A.; Yli-Pirilä, P.; Hakulinen, P.; Hillamo, R.; Mäki-Paakkanen, J.; Salonen, R.O.; et al. Toxicological properties of emission particles from heavy duty engines powered by conventional and bio-based diesel fuels and compressed natural gas. Part. Fibre Toxicol. 2012, 9, 37. [Google Scholar] [CrossRef]
- Brito, J.M.; Belotti, L.; Toledo, A.C.; Antonangelo, L.; Silva, F.S.; Alvim, D.S.; Andre, P.A.; Saldiva, P.H.N.; Rivero, D.H.R.F. Acute Cardiovascular and Inflammatory Toxicity Induced by Inhalation of Diesel and Biodiesel Exhaust Particles. Toxicol. Sci. 2010, 116, 67–78. [Google Scholar] [CrossRef]
- De Brito, J.M.; Mauad, T.; Cavalheiro, G.F.; Yoshizaki, K.; de André, P.A.; Lichtenfels, A.J.F.C.; Guimarães, E.T.; Rivero, D.H.R.F.; Antonangelo, L.; Oliveira, L.B.; et al. Acute exposure to diesel and sewage biodiesel exhaust causes pulmonary and systemic inflammation in mice. Sci. Total Environ. 2018, 628–629, 1223–1233. [Google Scholar] [CrossRef]
- Gavett, S.H.; Wood, C.E.; Williams, M.A.; Cyphert, J.M.; Boykin, E.H.; Daniels, M.J.; Copeland, L.B.; King, C.; Krantz, T.Q.; Richards, J.H.; et al. Soy biodiesel emissions have reduced inflammatory effects compared to diesel emissions in healthy and allergic mice. Inhal. Toxicol. 2015, 27, 533–544. [Google Scholar] [CrossRef]
- Farraj, A.K.; Haykal-Coates, N.; Winsett, D.W.; Gilmour, M.I.; King, C.; Krantz, Q.T.; Richards, J.; Hazari, M.S. Comparative electrocardiographic, autonomic and systemic inflammatory responses to soy biodiesel and petroleum diesel emissions in rats. Inhal. Toxicol. 2015, 27, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Hazari, M.S.; Haykal-Coates, N.; Winsett, D.W.; King, C.; Krantz, Q.T.; Gilmour, M.I.; Farraj, A.K. The effects of B0, B20, and B100 soy biodiesel exhaust on aconitine-induced cardiac arrhythmia in spontaneously hypertensive rats. Inhal. Toxicol. 2015, 27, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Bass, V.L.; Schladweiler, M.C.; Nyska, A.; Thomas, R.F.; Miller, D.B.; Krantz, T.; King, C.; Gilmour, M.I.; Ledbetter, A.D.; Richards, J.E.; et al. Comparative cardiopulmonary toxicity of exhausts from soy-based biofuels and diesel in healthy and hypertensive rats. Inhal. Toxicol. 2015, 27, 545–556. [Google Scholar] [CrossRef]
- Douki, T.; Corbière, C.; Preterre, D.; Martin, P.J.; Lecureur, V.; André, V.; Landkocz, Y.; Pottier, I.; Keravec, V.; Fardel, O.; et al. Comparative study of diesel and biodiesel exhausts on lung oxidative stress and genotoxicity in rats. Environ. Pollut. 2018, 235, 514–524. [Google Scholar] [CrossRef]
- Valand, R.; Magnusson, P.; Dziendzikowska, K.; Gajewska, M.; Wilczak, J.; Oczkowski, M.; Kamola, D.; Królikowski, T.; Kruszewski, M.; Lankoff, A.; et al. Gene expression changes in rat brain regions after 7- and 28 days inhalation exposure to exhaust emissions from 1st and 2nd generation biodiesel fuels—The FuelHealth project. Inhal. Toxicol. 2018, 30, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, P.; Dziendzikowska, K.; Oczkowski, M.; Øvrevik, J.; Eide, D.M.; Brunborg, G.; Gutzkow, K.B.; Instanes, C.; Gajewska, M.; Wilczak, J.; et al. Lung effects of 7- and 28-day inhalation exposure of rats to emissions from 1st and 2nd generation biodiesel fuels with and without particle filter—The FuelHealth project. Environ. Toxicol. Pharmacol. 2019, 67, 8–20. [Google Scholar] [CrossRef]
- Dziendzikowska, K.; Gajewska, M.; Wilczak, J.; Mruk, R.; Oczkowski, M.; Żyła, E.; Królikowski, T.; Stachoń, M.; Øvrevik, J.; Myhre, O.; et al. The effects of 1st and 2nd generation biodiesel exhaust exposure on hematological and biochemical blood indices of Fisher344 male rats—The FuelHealth project. Environ. Toxicol. Pharmacol. 2018, 63, 34–47. [Google Scholar] [CrossRef]
