Biological Use of Nanostructured Silica-Based Materials Functionalized with Metallodrugs: The Spanish Perspective
Abstract
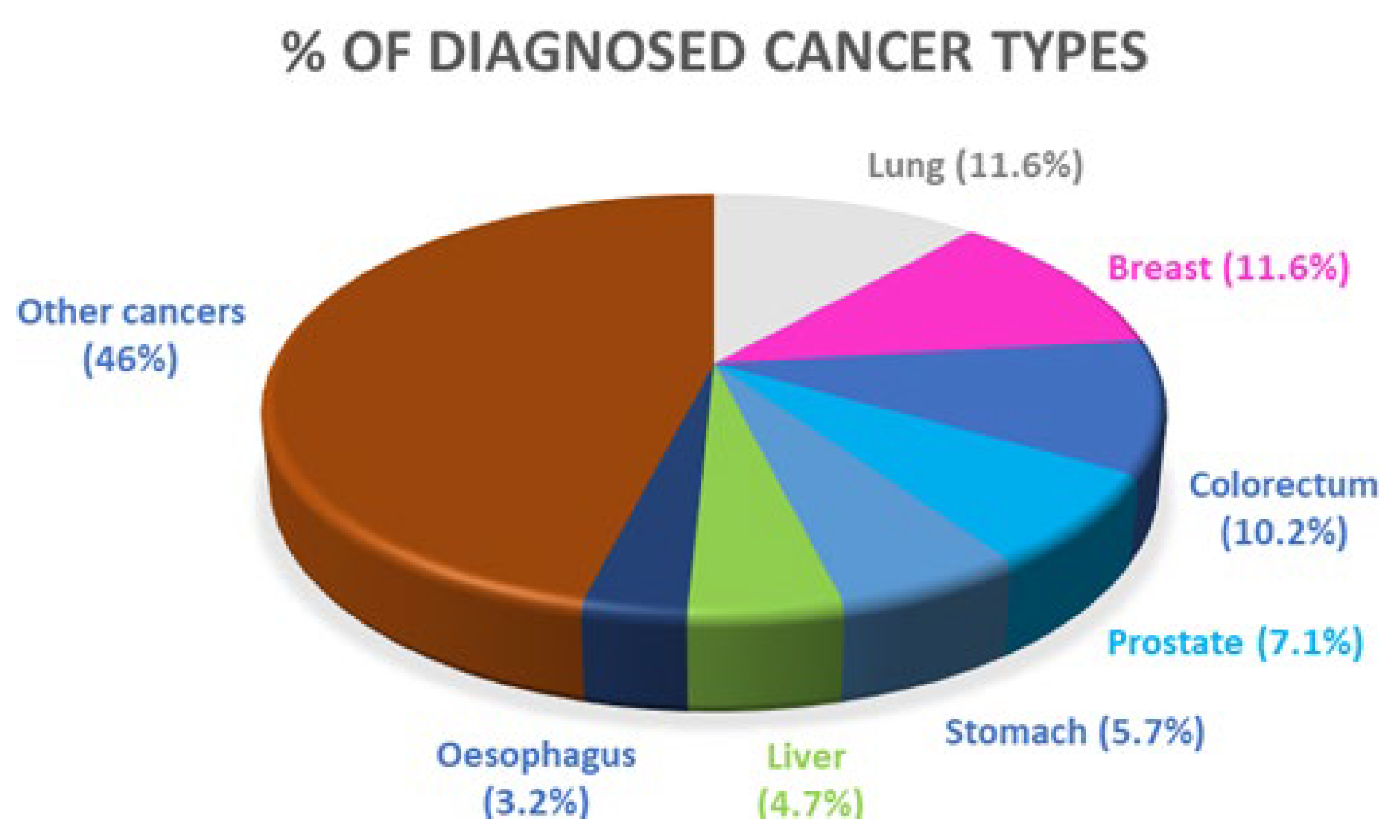
1. Introduction
2. Metallodrugs as Biological Agents
3. Application of Metallodrugs in Silica-Based Materials
3.1. Anticancer Therapy
3.1.1. Platinum Compounds
3.1.2. Titanium Compounds
3.1.3. Tin Compounds
3.1.4. Other Metals in Cancer
3.2. Antibacterial Activity
3.2.1. Silver
3.2.2. Copper
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, W.; Leung, S.S.; To, K.K. Updates on the Use of Liposomes for Active Tumor Targeting in Cancer Therapy. Nanomedicine 2019, 15, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. (Chezy) Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic: An Update. Bioeng. Transl. Med. 2019, 4, 10143. [Google Scholar] [CrossRef]
- Passero, F.C.; Grapsa, D.; Syrigos, K.N.; Saif, M.W. The Safety and Efficacy of Onivyde (Irinotecan Liposome Injection) for the Treatment of Metastatic Pancreatic Cancer Following Gemcitabine-Based Therapy. Expert Rev. Anticancer Ther. 2016, 16, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Krauss, A.C.; Gao, X.; Li, L.; Manning, M.L.; Patel, P.; Fu, W.; Janoria, K.G.; Gieser, G.; Bateman, D.A.; Przepiorka, D.; et al. FDA Approval Summary: (Daunorubicin and Cytarabine) Liposome for Injection for the Treatment of Adults with High-Risk Acute Myeloid Leukemia. Clin. Cancer Res. 2019, 25, 2685–2690. [Google Scholar] [CrossRef]
- Yuan-Yu, H. Approval of The First-Ever RNAi Therapeutics and Its Technological Development History. Prog. Biochem. Biophys. 2019, 46, 313–322. [Google Scholar] [CrossRef]
- Attwood, C. FDA Approve Abraxane for Pancreatic Cancer. Expert Rev. Clin. Pharmacol. 2013, 6, 602. [Google Scholar]
- Mioc, A.; Mioc, M.; Ghiulai, R.; Voicu, M.; Racoviceanu, R.; Trandafirescu, C.; Dehelean, C.; Coricovac, D.; Soica, C. Gold Nanoparticles as Targeted Delivery Systems and Theranostic Agents in Cancer Therapy. Curr. Med. Chem. 2019, 26, 6493–6513. [Google Scholar] [CrossRef]
