Natriuretic Peptides: It Is Time for Guided Therapeutic Strategies Based on Their Molecular Mechanisms
Abstract
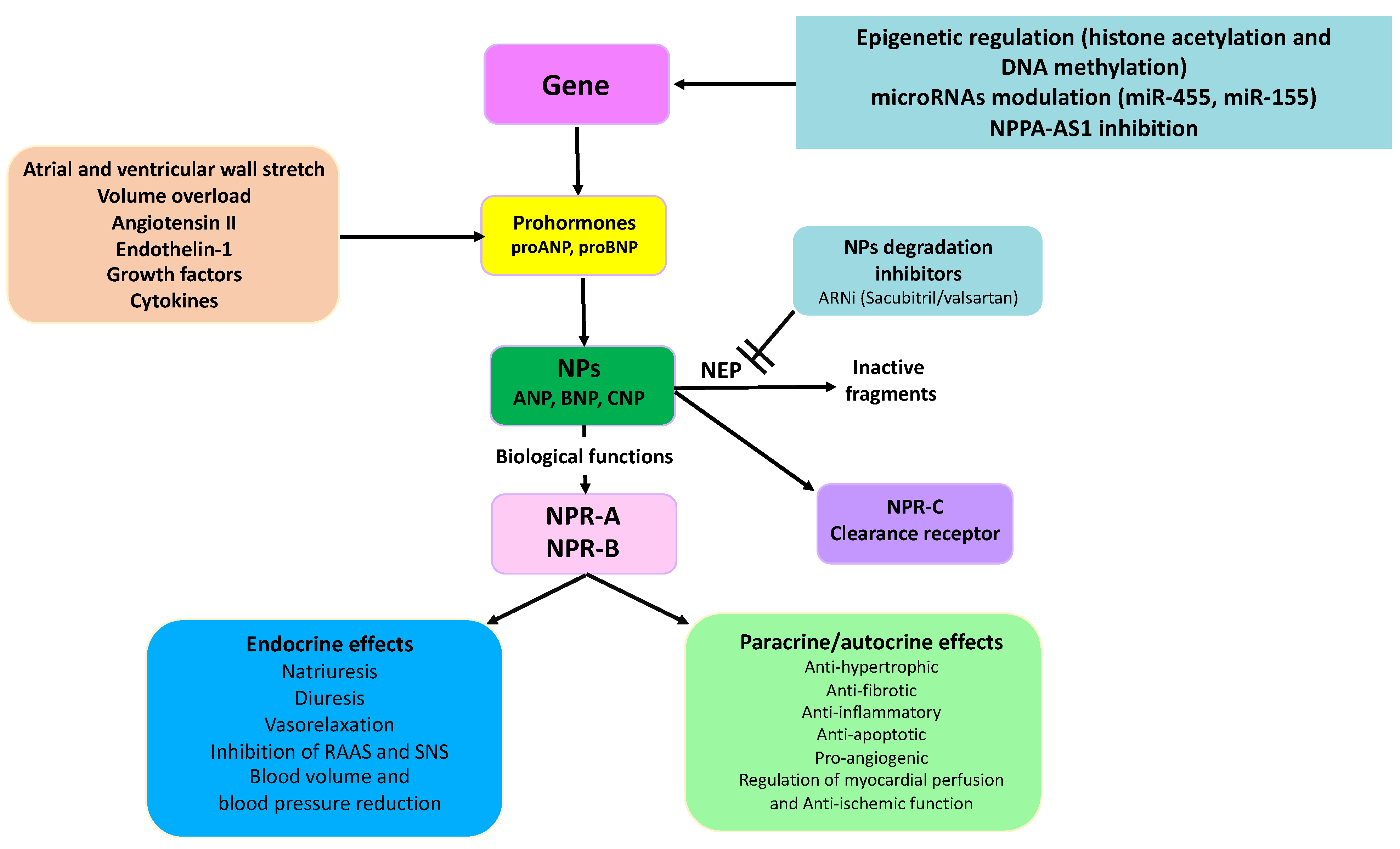
1. Regulation of Natriuretic Peptide Synthesis and Secretion
2. Natriuretic Peptide Receptors
3. The Key Role of Natriuretic Peptides as Clinical Biomarkers in Cardiovascular Diseases
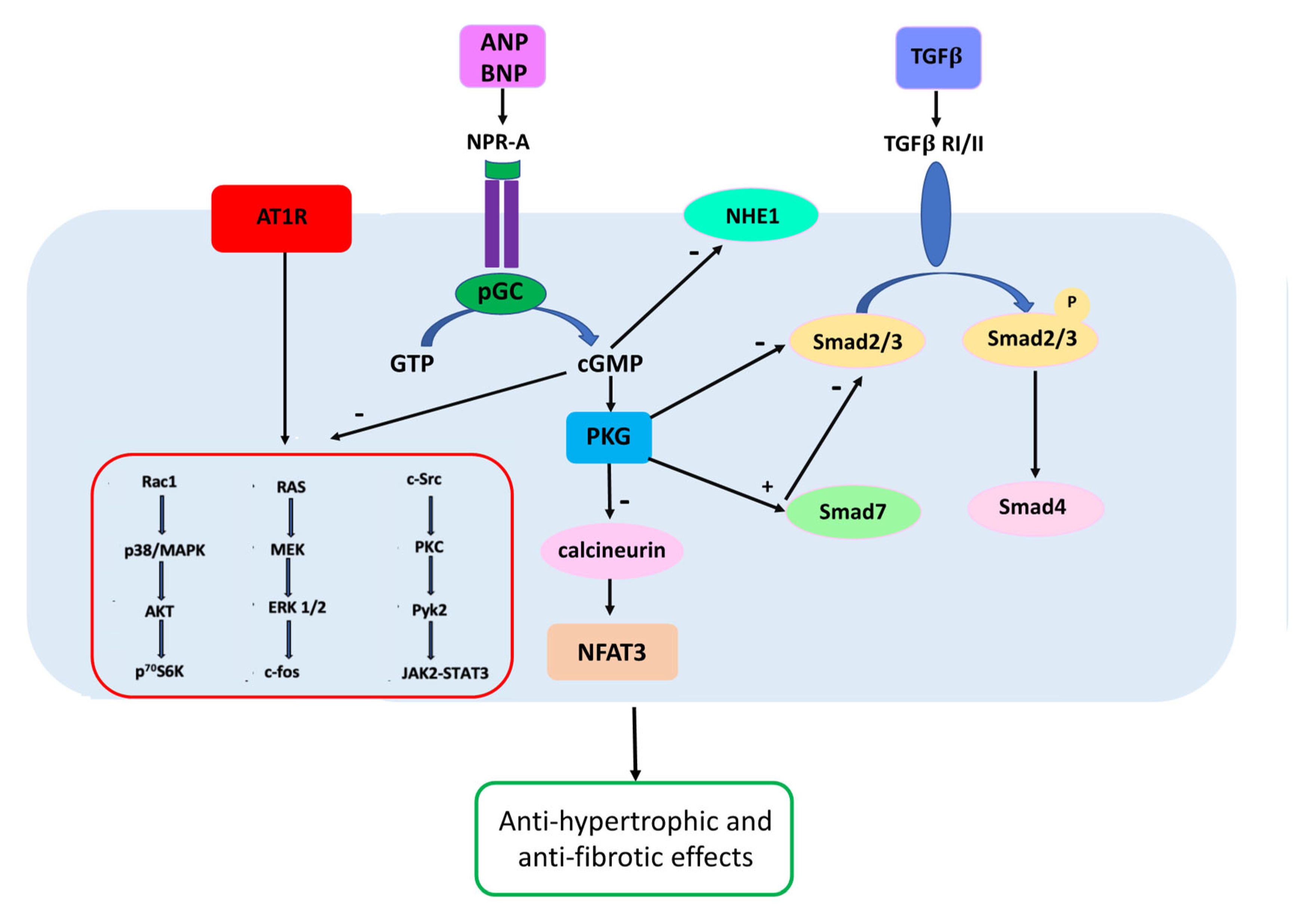
4. Therapeutic Implications of the Regulation of Natriuretic Peptide Levels and Activity
5. Novel NP-Based Therapeutic Strategies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Volpe, M.; Gallo, G.; Rubattu, S. Endocrine functions of the heart: From bench to bedside. Eur. Heart J. 2023, 44, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Rubattu, S.; Burnett, J., Jr. Natriuretic peptides in cardiovascular diseases: Current use and perspectives. Eur. Heart J. 2014, 35, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R.; Gardner, D.G.; Samson, W.K. Natriuretic peptides. N. Engl. J. Med. 1998, 339, 321–328. [Google Scholar]
- Pandey, K.N. Biology of natriuretic peptides and their receptors. Peptides 2005, 26, 901–932. [Google Scholar] [CrossRef]
- Forte, M.; Madonna, M.; Schiavon, S.; Valenti, V.; Versaci, F.; Zoccai, G.B.; Frati, G.; Sciarretta, S. Cardiovascular Pleiotropic Effects of Natriuretic Peptides. Int. J. Mol. Sci. 2019, 20, 3874. [Google Scholar] [CrossRef]
- Ogawa, T.; Vatta, M.; Bruneau, B.G.; de Bold, A.J. Characterization of natriuretic peptide production by adult heart atria. Am. J. Physiol. 1999, 276, H1977–H1986. [Google Scholar] [CrossRef]
- Lugnier, C.; Meyer, A.; Charloux, A.; Andrès, E.; Gény, B.; Talha, S. The Endocrine Function of the Heart: Physiology and Involvements of Natriuretic Peptides and Cyclic Nucleotide Phosphodiesterases in Heart Failure. J. Clin. Med. 2019, 8, 1746. [Google Scholar] [CrossRef]
- Mangat, H.; de Bold, A.J. Stretch-induced atrial natriuretic factor release utilizes a rapidly depleting pool of newly synthesized hormone. Endocrinology 1993, 133, 1398–1403. [Google Scholar] [CrossRef]
- Dietz, J.R. Mechanisms of atrial natriuretic peptide secretion from the atrium. Cardiovasc. Res. 2005, 68, 8–17. [Google Scholar] [CrossRef]
- Semenov, A.G.; Tamm, N.N.; Seferian, K.R.; Postnikov, A.B.; Karpova, N.S.; Serebryanaya, D.V.; Koshkina, E.V.; Krasnoselsky, M.I.; Katrukha, A.G. Processing of pro-B-type natriuretic peptide: Furin and corin as candidate convertases. Clin. Chem. 2010, 56, 1166–1176. [Google Scholar] [CrossRef]
- Gupta, D.K.; Daniels, L.B.; Cheng, S.; deFilippi, C.R.; Criqui, M.H.; Maisel, A.S.; Lima, J.A.; Bahrami, H.; Greenland, P.; Cushman, M.; et al. Differences in Natriuretic Peptide Levels by Race/Ethnicity (From the Multi-Ethnic Study of Atherosclerosis). Am. J. Cardiol. 2017, 120, 1008–1015. [Google Scholar] [CrossRef]
- Patel, N.; Russell, G.K.; Musunuru, K.; Gutierrez, O.M.; Halade, G.; Kain, V.; Lv, W.; Prabhu, S.D.; Margulies, K.B.; Cappola, T.P.; et al. Race, Natriuretic Peptides, and High-Carbohydrate Challenge: A Clinical Trial. Circ. Res. 2019, 125, 957–968. [Google Scholar] [CrossRef]
- Luchner, A.; Stevens, T.L.; Borgeson, D.D.; Redfield, M.; Wei, C.M.; Porter, J.G.; Burnett, J.C., Jr. Differential atrial and ventricular expression of myocardial BNP during evolution of heart failure. Am. J. Physiol. 1998, 274, H1684–H1689. [Google Scholar] [CrossRef]
- Frustaci, A.; Russo, M.A.; Chimenti, C. Diagnostic contribution of left ventricular endomyocardial biopsy in patients with clinical phenotype of hypertrophic cardiomyopathy. Hum. Pathol. 2013, 44, 133–141. [Google Scholar] [CrossRef]
- Murakami, Y.; Shimada, T.; Inoue, S.; Shimizu, H.; Ohta, Y.; Katoh, H.; Nakamura, K.; Ishibashi, Y. New insights into the mechanism of the elevation of plasma brain natriuretic polypeptide levels in patients with left ventricular hypertrophy. Can. J. Cardiol. 2002, 18, 1294–1300. [Google Scholar]
- Ogawa, T.; Veinot, J.P.; Davies, R.A.; Haddad, H.; Smith, S.J.; Masters, R.G. Neuroendocrine profiling of humans receiving cardiac allografts. J. Heart Lung Transplant. 2005, 24, 1046–1054. [Google Scholar] [CrossRef]
