A Dual Coverage Monitoring of the Bile Acids Profile in the Liver–Gut Axis throughout the Whole Inflammation-Cancer Transformation Progressive: Reveal Hepatocellular Carcinoma Pathogenesis
Abstract
1. Introduction
2. Results
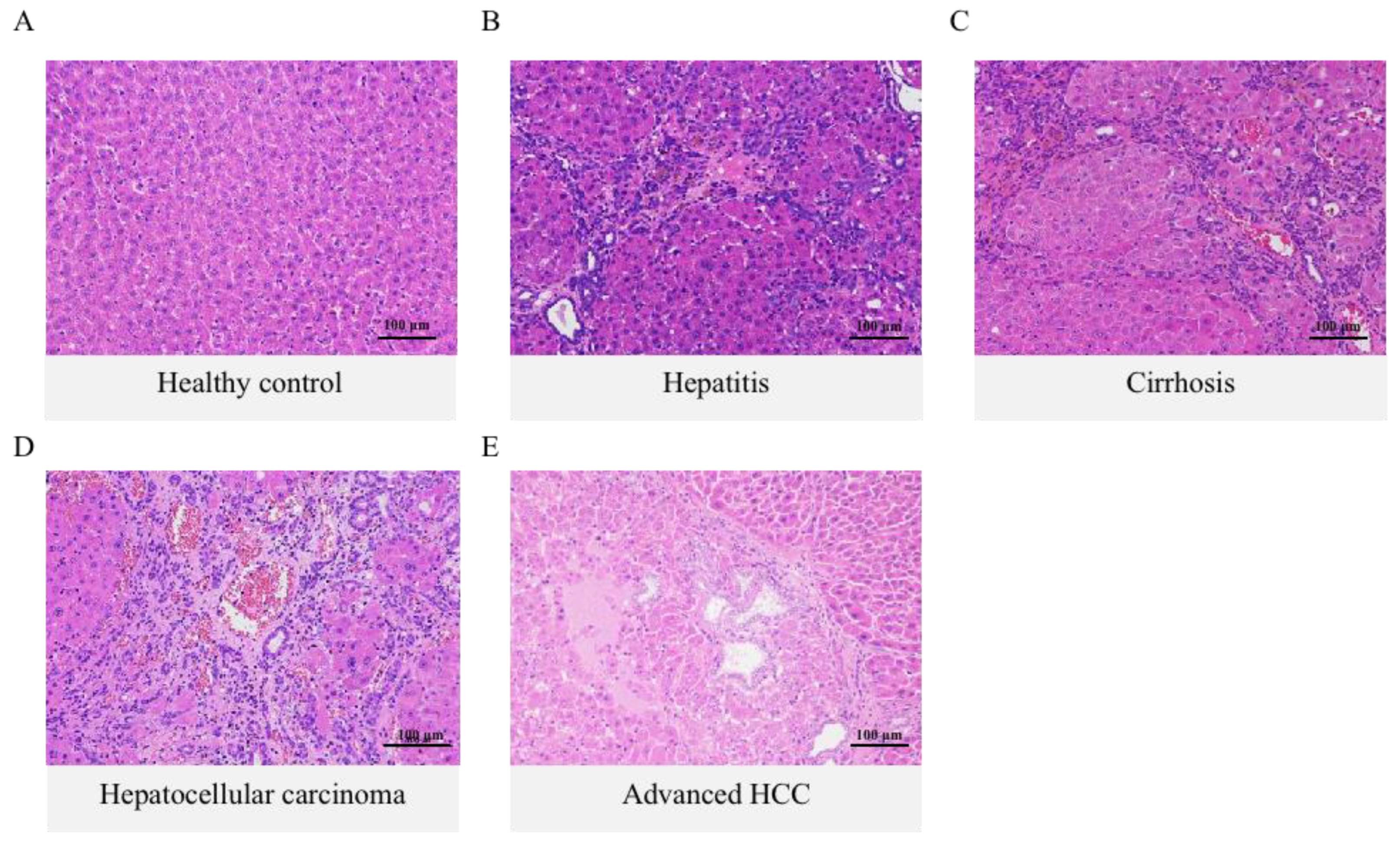
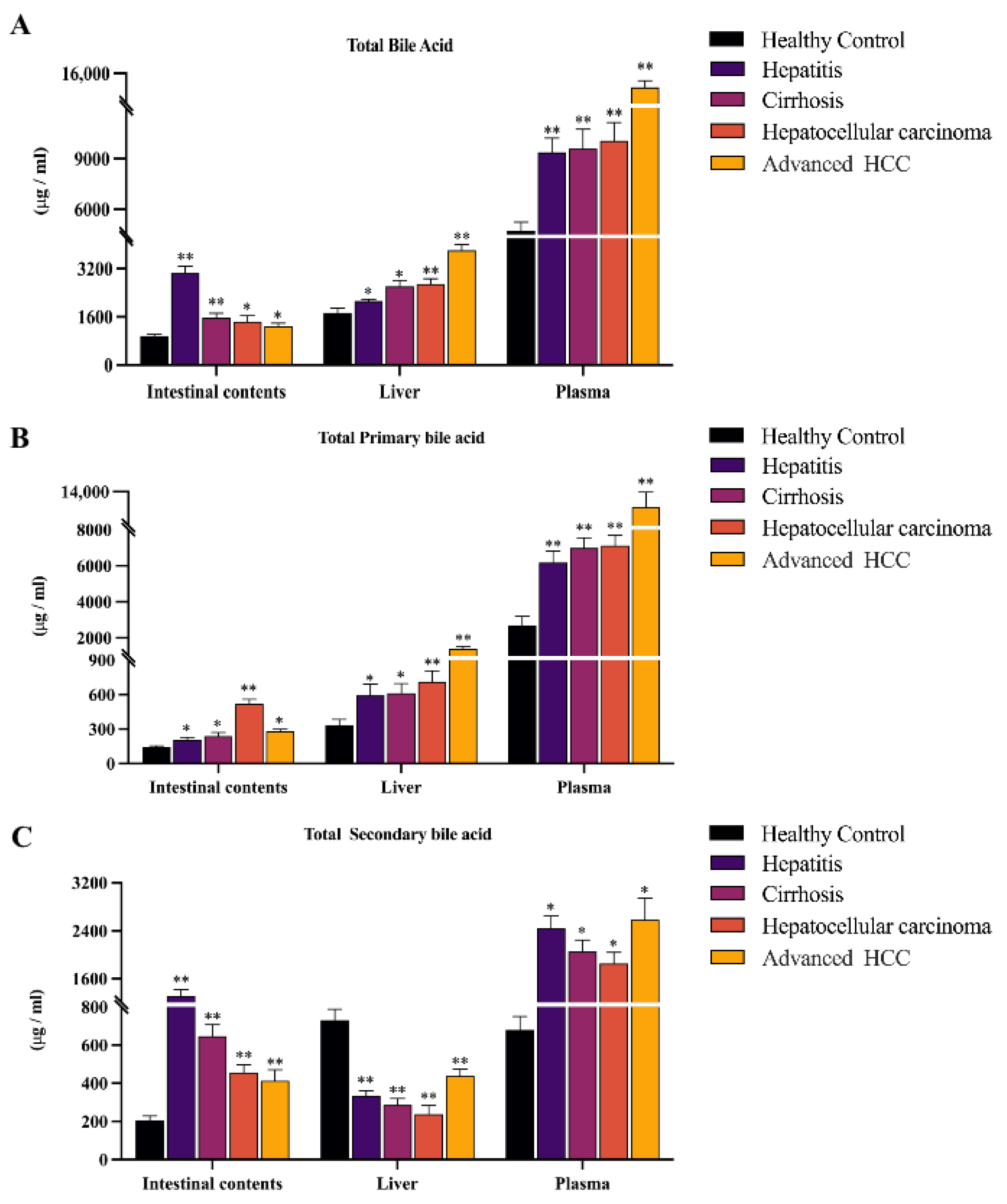
2.1. Histology Assessment and Total Bile Acid Features in the Inflammation-Cancer Transformation Process
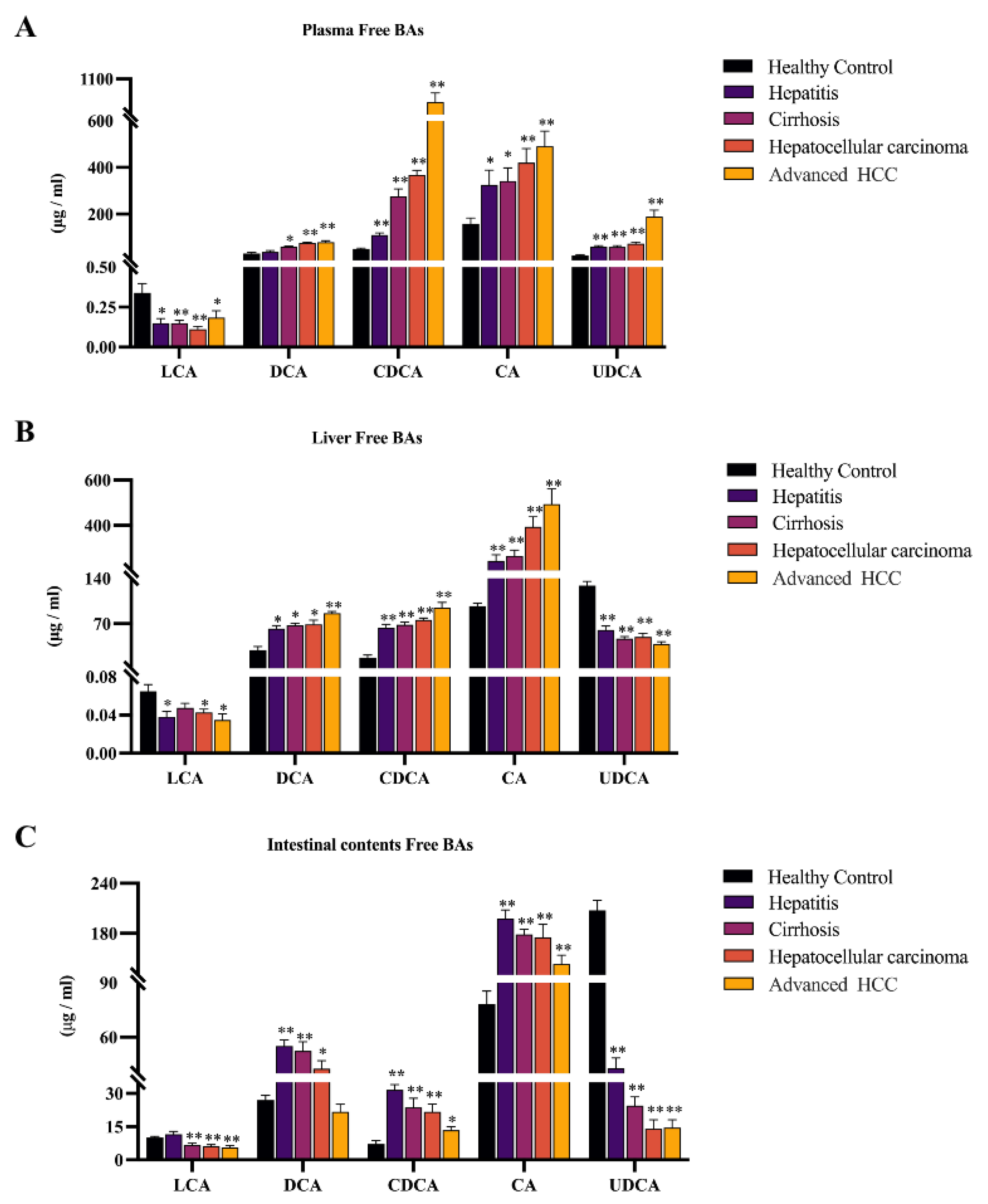
2.2. Observing Liver–Gut Axis BAs Environment and Screening HCC Biomarkers for Early Diagnosis
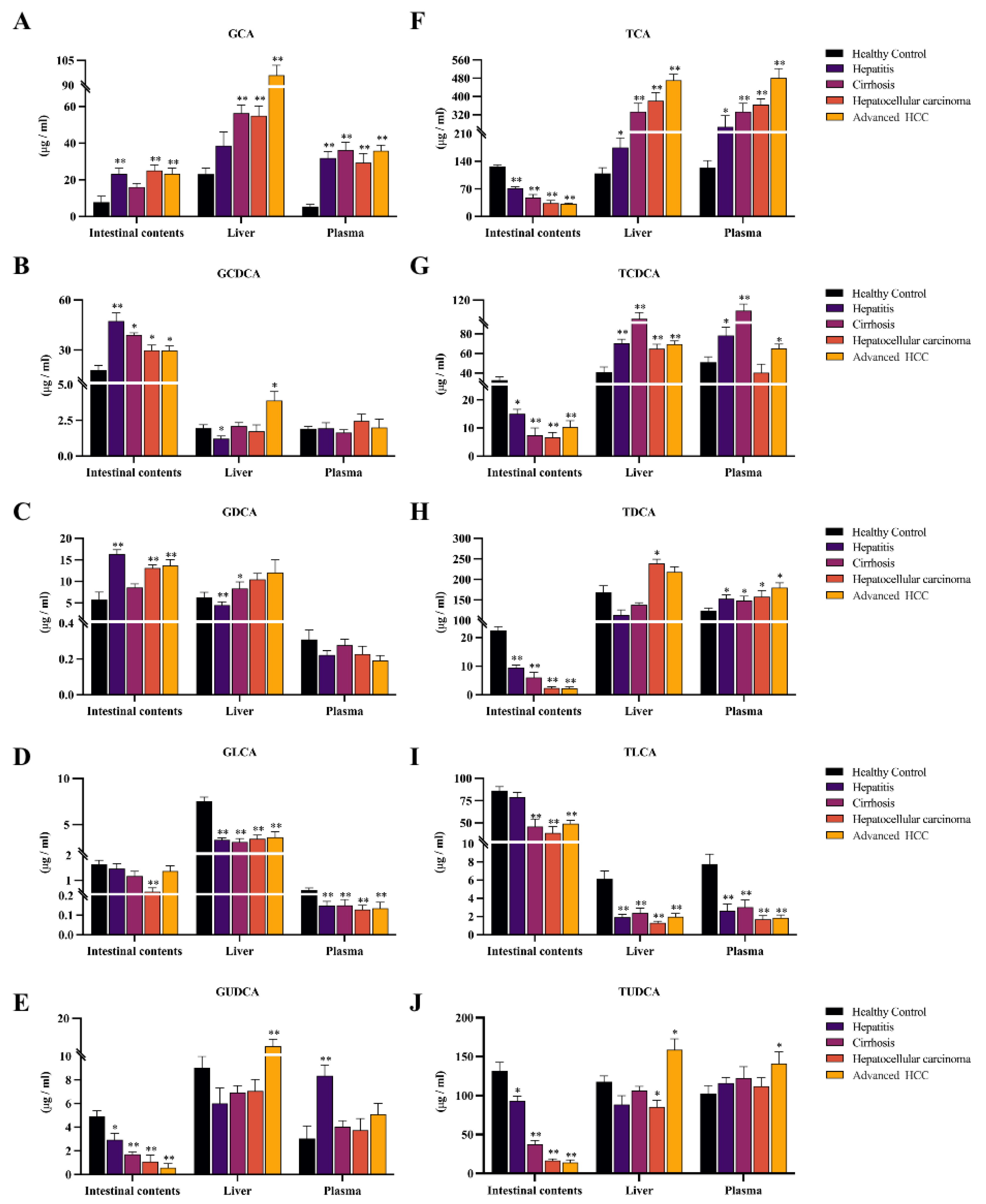
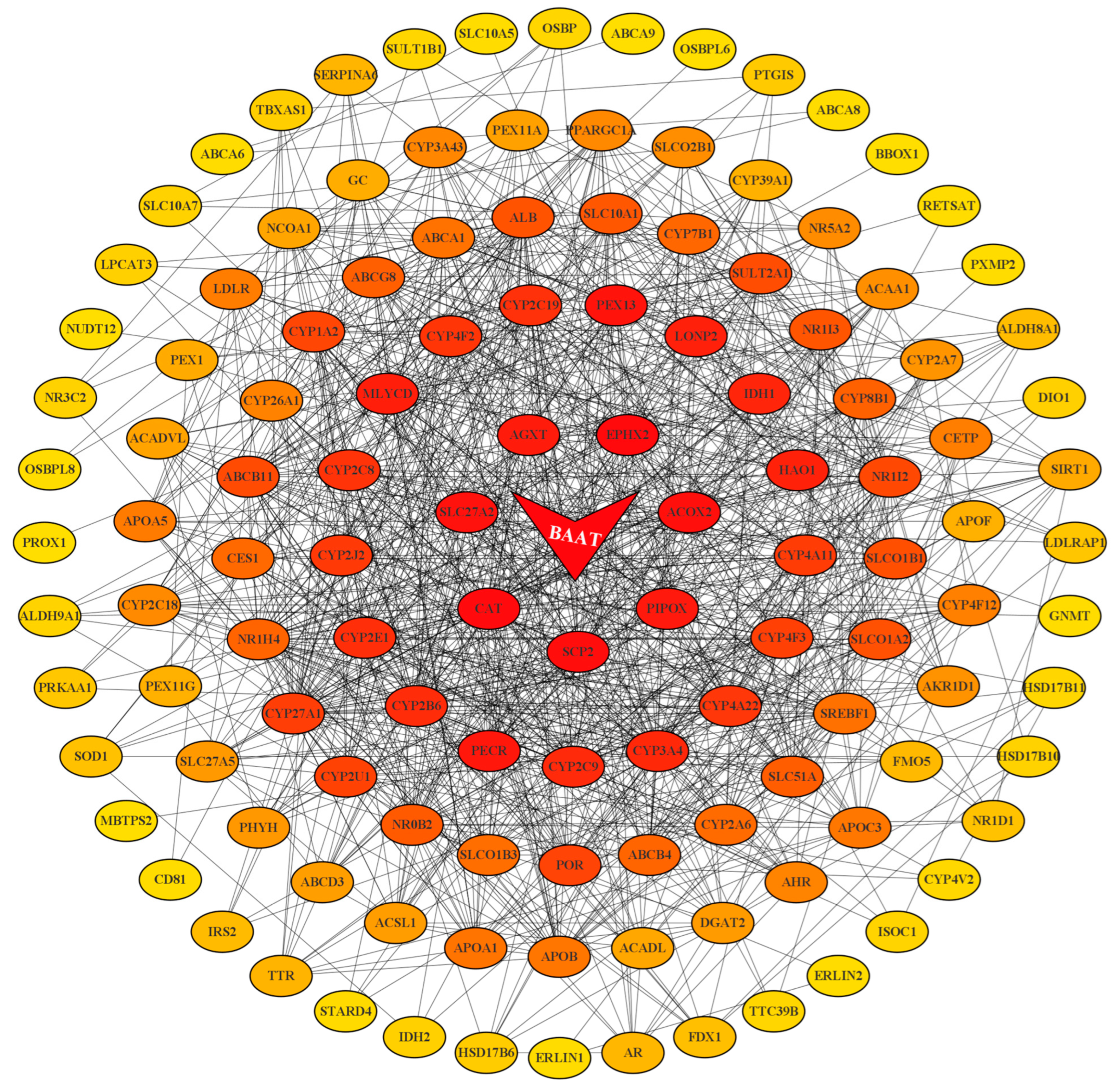
2.3. BAAT was Associated with Altered Composition of the Intestinal BA Pool and Disruption of Enterohepatic Circulation
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Histopathological Analysis
4.4. UFLC-MS/MS Conditions for Quantitation of BAs
4.5. Sample Collection and Pretreatment
4.6. Gene Enrichment Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| BA | Bile acid |
| BAAT | Bile acid-CoA:amino acid N-acyltransferase |
| DEN | N-nitrosodiethylamine |
| OSTα/OSTβ | Organic solute transporter-alpha/beta |
| BSEP | Bile salt output pump |
| MRP2 | Multidrug resistance-associated protein 2 |
| NTCP | Sodium bile acid/taurocholic synergistic polypeptide |
| OATP | Organic anion transport peptide |
| PCA | Principal component analysis |
| OPLS-DA | Orthogonal partial least squares discrimination analysis |
| BLDA | Bayesian linear discriminant analysis |
| GSEA | Gene set enrichment analysis |
| TCGA | The cancer genome atlas |
| FDR | False discovery rate |
| NES | Normalized enrichment score |
| PPI | Protein-protein Interaction |
| MCC | Maximal clique centrality |
| DEABA | N,N-diethyl-1,4-butanediamine |
| UPLC-MS/MS | Ultra-performance liquid chromatography—tandem mass spectrometer |
| MS | Mass spectrometer |
| ESI | Electrospray ionization |
| MRM | Multiple reaction monitoring |
References
- Konyn, P.; Ahmed, A.; Kim, D. Current epidemiology in hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1295–1307. [Google Scholar] [CrossRef]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Hytiroglou, P.; Bioulac-Sage, P.; Theise, N.D.; Sempoux, C. Etiology, Pathogenesis, Diagnosis, and Practical Implications of Hepatocellular Neoplasms. Cancers 2022, 14, 3670. [Google Scholar] [CrossRef] [PubMed]