- Magnusson, P.; Oczkowski, M.; Øvrevik, J.; Gajewska, M.; Wilczak, J.; Biedrzycki, J.; Dziendzikowska, K.; Kamola, D.; Królikowski, T.; Kruszewski, M.; et al. No adverse lung effects of 7- and 28-day inhalation exposure of rats to emissions from petrodiesel fuel containing 20% rapeseed methyl esters (B20) with and without particulate filter—The FuelHealth project. Inhal. Toxicol. 2017, 29, 206–218. [Google Scholar] [CrossRef]
- EERE, Energy Efficiency & Renewable Energy, Alternate Fuels Data Centre. US Department of Energy 2020.
- ASTM International. ASTM D7467-20a, Standard Specification for Diesel Fuel Oil, Biodiesel Blend (B6 to B20); ASTM: West Conshohocken, PA, USA, 2020. [Google Scholar] [CrossRef]
- Hamje, H.D.; Hass, H.; Lonza, L.; Maas, H.; Reid, A.; Rose, K.; Venderbosch, T. EU renewable energy targets in 2020: Revised analysis of scenarios for transport fuels. Sci. Policy Rep. Jt. Res. Cent. Eur. Comm. 2014, 27. [Google Scholar] [CrossRef]
- Landwehr, K.R.; Hillas, J.; Mead-Hunter, R.; Brooks, P.; King, A.; O’Leary, R.A.; Kicic, A.; Mullins, B.J.; Larcombe, A.N. Fuel feedstock determines biodiesel exhaust toxicity in a human airway epithelial cell exposure model. J. Hazard. Mater. 2021, 420, 126637. [Google Scholar] [CrossRef]
- Hawley, B.; L’Orange, C.; Olsen, D.B.; Marchese, A.J.; Volckens, J. Oxidative stress and aromatic hydrocarbon response of human bronchial epithelial cells exposed to petro- or biodiesel exhaust treated with a diesel particulate filter. Toxicol. Sci. Off. J. Soc. Toxicol. 2014, 141, 505–514. [Google Scholar] [CrossRef]
- Ackland, M.L.; Zou, L.; Freestone, D.; Van De Waasenburg, S.; Michalczyk, A.A. Diesel exhaust particulate matter induces multinucleate cells and zinc transporter-dependent apoptosis in human airway cells. Immunol. Cell Biol. 2007, 85, 617–622. [Google Scholar] [CrossRef]
- Hemmingsen, J.G.; Møller, P.; Nøjgaard, J.K.; Roursgaard, M.; Loft, S. Oxidative Stress, Genotoxicity, And Vascular Cell Adhesion Molecule Expression in Cells Exposed to Particulate Matter from Combustion of Conventional Diesel and Methyl Ester Biodiesel Blends. Environ. Sci. Technol. 2011, 45, 8545–8551. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2020–2029; FAO: Rome, Italy, 2020. [Google Scholar]
- ARENA (Australian Renewable Energy Agency), Clean Energy Finance Corporation. Biofuels and Transport: An Australian Opportunity; ARENA (Australian Renewable Energy Agency): Canberra, Australia, 2018. [Google Scholar]
- Landwehr, K.R.; Hillas, J.; Mead-Hunter, R.; King, A.; O’Leary, R.A.; Kicic, A.; Mullins, B.J.; Larcombe, A.N. Toxicity of different biodiesel exhausts in primary human airway epithelial cells grown at air-liquid interface. Sci. Total Environ. 2022, 832, 155016. [Google Scholar] [CrossRef]
- Amanatidis, S.; Ntziachristos, L.; Giechaskiel, B.; Bergmann, A.; Samaras, Z. Impact of Selective Catalytic Reduction on Exhaust Particle Formation over Excess Ammonia Events. Environ. Sci. Technol. 2014, 48, 11527–11534. [Google Scholar] [CrossRef]
- SafeWork Australia. Workplace Exposure Standards for Airborne Contaminants; SafeWork Australia: Canberra, Australia, 2019. [Google Scholar]
- European Agency for Safety and Health at Work. Workplace Exposure Standards for Airborne Contaminants. Available online: https://osha.europa.eu/en/themes/dangerous-substances/practical-tools-dangerous-substances/workplace-exposure-standards (accessed on 22 April 2021).