- Masters, R.G.; Davies, R.A.; Veinot, J.P.; Hendry, P.J.; Smith, S.J.; de Bold, A.J. Discoordinate modulation of natriuretic peptides during acute cardiac allograft rejection in humans. Circulation 1999, 100, 287–291. [Google Scholar] [CrossRef]
- Kim, M.; Platt, M.J.; Shibasaki, T.; Quaggin, S.E.; Backx, P.H.; Seino, S.; Simpson, J.A.; Drucker, D.J. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat. Med. 2013, 19, 567–575. [Google Scholar] [CrossRef]
- Fish-Trotter, H.; Ferguson, J.F.; Patel, N.; Arora, P.; Allen, N.B.; Bachmann, K.N. Inflammation and Circulating Natriuretic Peptide Levels. Circ. Heart Fail. 2020, 13, e006570. [Google Scholar] [CrossRef]
- Richards, A.M. The renin-angiotensin-aldosterone system and the cardiac natriuretic peptides. Heart 1996, 76 (Suppl. 3), 36–44. [Google Scholar] [CrossRef] [PubMed]
- Undank, S.; Kaiser, J.; Sikimic, J.; Düfer, M.; Krippeit-Drews, P.; Drews, G. Atrial Natriuretic Peptide Affects Stimulus-Secretion Coupling of Pancreatic β-Cells. Diabetes 2017, 66, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
- Clerk, A.; Pham, F.H.; Fuller, S.J.; Sahai, E.; Aktories, K.; Marais, R.; Marshall, C.; Sugden, P.H. Regulation of mitogen-activated protein kinases in cardiac myocytes through the small G protein Rac1. Mol. Cell. Biol. 2001, 21, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.A.; Poizat, C. Epigenetics and chromatin remodeling in adult cardiomyopathy. J. Pathol. 2013, 231, 147–157. [Google Scholar] [CrossRef]
- Hohl, M.; Wagner, M.; Reil, J.C.; Müller, S.A.; Tauchnitz, M.; Zimmer, A.M.; Lehmann, L.; Thiel, G.; Böhm, M.; Backs, J.; et al. HDAC4 controls histone methylation in response to elevated cardiac load. J. Clin. Investig. 2013, 123, 1359–1370. [Google Scholar] [CrossRef]
- Rosales, W.; Lizcano, F. The Histone Demethylase JMJD2A Modulates the Induction of Hypertrophy Markers in iPSC-Derived Cardiomyocytes. Front. Genet. 2018, 9, 9–14. [Google Scholar] [CrossRef]
- Ito, E.; Miyagawa, S.; Fukushima, S.; Yoshikawa, Y.; Saito, S.; Saito, T.; Harada, A.; Takeda, M.; Kashiyama, N.; Nakamura, Y.; et al. Histone Modification Is Correlated with Reverse Left Ventricular Remodeling in Nonischemic Dilated Cardiomyopathy. Ann. Thorac. Surg. 2017, 104, 1531–1539. [Google Scholar] [CrossRef]
- Arora, S.; Rana, R.; Chhabra, A.; Jaiswal, A.; Rani, V. miRNA-transcription factor interactions: A combinatorial regulation of gene expression. Mol. Genet. Genom. 2013, 288, 77–87. [Google Scholar] [CrossRef]
- Arora, P.; Wu, C.; Hamid, T.; Arora, G.; Agha, O.; Allen, K. Acute Metabolic Influences on the Natriuretic Peptide System in Humans. J. Am. Coll. Cardiol. 2016, 67, 804–812. [Google Scholar] [CrossRef]
- Wu, C.; Arora, P.; Agha, O.; Hurst, L.A.; Allen, K.; Nathan, D.I.; Hu, D.; Jiramongkolchai, P.; Smith, J.G.; Melander, O.; et al. Novel MicroRNA Regulators of Atrial Natriuretic Peptide Production. Mol. Cell. Biol. 2016, 36, 1977–1987. [Google Scholar] [CrossRef]
- Newton-Cheh, C.; Larson, M.G.; Vasan, R.S.; Levy, D.; Bloch, K.D.; Surti, A.; Guiducci, C.; Kathiresan, S.; Benjamin, E.; Struck, J.; et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat. Genet. 2009, 41, 348–353. [Google Scholar] [CrossRef]
- Cannone, V.; Boerrigter, G.; Cataliotti, A.; Costello-Boerrigter, L.C.; Olson, T.M.; McKie, P.M.; Heublein, D.M.; Lahr, B.D.; Bailey, K.R.; Averna, M.; et al. A genetic variant of the atrial natriuretic peptide gene is associated with cardiometabolic protection in the general community. J. Am. Coll. Cardiol. 2011, 58, 629–633. [Google Scholar] [CrossRef]
- Cannone, V.; Cefalu’, A.B.; Noto, D.; Scott, C.G.; Bailey, K.R.; Cavera, G.; Pagano, M.; Sapienza, M.; Averna, M.R.; Burnett, J.C., Jr. The atrial natriuretic peptide genetic variant rs5068 is associated with a favorable cardiometabolic phenotype in a Mediterranean population. Diabetes Care 2013, 36, 2850–2856. [Google Scholar] [CrossRef]