- Petruzziello, A. Epidemiology of Hepatitis B Virus (HBV) and Hepatitis C Virus (HCV) Related Hepatocellular Carcinoma. Open Virol. J. 2018, 12, 26–32. [Google Scholar] [CrossRef]
- Kanda, T.; Goto, T.; Hirotsu, Y.; Moriyama, M.; Omata, M. Molecular Mechanisms Driving Progression of Liver Cirrhosis towards Hepatocellular Carcinoma in Chronic Hepatitis B and C Infections: A Review. Int. J. Mol. Sci. 2019, 20, 1358. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhu, J.; Ge, Z.; Yu, C.; Tian, T.; Wang, H.; Han, J.; Shen, H.; Dai, J.; Lu, J.; et al. Spontaneous Seroclearance of Hepatitis B Surface Antigen and Risk of Hepatocellular Carcinoma. Clin. Gastroenterol. Hepatol. 2019, 17, 1204–1206. [Google Scholar] [CrossRef] [PubMed]
- Quante, M.; Bhagat, G.; Abrams, J.A.; Marache, F.; Good, P.; Lee, M.D.; Lee, Y.; Friedman, R.; Asfaha, S.; Dubeykovskaya, Z.; et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 2012, 21, 36–51. [Google Scholar] [CrossRef]
- Bernstein, C.; Holubec, H.; Bhattacharyya, A.K.; Nguyen, H.; Payne, C.M.; Zaitlin, B.; Bernstein, H. Carcinogenicity of deoxycholate, a secondary bile acid. Arch. Toxicol. 2011, 85, 863–871. [Google Scholar] [CrossRef]
- Lozano, E.; Sanchez-Vicente, L.; Monte, M.J.; Hetrraez, E.; Briz, O.; Banales, J.M.; Marin, J.J.; Macias, R.I. Cocarcinogenic effects of intrahepatic bile acid accumulation in cholangiocarcinoma development. Mol. Cancer Res. 2014, 12, 91–100. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef]
- Duan, Z.; Yang, T.; Li, L.; Wang, X.; Weti, C.; Xia, Z.; Chai, Y.; Huang, X.; Zhang, L.; Jiang, Z. Comparison of bile acids profiles in the enterohepatic circulation system of mice and rats. J. Steroid Biochem. Mol. Biol. 2022, 220, 106100. [Google Scholar] [CrossRef] [PubMed]
