Interplay between Learning and Voluntary Wheel Running in Male C57BL/6NCrl Mice
Abstract
1. Introduction
2. Results
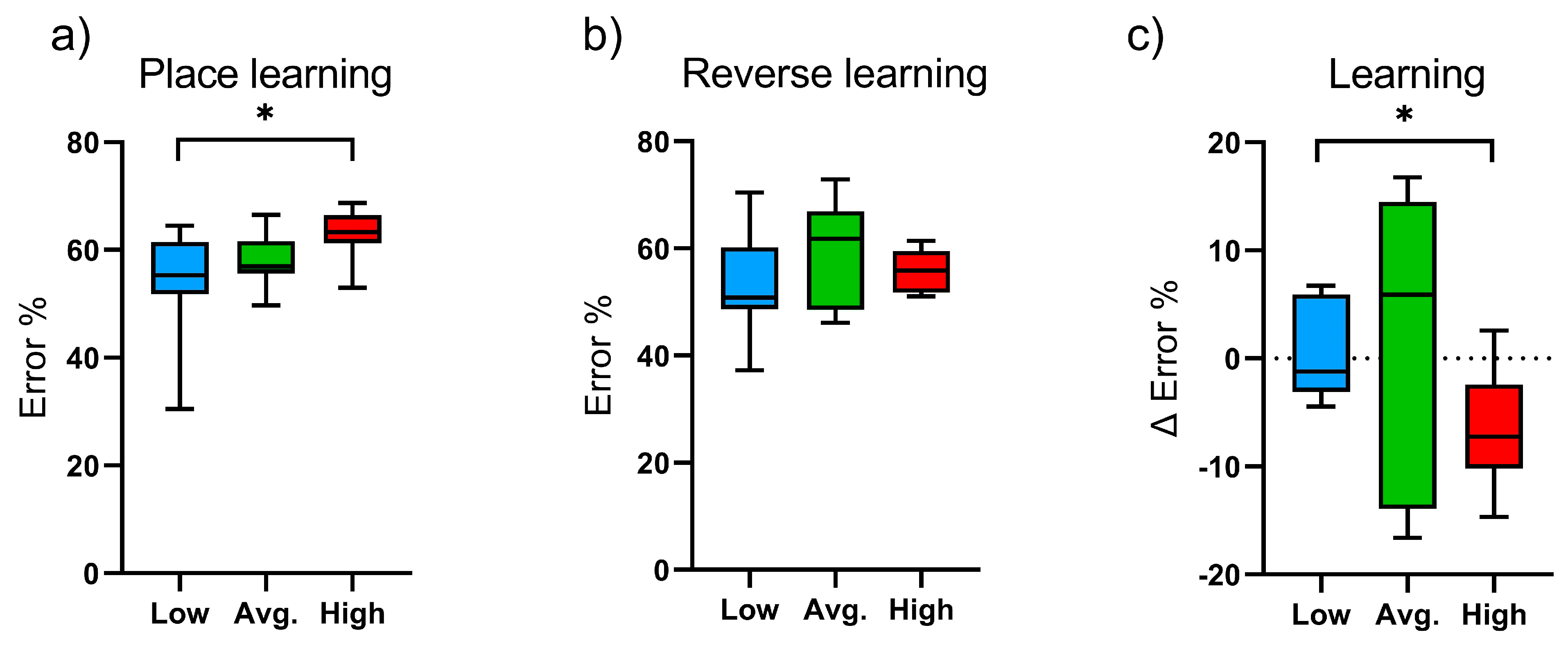
2.1. Learning and Cognitive Function: IntelliCage Results
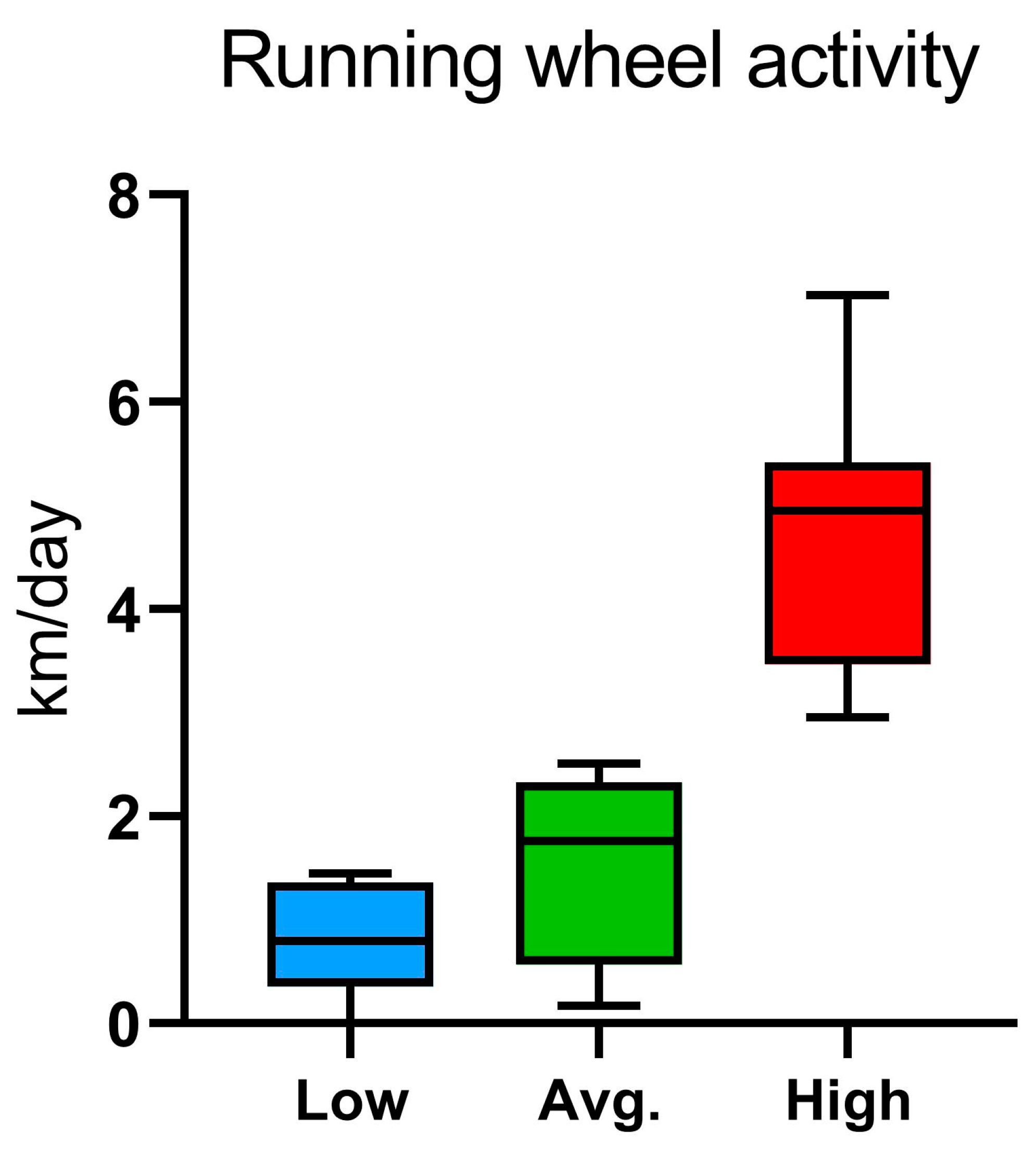
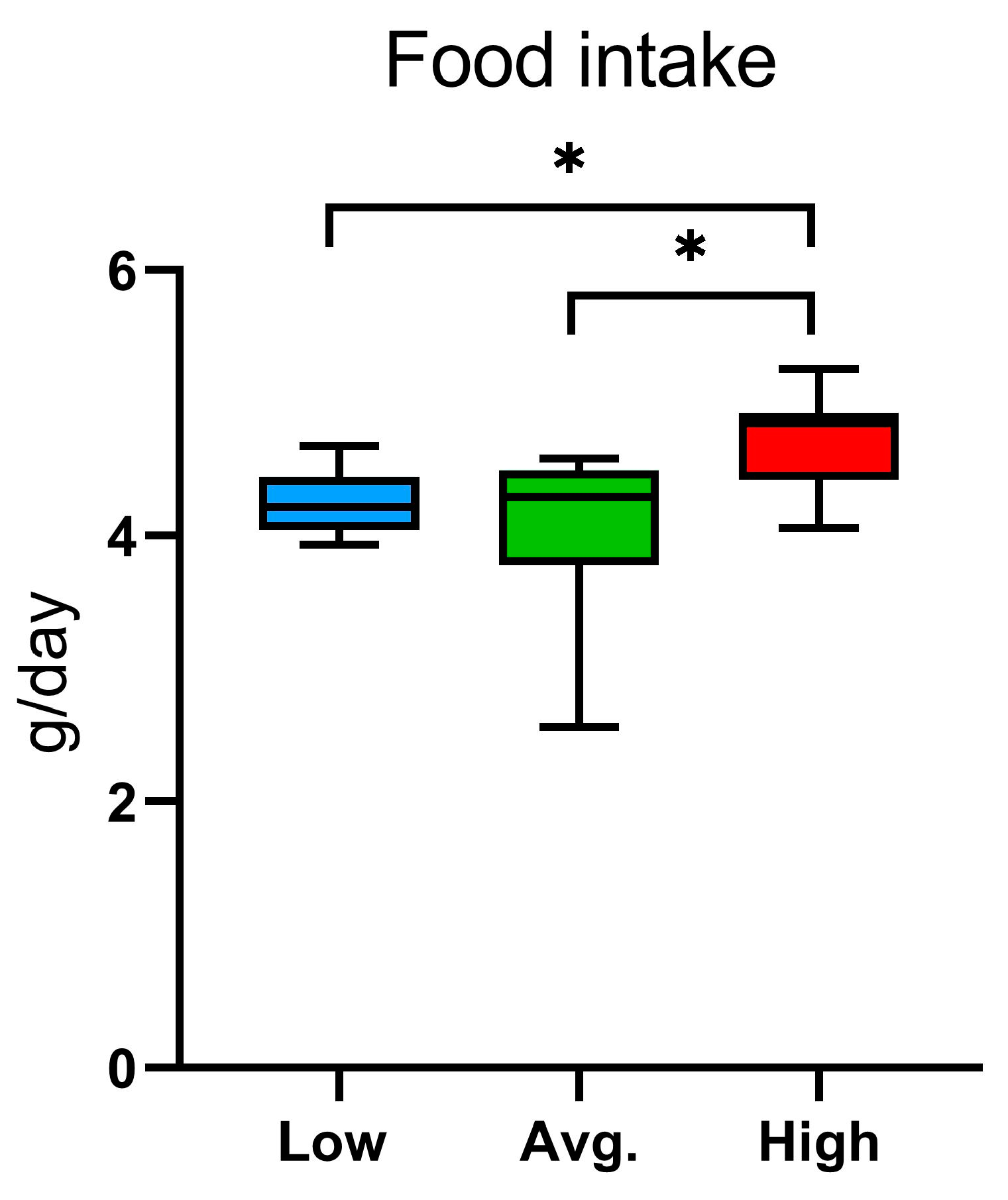
2.2. Voluntary Wheel Running, Home-Cage Activity, and Metabolic Parameters: PhenoMaster Results
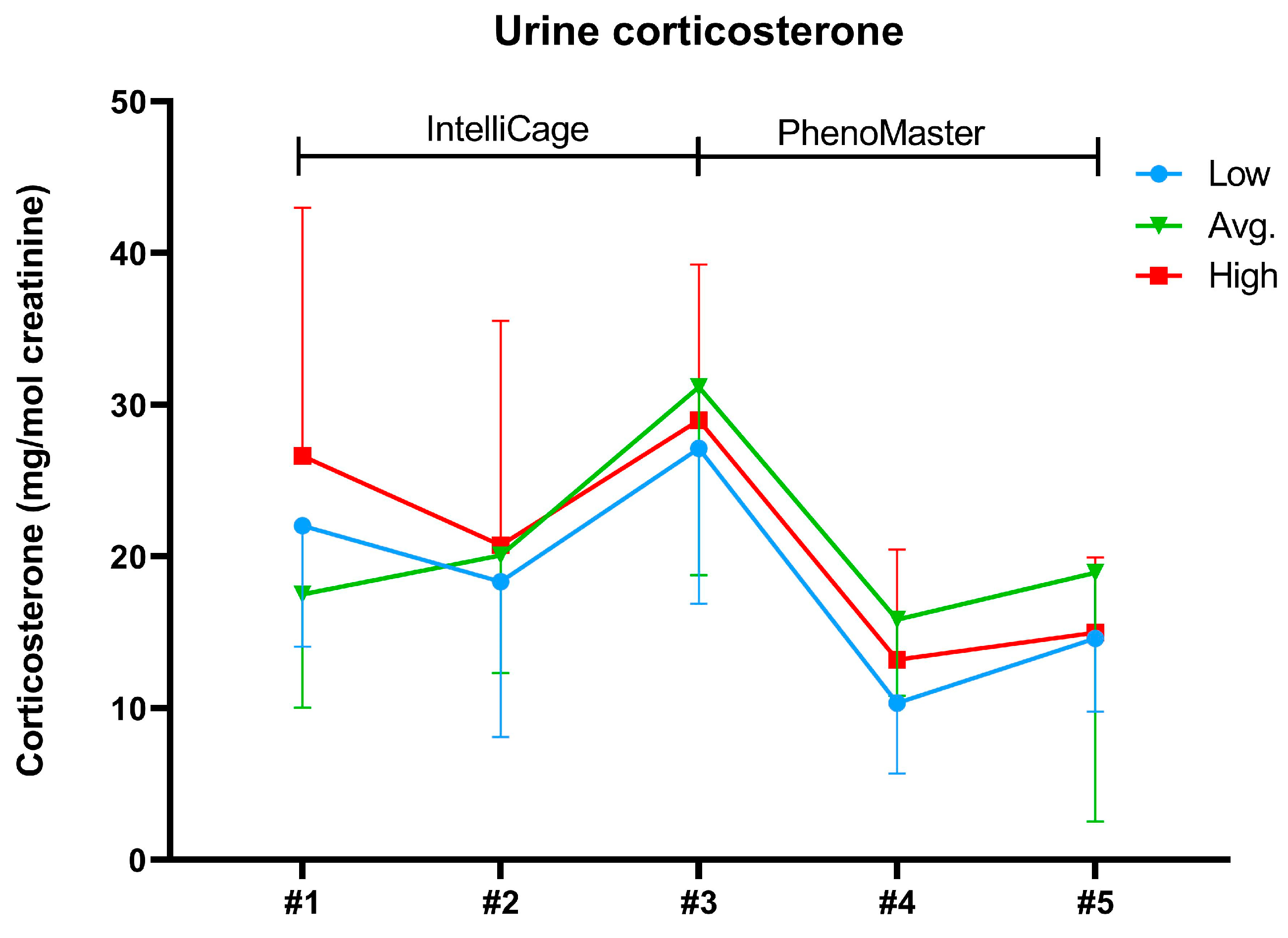
2.3. Body Weights and Urine Corticosterone
3. Discussion
4. Materials and Methods
4.1. IntelliCage Measurements
4.2. PhenoMaster Monitoring and Voluntary Wheel Running
4.3. Body Weights and Urine Collection for Corticosterone and Creatinine Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Liang, A.; Guan, F.; Fan, R.; Chi, L.; Yang, B. Regular Treadmill Running Improves Spatial Learning and Memory Performance in Young Mice through Increased Hippocampal Neurogenesis and Decreased Stress. Brain Res. 2013, 1531, 1–8. [Google Scholar] [CrossRef]
- Mustroph, M.L.; Chen, S.; Desai, S.C.; Cay, E.B.; DeYoung, E.K.; Rhodes, J.S. Aerobic Exercise Is the Critical Variable in an Enriched Environment That Increases Hippocampal Neurogenesis and Water Maze Learning in Male C57BL/6J Mice. Neuroscience 2012, 219, 62–71. [Google Scholar] [CrossRef]
- Nokia, M.S.; Lensu, S.; Ahtiainen, J.P.; Johansson, P.P.; Koch, L.G.; Britton, S.L.; Kainulainen, H. Physical Exercise Increases Adult Hippocampal Neurogenesis in Male Rats Provided It Is Aerobic and Sustained. J. Physiol. 2016, 594, 1855–1873. [Google Scholar] [CrossRef]