- Celik, S.; Sadegh, M.K.; Morley, M.; Roselli, C.; Ellinor, P.T.; Cappola, T.; Smith, J.G.; Gidlof, O. Antisense regulation of atrial natriuretic peptide expression. JCI Insight 2019, 4, e130978. [Google Scholar] [CrossRef]
- Pandey, K.N. Molecular Signaling Mechanisms and Function of Natriuretic Peptide Receptor-A in the Pathophysiology of Cardiovascular Homeostasis. Front. Physiol. 2021, 12, 693099. [Google Scholar] [CrossRef]
- Airhart, N.; Yang, Y.F.; Roberts, C.T., Jr.; Silberbach, M. Atrial natriuretic peptide induces natriuretic peptide receptor-cGMP-dependent protein kinase interaction. J. Biol. Chem. 2003, 278, 38693–38698. [Google Scholar] [CrossRef]
- Moyes, A.J.; Khambata, R.S.; Villar, I.; Bubb, K.J.; Baliga, R.S.; Lumsden, N.G.; Xiao, F.; Gane, P.; Rebstock, A.-S.; Worthington, R.J.; et al. Endothelial C-type natriuretic peptide maintains vascular homeostasis. J. Clin. Investig. 2014, 124, 4039–4051. [Google Scholar] [CrossRef]
- Bayes-Genis, A.; Barallat, J.; Richards, A.M. A Test in Context: Neprilysin: Function, Inhibition, and Biomarker. J. Am. Coll. Cardiol. 2016, 68, 639–653. [Google Scholar] [CrossRef]
- Supaporn, T.; Sandberg, S.M.; Borgeson, D.D.; Heublein, D.M.; Luchner, A.; Wei, C.M.; Dousa, T.P.; Burnett, J.C., Jr. Blunted cGMP response to agonists and enhanced glomerular cyclic 3′,5′-nucleotide phosphodiesterase activities in experimental congestive heart failure. Kidney Int. 1996, 50, 1718–1725. [Google Scholar] [CrossRef]
- Francis, S.H.; Busch, J.L.; Corbin, J.D.; Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef]
- Inserte, J.; Garcia-Dorado, D. The cGMP/PKG pathway as a common mediator of cardioprotection: Translatability and mechanism. Br. J. Pharmacol. 2015, 172, 1996–2009. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Kuwahara, K.; Nishikimi, T.; Nakagawa, Y.; Kinoshita, H.; Minami, T. Endothelium-Derived C-Type Natriuretic Peptide Contributes to Blood Pressure Regulation by Maintaining Endothelial Integrity. Hypertension 2017, 69, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Kavurma, M.M.; Bursill, C.; Stanley, C.P.; Passam, F.; Cartland, S.P.; Patel, S.; Loa, J.; Figtree, G.A.; Golledge, J.; Aitken, S.; et al. Endothelial cell dysfunction: Implications for the pathogenesis of peripheral artery disease. Front. Cardiovasc. Med. 2022, 9, 1054576. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Gheorghiade, M.; Borlaug, B.A.; Pieske, B.; Vaduganathan, M.; Burnett, J.C., Jr.; Roessig, L.; Stasch, J.P.; Solomon, S.D.; Paulus, W.J.; et al. The cGMP signaling pathway as a therapeutic target in heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2013, 2, e000536. [Google Scholar] [CrossRef] [PubMed]
- Inserte, J.; Hernando, V.; Ruiz-Meana, M.; Poncelas-Nozal, M.; Fernandez, C.; Agullo, L.; Sartorio, C.; Vilardosa, U.; Garcia-Dorado, D. Delayed phospholamban phosphorylation in post-conditioned heart favours Ca2+ normalization and contributes to protection. Cardiovasc. Res. 2014, 103, 542–553. [Google Scholar] [CrossRef]
- Dorey, T.W.; Mackasey, M.; Jansen, H.J.; McRae, M.D.; Bohne, L.J.; Liu, Y.; Belke, D.D.; Atkinson, L.; Rose, R.A. Natriuretic peptide receptor B maintains heart rate and sinoatrial node function via cyclic GMP-mediated signalling. Cardiovasc. Res. 2022, 118, 1917–1931. [Google Scholar] [CrossRef]
- Calvieri, C.; Rubattu, S.; Volpe, M. Molecular mechanisms underlying cardiac antihypertrophic and antifibrotic effects of natriuretic peptides. J. Mol. Med. 2012, 90, 5–13. [Google Scholar] [CrossRef]
- Kiemer, A.K.; Weber, N.C.; Vollmar, A.M. Induction of IkappaB: Atrial natriuretic peptide as a regulator of the NF-kappaB pathway. Biochem. Biophys. Res. Commun. 2002, 295, 1068–1076. [Google Scholar] [CrossRef]
- Rubattu, S.; Gallo, G.; Volpe, M. A Contemporary View of Natriuretic Peptides in the SARS-CoV-2 Era. Front. Physiol. 2021, 12, 643721. [Google Scholar] [CrossRef]
- John, S.W.; Krege, J.H.; Oliver, P.M.; Hagaman, J.R.; Hodgin, J.B.; Pang, S.C.; Smithies, O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science 1995, 267, 679–681. [Google Scholar] [CrossRef]