- Yamada, M.; Foote, M.; Prow, T.W. Therapeutic Gold, Silver, and Platinum Nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 428–445. [Google Scholar] [CrossRef]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef]
- Sharma, A.; Goyal, A.K.; Rath, G. Recent Advances in Metal Nanoparticles in Cancer Therapy. J. Drug Target. 2018, 26, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, Y.; Wang, Y.; Jiang, H.; Wang, X. Advances and Challenges in Metallic Nanomaterial Synthesis and Antibacterial Applications. J. Mater. Chem. B 2020, 8, 4764–4777. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-K.; Kim, J.-C.; Park, H.-J.; Sul, O.-J.; Lee, M.-H.; Kim, J.-S.; Choi, H.-S. Platinum Nanoparticles Reduce Ovariectomy-Induced Bone Loss by Decreasing Osteoclastogenesis. Exp. Mol. Med. 2012, 44, 432. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, J.; Han, Q.; Tao, R.; Liu, F.; Wang, W. Toxicity and Bioactivity of Cobalt Nanoparticles on the Monocytes. Orthop. Surg. 2015, 7, 168–173. [Google Scholar] [CrossRef]
- Van Hengel, I.A.J.; Tierolf, M.W.A.M.; Valerio, V.P.M.; Minneboo, M.; Fluit, A.C.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Self-Defending Additively Manufactured Bone Implants Bearing Silver and Copper Nanoparticles. J. Mater. Chem. B 2020, 8, 1589–1602. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Sadler, P.J. Dicarba-Closo-Dodecarborane-Containing Half-Sandwich Complexes of Ruthenium, Osmium, Rhodium and Iridium: Biological Relevance and Synthetic Strategies. Chem. Soc. Rev. 2012, 41, 3264–3279. [Google Scholar] [CrossRef]
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef]
- Gurruchaga-Pereda, J.; Martínez, Á.; Terenzi, A.; Salassa, L. Anticancer Platinum Agents and Light. Inorg. Chim. Acta 2019, 495, 118981. [Google Scholar] [CrossRef]