- Van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running Enhances Neurogenesis, Learning, and Long-Term Potentiation in Mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431. [Google Scholar] [CrossRef] [PubMed]
- Brockett, A.T.; LaMarca, E.A.; Gould, E. Physical Exercise Enhances Cognitive Flexibility as Well as Astrocytic and Synaptic Markers in the Medial Prefrontal Cortex. PLoS ONE 2015, 10, e0124859. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, D. Physical Activity and Cognition across the Life Span. In Physical Activity Effects on the Anthropological Status of Children, Youth and Adults; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016; pp. 257–280. [Google Scholar]
- Law, C.K.; Lam, F.M.; Chung, R.C.; Pang, M.Y. Physical Exercise Attenuates Cognitive Decline and Reduces Behavioural Problems in People with Mild Cognitive Impairment and Dementia: A Systematic Review. J. Physiother. 2020, 66, 9–18. [Google Scholar] [CrossRef] [PubMed]
- De Souto Barreto, P.; Demougeot, L.; Vellas, B.; Rolland, Y. Exercise Training for Preventing Dementia, Mild Cognitive Impairment, and Clinically Meaningful Cognitive Decline: A Systematic Review and Meta-Analysis. J. Gerontol.—Ser. A Biol. Sci. Med. Sci. 2018, 73, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.D. Domains of Cognition and Their Assessment. Dialogues Clin. Neurosci. 2019, 21, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef]
- Luo, B.; Liu, P.; Liu, X. Vascular Cognitive Impairment. Chin. J. Neurol. 2021, 54, 267–271. [Google Scholar] [CrossRef]
- Hugo, J.; Ganguli, M. Dementia and Cognitive Impairment. Epidemiology, Diagnosis, and Treatment. Clin. Geriatr. Med. 2014, 30, 421–442. [Google Scholar] [CrossRef] [PubMed]