- Scott, N.J.; Ellmers, L.J.; Lainchbury, J.G.; Maeda, N.; Smithies, O.; Richards, A.M.; Cameron, V.A. Influence of natriuretic peptide receptor-1 on survival and cardiac hypertrophy during development. Biochim. Biophys. Acta 2009, 1792, 1175–1184. [Google Scholar] [CrossRef]
- Rubattu, S.; Bigatti, G.; Evangelista, A.; Lanzani, C.; Stanzione, R.; Zagato, L. Association of atrial natriuretic peptide and type a natriuretic peptide receptor gene polymorphisms with left ventricular mass in human essential hypertension. J. Am. Coll. Cardiol. 2006, 48, 499–505. [Google Scholar] [CrossRef]
- Tamura, N.; Ogawa, Y.; Chusho, H.; Nakamura, K.; Nakao, K.; Suda, M.; Kasahara, M.; Hashimoto, R.; Katsuura, G.; Mukoyama, M.; et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc. Natl. Acad. Sci. USA 2000, 97, 4239–4244. [Google Scholar] [CrossRef]
- Rubattu, S.; Stanzione, R.; Cotugno, M.; Bianchi, F.; Marchitti, S.; Forte, M. Epigenetic control of natriuretic peptides: Implications for health and disease. Cell. Mol. Life Sci. 2020, 77, 5121–5130. [Google Scholar] [CrossRef]
- Cataliotti, A.; Tonne, J.M.; Bellavia, D.; Martin, F.L.; Oehler, E.A.; Harders, G.E.; Campbell, J.M.; Peng, K.W.; Russell, S.J.; Malatino, L.S.; et al. Long-term cardiac pro-B-type natriuretic peptide gene delivery prevents the development of hypertensive heart disease in spontaneously hypertensive rats. Circulation 2011, 123, 1297–1305. [Google Scholar] [CrossRef]
- Ly, O.T.; Chen, H.; Brown, G.E.; Hong, L.; Wang, X.; Han, Y.D.; Pavel, M.A.; Sridhar, A.; Maienschein-Cline, M.; Chalazan, B.; et al. Mutant ANP induces mitochondrial and ion channel remodeling in a human iPSC-derived atrial fibrillation model. JCI Insight 2022, 7, e155640. [Google Scholar] [CrossRef]
- Abraham, R.L.; Yang, T.; Blair, M.; Roden, D.M.; Darbar, D. Augmented potassium current is a shared phenotype for two genetic defects associated with familial atrial fibrillation. J. Mol. Cell. Cardiol. 2010, 48, 181–190. [Google Scholar] [CrossRef]
- Forte, M.; Marchitti, S.; Di Nonno, F.; Stanzione, R.; Schirone, L.; Cotugno, M.; Bianchi, F.; Schiavon, S.; Raffa, S.; Ranieri, D.; et al. NPPA/atrial natriuretic peptide is an extracellular modulator of autophagy in the heart. Autophagy 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nishikimi, T.; Kuwahara, K.; Fujishima, A.; Oka, S.; Tsutamoto, T.; Kinoshita, H.; Nakao, K.; Cho, K.; Inazumi, H.; et al. MiR30-GALNT1/2 Axis-Mediated Glycosylation Contributes to the Increased Secretion of Inactive Human Prohormone for Brain Natriuretic Peptide (proBNP) from Failing Hearts. J. Am. Heart Assoc. 2017, 6, e003601. [Google Scholar] [CrossRef]
- Rose, R.A.; Giles, W.R. Natriuretic peptide C receptor signalling in the heart and vasculature. J. Physiol. 2008, 586, 353–366. [Google Scholar] [CrossRef]
- Preedy, M.E.J. Cardiac Cyclic Nucleotide Phosphodiesterases: Roles and Therapeutic Potential in Heart Failure. Cardiovasc. Drugs Ther. 2020, 34, 401–417. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Straus, S.E.; Farkouh, M.E.; Austin, P.C.; Taljaard, M.; Chong, A.; Fahim, C.; Poon, S.; Cram, P.; Smith, S.; et al. Trial of an Intervention to Improve Acute Heart Failure Outcomes. N. Engl. J. Med. 2023, 388, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Pedicino, D.; Volpe, M. Improving care for heart failure patients by COACHing clinicians to use decision-support tools. Eur. Heart J. 2023, in press. [Google Scholar]
- Miller, W.L.; Hartman, K.A.; Grill, D.E.; Struck, J.; Bergmann, A.; Jaffe, A.S. Serial measurements of midregion proANP and copeptin in ambulatory patients with heart failure: Incremental prognostic value of novel biomarkers in heart failure. Heart 2012, 98, 389–394. [Google Scholar] [CrossRef]