- Rocamora-Reverte, L.; Carrasco-García, E.; Ceballos-Torres, J.; Prashar, S.; Kaluđerović, G.N.; Ferragut, J.A.; Gómez-Ruiz, S. Study of the Anticancer Properties of Tin(IV) Carboxylate Complexes on a Panel of Human Tumor Cell Lines. ChemMedChem 2012, 7, 301–310. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; Stanojković, T.P.; Kaluđerović, G.N. Synthesis, Characterization, Biological Studies and In Vitro Cytotoxicity on Human Cancer Cell Lines of Titanium(IV) and Tin(IV) Derivatives with the α,A′-Dimercapto-o-Xylene Ligand. Appl. Organomet. Chem. 2012, 26, 383–389. [Google Scholar] [CrossRef]
- Rodríguez-Prieto, T.; Michlewska, S.; Hołota, M.; Ionov, M.; de la Mata, F.J.; Cano, J.; Bryszewska, M.; Gómez, R. Organometallic Dendrimers Based on Ruthenium(II) N-Heterocyclic Carbenes and Their Implication as Delivery Systems of Anticancer Small Interfering RNA. J. Inorg. Biochem. 2021, 223, 111540. [Google Scholar] [CrossRef] [PubMed]
- Kaluđerović, M.R.; Gómez-Ruiz, S.; Gallego, B.; Hey-Hawkins, E.; Paschke, R.; Kaluđerović, G.N. Anticancer Activity of Dinuclear Gallium(III) Carboxylate Complexes. Eur. J. Med. Chem. 2010, 45, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Quero, J.; Ruighi, F.; Osada, J.; Gimeno, M.C.; Cerrada, E.; Rodriguez-Yoldi, M.J. Gold(I) Complexes Bearing Alkylated 1,3,5-Triaza-7-Phosphaadamantane Ligands as Thermoresponsive Anticancer Agents in Human Colon Cells. Biomedicines 2021, 9, 1848. [Google Scholar] [CrossRef]
- Gascón, E.; Maisanaba, S.; Otal, I.; Valero, E.; Repetto, G.; Jones, P.G.; Jiménez, J. (Amino)Cyclophosphazenes as Multisite Ligands for the Synthesis of Antitumoral and Antibacterial Silver(I) Complexes. Inorg. Chem. 2020, 59, 2464–2483. [Google Scholar] [CrossRef]