- Gadaleta, R.M.; van Erpecum, K.J.; Oldenburg, B.; Willemsen, E.C.L.; Renooij, W.; Murzilli, S.; Klomp, L.W.J.; Siersema, P.D.; Schipper, M.E.; Danese, S.; et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 2011, 60, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Knisely, A.S.; Strautnieks, S.S.; Meier, Y.; Stieger, B.; Byrne, J.A.; Portmann, B.C.; Bull, L.N.; Pawlikowska, L.; Bilezikçi, B.; Özçay, F.; et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 2006, 44, 478–486. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Cravetto, C.; Molino, G.; Belforte, G.; Bona, B. Simulation of the metabolism and enterohepatic circulation of endogenous deoxycholic acid in humans using a physiologic pharmacokinetic model for bile acid metabolism. Gastroenterology 1987, 93, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shang, X.; Wan, X.; Xiang, X.; Mao, Q.; Detng, G.; Wu, Y. Increased hepatocellular carcinoma risk in chronic hepatitis B patients with persistently elevated serum total bile acid: A retrospective cohort study. Sci. Rep. 2016, 6, 38180. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Daita, K.; Joyce, A.; Mirshahi, F.; Santhekadur, P.K.; Cazanave, S.; Luketic, V.A.; Siddiqui, M.S.; Boyett, S.; Min, H.-K.; et al. The presence and severity of nonalcoholic steatohepatitis is associated with specific changes in circulating bile acids. Hepatology 2018, 67, 534–548. [Google Scholar] [CrossRef]
- Li, J.; Dawson, P.A. Animal models to study bile acid metabolism. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 895–911. [Google Scholar] [CrossRef]
- Alnouti, Y.; Csanaky, I.L.; Klaassen, C.D. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2008, 873, 209–217. [Google Scholar] [CrossRef]
- Pellicoro, A.; van den Heuvel, F.A.; Geuken, M.; Moshage, H.; Jansen, P.L.; Faber, K.N. Human and rat bile acid-CoA:amino acid N-acyltransferase are liver-specific peroxisomal enzymes: Implications for intracellular bile salt transport. Hepatology 2007, 45, 340–348. [Google Scholar] [CrossRef]
- He, D.; Barnes, S.; Falany, C.N. Rat liver bile acid CoA:amino acid N-acyltransferase: Expression, characterization, and peroxisomal localization. J. Lipid Res. 2003, 44, 2242–2249. [Google Scholar] [CrossRef]
- Falany, C.N.; Johnson, M.R.; Barnes, S.; Diasio, R.B. Glycine and taurine conjugation of bile acids by a single enzyme. Molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J. Biol. Chem. 1994, 269, 19375–19379. [Google Scholar] [CrossRef]
