Cytogenetic Damage Induced by Radioiodine Therapy: A Follow-Up Case Study
Abstract
1. Introduction
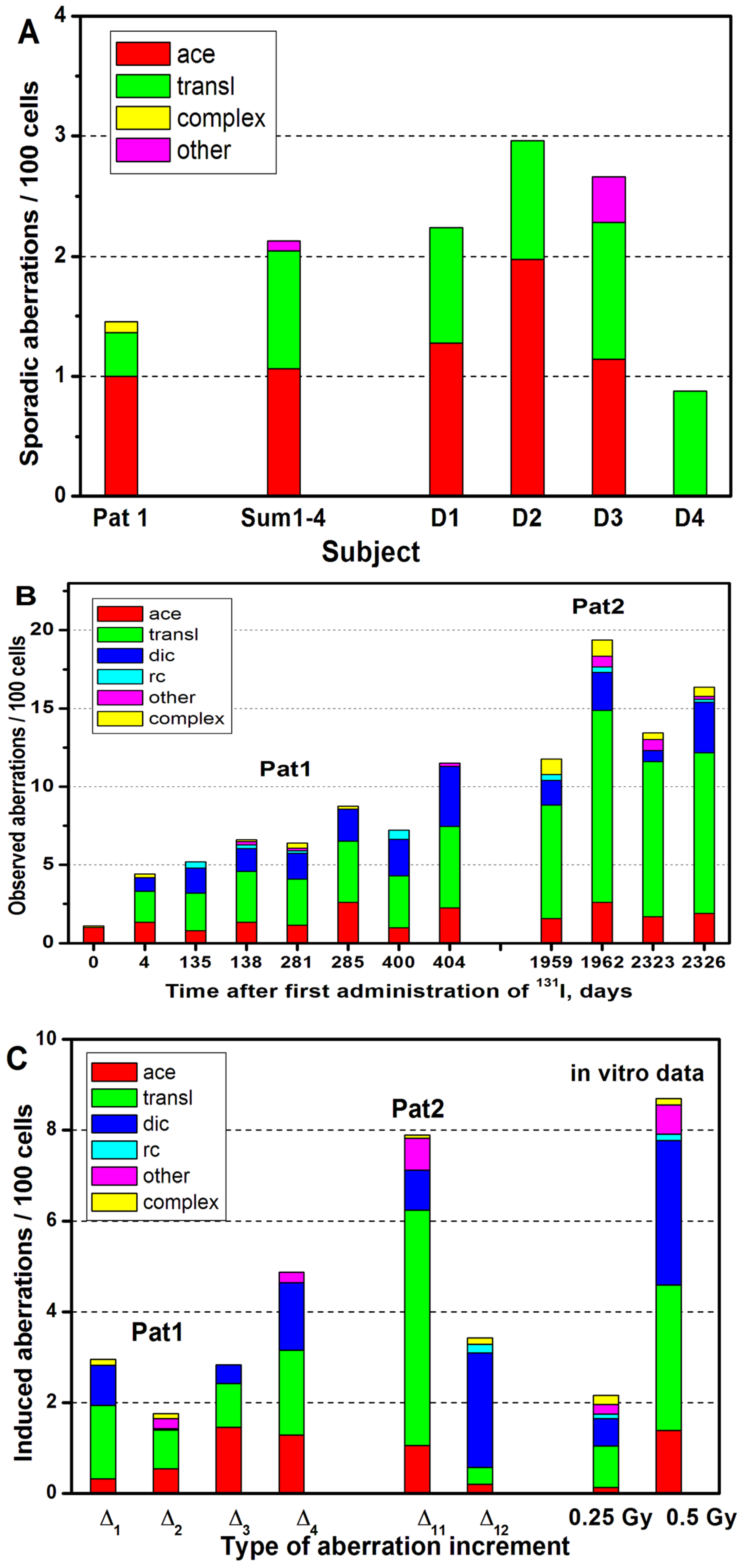
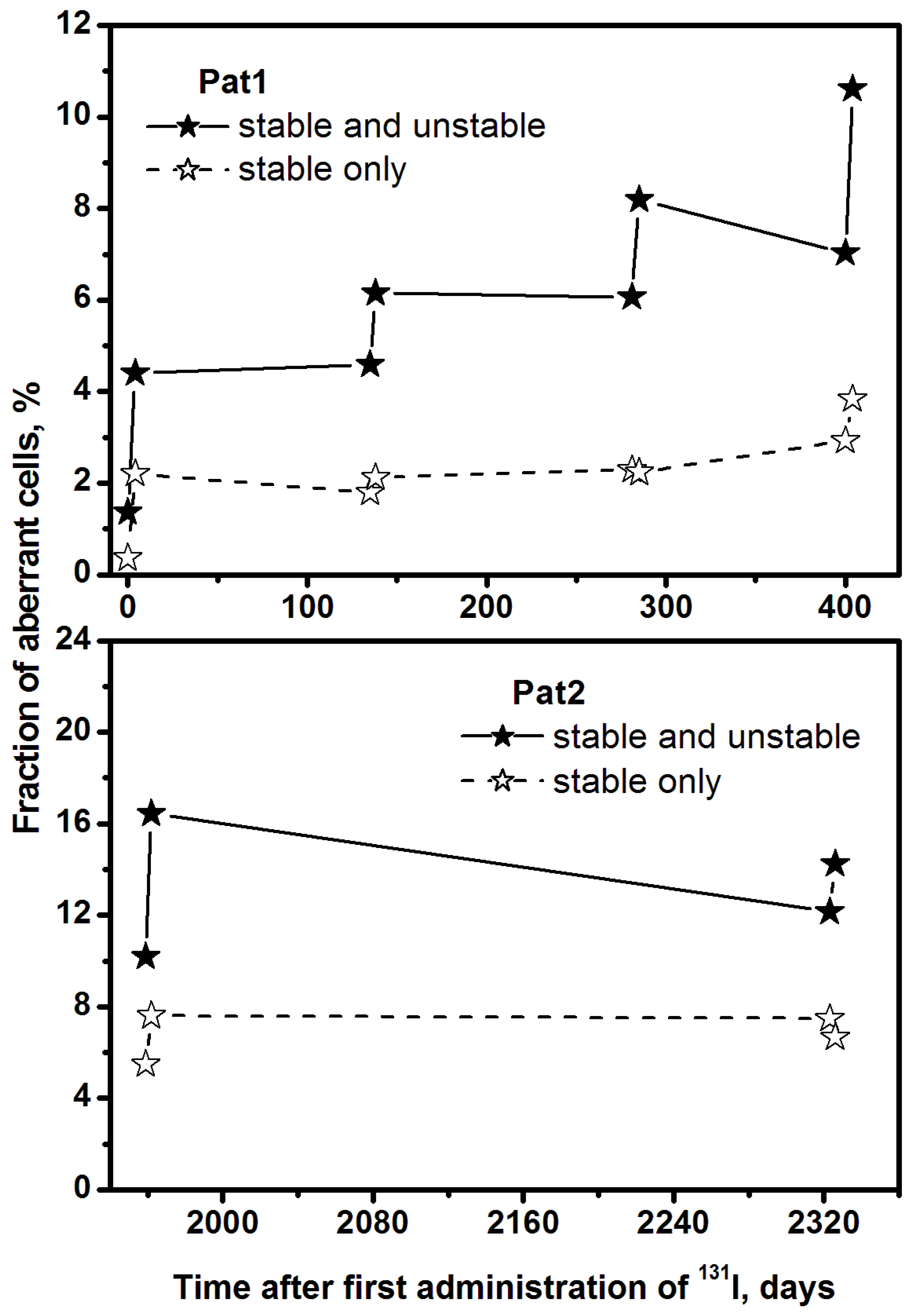
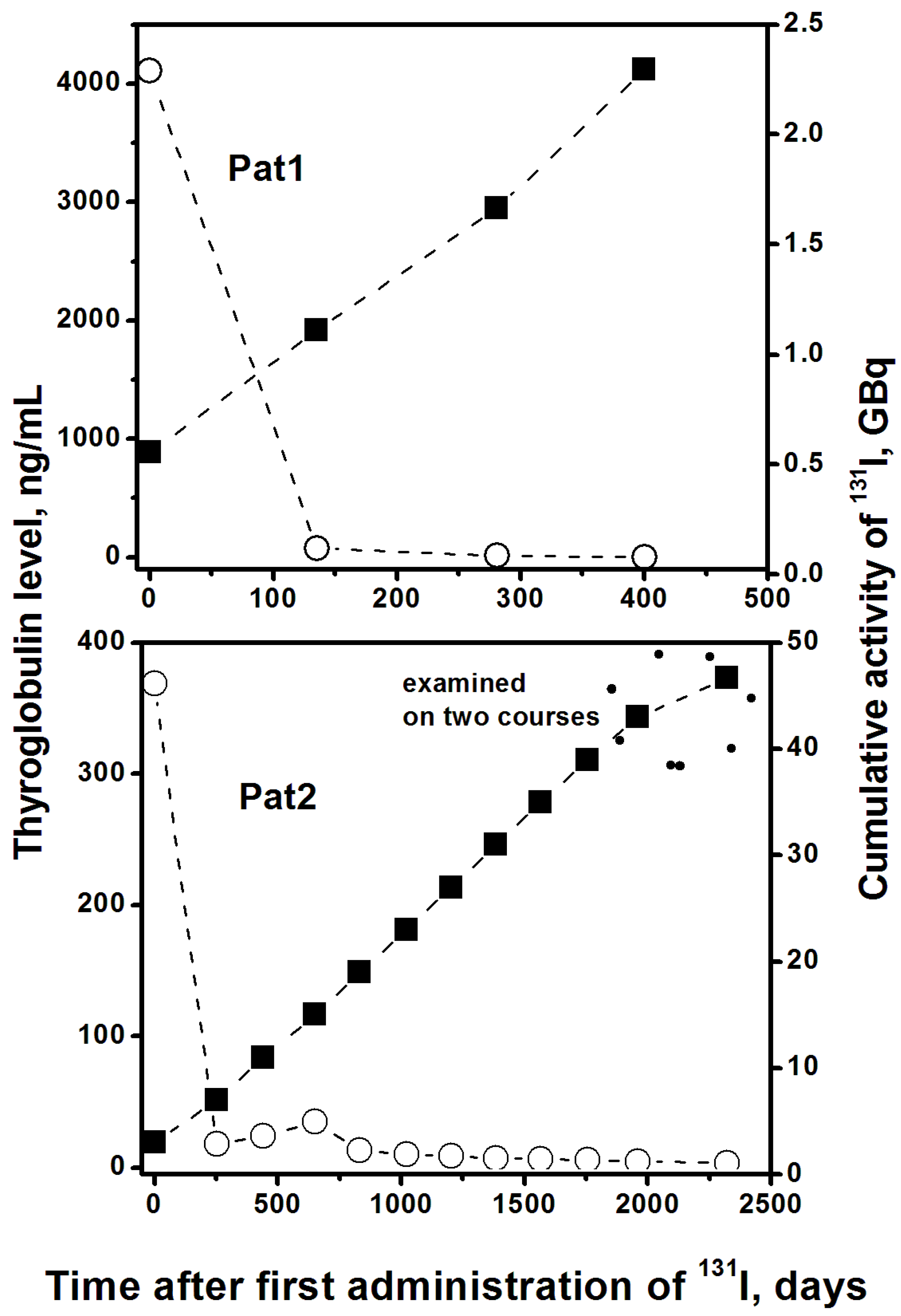
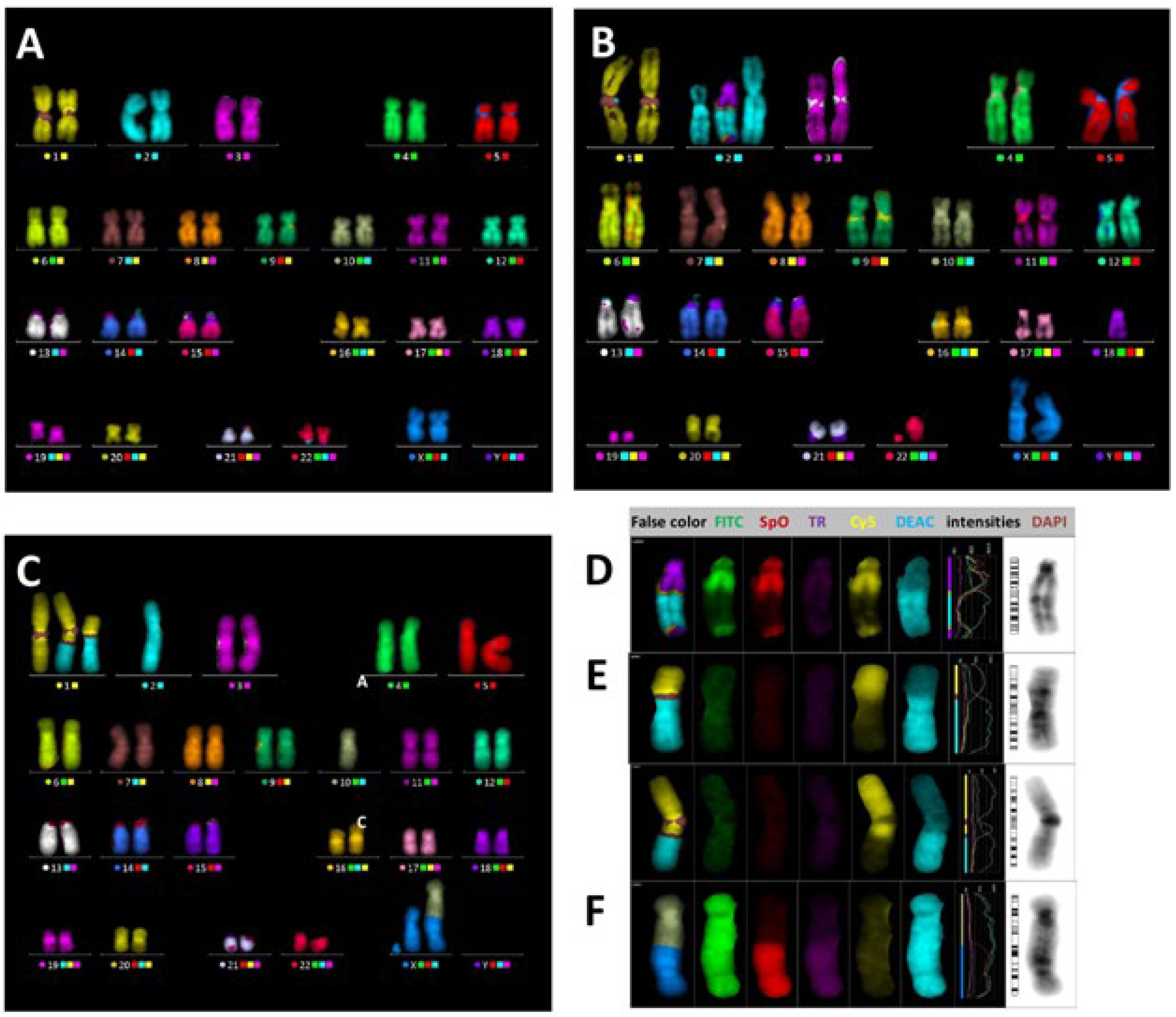
2. Results
3. Discussion
4. Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeong, C.H.; Cheng, M.; Ng, K.H. Therapeutic radionuclides in nuclear medicine: Current and future prospects. Review. J. Zhejiang Univ.-Sci. B (Biomed. Biotechnol.) 2014, 15, 845–863. [Google Scholar] [CrossRef]
- Sawka, A.M.; Thabane, L.; Parlea, L.; Ibrahim-Zada, I.; Tsang, R.W.; Brierley, J.D.; Straus, S.; Ezzat, S.; Goldstein, D.P. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: A systematic review and meta-analysis. Thyroid 2009, 19, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Van Nostrand, D. The benefits and risks of I-131 therapy in patients with well-differentiated thyroid cancer. Thyroid 2009, 19, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Benua, R.S.; Leeper, R.D. A Method and Rationale for Treating Thyroid Carcinoma with the Largest Safe Dose of I-131. In Frontiers of Thyroidology, Vol. II; Medeiros-Neto, G.A., Gaitan, E., Eds.; Plenum: New York, NY, USA, 1986; pp. 1317–1321. [Google Scholar]
- Haenscheid, H.; Lassmann, M.; Luster, M.; Thomas, S.R.; Pacini, F.; Ceccarelli, C.; Ladenson, P.W.; Wahl, R.L.; Schlumberger, M.; Ricard, M.; et al. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: Procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J. Nucl. Med. 2006, 47, 648–654. [Google Scholar]
- Buckley, S.E.; Chittenden, S.J.; Saran, F.H.; Meller, S.T.; Flux, G.F. Whole-body dosimetry for individualized treatment planning of 131-I-MIBG radionuclide therapy for neuroblastoma. J. Nucl. Med. 2009, 50, 1518–1524. [Google Scholar] [CrossRef]