- da Costa Daniele, T.M.; de Bruin, P.F.C.; de Matos, R.S.; de Bruin, G.S.; Maia Chaves, C.; de Bruin, V.M.S. Exercise Effects on Brain and Behavior in Healthy Mice, Alzheimer’s Disease and Parkinson’s Disease Model—A Systematic Review and Meta-Analysis. Behav. Brain Res. 2020, 383, 112488. [Google Scholar] [CrossRef] [PubMed]
- Hölter, S.M.; Garrett, L.; Einicke, J.; Sperling, B.; Dirscherl, P.; Zimprich, A.; Fuchs, H.; Gailus-Durner, V.; Hrabě de Angelis, M.; Wurst, W. Assessing Cognition in Mice. Curr. Protoc. Mouse Biol. 2015, 5, 331–358. [Google Scholar] [CrossRef] [PubMed]
- Commons, K.G.; Cholanians, A.B.; Babb, J.A.; Ehlinger, D.G. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem. Neurosci. 2017, 8, 955–960. [Google Scholar] [CrossRef]
- Himanshu; Dharmila; Sarkar, D.; Nutan. A Review of Behavioral Tests to Evaluate Different Types of Anxiety and Anti-Anxiety Effects. Clin. Psychopharmacol. Neurosci. 2020, 18, 341. [Google Scholar] [CrossRef]
- Kim, D.H.; Jang, Y.S.; Jeon, W.K.; Han, J.S. Assessment of Cognitive Phenotyping in Inbred, Genetically Modified Mice, and Transgenic Mouse Models of Alzheimer’s Disease. Exp. Neurobiol. 2019, 28, 146–157. [Google Scholar] [CrossRef]
- Kiryk, A.; Janusz, A.; Zglinicki, B.; Turkes, E.; Knapska, E.; Konopka, W.; Lipp, H.P.; Kaczmarek, L. IntelliCage as a Tool for Measuring Mouse Behavior—20 Years Perspective. Behav. Brain Res. 2020, 388, 112620. [Google Scholar] [CrossRef]
- Kahnau, P.; Guenther, A.; Boon, M.N.; Terzenbach, J.D.; Hanitzsch, E.; Lewejohann, L.; Brust, V. Lifetime Observation of Cognition and Physiological Parameters in Male Mice. Front. Behav. Neurosci. 2021, 15, 709775. [Google Scholar] [CrossRef]