- Roberts, S.K.; Majeed, A.; Kemp, W. Controversies in the Management of Hepatitis B: Hepatocellular Carcinoma. Clin. Liver Dis. 2021, 25, 785–803. [Google Scholar] [CrossRef]
- Harris, P.S.; Hansen, R.M.; Gray, M.E.; Massoud, O.I.; McGuire, B.M.; Shoreibah, M.G. Hepatocellular carcinoma surveillance: An evidence-based approach. World J. Gastroenterol. 2019, 25, 1550–1559. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Yin, P.; Hua, R.; Tan, Y.; Li, Z.; Qiu, G.; Yin, Z.; Xie, X.; Wang, X.; Chen, W.; et al. A Large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma. Hepatology 2018, 67, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Ticho, A.L.; Malhotra, P.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. Intestinal Absorption of Bile Acids in Health and Disease. Compr. Physiol. 2019, 10, 21–56. [Google Scholar] [CrossRef] [PubMed]
- Kurma, K.; Manches, O.; Chuffart, F.; Sturm, N.; Gharzeddine, K.; Zhang, J.; Mercey-Ressejac, M.; Rousseaux, S.; Millet, A.; Lerat, H.; et al. DEN-Induced Rat Model Reproduces Key Features of Human Hepatocellular Carcinoma. Cancers 2021, 13, 4981. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.F.; Wu, Z.H.; Wei, Y.J.; Shu, L.; Peng, Y.R. Hepatic inflammation-fibrosis-cancer axis in the rat hepatocellular carcinoma induced by diethylnitrosamine. J. Cancer Res. Clin. Oncol. 2017, 143, 821–834. [Google Scholar] [CrossRef]
- Tan, Y.; Yin, P.; Tang, L.; Xing, W.; Huang, Q.; Cao, D.; Zhao, X.; Wang, W.; Lu, X.; Xu, Z.; et al. Metabolomics study of stepwise hepatocarcinogenesis from the model rats to patients: Potential biomarkers effective for small hepatocellular carcinoma diagnosis. Mol. Cell. Proteom. 2012, 11, 010694. [Google Scholar] [CrossRef]
- Tsuboyama, T.; Onishi, H.; Kim, T.; Akita, H.; Hori, M.; Tatsumi, M.; Nakamoto, A.; Nagano, H.; Matsuura, N.; Wakasa, K.; et al. Hepatocellular carcinoma: Hepatocyte-selective enhancement at gadoxetic acid-enhanced MR imaging–correlation with expression of sinusoidal and canalicular transporters and bile accumulation. Radiology 2010, 255, 824–833. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef]