- Stabin, M.G.; Brill, A.B. State of the art in nuclear medicine dose assessment. Semin. Nucl. Med. 2008, 38, 308–320. [Google Scholar] [CrossRef] [PubMed]
- M’Kacher, R.; Schlumberger, M.; Legal, J.-D.; Violot, D.; Béron-Gaillard, N.; Gaussen, A.; Parmentier, P. Biologic dosimetry in thyroid cancer patients after repeated treatments with iodine-131. J. Nucl. Med. 1998, 39, 825–829. [Google Scholar] [PubMed]
- Monsieurs, M.A.; Thierens, H.M.; Vral, A.; Brans, B.; De Ridder, L.; Dierckx, R.A. Patient dosimetry after 131-I-MIBG therapy for neuroblastoma and carcinoid tumours. Nucl. Med. Commun. 2001, 22, 367–374. [Google Scholar] [CrossRef]
- Serna, A.; Alcaraz, M.; Navarro, J.L.; Acevedo, C.; Vicente, V.; Canteras, M. Biological dosimetry and Bayesian analysis of chromosomal damage in thyroid cancer patients. Radiat. Prot. Dosim. 2008, 129, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Kim, B. An increased relative but small absolute risk of leukemia can be attributed to I-131 ablation. Clin. Thyroidol. 2019, 31, 30–32. [Google Scholar] [CrossRef]
- Seo, G.H.; Cho, Y.Y.; Chung, J.H.; Kim, S.W. Increased risk of leukemia after radioactive iodine therapy in patients with thyroid cancer: A nationwide, population-based study in Korea. Thyroid 2015, 25, 927–934. [Google Scholar] [CrossRef]
- Sotnik, N.V.; Osovets, S.V.; Scherthan, H.; Azizova, T.V. mFISH analysis of chromosome aberrations in workers occupationally exposed to mixed radiation. Radiat. Environ. Biophys. 2014, 53, 347–354. [Google Scholar] [CrossRef]
- IAEA. Cytogenetic Analysis for Radiation Dose Assessment: A Manual; Technical Reports Series, No 405; IAEA: Vienna, Austria, 2001. [Google Scholar]