- Mifflin, M.A.; Winslow, W.; Surendra, L.; Tallino, S.; Vural, A.; Velazquez, R. Sex Differences in the IntelliCage and the Morris Water Maze in the APP/PS1 Mouse Model of Amyloidosis. Neurobiol. Aging 2021, 101, 130–140. [Google Scholar] [CrossRef]
- van Klinken, J.B.; van den Berg, S.A.A.; Havekes, L.M.; Willems Van Dijk, K. Estimation of Activity Related Energy Expenditure and Resting Metabolic Rate in Freely Moving Mice from Indirect Calorimetry Data. PLoS ONE 2012, 7, e36162. [Google Scholar] [CrossRef]
- Goh, J.; Ladiges, W. Voluntary Wheel Running in Mice. Curr. Protoc. Mouse Biol. 2015, 5, 283–290. [Google Scholar] [CrossRef]
- Allen, D.L.; Harrison, B.C.; Maass, A.; Bell, M.L.; Byrnes, W.C.; Leinwand, L.A. Cardiac and Skeletal Muscle Adaptations to Voluntary Wheel Running in the Mouse. J. Appl. Physiol. 2001, 90, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Cui, X.X.; Huang, M.T.; Liu, Y.; Shih, W.J.; Lin, Y.; Lu, Y.P.; Wagner, G.C.; Conney, A.H. Inhibitory Effect of Voluntary Running Wheel Exercise on the Growth of Human Pancreatic Panc-1 and Prostate PC-3 Xenograft Tumors in Immunodeficient Mice. Oncol. Rep. 2008, 19, 1583–1588. [Google Scholar] [PubMed]
- Morgan, J.A.; Singhal, G.; Corrigan, F.; Jaehne, E.J.; Jawahar, M.C.; Baune, B.T. The Effects of Aerobic Exercise on Depression-like, Anxiety-like, and Cognition-like Behaviours over the Healthy Adult Lifespan of C57BL/6 Mice. Behav. Brain Res. 2018, 337, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Wu, C.; He, X.F.; Wu, D.; He, X.; Liang, F.Y.; Dai, G.Y.; Pei, Z.; Xu, G.Q.; Lan, Y. Effects of Voluntary Wheel-Running Types on Hippocampal Neurogenesis and Spatial Cognition in Middle-Aged Mice. Front. Cell. Neurosci. 2018, 12, 177. [Google Scholar] [CrossRef]