- Occupational Safety and Health Administration. Permissible Exposure Limits–Annotated Tables. Available online: https://www.osha.gov/annotated-pels/table-z-1 (accessed on 22 April 2021).
- SafeWork Australia. Confined spaces Code of Practice; SafeWork Australia: Canberra, Australia, 2018. [Google Scholar]
- European Union Directive 2004/37/EC of the European Parliament and of the Council of 29 April 2004 on the protection of workers from the risks related to exposure to carcinogens or mutagens at work. Off. J. Eur. Union. 2004, 47, 50–76.
- European Union Directive (EU) 2019/130 of the European Parliament and of the Council of 16 January 2019 amending Directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens or mutagens at work (Text with EEA relevance). Off. J. Eur. Union 2019, 30, 112.
- Mine Safety and Health Administration. Exposure of Underground Miners to Diesel Exhaust; Mine Safety and Health Administration: Arlington, VA, USA, 2016. [Google Scholar]
- Australian Institute of Occupational Hygienists. Diesel Particulate Matter and Occupational Health Issues-Position Paper; Australian Institute of Occupational Hygienists: Tullamarine, Australia, 2017. [Google Scholar]
- Unosson, J.; Kabéle, M.; Boman, C.; Nyström, R.; Sadiktsis, I.; Westerholm, R.; Mudway, I.S.; Purdie, E.; Raftis, J.; Miller, M.R.; et al. Acute cardiovascular effects of controlled exposure to dilute Petrodiesel and biodiesel exhaust in healthy volunteers: A crossover study. Part. Fibre Toxicol. 2021, 18, 22. [Google Scholar] [CrossRef]
- Landwehr, K.R.; Hillas, J.; Mead-Hunter, R.; O’Leary, R.A.; Kicic, A.; Mullins, B.J.; Larcombe, A.N. Soy Biodiesel Exhaust is More Toxic than Mineral Diesel Exhaust in Primary Human Airway Epithelial Cells. Environ. Sci. Technol. 2019, 53, 11437–11446. [Google Scholar] [CrossRef]
- Hesterberg, T.W.; Bunn, W.B.; McClellan, R.O.; Hamade, A.K.; Long, C.M.; Valberg, P.A. Critical review of the human data on short-term nitrogen dioxide (NO2) exposures: Evidence for NO2 no-effect levels. Crit. Rev. Toxicol. 2009, 39, 743–781. [Google Scholar] [CrossRef]
- Larcombe, A.N.; Janka, M.A.; Mullins, B.J.; Berry, L.J.; Bredin, A.; Franklin, P.J. The effects of electronic cigarette aerosol exposure on inflammation and lung function in mice. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017, 313, L67–L79. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Cras, T.D.L.; Larcombe, A.N.; Zosky, G.R.; Elliot, J.G.; James, A.L.; Noble, P.B. Independent and combined effects of airway remodelling and allergy on airway responsiveness. Clin. Sci. 2018, 132, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.A.; Gualano, R.C.; Zosky, G.R.; Atkins, C.L.; Turner, D.J.; Colasurdo, G.N.; Sly, P.D. Hyperresponsiveness to inhaled but not intravenous methacholine during acute respiratory syncytial virus infection in mice. Respir. Res. 2005, 6, 142. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.L.; Mangum, J.B.; DeLorme, M.P.; Everitt, J.I. Ultrafine Carbon Black Particles Enhance Respiratory Syncytial Virus-Induced Airway Reactivity, Pulmonary Inflammation, and Chemokine Expression. Toxicol. Sci. 2003, 72, 339–346. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Salam, S.; Zia, S.; Marzouqi, F.; Al-Dhaheri, A.; Subramaniyan, D.; Dhanasekaran, S.; Yasin, J.; Ali, B.H.; Kazzam, E.E. Contrasting actions of diesel exhaust particles on the pulmonary and cardiovascular systems and the effects of thymoquinone. Br. J. Pharm. 2011, 164, 1871–1882. [Google Scholar] [CrossRef]
- Brandt, E.B.; Biagini Myers, J.M.; Acciani, T.H.; Ryan, P.H.; Sivaprasad, U.; Ruff, B.; LeMasters, G.K.; Bernstein, D.I.; Lockey, J.E.; LeCras, T.D.; et al. Exposure to allergen and diesel exhaust particles potentiates secondary allergen-specific memory responses, promoting asthma susceptibility. J. Allergy Clin. Immunol. 2015, 136, 295–303.e297. [Google Scholar] [CrossRef]
- Brandt, E.B.; Kovacic, M.B.; Lee, G.B.; Gibson, A.M.; Acciani, T.H.; Le Cras, T.D.; Ryan, P.H.; Budelsky, A.L.; Khurana Hershey, G.K. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J. Allergy Clin. Immunol. 2013, 132, 1194–1204.e1192. [Google Scholar] [CrossRef]
- Nordenhäll, C.; Pourazar, J.; Ledin, M.-C.; Levin, J.-O.; Sandström, T.; Ädelroth, E. Diesel exhaust enhances airway responsiveness in asthmatic subjects. Eur. Respir. J. 2001, 17, 909–915. [Google Scholar] [CrossRef]
- Carlsten, C.; MacNutt, M.J.; Zhang, Z.; Sava, F.; Pui, M.M. Anti-Oxidant N-Acetylcysteine Diminishes Diesel Exhaust-Induced Increased Airway Responsiveness in Person with Airway Hyper-Reactivity. Toxicol. Sci. 2014, 139, 479–487. [Google Scholar] [CrossRef]
- Alvarez-Simón, D.; Muñoz, X.; Gómez-Ollés, S.; de Homdedeu, M.; Untoria, M.-D.; Cruz, M.-J. Effects of diesel exhaust particle exposure on a murine model of asthma due to soybean. PLoS ONE 2017, 12, e0179569. [Google Scholar] [CrossRef]
- Khalek, I.A.; Bougher, T.L.; Merritt, P.M.; Zielinska, B. Regulated and Unregulated Emissions from Highway Heavy-Duty Diesel Engines Complying with U.S. Environmental Protection Agency 2007 Emissions Standards. J. Air Waste Manag. Assoc. 2011, 61, 427–442. [Google Scholar] [CrossRef]
- Hesterberg, T.W.; Long, C.M.; Sax, S.N.; Lapin, C.A.; McClellan, R.O.; Bunn, W.B.; Valberg, P.A. Particulate Matter in New Technology Diesel Exhaust (NTDE) is Quantitatively and Qualitatively Very Different from that Found in Traditional Diesel Exhaust (TDE). J. Air Waste Manag. Assoc. 2011, 61, 894–913. [Google Scholar] [CrossRef]
- Karavalakis, G.; Stournas, S.; Bakeas, E. Light vehicle regulated and unregulated emissions from different biodiesels. Sci. Total Environ. 2009, 407, 3338–3346. [Google Scholar] [CrossRef]
- Kisin, E.R.; Shi, X.C.; Keane, M.J.; Bugarski, A.B.; Shvedova, A.A. Mutagenicity of biodiesel or diesel exhaust particles and the effect of engine operating conditions. J. Environ. Eng. Ecol. Sci. 2013, 2. [Google Scholar] [CrossRef]
- Bernhard, W. Lung surfactant: Function and composition in the context of development and respiratory physiology. Ann. Anat. = Anat. Anz. Off. Organ Anat. Ges. 2016, 208, 146–150. [Google Scholar] [CrossRef]