- Kullak-Ublick, G.A.; Stieger, B.; Meier, P.J. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology 2004, 126, 322–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, X.; Valanejad, L.; Vasilenko, A.; More, V.; Qiu, X.; Chen, W.; Lai, Y.; Slitt, A.; Stoner, M.; et al. Bile salt export pump is dysregulated with altered farnesoid X receptor isoform expression in patients with hepatocellular carcinoma. Hepatology 2013, 57, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, F.; Patterson, A.D.; Wang, Y.; Krausz, K.W.; Neale, G.; Thomas, S.; Nachagari, D.; Vogel, P.; Vore, M.; et al. Abcb11 deficiency induces cholestasis coupled to impaired β-fatty acid oxidation in mice. J. Biol. Chem. 2012, 287, 24784–24794. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.Y.; Ouyang, X.; Chen, Y.; Soroka, C.J.; Wang, J.; Metnnone, A.; Wang, Y.; Mehal, W.Z.; Jain, D.; Boyer, J.L. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight 2017, 2, e90780. [Google Scholar] [CrossRef]
- Meier, P.J.; Stieger, B. Bile salt transporters. Annu. Rev. Physiol. 2002, 64, 635–661. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Q.; Ding, M.; Yao, J.; Guo, Y.; Yan, W.; Yu, S.; Shen, Q.; Huang, M.; Zheng, Y.; et al. Alcohol triggered bile acid disequilibrium by suppressing BSEP to sustain hepatocellular carcinoma progression. Chem. Biol. Interact. 2022, 356, 109847. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhang, X.; Liu, J.; Zhang, Q.; Zhao, Y.; Peng, J.; Feng, Q.; Dai, J.; Sun, S.; et al. Gut Microbial Dysbiosis Is Associated with Altered Hepatic Functions and Serum Metabolites in Chronic Hepatitis B Patients. Front. Microbiol. 2017, 8, 2222. [Google Scholar] [CrossRef]
- Dawson, P.A.; Hubbert, M.; Haywood, J.; Craddock, A.L.; Zerangue, N.; Christian, W.V.; Ballatori, N. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J. Biol. Chem. 2005, 280, 6960–6968. [Google Scholar] [CrossRef]
- Sultan, M.; Rao, A.; Elpeleg, O.; Vaz, F.M.; Abu-Libdeh, B.Y.; Karpen, S.J.; Dawson, P.A. Organic solute transporter-β (SLC51B) deficiency in two brothers with congenital diarrhea and features of cholestasis. Hepatology 2018, 68, 590–598. [Google Scholar] [CrossRef]