- Jia, X.; Al Rifai, M.; Hoogeveen, R.; Echouffo-Tcheugui, J.B.; Shah, A.M.; Ndumele, C.E.; Virani, S.S.; Bozkurt, B.; Selvin, E.; Ballantyne, C.M.; et al. Association of Long-term Change in N-Terminal Pro-B-Type Natriuretic Peptide with Incident Heart Failure and Death. JAMA Cardiol. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Enia, G.; Mallamaci, F.; Benedetto, F.A.; Panuccio, V.; Parlongo, S.; Cutrupi, S.; Giacone, G.; Cottini, E.; Tripepi, G.; Malatino, L.S.; et al. Long-term CAPD patients are volume expanded and display more severe left ventricular hypertrophy than haemodialysis patients. Nephrol. Dial. Transplant. 2001, 16, 1459–1464. [Google Scholar] [CrossRef]
- Cataliotti, A.; Malatino, L.S.; Jougasaki, M.; Zoccali, C.; Castellino, P.; Giacone, G.; Bellanuova, I.; Tripepi, R.; Seminara, G.; Parlongo, S.; et al. Circulating natriuretic peptide concentrations in patients with end-stage renal disease: Role of brain natriuretic peptide as a biomarker for ventricular remodeling. Mayo Clin. Proc. 2001, 76, 1111–1119. [Google Scholar] [CrossRef]
- Mallamaci, F.; Zoccali, C.; Tripepi, G.; Benedetto, F.A.; Parlongo, S.; Cataliotti, A.; Cutrupi, S.; Giacone, G.; Bellanuova, I.; Stancanelli, B.; et al. The Cardiovascular Risk Extended Evaluation. Diagnostic potential of cardiac natriuretic peptides in dialysis patients. Kidney Int. 2001, 59, 1559–1566. [Google Scholar] [CrossRef]
- Paget, V.; Legedz, L.; Gaudebout, N.; Girerd, N.; Bricca, G.; Milon, H.; Vincent, M.; Lantelme, P. N-terminal pro-brain natriuretic peptide: A powerful predictor of mortality in hypertension. Hypertension 2011, 57, 702–709. [Google Scholar] [CrossRef]
- Belluardo, P.; Cataliotti, A.; Bonaiuto, L.; Giuffrè, E.; Maugeri, E.; Noto, P.; Orlando, G.; Raspa, G.; Piazza, B.; Babuin, L.; et al. Lack of activation of molecular forms of the BNP system in human grade 1 hypertension and relationship to cardiac hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1529–H1535. [Google Scholar] [CrossRef]
- Macheret, F.; Heublein, D.; Costello-Boerrigter, L.C.; Boerrigter, G.; McKie, P.; Bellavia, D.; Mangiafico, S.; Ikeda, Y.; Bailey, K.; Scott, C.G.; et al. Human hypertension is characterized by a lack of activation of the antihypertensive cardiac hormones ANP and BNP. J. Am. Coll. Cardiol. 2012, 60, 1558–1565. [Google Scholar] [CrossRef]
- Hijazi, Z.; Lindahl, B.; Oldgren, J.; Andersson, U.; Lindbäck, J.; Granger, C.B.; Alexander, J.H.; Gersh, B.J.; Hanna, M.; Harjola, V.P.; et al. Repeated Measurements of Cardiac Biomarkers in Atrial Fibrillation and Validation of the ABC Stroke Score Over Time. J. Am. Heart Assoc. 2017, 6, e004851. [Google Scholar] [CrossRef]
- Barbato, E.; Rubattu, S.; Bartunek, J.; Berni, A.; Sarno, G.; Vanderheyden, M.; Delrue, L.; Zardi, D.; Pace, B.; De Bruyne, B.; et al. Human coronary atherosclerosis modulates cardiac natriuretic peptide release. Atherosclerosis 2009, 206, 258–264. [Google Scholar] [CrossRef]
- Barbato, E.; Bartunek, J.; Marchitti, S.; Mangiacapra, F.; Stanzione, R.; Delrue, L.; Cotugno, M.; Di Castro, S.; De Bruyne, B.; Wijns, W.; et al. NT-proANP circulating level is a prognostic marker in stable ischemic heart disease. Int. J. Cardiol. 2012, 155, 311–312. [Google Scholar] [CrossRef]
- Stewart, R.A.H.; Kirby, A.; White, H.D.; Marschner, S.L.; West, M.; Thompson, P.L.; Sullivan, D.; Janus, E.; Hunt, D.; Kritharides, L.; et al. B-Type Natriuretic Peptide and Long-Term Cardiovascular Mortality in Patients with Coronary Heart Disease. J. Am. Heart Assoc. 2022, 11, e024616. [Google Scholar] [CrossRef]
- Gallo, G.; Presta, V.; Volpe, M.; Rubattu, S. Molecular and clinical implications of natriuretic peptides in aortic valve stenosis. J. Mol. Cell. Cardiol. 2019, 129, 266–271. [Google Scholar] [CrossRef]
- Gallo, G.; Forte, M.; Stanzione, R.; Cotugno, M.; Bianchi, F.; Marchitti, S.; Berni, A.; Volpe, M.; Rubattu, S. Functional Role of Natriuretic Peptides in Risk Assessment and Prognosis of Patients with Mitral Regurgitation. J. Clin. Med. 2020, 9, 1348. [Google Scholar] [CrossRef]
- Helgeson, S.A.; Imam, J.S.; Moss, J.E.; Hodge, D.O.; Burger, C.D. Comparison of Brain Natriuretic Peptide Levels to Simultaneously Obtained Right Heart Hemodynamics in Stable Outpatients with Pulmonary Arterial Hypertension. Diseases 2018, 6, 33. [Google Scholar] [CrossRef]