- Toth, L.A. The Influence of the Cage Environment on Rodent Physiology and Behavior: Implications for Reproducibility of Pre-Clinical Rodent Research. Exp. Neurol. 2015, 270, 72–77. [Google Scholar] [CrossRef]
- Meijer, J.H.; Robbers, Y. Wheel Running in the Wild. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140210. [Google Scholar] [CrossRef]
- Svensson, M.; Rosvall, P.; Boza-Serrano, A.; Andersson, E.; Lexell, J.; Deierborg, T. Forced Treadmill Exercise Can Induce Stress and Increase Neuronal Damage in a Mouse Model of Global Cerebral Ischemia. Neurobiol. Stress 2016, 5, 8–18. [Google Scholar] [CrossRef]
- Novak, C.M.; Burghardt, P.R.; Levine, J.A. The Use of a Running Wheel to Measure Activity in Rodents: Relationship to Energy Balance, General Activity, and Reward. Neurosci. Biobehav. Rev. 2012, 36, 1001–1014. [Google Scholar] [CrossRef]
- De Bono, J.P.; Adlam, D.; Paterson, D.J.; Channon, K.M. Novel Quantitative Phenotypes of Exercise Training in Mouse Models. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2006, 290, R926–R934. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, J.T.; Turner, M.J.; Daves, M.; Vordermark, A.; Kleeberger, S.R. Genetic Influence on Daily Wheel Running Activity Level. Physiol. Genom. 2005, 19, 270–276. [Google Scholar] [CrossRef]