- Pires, A.S.; Batista, J.; Murtinho, D.; Nogueira, C.; Karamysheva, A.; Luísa Ramos, M.; Milne, B.F.; Tavares, N.T.; Gonçalves, J.; Gonçalves, A.C.; et al. Synthesis, Characterization and Evaluation of the Antibacterial and Antitumor Activity of HalogenatedSalen Copper (II) Complexes Derived from Camphoric Acid. Appl. Organomet. Chem. 2020, 34, e5569. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, L.-Y.; Mao, Z.-W.; Zou, T. Approaches towards Understanding the Mechanism-of-Action of Metallodrugs. Coord. Chem. Rev. 2022, 453, 214311. [Google Scholar] [CrossRef]
- Lei, Q.; Guo, J.; Noureddine, A.; Wang, A.; Wuttke, S.; Brinker, C.J.; Zhu, W. Sol–Gel-Based Advanced Porous Silica Materials for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 1909539. [Google Scholar] [CrossRef]
- Florensa, M.; Llenas, M.; Medina-Gutiérrez, E.; Sandoval, S.; Tobías-Rossell, G. Key Parameters for the Rational Design, Synthesis, and Functionalization of Biocompatible Mesoporous Silica Nanoparticles. Pharmaceutics 2022, 14, 2703. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef]
- Singh, P.; Srivastava, S.; Singh, S.K. Nanosilica: Recent Progress in Synthesis, Functionalization, Biocompatibility, and Biomedical Applications. ACS Biomater. Sci. Eng. 2019, 5, 4882–4898. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous Silica Nanoparticles for Drug and Gene Delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Iturrioz-Rodríguez, N.; Correa-Duarte, M.A.; Fanarraga, M.L. Controlled Drug Delivery Systems for Cancer Based on Mesoporous Silica Nanoparticles. Int. J. Nanomed. 2019, 14, 3389–3401. [Google Scholar] [CrossRef] [PubMed]
- Gulla, S.; Lomada, D.; Srikanth, V.V.S.S.; Shankar, M.V.; Reddy, K.R.; Soni, S.; Reddy, M.C. Chapter 11—Recent Advances in Nanoparticles-Based Strategies for Cancer Therapeutics and Antibacterial Applications. In Methods in Microbiology; Nanotechnology; Gurtler, V., Ball, A.S., Soni, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 46, pp. 255–293. [Google Scholar]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.A.; Prashar, S.; Shreaz, S.; Gómez-Ruiz, S. Nanostructured Materials Functionalized with Metal Complexes: In Search of Alternatives for Administering Anticancer Metallodrugs. Coord. Chem. Rev. 2016, 312, 67–98. [Google Scholar] [CrossRef]