- Cornforth, M.N. Analyzing radiation-induced complex chromosome rearrangements by combinatorial painting. Radiat. Res. 2001, 155, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Khvostunov, I.K.; Saenko, V.A.; Krylov, V.V.; Rodichev, A.A.; Yamashita, S. Cytogenetic biodosimetry and dose-rate effect after radioiodine therapy for thyroid cancer. Radiat. Environ. Biophys. 2017, 56, 213–226. [Google Scholar] [CrossRef]
- Estevao-Costa, J.; Gil-da-Costa, J.; Medina, A.M.; Sobrinho-Simoes, M. Thyroid carcinoma in a newborn: Clinical challenges in managing the first recorded case. Med. Pediatr. Oncol. 2000, 34, 290–292. [Google Scholar] [CrossRef]
- Dottorini, M.E.; Vignati, A.; Mazzucchelli, L.; Lomuscio, G.; Colombo, L. Differentiated thyroid carcinoma in children and adolescents: A 37-year experience in 85 patients. J. Nucl. Med. 1997, 38, 669–676. [Google Scholar]
- Sigurdson, A.J.; Ha, M.; Hauptmann, M.; Bhatti, P.; Sram, R.J.; Beskid, O.; Tawn, E.J.; Whitehouse, C.A.; Lindholm, C.; Nakano, M.; et al. International study of factors affecting human chromosome translocations. Mutat. Res. 2008, 652, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Bonassi, S.; Znaor, A.; Norppa, H.; Hagmar, L. Chromosomal aberrations and risk of cancer in humans: An epidemiologic perspective. Cytogenet. Genome Res. 2004, 104, 376–382. [Google Scholar] [CrossRef]
- Stephan, G.; Schneider, K.; Panzer, W.; Walsh, L.; Oestreicher, U. Enhanced yield of chromosome aberrations after CT examinations in pediatric patients. Int. J. Radiat. Biol. 2007, 83, 281–287. [Google Scholar] [CrossRef]
- Livingston, G.K.; Ryan, T.L.; Smith, T.L.; Escalona, M.B.; Foster, A.E.; Balajee, A.S. Detection of simple, complex and clonal chromosome translocations induced by internal radioiodine exposure: A cytogenetic follow-up case study after 25 years. Cytogenet. Genome Res. 2019, 159, 169–181. [Google Scholar] [CrossRef]