- Bartling, B.; Al-Robaiy, S.; Lehnich, H.; Binder, L.; Hiebl, B.; Simm, A. Sex-Related Differences in the Wheel-Running Activity of Mice Decline with Increasing Age. Exp. Gerontol. 2017, 87, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Sibold, J.S.; Hammack, S.E.; Falls, W.A. C57 Mice Increase Wheel-Running Behavior Following Stress: Preliminary Findings. Percept. Mot. Skills 2011, 113, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.D.; Chambers, J.B.; Henderson, R.P.; Rashotte, M.E.; Overton, J.M. Cardiovascular Responses to Caloric Restriction and Thermoneutrality in C57BL/6J Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.H.; Gass, P.; Fuss, J. Resting Is Rusting: A Critical View on Rodent Wheel-Running Behavior. Neuroscientist 2014, 20, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Pillay, N. Is Wheel Running a Re-Directed Stereotypic Behaviour in Striped Mice Rhabdomys Dilectus? Appl. Anim. Behav. Sci. 2018, 204, 113–121. [Google Scholar] [CrossRef]
- Mason, G.; Würbel, H. What Can Be Learnt from Wheel-Running by Wild Mice, and How Can We Identify When Wheel-Running Is Pathological? Proc. R. Soc. B Biol. Sci. 2016, 283, 20150738. [Google Scholar] [CrossRef]
- Swallow, J.G.; Carter, P.A.; Garland, T. Artificial Selection for Increased Wheel-Running Behavior in House Mice. Behav. Genet. 1998, 28, 227–237. [Google Scholar] [CrossRef]
- Thompson, Z.; Kolb, E.M.; Garland, T. High-Runner Mice Have Reduced Incentive Salience for a Sweet-Taste Reward When Housed with Wheel Access. Behav. Process. 2018, 146, 46–53. [Google Scholar] [CrossRef]
- Guo, S.; Huang, Y.; Zhang, Y.; Huang, H.; Hong, S.; Liu, T. Impacts of Exercise Interventions on Different Diseases and Organ Functions in Mice. J. Sport Health Sci. 2020, 9, 53–73. [Google Scholar] [CrossRef]
