Hyperbaric Oxygenation Prevents Loss of Immature Neurons in the Adult Hippocampal Dentate Gyrus Following Brain Injury
Abstract
1. Introduction
2. Results
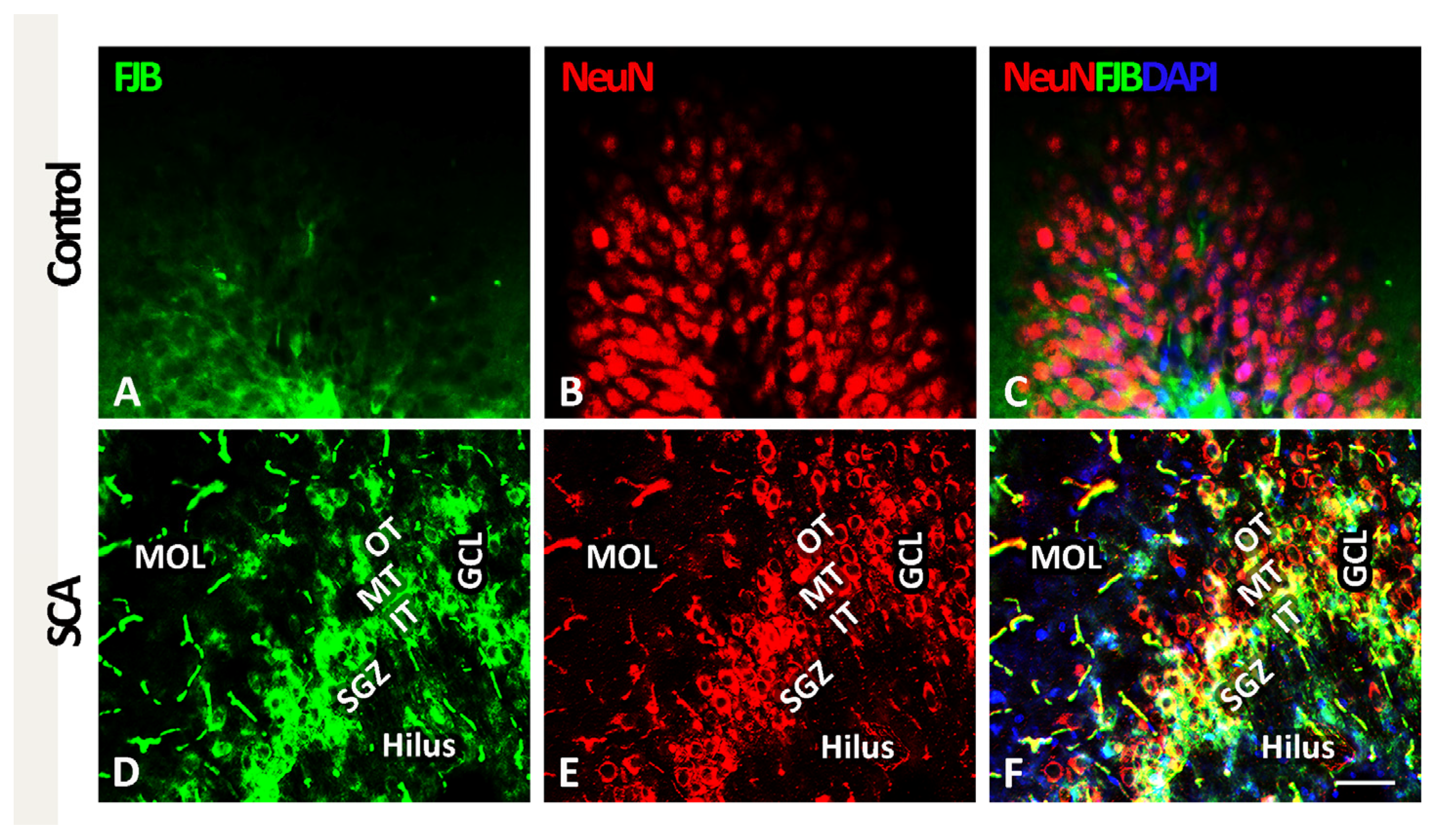
2.1. SCA Leads to Layer-Specific Neurodegeneration in the Hippocampal DG
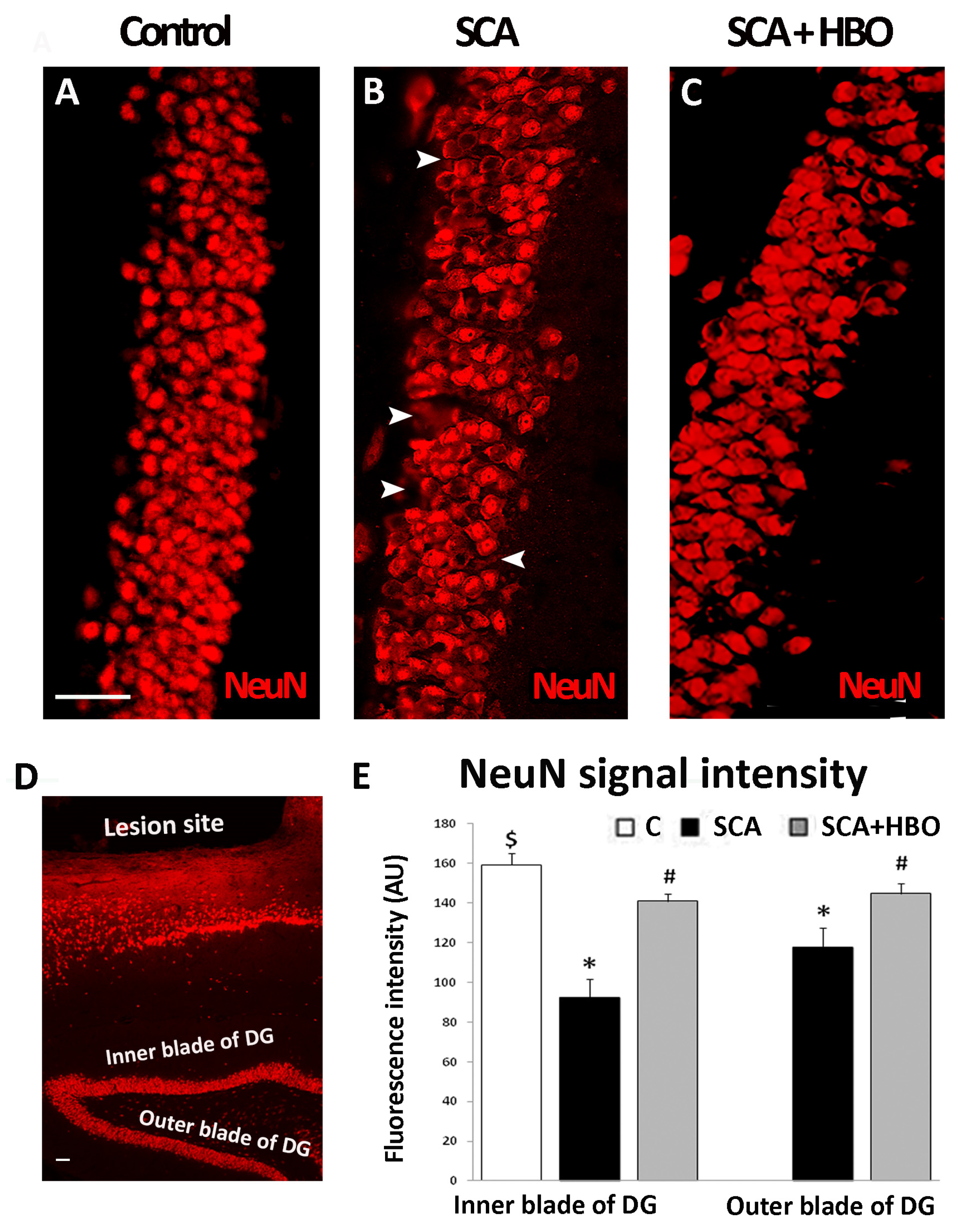
2.2. HBOT Prevents/Ameliorates SCA-Provoked Loss of Neurons in the GCL of the Hippocampal DG
2.3. Cell Type of Neurons Undergoing Neurodegeneration in the Hippocampal DG Following SCA Injury and HBOT
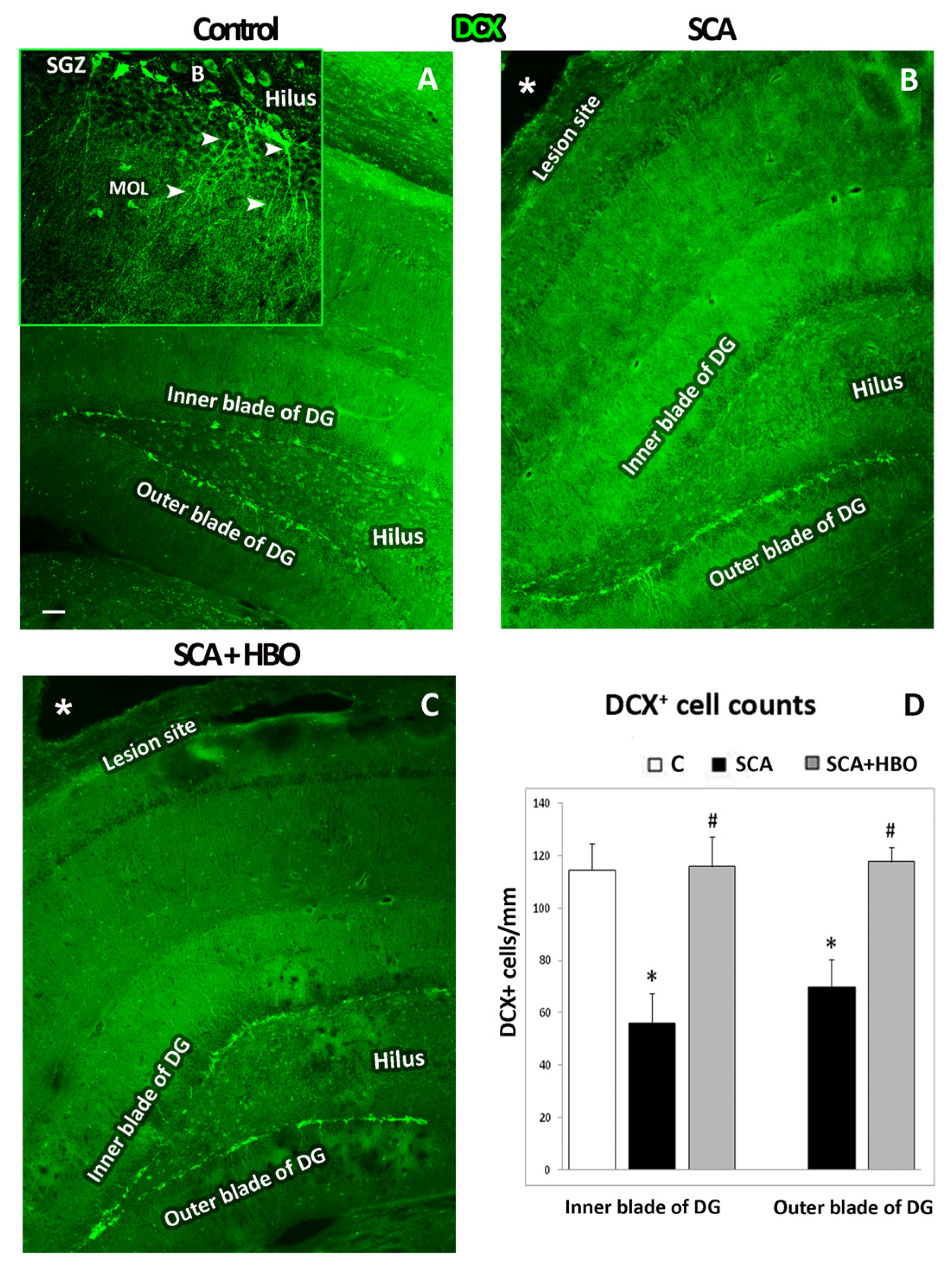
2.3.1. HBOT Prevents Loss of DCX-Positive Newborn Immature Neurons in the GCL of the Hippocampal DG Following SCA Injury
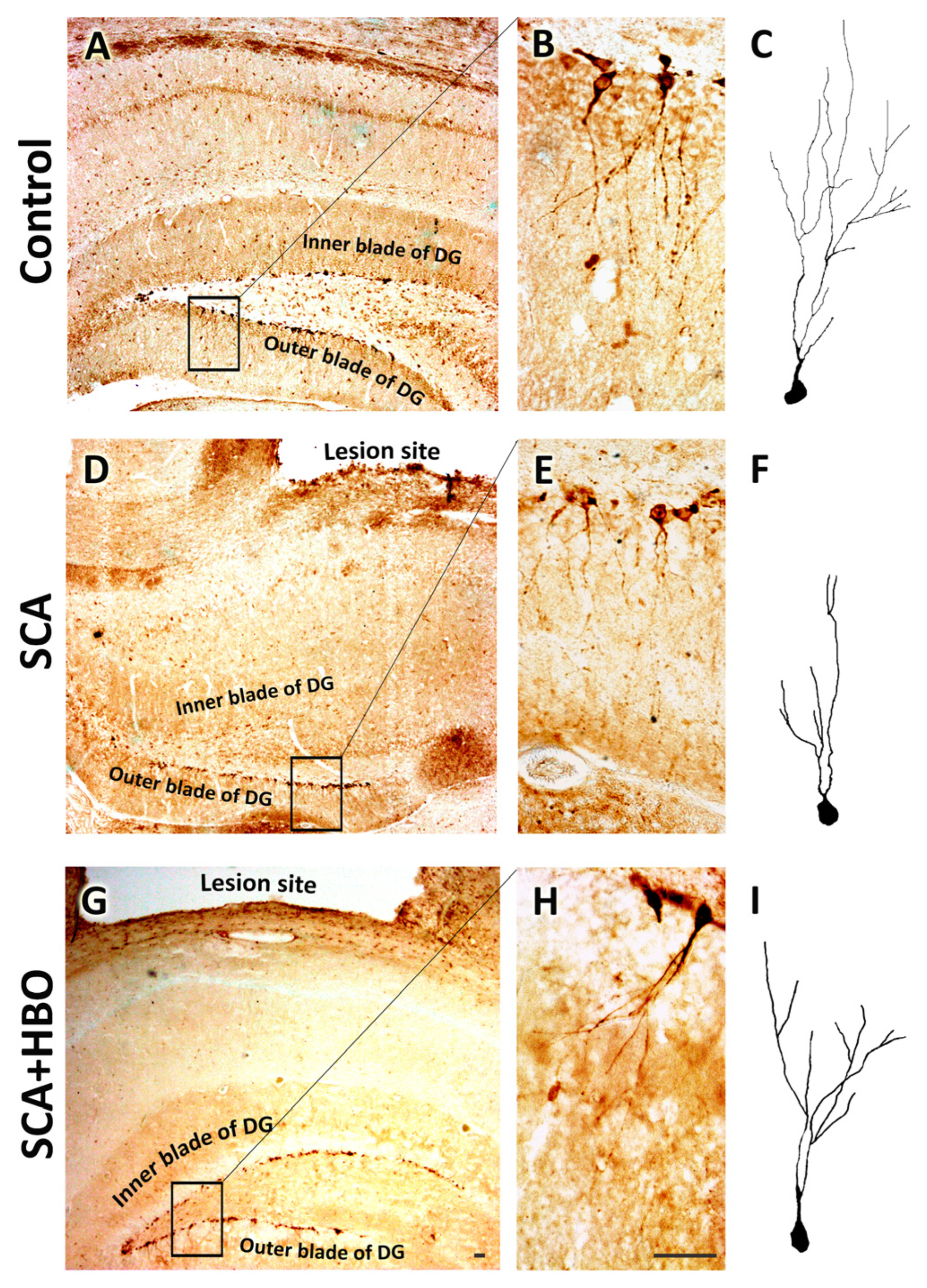
2.3.2. HBOT Prevents SCA-Caused Neuronal Loss and Dendrite Degeneration of Newborn Immature Neurons in the SGZ of the Hippocampal DG
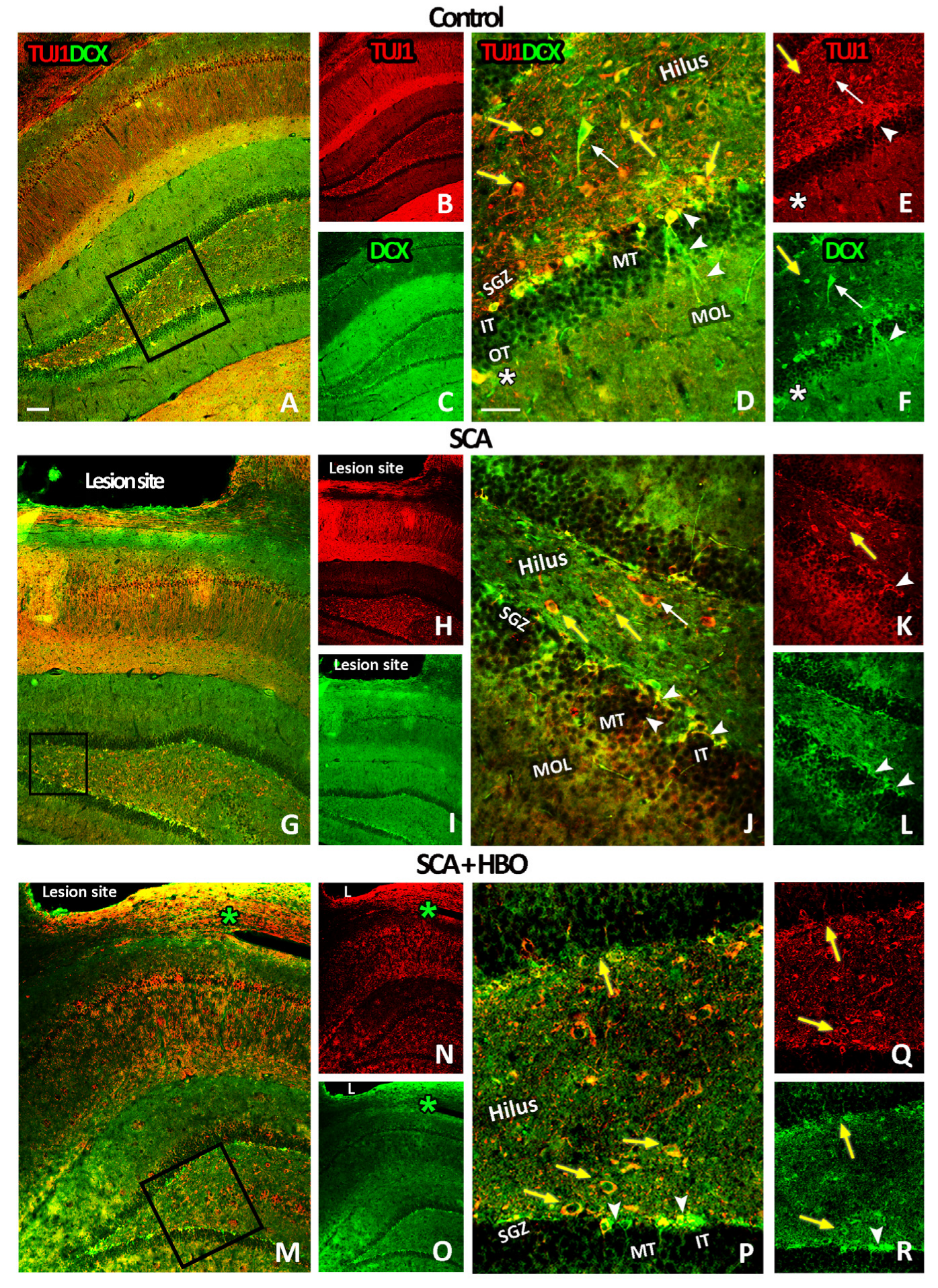
2.3.3. HBOT Prevents Loss of DCX/TUJ1-Positive Newborn Immature Neurons in the GCL of the Hippocampal DG Following SCA Injury
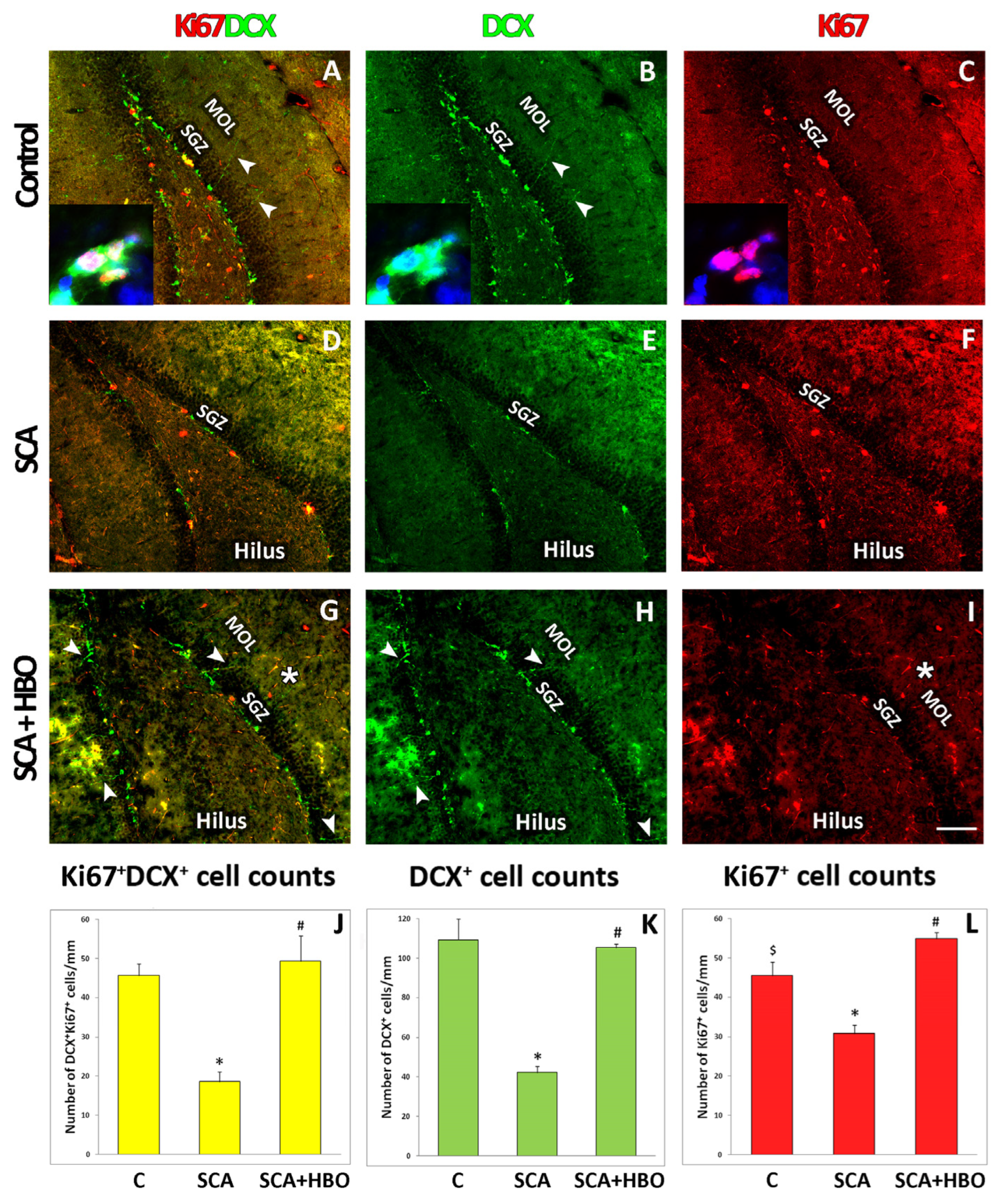
2.4. Proliferation of Ki67-Positive Newborn Immature Neurons Co-Labeled with DCX in the SGZ of the Hippocampal DG Following SCA Injury and HBOT
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgical Procedure
4.3. Hyperbaric Oxygen Treatment
4.4. Brain Tissue Preparation
4.5. Immunohistochemistry and Immunofluorescence Staining
4.6. Quantification of Immunoreactive Cells
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Owji, S.; Shoja, M.M. The History of Discovery of Adult Neurogenesis. Clin. Anat. 2020, 33, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, C. Adult mammalian neurogenesis and motivated behaviors. Integr. Zool. 2018, 13, 655–672. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, C.; Parolisi, R.; Bonfanti, L. Brain Structural Plasticity: From Adult Neurogenesis to Immature Neurons. Front. Neurosci. 2020, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Mensching, L.; Djogo, N.; Keller, C.; Rading, S.; Karsak, M. Stable adult hippocampal neurogenesis in cannabinoid receptor CB2 deficient mice. Int. J. Mol. Sci. 2019, 20, 3759. [Google Scholar] [CrossRef]
- Yu, T.S.; Washington, P.M.; Kernie, S.G. Injury-Induced Neurogenesis: Mechanisms and Relevance. Neuroscientist 2016, 22, 61–71. [Google Scholar] [CrossRef]