- Nakamura, N.; Nakano, M.; Kodama, Y.; Ohtaki, K.; Cologne, J.; Awa, A.A. Prediction of clonal chromosome aberration frequency in human blood lymphocytes. Radiat. Res. 2004, 161, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Hartel, C.; Nasonova, E.; Fuss, M.C.; Nikoghosyan, A.V.; Debus, J.; Ritter, S. Persistence of radiation-induced aberrations in patients after radiotherapy with C-ions and IMRT. Clin. Transl. Radiat. Oncol. 2018, 13, 57–63. [Google Scholar] [CrossRef]
- Loucas, B.D.; Shuryak, I.; Cornforth, M.N. Three-color chromosome painting as seen through the eyes of mFISH: Another look at radiation-induced exchanges and their conversion to whole-genome equivalency. Front. Oncol. 2016, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Greulich, K.M.; Kreja, L.; Heinze, B.; Rhein, A.P.; Weier, H.-U.G.; Bruckner, M.; Fuchs, P.; Molls, M. Rapid detection of radiation-induced chromosomal aberrations in lymphocytes and hematopoietic progenitor cells by mFISH. Mutat. Res. 2000, 452, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Hartel, C.; Nikoghosyan, A.; Durante, M.; Sommer, S.; Nasonova, E.; Fournier, C.; Lee, R.; Debus, J.; Schulz-Ertner, D.; Ritter, S. Chromosomal aberrations in peripheral blood lymphocytes of prostate cancer patients treated with IMRT and carbon ions. Radiother. Oncol. 2010, 95, 73–78. [Google Scholar] [CrossRef]
- McKenna, M.J.; Robinson, E.; Taylor, L.; Tompkins, C.; Cornforth, M.N.; Simon, S.L.; Bailey, S.M. Chromosome Translocations, Inversions and Telomere Length for Retrospective Biodosimetry on Exposed U.S. Atomic Veterans. Radiat. Res. 2019, 191, 311–322. [Google Scholar] [CrossRef]