- U.S. Department of Health & Human Services (HHS). Available online: https://www.hhs.gov/index.html (accessed on 26 December 2022).
- Ortega, E.; Vigueras, G.; Ballester, F.J.; Ruiz, J. Targeting Translation: A Promising Strategy for Anticancer Metallodrugs. Coord. Chem. Rev. 2021, 446, 214129. [Google Scholar] [CrossRef]
- Garg, A.D.; More, S.; Rufo, N.; Mece, O.; Sassano, M.L.; Agostinis, P.; Zitvogel, L.; Kroemer, G.; Galluzzi, L. Trial Watch: Immunogenic Cell Death Induction by Anticancer Chemotherapeutics. Oncoimmunology 2017, 6, 1386829. [Google Scholar] [CrossRef]
- Xiao, H.; Yan, L.; Dempsey, E.M.; Song, W.; Qi, R.; Li, W.; Huang, Y.; Jing, X.; Zhou, D.; Ding, J.; et al. Recent Progress in Polymer-Based Platinum Drug Delivery Systems. Prog. Polym. Sci. 2018, 87, 70–106. [Google Scholar] [CrossRef]
- Di Pasqua, A.J.; Wallner, S.; Kerwood, D.J.; Dabrowiak, J.C. Adsorption of the Pt(II) Anticancer Drug Carboplatin by Mesoporous Silica. Chem. Biodivers. 2009, 6, 1343–1349. [Google Scholar] [CrossRef]
- Tao, Z.; Toms, B.; Goodisman, J.; Asefa, T. Mesoporous Silica Microparticles Enhance the Cytotoxicity of Anticancer Platinum Drugs. ACS Nano 2010, 4, 789–794. [Google Scholar] [CrossRef] [PubMed]
- López, T.; Islas, E.O.; Alvarez, M.; González, R.D. Nanostructured Pt(NH3)4Cl2/SiO2 for Nanomedicine: Catalytic Degradation of DNA in Cancer Cells. Nano Rev. 2011, 2, 5461. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Cheng, S.-H.; Liao, W.-N.; Wei, P.-R.; Sung, P.-J.; Weng, C.-F.; Lee, C.-H. Mesoporous Silica Nanoparticles for the Improved Anticancer Efficacy of Cis-Platin. Int. J. Pharm. 2012, 429, 138–147. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Xiao, H.; Kuang, H.; Xie, Z.; Chen, X.; Jing, X.; Huang, Y. Synthesis of Mesoporous Silica Nanoparticle–Oxaliplatin Conjugates for Improved Anticancer Drug Delivery. Colloids Surf. B Biointerfaces 2014, 117, 75–81. [Google Scholar] [CrossRef]