- Rodríguez-Nogales, A.; Algieri, F.; Vezza, T.; Garrido-Mesa, J.; Molina-Tijeras, J.A.; Rodríguez-Cabezas, M.E.; Utrilla, M.P.; Pischel, I.; Gálvez, J. Calcium Pyruvate Exerts Beneficial Effects in an Experimental Model of Irritable Bowel Disease Induced by DCA in Rats. Nutrients 2019, 11, 140. [Google Scholar] [CrossRef]
- Fukui, H. Role of Gut Dysbiosis in Liver Diseases: What Have We Learned So Far? Diseases 2019, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Schwabe, R.F. The gut microbiome and liver cancer: Mechanisms and clinical translation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 527–539. [Google Scholar] [CrossRef]
- Funabashi, M.; Grove, T.L.; Wang, M.; Varma, Y.; McFadden, M.E.; Brown, L.C.; Guo, C.; Higginbottom, S.; Almo, S.C.; Fischbach, M.A. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature 2020, 582, 566–570. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Attili, A.F.; Angelico, M.; Cantafora, A.; Alvaro, D.; Capocaccia, L. Bile acid-induced liver toxicity: Relation to the hydrophobic-hydrophilic balance of bile acids. Med. Hypotheses 1986, 19, 57–69. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Kanno, K.; Pham, Q.T.; Kikuchi, Y.; Kakimoto, M.; Kobayashi, T.; Otani, Y.; Kishikawa, N.; Miyauchi, M.; Arihiro, K.; et al. Senescent hepatic stellate cells caused by deoxycholic acid modulates malignant behavior of hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2020, 146, 3255–3268. [Google Scholar] [CrossRef]
- Hu, Y.; Chau, T.; Liu, H.X.; Liao, D.; Keane, R.; Nie, Y.; Yang, H.; Wan, Y.J.Y. Bile acids regulate nuclear receptor (Nur77) expression and intracellular location to control proliferation and apoptosis. Mol. Cancer Res. 2015, 13, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef]
- Xie, A.J.; Mai, C.T.; Zhu, Y.Z.; Liu, X.C.; Xie, Y. Bile acids as regulatory molecules and potential targets in metabolic diseases. Life Sci. 2021, 287, 120152. [Google Scholar] [CrossRef]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018, 18, 71–87. [Google Scholar] [CrossRef]
- Xiao, Z.L.; Biancani, P.; Carey, M.C.; Behar, J. Hydrophilic but not hydrophobic bile acids prevent gallbladder muscle dysfunction in acute cholecystitis. Hepatology 2003, 37, 1442–1450. [Google Scholar] [CrossRef]