- Gregoire, E.; Roy, L.; Buard, V.; Delbos, M.; Durand, V.; Martin-Bodiot, C.; Voisin, P.; Sorokine-Durm, I.; Vaurijoux, A.; Voisin, P.; et al. Twenty years of FISH-based translocation analysis for retrospective ionizing radiation biodosimetry. Int. J. Radiat. Biol. 2018, 94, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Balajee, A.S.; Hadjidekova, V. Retrospective cytogenetic analysis of unstable and stable chromosome aberrations in the victims of radiation accident in Bulgaria. Mutat. Res.—Genet. Toxicol. Environ. Mutagen. 2021, 861–862, 503295. [Google Scholar] [CrossRef] [PubMed]
- Borgmann, K.; Roeper, B.; Abd El-Awady, R.; Brackrock, S.; Bigalkee, M.; Dork, T.; Alberti, W.; Dikomey, E.; Dahm-Daphi, J. Indicators of late normal tissue response after radiotherapy for head and neck cancer: Fibroblasts, lymphocytes, genetics, DNA repair, and chromosome aberrations. Radiother. Oncol. 2002, 64, 141–152. [Google Scholar] [CrossRef]
| Patient 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 4 | Day 135 | Day 138 | Day 281 | Day 285 | Day 400 | Day 404 | |
| Conventional analysis using Giemsa painting | ||||||||
| Number of cells: | 1068 | 1000 | 500 | 550 | 500 | 400 | 500 | 500 |
| aberrant cells | 3 | 33 | 14 | 42 | 16 | 26 | 29 | 47 |
| acentrics | 1 | 20 | 8 | 27 | 16 | 16 | 16 | 20 |
| centric rings | 0 | 5 | 0 | 4 | 0 | 5 | 3 | 6 |
| dicentrics | 3 | 12 | 6 | 15 | 4 | 8 | 11 | 26 |
| FISH analysis using selective painting of chromosomes 2, 4, 12 | ||||||||
| Number of cells: | - | - | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 |
| reciprocal translocations (tc) | - | - | 8 | 11 | 5 | 8 | 5 | 8 |
| non-reciprocal translocations (ti) | - | - | 2 | 3 | 5 | 6 | 1 | 4 |
| deletions | - | - | 5 | 9 | 3 | 7 | 4 | 6 |
| mFISH analysis using whole genome painting | ||||||||
| Number of cells: | 1102 | 454 | 501 | 893 | 610 | 537 | 512 | 443 |
| aberrant cells | 15 | 20 | 23 | 55 | 37 | 44 | 36 | 47 |
| stable aberrant cells | 4 | 10 | 9 | 19 | 14 | 12 | 15 | 17 |
| reciprocal translocations | 4 | 9 | 10 | 22 | 14 | 15 | 16 | 18 |