- Chakraborty, M.; Kotecha, S. Pulmonary surfactant in newborn infants and children. Breathe 2013, 9, 476–488. [Google Scholar] [CrossRef]
- Boylen, C.E.; Sly, P.D.; Zosky, G.R.; Larcombe, A.N. Physiological and inflammatory responses in an anthropomorphically relevant model of acute diesel exhaust particle exposure are sex and dose-dependent. Inhal. Toxicol. 2011, 23, 906–917. [Google Scholar] [CrossRef]
- Dai, Y.; Ren, D.; Bassig, B.A.; Vermeulen, R.; Hu, W.; Niu, Y.; Duan, H.; Ye, M.; Meng, T.; Xu, J.; et al. Occupational exposure to diesel engine exhaust and serum cytokine levels. Environ. Mol. Mutagen. 2018, 59, 144–150. [Google Scholar] [CrossRef]
- Gowdy, K.M.; Krantz, Q.T.; King, C.; Boykin, E.; Jaspers, I.; Linak, W.P.; Gilmour, M.I. Role of oxidative stress on diesel-enhanced influenza infection in mice. Part. Fibre Toxicol. 2010, 7, 34. [Google Scholar] [CrossRef]
- Zarcone, M.C.; van Schadewijk, A.; Duistermaat, E.; Hiemstra, P.S.; Kooter, I.M. Diesel exhaust alters the response of cultured primary bronchial epithelial cells from patients with chronic obstructive pulmonary disease (COPD) to non-typeable Haemophilus influenzae. Respir. Res. 2017, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Shears, R.K.; Jacques, L.C.; Naylor, G.; Miyashita, L.; Khandaker, S.; Lebre, F.; Lavelle, E.C.; Grigg, J.; French, N.; Neill, D.R.; et al. Exposure to diesel exhaust particles increases susceptibility to invasive pneumococcal disease. J. Allergy Clin. Immunol. 2020, 145, 1272–1284.e1276. [Google Scholar] [CrossRef] [PubMed]
- Larcombe, A.N.; Foong, R.E.; Boylen, C.E.; Zosky, G.R. Acute diesel exhaust particle exposure increases viral titre and inflammation associated with existing influenza infection, but does not exacerbate deficits in lung function. Influenza Other Respir. Viruses 2012, 7, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, E.A.; Noah, T.L.; Zhou, H.; Chehrazi, C.; Robinette, C.; Diaz-Sanchez, D.; Müller, L.; Jaspers, I. Diesel exposure suppresses natural killer cell function and resolution of eosinophil inflammation: A randomized controlled trial of exposure in allergic rhinitics. Part. Fibre Toxicol. 2016, 13, 24. [Google Scholar] [CrossRef]
- Hsia, C.C.; Hyde, D.M.; Ochs, M.; Weibel, E.R. An Official Research Policy Statement of the American Thoracic Society/European Respiratory Society: Standards for Quantitative Assessment of Lung Structure. Am. J. Respir. Crit. Care Med. 2010, 181, 394–418. [Google Scholar] [CrossRef]
- Crowley, G.; Kwon, S.; Caraher, E.J.; Haider, S.H.; Lam, R.; Batra, P.; Melles, D.; Liu, M.; Nolan, A. Quantitative lung morphology: Semi-automated measurement of mean linear intercept. BMC Pulm. Med. 2019, 19, 206. [Google Scholar] [CrossRef]
- Mitzner, W. Use of mean airspace chord length to assess emphysema. J. Appl. Physiol. 2008, 105, 1980–1981. [Google Scholar] [CrossRef]