- Lazaridis, K.N.; Gores, G.J.; Lindor, K.D. Ursodeoxycholic acid ‘mechanisms of action and clinical use in hepatobiliary disorders’. J. Hepatol. 2001, 35, 134–146. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, R.; Zheng, X.; Lei, S.; Huang, F.; Xie, G.; Kwee, S.; Yu, H.; Farrar, C.; Sun, B.; et al. Ursodeoxycholic acid accelerates bile acid enterohepatic circulation. Br. J. Pharm. 2019, 176, 2848–2863. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandusse, S.; Jimenez-Sanchez, G.; Koster, J.; Denis, S.; Van Roermund, C.W.; Silva-Zolezzi, I.; Moser, A.B.; Visser, W.F.; Gulluoglu, M.; Durmaz, O.; et al. A novel bile acid biosynthesis defect due to a deficiency of peroxisomal ABCD3. Hum. Mol. Genet. 2015, 24, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Rembacz, K.P.; Woudenberg, J.; Hoekstra, M.; Jonkers, E.Z.; Van Den Heuvel, F.A.; Buist-Homan, M.; Woudenberg-Vrenken, T.E.; Rohacova, J.; Marin, M.L.; Miranda, M.A.; et al. Unconjugated bile salts shuttle through hepatocyte peroxisomes for taurine conjugation. Hepatology 2010, 52, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Kirilenko, B.M.; Hagey, L.R.; Barnes, S.; Falany, C.N.; Hiller, M. Evolutionary Analysis of Bile Acid-Conjugating Enzymes Reveals a Complex Duplication and Reciprocal Loss History. Genome Biol. Evol. 2019, 11, 3256–3268. [Google Scholar] [CrossRef]
- Alrehaili, B.D.; Lee, M.; Takahashi, S.; Novak, R.; Rimal, B.; Boehme, S.; Trammell, S.A.J.; Grevengoed, T.J.; Kumar, D.; Alnouti, Y.; et al. Bile acid conjugation deficiency causes hypercholanemia, hyperphagia, islet dysfunction, and gut dysbiosis in mice. Hepatol. Commun. 2022, 6, 2765–2780. [Google Scholar] [CrossRef]
- Tong, M.; Zhang, Q.; Zhang, Y.; Xing, L.; Bi, K.; Li, Q. A convenient and efficient 4-(diethylamino)-butylamine-labeled polarity-response-homodispersed strategy for absolute quantification of carboxyl submetabolome: Monitoring the whole progressive course of hepatocellular carcinoma. J. Chromatogr. A 2022, 1683, 463504. [Google Scholar] [CrossRef]
- Granito, A.; Muratori, L.; Lalanne, C.; Quarneti, C.; Ferri, S.; Guidi, M.; Lenzi, M.; Muraltori, P. Hepatocellular carcinoma in viral and autoimmune liver diseases: Role of CD4+ CD25+ Foxp3+ regulatory T cells in the immune microenvironment. World J. Gastroenterol. 2021, 27, 2994–3009. [Google Scholar] [CrossRef]