- Benza, R.L.; Miller, D.P.; Gomberg-Maitland, M.; Frantz, R.P.; Foreman, A.J.; Coffey, C.S.; Frost, A.; Barst, R.J.; Badesch, D.B.; Elliott, C.G.; et al. Predicting survival in pulmonary arterial hypertension: Insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010, 122, 164–172. [Google Scholar] [CrossRef]
- Romaniello, A.; Rubattu, S.; Gigante, A.; Simonelli, F.; Grimaldi, M.C.; D’Angelo, A.; Alunni, D.; Sada, L.; Gasperini, M.L.; Marchitti, S.; et al. Atrial natriuretic peptide predicts disease progression and digital ulcers development in systemic sclerosis patients. J. Cardiovasc. Med. 2019, 20, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Romaniello, A.; Rubattu, S.; Vaiarello, V.; Gigante, A.; Volpe, M.; Rosato, E. Circulating NT-proANP level is a predictor of mortality for systemic sclerosis: A retrospective study of an Italian cohort. Expert Rev. Clin. Immunol. 2021, 17, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Mallamaci, F.; Benedetto, F.A.; Tripepi, G.; Parlongo, S.; Cataliotti, A.; Cutrupi, S.; Giacone, G.; Bellanuova, I.; Cottini, E.; et al. Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J. Am. Soc. Nephrol. 2001, 12, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau , J.L.; Shi , V.C.; Solomon , S.D.; Swedberg , K.; et al. PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Zile, M.R.; Claggett, B.L.; Prescott, M.F.; McMurray, J.J.; Packer, M.; Rouleau, J.L.; Swedberg, K.; Desai, A.S.; Gong, J.; Shi, V.C.; et al. Prognostic Implications of Changes in N-Terminal Pro-B-Type Natriuretic Peptide in Patients with Heart Failure. J. Am. Coll. Cardiol. 2016, 68, 2425–2436. [Google Scholar] [CrossRef]
- Myhre, P.L.; Vaduganathan, M.; Claggett, B.; Packer, M.; Desai, A.S.; Rouleau, J.L.; Zile, M.R.; Swedberg, K.; Lefkowitz, M.; Shi, V.; et al. B-Type Natriuretic Peptide during Treatment with Sacubitril/Valsartan: The PARADIGM-HF Trial. J. Am. Coll. Cardiol. 2019, 73, 1264–1272. [Google Scholar] [CrossRef]
- Senni, M.; Wachter, R.; Witte, K.K.; Straburzynska-Migaj, E.; Belohlavek, J.; Fonseca, C.; Mueller, C.; Lonn, E.; Chakrabarti, A.; Bao, W.; et al. TRANSITION Investigators. Initiation of sacubitril/valsartan shortly after hospitalisation for acutely decompensated heart failure in patients with newly diagnosed (de novo) heart failure: A subgroup analysis of the TRANSITION study. Eur. J. Heart Fail. 2020, 22, 303–312. [Google Scholar] [CrossRef]
- Berg, D.D.; Samsky, M.D.; Velazquez, E.J.; Duffy, C.I.; Gurmu, Y.; Braunwald, E.; Morrow, D.A.; DeVore, A.D. 2 Efficacy and Safety of Sacubitril/Valsartan in High-Risk Patients in the PIONEER-HF Trial. Circ. Heart Fail. 2021, 14, e007034. [Google Scholar] [CrossRef]
- Senni, M.; McMurray, J.J.; Wachter, R.; McIntyre, H.F.; Reyes, A.; Majercak, I.; Andreka, P.; Shehova-Yankova, N.; Anand, I.; Yilmaz, M.B.; et al. Initiating sacubitril/valsartan (LCZ696) in heart failure: Results of TITRATION, a double-blind, randomized comparison of two uptitration regimens. Eur. J. Heart Fail. 2016, 18, 1193–1202. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P. PARAGON-HF Investigators and Committees. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef]
- Solomon, S.D.; Vaduganathan, M.; LClaggett, B.; Packer, M.; Zile, M.; Swedberg, K.; Rouleau, J.; Pfeffer, M.A.; Desai, A.; Lund, L.H.; et al. Sacubitril/Valsartan Across the Spectrum of Ejection Fraction in Heart Failure. Circulation 2020, 141, 352–361. [Google Scholar] [CrossRef]
- Volpe, M.; Bauersachs, J.; Bayés-Genís, A.; Butler, J.; Cohen-Solal, A.; Gallo, G.; Deichl, A.S.; Khan, M.S.; Battistoni, A.; Pieske, B.; et al. Sacubitril/valsartan for the management of heart failure: A perspective viewpoint on current evidence. Int. J. Cardiol. 2021, 327, 138–145. [Google Scholar] [CrossRef]