- Kempermann, G.; Gage, F.H.; Aigner, L.; Song, H.; Curtis, M.A.; Thuret, S.; Kuhn, H.G.; Jessberger, S.; Frankland, P.W.; Cameron, H.A.; et al. Human Adult Neurogenesis: Evidence and Remaining Questions. Cell Stem Cell 2018, 23, 25–30. [Google Scholar] [CrossRef]
- Rubiano, A.M.; Carney, N.; Chesnut, R.; Puyana, J.C. Global neurotrauma research challenges and opportunities. Nature 2015, 527, S193–S197. [Google Scholar] [CrossRef]
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef]
- Brkić, P.; Sanja, P.; Danijela, K.; Jovanović, T. Hyperbaric oxygenation as an adjuvant therapy for traumatic brain injury: A review of literature. Period. Biol. 2014, 116, 29–36. [Google Scholar]
- Parabucki, A.B.; Božić, I.D.; Bjelobaba, I.M.; Lavrnja, I.C.; Brkić, P.D.; Jovanović, T.S.; Savić, D.Z.; Stojiljković, M.B.; Peković, S.M. Hyperbaric oxygenation alters temporal expression pattern of superoxide dismutase 2 after cortical stab injury in rats. Croat. Med. J. 2012, 53, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Pekovic, S.; Subasic, S.; Nedeljkovic, N.; Bjelobaba, I.; Filipovic, R.; Milenkovic, I.; Lavrnja, I.; Stojkov, D.; Jovanovic, S.; Rakic, L.; et al. Molecular basis of brain injury and repair. In Neurobiological Studies—From Genes to Behavior; Ruzdijic, S., Rakic, L.J., Eds.; Research Signpost: Kerala, India, 2006; pp. 143–165. ISBN 81-308-0107-8. [Google Scholar]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef] [PubMed]
- Terranova, J.I.; Ogawa, S.K.; Kitamura, T. Adult hippocampal neurogenesis for systems consolidation of memory. Behav. Brain Res. 2019, 372, 112035. [Google Scholar] [CrossRef] [PubMed]
- Margulies, S.; Hicks, R. Combination therapies for traumatic brain injury: Prospective considerations. J. Neurotrauma 2009, 26, 925–939. [Google Scholar] [CrossRef]
- Pekovic, S.; Dacic, S.; Krstic, D.; Jeremic, R.; Djelic, M.; Brkic, P. Hyperbaric oxygen therapy in traumatic brain injury: Cellular and molecular mechanisms. In Hyperbaric Oxygen Treatment in Research and Clinical Practice—Mechanisms of Action in Focus; Drenjančević, I., Ed.; InTechOpen: Rijeka, Croatia, 2018; pp. 25–46. ISBN 978-953-51-5916-2. [Google Scholar] [CrossRef]
- De Wolde, S.D.; Hulskes, R.H.; Weenink, R.P.; Hollmann, M.W.; Van Hulst, R.A. The effects of hyperbaric oxygenation on oxidative stress, inflammation and angiogenesis. Biomolecules 2021, 11, 1210. [Google Scholar] [CrossRef]