- Tanoue, T.; Atarashi, K.; Honda, K. Development and maintenance of intestinal regulatory T cells. Nat. Rev. Immunol. 2016, 16, 295–309. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef] [PubMed]
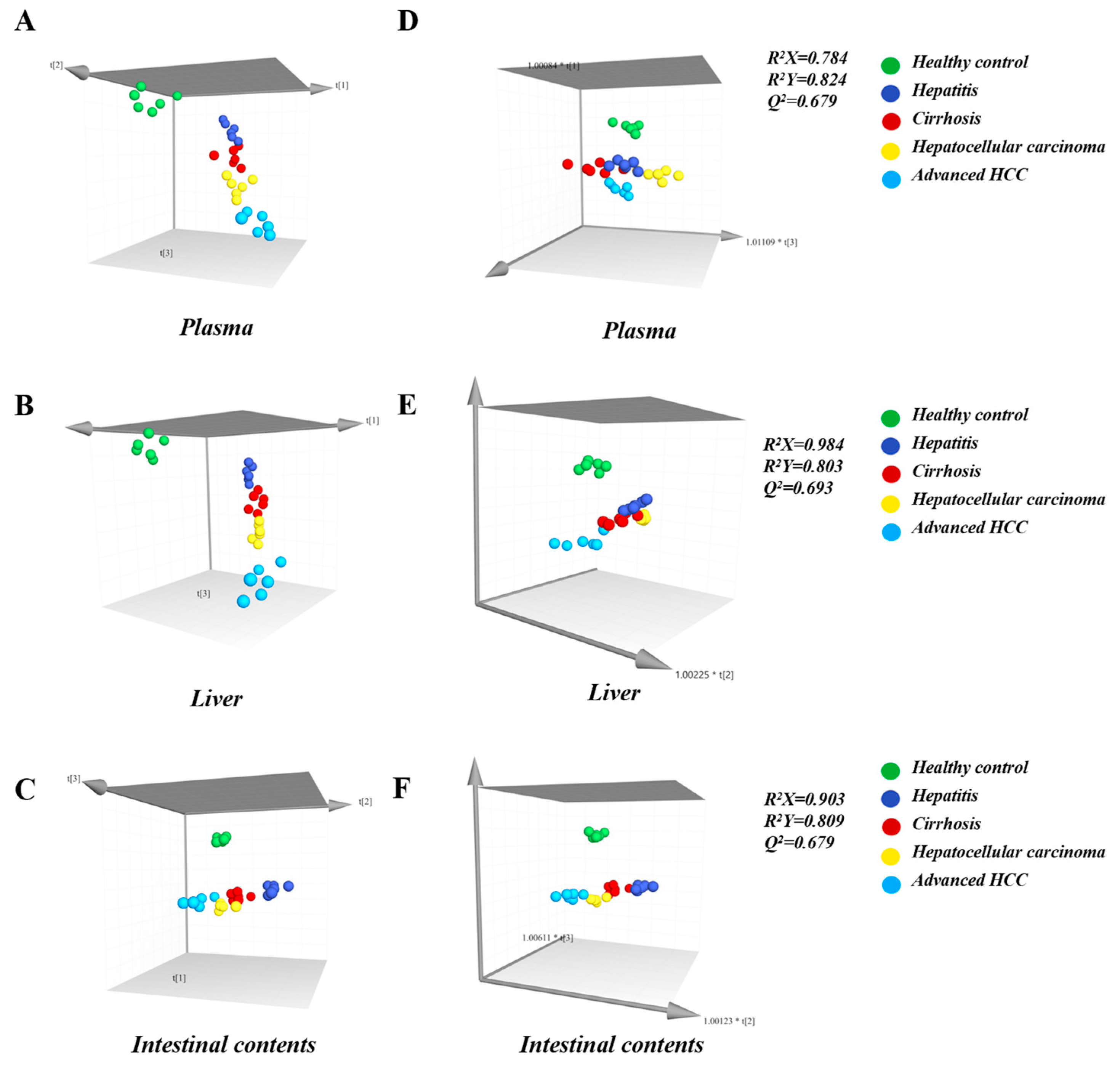
| Health Control | Hepatitis | Cirrhosis | Hepatocellular Carcinoma | Advanced HCC | |
|---|---|---|---|---|---|
| CDCA | 0.006 | 0.041 | 0.126 | 0.169 | 0.489 |
| LCA | 28.368 | 8.839 | 16.390 | 6.180 | 9.522 |
| UDCA | −0.026 | 0.058 | 0.018 | 0.038 | 0.097 |
| GLCA | 37.047 | 3.851 | −4.336 | −5.770 | −32.010 |
| Constant | −17.575 | −6.589 | −20.411 | −34.025 | −264.774 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, L.; Zhang, Y.; Li, S.; Tong, M.; Bi, K.; Zhang, Q.; Li, Q. A Dual Coverage Monitoring of the Bile Acids Profile in the Liver–Gut Axis throughout the Whole Inflammation-Cancer Transformation Progressive: Reveal Hepatocellular Carcinoma Pathogenesis. Int. J. Mol. Sci. 2023, 24, 4258. https://doi.org/10.3390/ijms24054258
Xing L, Zhang Y, Li S, Tong M, Bi K, Zhang Q, Li Q. A Dual Coverage Monitoring of the Bile Acids Profile in the Liver–Gut Axis throughout the Whole Inflammation-Cancer Transformation Progressive: Reveal Hepatocellular Carcinoma Pathogenesis. International Journal of Molecular Sciences. 2023; 24(5):4258. https://doi.org/10.3390/ijms24054258
Chicago/Turabian StyleXing, Luwen, Yiwen Zhang, Saiyu Li, Minghui Tong, Kaishun Bi, Qian Zhang, and Qing Li. 2023. "A Dual Coverage Monitoring of the Bile Acids Profile in the Liver–Gut Axis throughout the Whole Inflammation-Cancer Transformation Progressive: Reveal Hepatocellular Carcinoma Pathogenesis" International Journal of Molecular Sciences 24, no. 5: 4258. https://doi.org/10.3390/ijms24054258
APA StyleXing, L., Zhang, Y., Li, S., Tong, M., Bi, K., Zhang, Q., & Li, Q. (2023). A Dual Coverage Monitoring of the Bile Acids Profile in the Liver–Gut Axis throughout the Whole Inflammation-Cancer Transformation Progressive: Reveal Hepatocellular Carcinoma Pathogenesis. International Journal of Molecular Sciences, 24(5), 4258. https://doi.org/10.3390/ijms24054258