- Zarcone, M.C.; Duistermaat, E.; Schadewijk, A.V.; Jedynska, A.; Hiemstra, P.S.; Kooter, I.M. Cellular response of mucociliary differentiated primary bronchial epithelial cells to diesel exhaust. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 311, L111–L123. [Google Scholar] [CrossRef]
- Larcombe, A.N.; Phan, J.A.; Kicic, A.; Perks, K.L.; Mead-Hunter, R.; Mullins, B.J. Route of exposure alters inflammation and lung function responses to diesel exhaust. Inhal. Toxicol. 2014, 26, 409–418. [Google Scholar] [CrossRef]
- Larcombe, A.N.; Zosky, G.R.; Bozanich, E.M.; Turner, D.J.; Hantos, Z.; Sly, P.D. Absence of cholinergic airway tone in normal BALB/c mice. Respir. Physiol. Neurobiol. 2008, 161, 223–229. [Google Scholar] [CrossRef]
- Knothe, G.; de Castro, M.E.G.; Razon, L.F. Methyl Esters (Biodiesel) from and Fatty Acid Profile of Gliricidia sepium Seed Oil. J. Am. Oil Chem. Soc. 2015, 92, 769–775. [Google Scholar] [CrossRef]
- Landwehr, K.R.; Hillas, J.; Mead-Hunter, R.; Brooks, P.; King, A.; O’Leary, R.A.; Kicic, A.; Mullins, B.J.; Larcombe, A.N. In Vitro primary human airway epithelial whole exhaust exposure. MethodsX 2021, 8, 101561. [Google Scholar] [CrossRef]
- Olfert, J.S.; Symonds, J.P.R.; Collings, N. The effective density and fractal dimension of particles emitted from a light-duty diesel vehicle with a diesel oxidation catalyst. J. Aerosol Sci. 2007, 38, 69–82. [Google Scholar] [CrossRef]
- Larcombe, A.N.; Foong, R.E.; Berry, L.J.; Zosky, G.R.; Sly, P.D. In utero cigarette smoke exposure impairs somatic and lung growth in BALB/c mice. Eur. Respir. J. 2011, 38, 932–938. [Google Scholar] [CrossRef]
- Jánosi, T.Z.; Adamicza, Á.; Zosky, G.R.; Asztalos, T.; Sly, P.D.; Hantos, Z. Plethysmographic estimation of thoracic gas volume in apneic mice. J. Appl. Physiol. 2006, 101, 454–459. [Google Scholar] [CrossRef]
- Peták, F.; Hantos, Z.; Adamicza, Á.; Asztalos, T.; Sly, P.D. Methacholine-induced bronchoconstriction in rats: Effects of intravenous vs. aerosol delivery. J. Appl. Physiol. 1997, 82, 1479–1487. [Google Scholar] [CrossRef]
- Sly, P.D.; Collins, R.A.; Thamrin, C.; Turner, D.J.; Hantos, Z. Volume dependence of airway and tissue impedances in mice. J. Appl. Physiol. 2003, 94, 1460–1466. [Google Scholar] [CrossRef]
- Limjunyawong, N.; Fallica, J.; Horton, M.R.; Mitzner, W. Measurement of the Pressure-volume Curve in Mouse Lungs. JoVE 2015, 27, e52376. [Google Scholar] [CrossRef]
- Larcombe, A.N.; Foong, R.E.; Bozanich, E.M.; Berry, L.J.; Garratt, L.W.; Gualano, R.C.; Jones, J.E.; Dousha, L.F.; Zosky, G.R.; Sly, P.D. Sexual dimorphism in lung function responses to acute influenza A infection. Influenza Other Respir Viruses 2011, 5, 334–342. [Google Scholar] [CrossRef]
- Foong, R.E.; Sly, P.D.; Larcombe, A.N.; Zosky, G.R. No role for neutrophil elastase in influenza-induced cellular recruitment, cytokine production or airway hyperresponsiveness in mice. Respir. Physiol. Neurobiol. 2010, 173, 164–170. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Larcombe, A.N.; Papini, M.G.; Chivers, E.K.; Berry, L.J.; Lucas, R.M.; Wyrwoll, C.S. Mouse Lung Structure and Function after Long-Term Exposure to an Atmospheric Carbon Dioxide Level Predicted by Climate Change Modeling. Environ. Health Perspect. 2021, 129, 017001. [Google Scholar] [CrossRef] [PubMed]