- Daly, S.; Thorpe, M.; Rockswold, S.; Hubbard, M.; Bergman, T.; Samadani, U.; Rockswold, G. Hyperbaric Oxygen Therapy in the Treatment of Acute Severe Traumatic Brain Injury: A Systematic Review. J. Neurotrauma 2018, 35, 623–629. [Google Scholar] [CrossRef]
- Manivannan, S.; Marei, O.; Elalfy, O.; Zaben, M. Neurogenesis after traumatic brain injury—The complex role of HMGB1 and neuroinflammation. Neuropharmacology 2021, 183, 108400. [Google Scholar] [CrossRef]
- Pantic, I.; Jeremic, R.; Dacic, S.; Pekovic, S.; Pantic, S.; Djelic, M.; Vitic, Z.; Brkic, P.; Brodski, C. Gray-Level Co-Occurrence Matrix Analysis of Granule Neurons of the Hippocampal Dentate Gyrus Following Cortical Injury. Microsc. Microanal. 2020, 26, 166–172. [Google Scholar] [CrossRef]
- Brkic, P.; Stojiljkovic, M.; Jovanovic, T.; Dacic, S.; Lavrnja, I.; Savic, D.; Parabucki, A.; Bjelobaba, I.; Rakic, L.; Pekovic, S. Hyperbaric oxygenation improves locomotor ability by enhancing neuroplastic responses after cortical ablation in rats. Brain Inj. 2012, 26, 1273–1284. [Google Scholar] [CrossRef]
- Hu, Q.; Manaenko, A.; Xu, T.; Guo, Z.; Tang, J.; Zhang, J.H. Hyperbaric oxygen therapy for traumatic brain injury: Bench-to-bedside. Med. Gas Res. 2016, 6, 102–110. [Google Scholar] [CrossRef]
- Shandley, S.; Wolf, E.G.; Schubert-Kabban, C.M.; Baugh, L.M.; Richards, M.F.; Prye, J.; Arizpe, H.M.; Kalns, J. Increased circulating stem cells and better cognitive performance in traumatic brain injury subjects following hyperbaric oxygen therapy. Undersea Hyperb. Med. 2017, 44, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Krafft, P.R.; Zhang, J.H. Hyperbaric oxygen therapy promotes neurogenesis: Where do we stand? Med. Gas Res. 2011, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Gast, D.; Kronenberg, G.; Yamaguchi, M.; Gage, F.H. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development 2003, 130, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Collombet, J.M.; Masqueliez, C.; Four, E.; Burckhart, M.F.; Bernabé, D.; Baubichon, D.; Lallement, G. Early reduction of NeuN antigenicity induced by soman poisoning in mice can be used to predict delayed neuronal degeneration in the hippocampus. Neurosci. Lett. 2006, 398, 337–342. [Google Scholar] [CrossRef]