- Li, H.; Yu, H.; Zhu, C.; Hu, J.; Du, M.; Zhang, F.; Yang, D. Cisplatin and Doxorubicin Dual-Loaded Mesoporous Silica Nanoparticles for Controlled Drug Delivery. RSC Adv. 2016, 6, 94160–94169. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, J.; Su, H.; Mu, G.; Sun, J.-H.; Tan, C.-P.; Liang, X.-J.; Ji, L.-N.; Mao, Z.-W. Co-Delivery of Cisplatin Prodrug and Chlorin E6 by Mesoporous Silica Nanoparticles for Chemo-Photodynamic Combination Therapy to Combat Drug Resistance. ACS Appl. Mater. Interfaces 2016, 8, 13332–13340. [Google Scholar] [CrossRef]
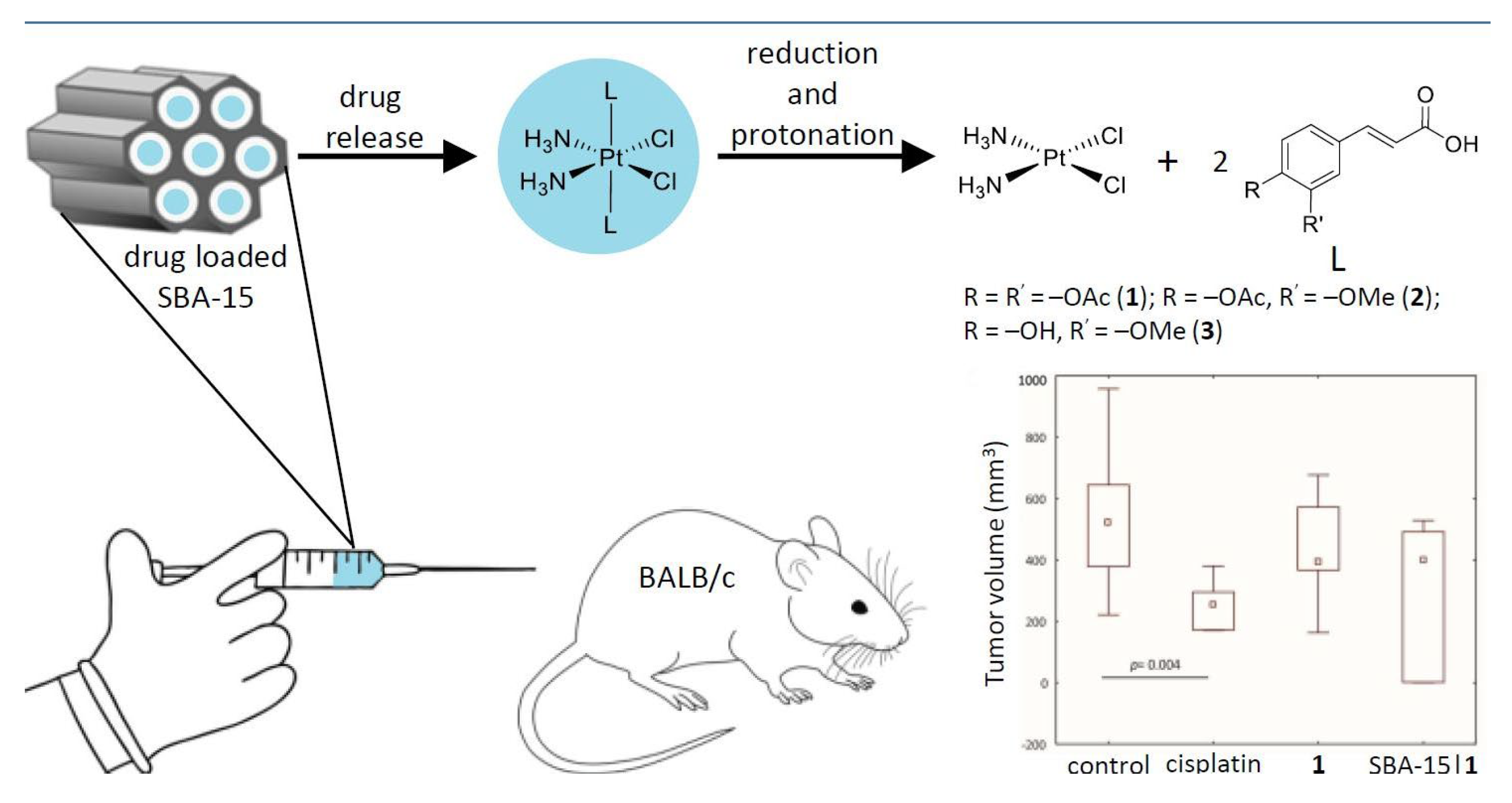
- Predarska, I.; Saoud, M.; Morgan, I.; Eichhorn, T.; Kaluđerović, G.N.; Hey-Hawkins, E. Cisplatin−cyclooxygenase Inhibitor Conjugates, Free and Immobilised in Mesoporous Silica SBA-15, Prove Highly Potent against Triple-Negative MDA-MB-468 Breast Cancer Cell Line. Dalton Trans. 2022, 51, 857–869. [Google Scholar] [CrossRef]
- Predarska, I.; Saoud, M.; Drača, D.; Morgan, I.; Komazec, T.; Eichhorn, T.; Mihajlović, E.; Dunđerović, D.; Mijatović, S.; Maksimović-Ivanić, D.; et al. Mesoporous Silica Nanoparticles Enhance the Anticancer Efficacy of Platinum(IV)-Phenolate Conjugates in Breast Cancer Cell Lines. Nanomaterials 2022, 12, 3767. [Google Scholar] [CrossRef]
- Del Hierro, I.; Pérez, Y.; Cruz, P.; Juárez, R. Pt and Ti Complexes Immobilized onto Mesoporous Silica Microspheres and Their Interaction with Molecules of Biological Interest. Eur. J. Inorg. Chem. 2017, 2017, 3030–3039. [Google Scholar] [CrossRef]
- Díez, P.; Lucena-Sánchez, E.; Escudero, A.; Llopis-Lorente, A.; Villalonga, R.; Martínez-Máñez, R. Ultrafast Directional Janus Pt–Mesoporous Silica Nanomotors for Smart Drug Delivery. ACS Nano 2021, 15, 4467–4480. [Google Scholar] [CrossRef]