- Wilhelmsson, U.; Kalm, M.; Pekna, M.; Pekny, M. Nestin Null Mice Show Improved Reversal Place Learning. Neurochem. Res. 2020, 45, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Dere, E.; Ronnenberg, A.; Tampe, B.; Arinrad, S.; Schmidt, M.; Zeisberg, E.; Ehrenreich, H. Cognitive, Emotional and Social Phenotyping of Mice in an Observer-Independent Setting. Neurobiol. Learn. Mem. 2018, 150, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Konhilas, J.P.; Maass, A.H.; Luckey, S.W.; Stauffer, B.L.; Olson, E.N.; Leinwand, L.A. Sex Modifies Exercise and Cardiac Adaptation in Mice. Am. J. Physiol. -Heart Circ. Physiol. 2004, 287, H2768–H2776. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, G.; Brito-Da-Silva, G.; Gandra, P.G. Voluntary Wheel Running: Patterns and Physiological Effects in Mice. Brazilian J. Med. Biol. Res. 2019, 52, e7830. [Google Scholar] [CrossRef]
- Binder, E.; Droste, S.K.; Ohl, F.; Reul, J.M.H.M. Regular Voluntary Exercise Reduces Anxiety-Related Behaviour and Impulsiveness in Mice. Behav. Brain Res. 2004, 155, 197–206. [Google Scholar] [CrossRef]
- Kolb, E.M.; Rezende, E.L.; Holness, L.; Radtke, A.; Lee, S.K.; Obenaus, A.; Garland, T. Mice Selectively Bred for High Voluntary Wheel Running Have Larger Midbrains: Support for the Mosaic Model of Brain Evolution. J. Exp. Biol. 2013, 216, 515–523. [Google Scholar] [CrossRef]
- Rhodes, J.S.; Jeffrey, S.; Girard, I.; Mitchell, G.S.; Van Praag, H.; Garland, T.; Gage, F.H. Exercise Increases Hippocampal Neurogenesis to High Levels but Does Not Improve Spatial Learning in Mice Bred for Increased Voluntary Wheel Running. Behav. Neurosci. 2003, 117, 1006. [Google Scholar] [CrossRef]