- James, A.L.; Hogg, J.C.; Dunn, L.A.; Paré, P.D. The use of the internal perimeter to compare airway size and to calculate smooth muscle shortening. Am. Rev. Respir. Dis. 1988, 138, 136–139. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 28 October 2022).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. The vegan package (versions 2.5-7). Community Ecol. Package 2020, 10, 719. [Google Scholar]
- Cooper, T.F.; Berkelmans, R.; Ulstrup, K.E.; Weeks, S.; Radford, B.; Jones, A.M.; Doyle, J.; Canto, M.; O’Leary, R.A.; van Oppen, M.J. Environmental factors controlling the distribution of symbiodinium harboured by the coral Acropora millepora on the Great Barrier Reef. PLoS ONE 2011, 6, e25536. [Google Scholar] [CrossRef]





| ULSD | Canola | Tallow | |
|---|---|---|---|
| O2 (%) | 19.45 (0.53) | 19.58 (0.43) * #### | 19.20 (0.49) **** #### |
| CO (ppm) | 0.99 (0.76) | 1.87 (0.90) | 2.06 (1.15) |
| CO2 (%) | 0.95 (0.35) | 0.84 (0.27) ** #### | 1.11 (0.32) **** #### |
| NOx (ppm) | 33.30 (14.52) | 24.73 (9.35) **** #### | 32.37 (13.37) #### |
| NO (ppm) | 28.23 (11.48) | 22.71 (8.42) **** ### | 27.11 (9.95) ### |
| NO2 (ppm) | 5.07 (3.27) | 2.17 (1.47) **** #### | 5.47 (3.87) #### |
| SO2 (ppm) | 1.64 (0.73) | 1.21 (0.53) **** # | 1.38 (0.72) ** # |
| Particle Mass Concentration (µg/m3) | 54.42 | 42.58 [0.78] | 50.17 [0.92] |
| Median Particle Size (nm) | 18 | 20 | 20 |
| Total Particle Number (particles/cm3) | 101,788 | 89,086 [0.88] | 98,418 [0.97] |
| Particle Number >23 nm (particles/cm3) | 39,035 (38.55%) | 39,141 (43.94%) | 41,191 (41.85%) |
| Particle Number <23 nm (particles/cm3) | 62,753 (61.65%) | 49,945 (56.06%) | 57,228 (58.15%) |
| FRC Lung Function Measure | Exposure | |||
|---|---|---|---|---|
| Air | ULSD | Canola | Tallow | |
| TGV (mL) | 0.291 (0.043) | 0.279 (0.042) | 0.328 (0.049) **###a | 0.283 (0.035) a |
| sRaw, hPa·s−1 | 108.3 (23.80) | 102.6 (20.82) | 120.0 (21.08) ##a | 103.6 (22.32) a |
| sG, hPa | 2267 (421) | 2281 (255) | 2865 (398) ****#### a | 2160 (322) a |
| sH, hPa | 9446 (1734) | 9656 (1248) | 11,362 (1716) ***##a | 9217 (1835) a |
| η | 0.243 (0.020) | 0.237 (0.015) | 0.253 (0.024) **####a | 0.236 (0.027) |
| BAL Mediator | 8 Days Exposure | |||
|---|---|---|---|---|
| Air | ULSD | Canola | Tallow | |
| IL-1α (pg/mL) | 2.407 (0.806) | 2.278 (0.967) | 2.805 (1.097) a | 1.976 (0.767) a |
| IL-2 (pg/mL) | 2.835 (1.129) | 2.642 (1.242) | 3.643 (1.458) #a | 2.176 (0.771) a |
| IL-4 (pg/mL) | 0.338 (0.120) | 0.305 (0.130) | 0.357 (0.176) a | 0.220 (0.091) *a |
| IL-5 (pg/mL) | 0.900 (0.401) | 0.697 (0.366) | 0.990 (0.417) | 0.795 (0.615) |
| IL-6 (pg/mL) | 0.937 (0.744) | 1.052 (0.743) | 0.207 (0.070) | 0.811 (0.722) |
| IL-9 (pg/mL) | 3.364 (2.069) | 3.351 (1.958) | 3.795 (2.239) | 2.749 (1.380) |
| IL-10 (pg/mL) | 4.540 (1.297) | 3.036 (1.365) * | 4.160 (1.940) a | 2.890 (1.461) **a |