- Amaral, D.G.; Scharfman, H.E.; Lavenex, P. The dentate gyrus: Fundamental neuroanatomical organization (dentate gyrus for dummies). Prog. Brain Res. 2007, 163, 3–22. [Google Scholar] [CrossRef]
- Couillard-Despres, S.; Winner, B.; Schaubeck, S.; Aigner, R.; Vroemen, M.; Weidner, N.; Bogdahn, U.; Winkler, J.; Kuhn, H.G.; Aigner, L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005, 21, 1–14. [Google Scholar] [CrossRef]
- Von Bohlen Und Halbach, O. Immunohistological markers for staging neurogenesis in adult hippocampus. Cell Tissue Res. 2007, 329, 409–420. [Google Scholar] [CrossRef]
- Korzhevskii, D.E.; Karpenko, M.N.; Kirik, O.V. Microtubule-Associated Proteins as Indicators of Differentiation and the Functional State of Nerve Cells. Neurosci. Behav. Physiol. 2012, 42, 215–222. [Google Scholar] [CrossRef]
- Kempermann, G. What Is Adult Hippocampal Neurogenesis Good for? Front. Neurosci. 2022, 16, 852680. [Google Scholar] [CrossRef]
- Chen, J. Selective Death of Newborn Neurons in Hippocampal Dentate Gyrus Following Moderate Experimental Traumatic Brain Injury. J. Neurosci. Res. 2008, 86, 2258–2270. [Google Scholar] [CrossRef]
- Gao, X.; Enikolopov, G.; Chen, J. Moderate traumatic brain injury promotes proliferation of quiescent neural progenitors in the adult hippocampus. Exp. Neurol. 2009, 219, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Coates, D.R.; Chin, J.M.; Chung, S.T.L. Forebrain Neurogenesis after Focal Ischemic and Traumatic Brain. Bone 2011, 23, 1–7. [Google Scholar] [CrossRef]
- Gao, X.; Deng, P.; Xu, Z.C.; Chen, J. Moderate traumatic brain injury causes acute dendritic and synaptic degeneration in the hippocampal dentate gyrus. PLoS ONE 2011, 6, e24566. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, X.; Michalski, S.; Zhao, S.; Chen, J. Traumatic Brain Injury Severity Affects Neurogenesis in Adult Mouse Hippocampus. J. Neurotrauma 2016, 33, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, L.B. Model of recovery of locomotor ability after sensorimotor cortex injury in rats. ILAR J. 2003, 44, 125–129. [Google Scholar] [CrossRef]
- Lavrnja, I.; Trifunovic, S.; Ajdzanovic, V.; Pekovic, S.; Bjelobaba, I.; Stojiljkovic, M.; Milosevic, V. Sensorimotor cortex ablation induces time-dependent response of ACTH cells in adult rats: Behavioral, immunohistomorphometric and hormonal study. Physiol. Behav. 2014, 125, 30–37. [Google Scholar] [CrossRef]