| non-reciprocal translocations | 0 | 0 | 2 | 7 | 4 | 6 | 1 | 5 |
| acentrics | 11 | 6 | 4 | 12 | 7 | 14 | 5 | 10 |
| centric rings | 0 | 0 | 1 | 2 | 1 | 0 | 3 | 0 |
| dicentrics | 0 | 4 | 8 | 13 | 10 | 11 | 12 | 17 |
| other simple exchanges * | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 1 |
| complex aberrations | 1 | 1 | 0 | 1 | 2 | 1 | 0 | 0 |
| Total breaks | 25 | 35 | 46 | 107 | 74 | 83 | 71 | 92 |
| Patient 2 | ||||||||
| Day 1959 | Day 1962 | Day 2323 | Day 2326 | |||||
| Conventional analysis using Giemsa painting | ||||||||
| Number of cells: | 500 ** | 514 ** | 657 | 500 | ||||
| aberrant cells | 18 | 43 | 51 | 31 | ||||
| acentrics | 8 | 28 | 33 | 14 | ||||
| centric rings | 5 | 4 | 4 | 3 | ||||
| dicentrics | 10 | 20 | 20 | 18 | ||||
| FISH analysis using selective painting of chromosomes 2, 4, 12 | ||||||||
| Number of cells: | - | 1000 | 914 | |||||
| reciprocal translocations | - | - | 16 | 16 | ||||
| non-reciprocal translocations | - | - | 12 | 10 | ||||
| deletions | - | - | 1 | 3 | ||||
| mFISH analysis using whole genome painting | ||||||||
| Number of cells: | 510 | 578 | 707 | 526 | ||||
| aberrant cells | 52 | 95 | 86 | 75 | ||||
| stable aberrant cells | 28 | 44 | 53 | 35 | ||||
| reciprocal translocations | 34 | 57 | 60 | 44 | ||||
| non-reciprocal translocations | 3 | 14 | 10 | 10 | ||||
| acentrics | 8 | 15 | 12 | 10 | ||||
| centric rings | 2 | 2 | 0 | 1 | ||||
| dicentrics | 8 | 14 | 5 | 17 | ||||
| other simple exchanges * | 0 | 4 | 5 | 1 | ||||
| complex aberrations | 5 | 6 | 3 | 3 | ||||
| Total breaks | 118 | 219 | 183 | 166 | ||||
| Aberrations detected by mFISH in healthy donors induced by 60Co γ-irradiation | ||||||||
| Dose, Gy | ||||||||
| 0 | 0.25 | 0.5 | ||||||
| Number of cells: | 1223 | 1003 | 693 | |||||