- Rhodes, J.S.; Gammie, S.C.; Garland, T. Neurobiology of Mice Selected for High Voluntary Wheel-Running Activity. Integr. Comp. Biol. 2005, 45, 438–455. [Google Scholar] [CrossRef]
- Kappel, S.; Hawkins, P.; Mendl, M.T. To Group or Not to Group? Good Practice for Housing Male Laboratory Mice. Animals 2017, 7, 88. [Google Scholar] [CrossRef]
- Kamakura, R.; Kovalainen, M.; Leppaluoto, J.; Herzig, K.H.; Makela, K.A. The Effects of Group and Single Housing and Automated Animal Monitoring on Urinary Corticosterone Levels in Male C57BL/6 Mice. Physiol. Rep. 2016, 4, e12703. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niiranen, L.; Stenbäck, V.; Tulppo, M.; Herzig, K.-H.; Mäkelä, K.A. Interplay between Learning and Voluntary Wheel Running in Male C57BL/6NCrl Mice. Int. J. Mol. Sci. 2023, 24, 4259. https://doi.org/10.3390/ijms24054259
Niiranen L, Stenbäck V, Tulppo M, Herzig K-H, Mäkelä KA. Interplay between Learning and Voluntary Wheel Running in Male C57BL/6NCrl Mice. International Journal of Molecular Sciences. 2023; 24(5):4259. https://doi.org/10.3390/ijms24054259
Chicago/Turabian StyleNiiranen, Laura, Ville Stenbäck, Mikko Tulppo, Karl-Heinz Herzig, and Kari A. Mäkelä. 2023. "Interplay between Learning and Voluntary Wheel Running in Male C57BL/6NCrl Mice" International Journal of Molecular Sciences 24, no. 5: 4259. https://doi.org/10.3390/ijms24054259
APA StyleNiiranen, L., Stenbäck, V., Tulppo, M., Herzig, K.-H., & Mäkelä, K. A. (2023). Interplay between Learning and Voluntary Wheel Running in Male C57BL/6NCrl Mice. International Journal of Molecular Sciences, 24(5), 4259. https://doi.org/10.3390/ijms24054259