- Köpf-Maier, P.; Köpf, H. Tumor Inhibition by Titanocene Complexes. Activity against B16 Melanoma and Colon 38 Carcinoma. Arzneimittelforschung 1987, 37, 532–534. [Google Scholar] [PubMed]
- O’Connor, K.; Gill, C.; Tacke, M.; Rehmann, F.-J.K.; Strohfeldt, K.; Sweeney, N.; Fitzpatrick, J.M.; Watson, R.W.G. Novel Titanocene Anti-Cancer Drugs and Their Effect on Apoptosis and the Apoptotic Pathway in Prostate Cancer Cells. Apoptosis 2006, 11, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Cini, M.; Bradshaw, T.D.; Woodward, S. Using Titanium Complexes to Defeat Cancer: The View from the Shoulders of Titans. Chem. Soc. Rev. 2017, 46, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Senthilnathan, D.; Vaideeswaran, S.; Venuvanalingam, P.; Frenking, G. Antitumor Activity of Bent Metallocenes: Electronic Structure Analysis Using DFT Computations. J. Mol. Model. 2011, 17, 465–475. [Google Scholar] [CrossRef]
- Koubkova, L.; Vyzula, R.; Karban, J.; Pinkas, J.; Ondrouskova, E.; Vojtesek, B.; Hrstka, R. Evaluation of Cytotoxic Activity of Titanocene Difluorides and Determination of Their Mechanism of Action in Ovarian Cancer Cells. Investig. New Drugs 2015, 33, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
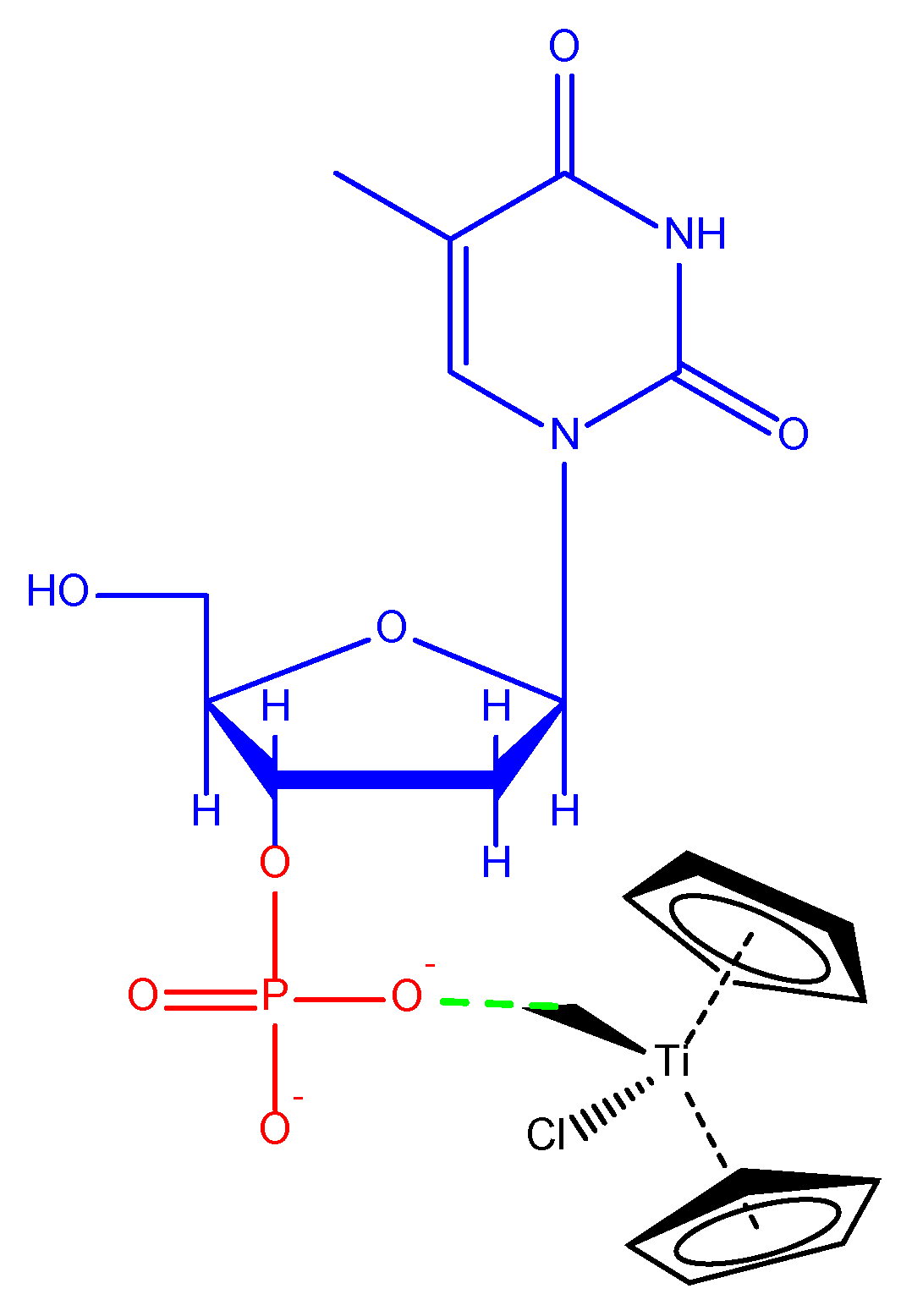
- Sun, H.; Li, H.; Weir, R.A.; Sadler, P.J. The First Specific TiIV–Protein Complex: Potential Relevance to Anticancer Activity of Titanocenes. Angew. Chem. Int. Ed. 1998, 37, 1577–1579. [Google Scholar] [CrossRef]
- Vincent, J.B.; Love, S. The Binding and Transport of Alternative Metals by Transferrin. Biochim. Biophys. Acta 2012, 1820, 362–378. [Google Scholar] [CrossRef]
- Eberle, R.P.; Schürch, S. Titanocene Binding to Oligonucleotides. J. Inorg. Biochem. 2018, 184, 1–7. [Google Scholar] [CrossRef]
- Christodoulou, C.V.; Eliopoulos, A.G.; Young, L.S.; Hodgkins, L.; Ferry, D.R.; Kerr, D.J. Anti-Proliferative Activity and Mechanism of Action of Titanocene Dichloride. Br. J. Cancer 1998, 77, 2088–2097. [Google Scholar] [CrossRef]