| IL-12(p40) (pg/mL) | 39.924 (16.53) | 37.253 (7.705) | 36.263 (8.970) | 43.284 (17.725) |
| IL-12(p70) (pg/mL) | 11.478 (2.881) | 8.457 (4.369) | 10.218 (4.897) | 7.948 (5.217) |
| IL-13 (pg/mL) | 17.758 (5.711) | 15.983 (6.676) | 21.243 (10.470) | 15.875 (6.435) |
| IL-17 (pg/mL) | 1.760 (0.343) | 1.464 (0.487) | 2.005 (0.864) #a | 1.198 (0.470) * |
| Eotaxin (pg/mL) | 5.084 (1.469) | 4.215 (1.585) | 5.359 (2.630) | 4.625 (1.226) |
| G-CSF (pg/mL) | 4.447 (2.192) | 3.342 (1.101) | 3.024 (0.009) *a | 4.707 (3.623) a |
| GM-CSF (pg/mL) | 4.494 (2.114) | 3.903 (2.182) | 4.725 (2.281) | 3.495 (1.313) |
| IFN-γ (pg/mL) | 3.201 (1.257) | 2.711 (1.083) | 3.152 (1.112) | 2.495 (1.140) |
| KC (pg/mL) | 39.244 (12.641) | 30.240 (7.664) * | 30.379 (2.714) *a | 41.86 (21.676) #a |
| MCP-1 (pg/mL) | 13.565 (8.868) | 13.646 (7.047) | 13.594 (8.997) | 14.862 (12.742) |
| MIP-1α (pg/mL) | 2.258 (0.584) | 1.545 (0.457) | 2.299 (1.297) a | 3.945 (3.918) *#a |
| MIP-1β (pg/mL) | 11.348 (4.568) | 8.628 (6.085) | 9.672 (6.022) | 8.876 (7.383) |
| RANTES (pg/mL) | 8.276 (2.247) | 7.515 (3.282) | 9.357 (3.376) a | 6.410 (2.617) a |
| TNF-α (pg/mL) | 8.913 (2.940) | 7.345 (2.861) | 9.451 (4.023) | 7.329 (1.951) |
| Measurement | Air | ULSD | Canola | Tallow |
|---|---|---|---|---|
| Total Wall Thickness (√area/Pbm) | 0.123 (0.015) | 0.130 (0.013) | 0.128 (0.013) | 0.127 (0.007) |
| True Wall Thickness (√area/Pbm) | 0.060 (0.007) | 0.057 (0.006) | 0.058 (0.005) | 0.058 (0.008) |
| Airway Smooth Muscle Thickness (√area/Pbm) | 0.036 (0.003) | 0.033 (0.003) | 0.033 (0.003) | 0.035 (0.004) |
| Epithelial Thickness (√area/Pbm) | 0.040 (0.003) | 0.039 (0.003) | 0.037 (0.002) | 0.038 (0.003) |
| Chord Length (µm) | 21.07 (2.31) | 21.89 (1.54) | 22.42 (2.39) | 23.33 (1.74) * |
| Collagen (%) | 2.718 (0.534) | 2.604 (0.230) | 2.456 (0.487) a | 3.262 (1.197) #a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landwehr, K.R.; Mead-Hunter, R.; O’Leary, R.A.; Kicic, A.; Mullins, B.J.; Larcombe, A.N. Respiratory Health Effects of In Vivo Sub-Chronic Diesel and Biodiesel Exhaust Exposure. Int. J. Mol. Sci. 2023, 24, 5130. https://doi.org/10.3390/ijms24065130
Landwehr KR, Mead-Hunter R, O’Leary RA, Kicic A, Mullins BJ, Larcombe AN. Respiratory Health Effects of In Vivo Sub-Chronic Diesel and Biodiesel Exhaust Exposure. International Journal of Molecular Sciences. 2023; 24(6):5130. https://doi.org/10.3390/ijms24065130
Chicago/Turabian StyleLandwehr, Katherine R., Ryan Mead-Hunter, Rebecca A. O’Leary, Anthony Kicic, Benjamin J. Mullins, and Alexander N. Larcombe. 2023. "Respiratory Health Effects of In Vivo Sub-Chronic Diesel and Biodiesel Exhaust Exposure" International Journal of Molecular Sciences 24, no. 6: 5130. https://doi.org/10.3390/ijms24065130
APA StyleLandwehr, K. R., Mead-Hunter, R., O’Leary, R. A., Kicic, A., Mullins, B. J., & Larcombe, A. N. (2023). Respiratory Health Effects of In Vivo Sub-Chronic Diesel and Biodiesel Exhaust Exposure. International Journal of Molecular Sciences, 24(6), 5130. https://doi.org/10.3390/ijms24065130