| aberrant cells | 26 | 43 | 69 | |||||
| stable aberrant cells | 10 | 15 | 23 | |||||
| reciprocal translocations | 10 | 15 | 25 | |||||
| non-reciprocal translocations | 2 | 4 | 4 | |||||
| acentrics | 13 | 12 | 17 | |||||
| centric rings | 0 | 1 | 1 | |||||
| dicentrics | 0 | 6 | 22 | |||||
| other simple exchanges * | 1 | 3 | 5 | |||||
| complex aberrations | 0 | 2 | 1 | |||||
| Patient 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Conventional analysis of (dic+rc) using Giemsa painting | ||||||||
| Days | Pre-treatment | T, h | Post-treatment | Increment ∆M ± SEM * | G(T) | Dose, Gy (CI **) | ||
| cells | M1 ± SEM * | cells | M2 ± SEM * | |||||
| 0–4 | 1068 | 0.28 ± 0.21 | 92 | 1000 | 1.70 ± 0.41 | 1.42 ± 0.62 | 0.246 | 0.52 (0–0.84) |
| 135–138 | 500 | 1.20 ± 0.49 | 68 | 550 | 3.45 ± 0.78 | 2.25 ± 1.27 | 0.251 | 0.70 (0–1.19) |
| 281–285 | 500 | 0.80 ± 0.40 | 92 | 400 | 3.25 ± 0.89 | 2.45 ± 1.29 | 0.246 | 0.74 (0–1.23) |
| 400–404 | 500 | 2.80 ± 0.74 | 92 | 500 | 6.40 ± 1.13 | 3.60 ± 1.87 | 0.246 | 0.95 (0–1.53) |
| FISH analysis of (tc+ti) using selective painting of chromosomes 2, 4, 12 | ||||||||
| Days | Pre-treatment | T, h | Post-treatment | Increment ∆F ± SEM * | G(T) | Dose, Gy (CI **) | ||
| cells | F1 ± SEM * | cells | F2 ± SEM ** | |||||
| 135–138 | 1000 | 3.19 ± 1.01 | 68 | 1000 | 4.47 ± 1.20 | 1.28 ± 2.21 | 0.251 | 0.57 (0–1.48) |
| 281–285 | 1000 | 3.19 ± 1.01 | 92 | 1000 | 4.47 ± 1.20 | 1.28 ± 2.21 | 0.246 | 0.57 (0–1.49) |
| 400–404 | 1000 | 1.92 ± 0.78 | 92 | 1000 | 3.83 ± 1.11 | 1.92 ± 1.87 | 0.246 | 0.75 (0–1.49) |
| Patient 2 | ||||||||
| Conventional analysis of (dic+rc) using Giemsa painting | ||||||||
| Days | Pre-treatment | T, h | Post-treatment | Increment ∆M ± SEM * | G(T) | Dose, Gy (CI **) | ||
| cells | M1 ± SEM * | cells | M2 ± SEM * | |||||