- Gallo, G.; Volpe, M.; Battistoni, A.; Russo, D.; Tocci, G.; Musumeci, M.B. Sacubitril/Valsartan as a Therapeutic Tool Across the Range of Heart Failure Phenotypes and Ejection Fraction Spectrum. Front. Physiol. 2021, 12, 652163. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Lewis, E.F.; Granger, C.B.; Køber, L.; Maggioni, A.P.; Mann, D.L.; McMurray, J.J.; Rouleau, J.L.; Solomon, S.D.; et al. PARADISE-MI Investigators and Committees. Angiotensin Receptor-Neprilysin Inhibition in Acute Myocardial Infarction. N. Engl. J. Med. 2021, 385, 1845–1855. [Google Scholar] [CrossRef]
- Berwanger, O.; Pfeffer, M.; Claggett, B.; Jering, K.S.; Maggioni, A.P.; Steg, P.G.; Mehran, R.; Lewis, E.F.; Zhou, Y.; van der Meer, P.; et al. Sacubitril/valsartan versus ramipril for patients with acute myocardial infarction: Win-ratio analysis of the PARADISE-MI trial. Eur. J. Heart Fail. 2022, 24, 1918–1927. [Google Scholar] [CrossRef]
- Januzzi, J.L.; Prescott, M.F.; Butler, J.; Felker, G.M.; Maisel, A.S.; McCague, K.; Camacho, A.; Pina, I.L.; Rocha, R.A.; Shah, A.M.; et al. PROVE-Hf Investigators. Association of change in N-terminal pro-B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA 2019, 322, 1085–1095. [Google Scholar] [CrossRef]
- Desai, A.S.; Solomon, S.D.; Shah, A.M.; Claggett, B.L.; Fang, J.C.; Izzo, J.; McCague, K.; Abbas, C.A.; Rocha, R.; Mitchell, G.F.; et al. Effect of Sacubitril-Valsartan vs. Enalapril on Aortic Stiffness in Patients with Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2019, 322, 1077–1084. [Google Scholar] [CrossRef]
- Zile, M.R.; O’Meara, E.; Claggett, B.; Prescott, M.F.; Solomon, S.D.; Swedberg, K.; Packer, M.; McMurray, J.J.V.; Shi, V.; Lefkowitz, M.; et al. Effects of Sacubitril/Valsartan on Biomarkers of Extracellular Matrix Regulation in Patients with HFrEF. J. Am. Coll. Cardiol. 2019, 73, 795–806. [Google Scholar] [CrossRef]
- Stasch, J.P.; Becker, E.M.; Alonso-Alija, C.; Apeler, H.; Dembowsky, K.; Feurer, A.; Gerzer, R.; Minuth, T.; Perzborn, E.; Pleiss, U.; et al. NO independent regulatory site on soluble guanylate cyclase. Nature 2001, 410, 212–215. [Google Scholar] [CrossRef]
- Schmidt, P.; Schramm, M.; Schroder, H.; Stasch, J.P. Mechanisms of nitric oxide independent activation of soluble guanylyl cyclase. Eur. J. Pharmacol. 2003, 468, 167–174. [Google Scholar] [CrossRef]
- Boerrigter, G.; Costello-Boerrigter, L.C.; Cataliotti, A.; Lapp, H.; Stasch, J.P.; Burnett, J.C., Jr. Targeting heme-oxidized soluble guanylate cyclase in experimental heart failure. Hypertension 2007, 49, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Rubattu, S.; Volpe, M. Targeting Cyclic Guanylate Monophosphate in Resistant Hypertension and Heart Failure: Are Sacubitril/Valsartan and Vericiguat Synergistic and Effective in Both Conditions? High Blood Press. Cardiovasc. Prev. 2021, 28, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Gallo, G. The Natriuretic Peptides for Hypertension Treatment. High Blood Press. Cardiovasc. Prev. 2022, 29, 15–21. [Google Scholar] [CrossRef]
- Tanaka, T.D.; Sawano, M.; Ramani, R.; Friedman, M.; Kohsaka, S. Acute heart failure management in the USA and Japan: Overview of practice patterns and review of evidence. ESC Heart Fail. 2018, 5, 931–947. [Google Scholar] [CrossRef]
- Ezekowitz, J.A.; Hernandez, A.F.; Starling, R.C.; Yancy, C.W.; Massie, B.; Hill, J.A.; Krum, H.; Diaz, R.; Ponikowski, P.; Metra, M.; et al. Standardizing care for acute decompensated heart failure in a large megatrial: The approach for the Acute Studies of Clinical Effectiveness of Nesiritide in Subjects with Decompensated Heart Failure (ASCEND-HF). Am. Heart J. 2009, 157, 219–228. [Google Scholar] [CrossRef]
- Lee, C.Y.; Huntley, B.K.; McCormick, D.J.; Ichiki, T.; Sangaralingham, S.J.; Lisy, O.; Burnett, J.C., Jr. Cenderitide: Structural requirements for the creation of a novel dual particulate guanylyl cyclase receptor agonist with renal-enhancing in vivo and ex vivo actions. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 98–105. [Google Scholar] [CrossRef]
- Chen, H.H.; Wan, S.H.; Iyer, S.R.; Cannone, V.; Sangaralingham, S.J.; Nuetel, J. First-in-Human Study of MANP: A Novel ANP (Atrial Natriuretic Peptide) Analog in Human Hypertension. Hypertension 2021, 78, 1859–1867. [Google Scholar] [CrossRef]