- Szele, F.G.; Alexander, C.; Chesselet, M.F. Expression of molecules associated with neuronal plasticity in the striatum after aspiration and thermocoagulatory lesions of the cerebral cortex in adult rats. J. Neurosci. 1995, 15, 4429–4448. [Google Scholar] [CrossRef]
- Pearlson, G.D.; Robinson, R.G. Suction lesions of the frontal cerebral cortex in the rat induce assymetrical behavioral and catecholaminergic responses. Brain Res. 1981, 218, 233–242. [Google Scholar] [CrossRef]
- Pulsinelli, W.A.; Brierley, J.B.; Plum, F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann. Neurol. 1982, 11, 491–498. [Google Scholar] [CrossRef]
- Saunders, D.E.; Howe, F.A.; Van den Boogaart, A.; McLean, M.A.; Griffiths, J.R.; Brown, M.M. Continuing ischemic damage after acute middle cerebral artery infarction in humans demonstrated by short-echo proton spectroscopy. Stroke 1995, 26, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Povlishock, J.T.; Erb, D.E.; Arstruc, J. Axonal response to traumatic brain injury: Reactive axonal change, deafferentation, and neuroplasticity. J. Neurotrauma 1992, 9, S189–S200. [Google Scholar]
- de Freitas, H.T.; da Silva, V.G.; Giraldi-Guimarães, A. Comparative study between bone marrowmononuclear fraction andmesenchymal stemcells treatment in sensorimotor recovery after focal cortical ablation in rats. Behav. Brain Funct. 2012, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Lavrnja, I.; Ajdzanovic, V.; Trifunovic, S.; Savic, D.; Milosevic, V.; Stojiljkovic, M.; Pekovic, S. Cortical ablation induces time-dependent changes in rat pituitary somatotrophs and upregulates growth hormone receptor expression in the injured cortex. J. Neurosci. Res. 2014, 92, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Colicos, M.A.; Dash, P.K. Apoptotic morphology of dentate gyrus granule cells following experimental cortical impact injury in rats: Possible role in spatial memory deficits. Brain Res. 1996, 739, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Grady, M.S.; Charleston, J.S.; Maris, D.; Witgen, B.M.; Lifshitz, J. Neuronal and Glial Cell Number in the Hippocampus after Experimental Traumatic Brain Injury: Analysis by Stereological Estimation. J. Neurotrauma 