| 1959–1962 | 500 | 3.00 ± 0.81 | 68 | 514 | 4.67 ± 1.01 | 1.67 ± 1.82 | 0.251 | 0.57 (0–1.23) |
| 2323–2326 | 657 | 3.65 ± 0.85 | 68 | 500 | 4.20 ± 0.94 | 0.55 ± 1.79 | 0.251 | 0.25 (0–1.05) |
| FISH analysis of (tc+ti) using selective painting of chromosomes 2, 4, 12 | ||||||||
| Days | Pre-treatment | T, h | Post-treatment | Increment ∆F ± SEM ** | G(T) | Dose, Gy (CI **) | ||
| cells | F1 ± SEM ** | cells | F2 ± SEM ** | |||||
| 2323–2326 | 1000 | 8.95 ± 1.69 | 68 | 914 | 9.09 ± 1.78 | 0.14 ± 3.47 | 0.251 | 0.10 (0–1.64) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khvostunov, I.K.; Nasonova, E.; Krylov, V.; Rodichev, A.; Kochetova, T.; Shepel, N.; Korovchuk, O.; Kutsalo, P.; Shegai, P.; Kaprin, A. Cytogenetic Damage Induced by Radioiodine Therapy: A Follow-Up Case Study. Int. J. Mol. Sci. 2023, 24, 5128. https://doi.org/10.3390/ijms24065128
Khvostunov IK, Nasonova E, Krylov V, Rodichev A, Kochetova T, Shepel N, Korovchuk O, Kutsalo P, Shegai P, Kaprin A. Cytogenetic Damage Induced by Radioiodine Therapy: A Follow-Up Case Study. International Journal of Molecular Sciences. 2023; 24(6):5128. https://doi.org/10.3390/ijms24065128
Chicago/Turabian StyleKhvostunov, Igor K., Elena Nasonova, Valeriy Krylov, Andrei Rodichev, Tatiana Kochetova, Natalia Shepel, Olga Korovchuk, Polina Kutsalo, Petr Shegai, and Andrei Kaprin. 2023. "Cytogenetic Damage Induced by Radioiodine Therapy: A Follow-Up Case Study" International Journal of Molecular Sciences 24, no. 6: 5128. https://doi.org/10.3390/ijms24065128
APA StyleKhvostunov, I. K., Nasonova, E., Krylov, V., Rodichev, A., Kochetova, T., Shepel, N., Korovchuk, O., Kutsalo, P., Shegai, P., & Kaprin, A. (2023). Cytogenetic Damage Induced by Radioiodine Therapy: A Follow-Up Case Study. International Journal of Molecular Sciences, 24(6), 5128. https://doi.org/10.3390/ijms24065128