- Cannone, V.; Burnett, J.C., Jr. Natriuretic Peptides and Blood Pressure Homeostasis: Implications for MANP, a Novel Guanylyl Cyclase a Receptor Activator for Hypertension. Front. Physiol. 2022, 12, 815796. [Google Scholar] [CrossRef]
- Volpe, M.; Gallo, G.; Rubattu, S. Novel ANP (Atrial Natriuretic Peptide)-Based Therapy for Hypertension: The Promising Role of a Disease Mechanism Targeted Approach. Hypertension 2021, 78, 1868–1870. [Google Scholar] [CrossRef]
- Chen, Y.; Iyer, S.R.; Nikolaev, V.O.; Naro, F.; Pellegrini, M.; Cardarelli, S.; Ma, X.; Lee, H.C.; Burnett, J.C., Jr. MANP Activation of the cGMP Inhibits Aldosterone via PDE2 and CYP11B2 in H295R Cells and in Mice. Hypertension 2022, 79, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Altara, R.; da Silva, G.J.J.; Frisk, M.; Spelta, F.; Zouein, F.A.; Louch, W.E.; Booz, G.W.; Cataliotti, A. Cardioprotective Effects of the Novel Compound Vastiras in a Preclinical Model of End-Organ Damage. Hypertension 2020, 75, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Cataliotti, A.; Boerrigter, G.; Costello-Boerrigter, L.C.; Schirger, J.A.; Tsuruda, T.; Heublein, D.M.; Chen, H.H.; Malatino, L.S.; Burnett, J.C., Jr. Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide-induced aldosterone activation in experimental heart failure. Circulation 2004, 109, 1680–1685. [Google Scholar] [CrossRef]
- Chen, Y.; Harty, G.J.; Huntley, B.K.; Iyer, S.R.; Heublein, D.M.; Harders, G.E.; Meems, L.; Pan, S.; Sangaralingham, S.J.; Ichiki, T.; et al. CRRL269: A novel designer and renal-enhancing pGC-A peptide activator. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R407–R414. [Google Scholar] [CrossRef] [PubMed]
- Meems, L.M.G.; Andersen, I.A.; Pan, S.; Harty, G.; Chen, Y.; Zheng, Y.; Harders, G.E.; Ichiki, T.; Heublein, D.M.; Iyer, S.R.; et al. Design, Synthesis, and Actions of an Innovative Bispecific Designer Peptide. Hypertension 2019, 73, 900–909. [Google Scholar] [CrossRef]
- Pan, S.; Chen, H.H.; Dickey, D.M.; Boerrigter, G.; Lee, C.; Kleppe, L.S.; Hall, J.L.; Lerman, A.; Redfield, M.M.; Potter, L.R.; et al. Biodesign of a renal-protective peptide based on alternative splicing of B-type natriuretic peptide. Proc. Natl. Acad. Sci. USA 2009, 106, 11282–11287. [Google Scholar] [CrossRef]
- Mezo, A.R.; McDonnell, K.A.; Low, S.C.; Song, J.; Reidy, T.J.; Lu, Q.; Amari, J.V.; Hoehn, T.; Peters, R.T.; Dumont, J.; et al. Atrial natriuretic peptide-Fc, ANP-Fc, fusion proteins: Semisynthesis, in vitro activity and pharmacokinetics in rats. Bioconjugate Chem. 2012, 23, 518–526. [Google Scholar] [CrossRef]
- Sangaralingham, S.J.; Whig, K.; Peddibhotla, S.; Kirby, R.J.; Sessions, H.E.; Maloney, P.R.; Hershberger, P.M.; Mose-Yates, H.; Hood, B.L.; Vasile, S.; et al. Discovery of small molecule guanylyl cyclase A receptor positive allosteric modulators. Proc. Natl. Acad. Sci. USA 2021, 118, e2109386118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, G.; Rubattu, S.; Autore, C.; Volpe, M. Natriuretic Peptides: It Is Time for Guided Therapeutic Strategies Based on Their Molecular Mechanisms. Int. J. Mol. Sci. 2023, 24, 5131. https://doi.org/10.3390/ijms24065131
Gallo G, Rubattu S, Autore C, Volpe M. Natriuretic Peptides: It Is Time for Guided Therapeutic Strategies Based on Their Molecular Mechanisms. International Journal of Molecular Sciences. 2023; 24(6):5131. https://doi.org/10.3390/ijms24065131
Chicago/Turabian StyleGallo, Giovanna, Speranza Rubattu, Camillo Autore, and Massimo Volpe. 2023. "Natriuretic Peptides: It Is Time for Guided Therapeutic Strategies Based on Their Molecular Mechanisms" International Journal of Molecular Sciences 24, no. 6: 5131. https://doi.org/10.3390/ijms24065131
APA StyleGallo, G., Rubattu, S., Autore, C., & Volpe, M. (2023). Natriuretic Peptides: It Is Time for Guided Therapeutic Strategies Based on Their Molecular Mechanisms. International Journal of Molecular Sciences, 24(6), 5131. https://doi.org/10.3390/ijms24065131