2003, 20, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Winston, C.N.; Chellappa, D.; Wilkins, T.; Barton, D.J.; Washington, P.M.; Loane, D.J.; Zapple, D.N.; Burns, M.P. Controlled cortical impact results in an extensive loss of dendritic spines that is not mediated by injury-induced amyloid-beta accumulation. J. Neurotrauma 2013, 30, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Becerra, A.P.; Logsdon, A.F.; Banks, W.A.; Ransom, C.B. Traumatic brain injury broadly affects GABaergic signaling in dentate gyrus granule cells. eNeuro 2021, 8, 1–15. [Google Scholar] [CrossRef]
- Baratz-Goldstein, R.; Toussia-Cohen, S.; Elpaz, A.; Rubovitch, V.; Pick, C.G. Immediate and delayed hyperbaric oxygen therapy as a neuroprotective treatment for traumatic brain injury in mice. Mol. Cell. Neurosci. 2017, 83, 74–82. [Google Scholar] [CrossRef]
- Kelly, K.M. Modeling traumatic brain injury and posttraumatic epilepsy. Epilepsy Curr. 2004, 4, 160–161. [Google Scholar] [CrossRef]
- Witgen, B.M.; Lifshitz, J.; Smith, M.L.; Schwarzbach, E.; Liang, S.L.; Grady, M.S.; Cohen, A.S. Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: A systems, network and cellular evaluation. Neuroscience 2005, 133, 1–15. [Google Scholar] [CrossRef]
- Markgraf, C.G.; Clifton, G.L.; Aguirre, M.; Chaney, S.F.; Bois, C.K.D.; Kennon, K.; Verma, N. Injury severity and sensitivity to treatment after controlled cortical impact in rats. J. Neurotrauma 2001, 18, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, H.L.; Capra, B.; Eidson, K.; Garcia, J.; Kennedy, D.; Uchida, T.; Parsley, M.; Cowart, J.; DeWitt, D.S.; Prough, D.S. Dose-dependent neuronal injury after traumatic brain injury. Brain Res. 2005, 1044, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Igarashi, T.; Ferriero, D.M.; Noble, L.J. Traumatic brain injury in the immature mouse brain: Characterization of regional vulnerability. Exp. Neurol. 2002, 176, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Ton, S.T.; Adamczyk, N.S.; Gerling, J.P.; Vaagenes, I.C.; Wu, J.Y.; Hsu, K.; O’Brien, T.E.; Tsai, S.Y.; Kartje, G.L. Dentate Gyrus Proliferative Responses After Traumatic Brain Injury and Binge Alcohol in Adult Rats. Neurosci. Insights 2020, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ngwenya, L.B.; Danzer, S.C. Impact of traumatic brain injury on neurogenesis. Front. Neurosci. 2019, 13, 1014. [Google Scholar] [CrossRef]
- Yu, T.S.; Zhang, G.; Liebl, D.J.; Kernie, S.G. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J. Neurosci. 2008, 28, 12901–12912. [Google Scholar] [CrossRef] [PubMed]
- Villasana, L.E.; Kim, K.N.; Westbrook, G.L.; Schnell, E. Functional integration of adult-born hippocampal neurons after traumatic brain injury. eNeuro 2015, 2, 1–17. [Google Scholar] [CrossRef]
- Namba, T.; Mochizuki, H.; Onodera, M.; Mizuno, Y.; Namiki, H.; Seki, T. The fate of neural progenitor cells expressing astrocytic and radial glial markers in the postnatal rat dentate gyrus. Eur. J. Neurosci. 2005, 22, 1928–1941. [Google Scholar] [CrossRef]
- Masuda, T.; Isobe, Y.; Aihara, N.; Furuyama, F.; Misumi, S.; Kim, T.S.; Nishino, H.; Hida, H. Increase in neurogenesis and neuroblast migration after a small intracerebral hemorrhage in rats. Neurosci. Lett. 2007, 425, 114–119. [Google Scholar] [CrossRef]
- Zheng, W.; Zhuge, Q.; Zhong, M.; Chen, G.; Shao, B.; Wang, H.; Mao, X.; Xie, L.; Jin, K. Neurogenesis in adult human brain after traumatic brain injury. J. Neurotrauma 2013, 30, 1872–1880. [Google Scholar] [CrossRef]
- Liu, Y.; Namba, T.; Liu, J.; Suzuki, R.; Shioda, S.; Seki, T. Glial fibrillary acidic protein-expressing neural progenitors give rise to immature neurons via early intermediate progenitors expressing both glial fibrillary acidic protein and neuronal markers in the adult hippocampus. Neuroscience 2010, 166, 241–251. [Google Scholar] [CrossRef]
- Wei, L.; Wang, J.; Cao, Y.; Ren, Q.; Zhao, L.; Li, X.; Wang, J. Hyperbaric oxygenation promotes neural stem cell proliferation and protects the learning and memory ability in neonatal hypoxic-ischemic brain damage. Int. J. Clin. Exp. Pathol. 2015, 8, 1752–1759. [Google Scholar]
- Brkic, P.; Mitrovic, A.; Rakic, M.; Grajic, M.; Jovanovic, T. Hyperbaric oxygen therapy of angiopathic changes in patients with inherited gene imbalance. Srpski Arhiv za Celokupno Lekarstvo 2007, 135, 669–671. [Google Scholar] [CrossRef]
- Puškaš, N.; Zaletel, I.; Stefanović, B.D.; Ristanović, D. Fractal dimension of apical dendritic arborization differs in the superficial and the deep pyramidal neurons of the rat cerebral neocortex. Neurosci. Lett. 2015, 589, 88–91. [Google Scholar] [CrossRef]
| C | SCA | SCA + HBO | p | |||
|---|---|---|---|---|---|---|
| SCA vs. C | SCA + HBO vs. C | SCA + HBO vs. SCA | ||||
| DTL | 261.08 ± 24.39 | 148.60 ± 21.25 | 211.51 ± 28.40 | <0.001 | <0.01 | <0.01 |
| ASL | 19.08 ± 1.70 | 25.41 ± 2.84 | 20.46 ± 3.67 | <0.01 | 0.69 | <0.05 |
| BP | 6.56 ± 0.96 | 2.57 ± 0.53 | 4.83 ± 0.35 | <0.001 | <0.01 | <0.001 |
| Antibody | Source | Dilution | Company |
|---|---|---|---|
| doublecortin | Goat | 1:200 | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
| TUJ1 | mouse | 1:400 | Abcam, Cambridge, MA, USA |
| NeuN | mouse | 1:200 | Milipore, Burlington, MA, USA |
| Ki67 | rabbit | 1:100 | Vector Laboratories, Burlingame, CA, USA |
| anti-goat HRP conjugated IgG | donkey | 1:200 | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
| anti-goat Alexa Fluor 488 | donkey | 1:200 | Invitrogen (Eugene, OR, USA) |
| anti-mouse Alexa Fluor 555 | donkey | 1:200 | Invitrogen (Eugene, OR, USA) |
| anti-rabbit Alexa Fluor 555 | donkey | 1:200 | Invitrogen (Eugene, OR, USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeremic, R.; Pekovic, S.; Lavrnja, I.; Bjelobaba, I.; Djelic, M.; Dacic, S.; Brkic, P. Hyperbaric Oxygenation Prevents Loss of Immature Neurons in the Adult Hippocampal Dentate Gyrus Following Brain Injury. Int. J. Mol. Sci. 2023, 24, 4261. https://doi.org/10.3390/ijms24054261
Jeremic R, Pekovic S, Lavrnja I, Bjelobaba I, Djelic M, Dacic S, Brkic P. Hyperbaric Oxygenation Prevents Loss of Immature Neurons in the Adult Hippocampal Dentate Gyrus Following Brain Injury. International Journal of Molecular Sciences. 2023; 24(5):4261. https://doi.org/10.3390/ijms24054261
Chicago/Turabian StyleJeremic, Rada, Sanja Pekovic, Irena Lavrnja, Ivana Bjelobaba, Marina Djelic, Sanja Dacic, and Predrag Brkic. 2023. "Hyperbaric Oxygenation Prevents Loss of Immature Neurons in the Adult Hippocampal Dentate Gyrus Following Brain Injury" International Journal of Molecular Sciences 24, no. 5: 4261. https://doi.org/10.3390/ijms24054261
APA StyleJeremic, R., Pekovic, S., Lavrnja, I., Bjelobaba, I., Djelic, M., Dacic, S., & Brkic, P. (2023). Hyperbaric Oxygenation Prevents Loss of Immature Neurons in the Adult Hippocampal Dentate Gyrus Following Brain Injury. International Journal of Molecular Sciences, 24(5), 4261. https://doi.org/10.3390/ijms24054261