- Curado, N.; Giménez, N.; Miachin, K.; Aliaga-Lavrijsen, M.; Cornejo, M.A.; Jarzecki, A.A.; Contel, M. Preparation of Titanocene–Gold Compounds Based on Highly Active Gold(I)-N-Heterocyclic Carbene Anticancer Agents: Preliminary In Vitro Studies in Renal and Prostate Cancer Cell Lines. ChemMedChem 2019, 14, 1086–1095. [Google Scholar] [CrossRef]
- Fernández-Vega, L.; Ruiz Silva, V.A.; Domínguez-González, T.M.; Claudio-Betancourt, S.; Toro-Maldonado, R.E.; Capre Maso, L.C.; Sanabria Ortiz, K.; Pérez-Verdejo, J.A.; Román González, J.; Rosado-Fraticelli, G.T.; et al. Evaluating Ligand Modifications of the Titanocene and Auranofin Moieties for the Development of More Potent Anticancer Drugs. Inorganics 2020, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, L.; Olasunkanmi, L.O.; Fadare, O.A. De Novo Design of Thioredoxin Reductase-Targeted Heterometallic Titanocene–Gold Compounds of Chlorambucil for Mechanistic Insights into Renal Cancer. Chem. Commun. 2019, 56, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Schilling, T.; Keppler, K.B.; Heim, M.E.; Niebch, G.; Dietzfelbinger, H.; Rastetter, J.; Hanauske, A.-R. Clinical Phase I and Pharmacokinetic Trial of the New Titanium Complex Budotitane. Investig. New Drugs 1995, 13, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Valadares, M.C.; Klein, S.I.; Zyngier, S.; Queiroz, M.L. Growth and Differentiation of Bone Marrow Hematopoietic Cells in Mice Bearing Ehrlich Ascite Tumor and Treated with Dicyclopentadienildichlorotitanium (IV). Int. J. Immunopharmacol. 1998, 20, 573–581. [Google Scholar] [CrossRef]
- Pérez-Quintanilla, D.; Gómez-Ruiz, S.; Zizak, Z.; Sierra, I.; Prashar, S.; del Hierro, I.; Fajardo, M.; Juranić, Z.D.; Kaluderović, G.N. A New Generation of Anticancer Drugs: Mesoporous Materials Modified with Titanocene Complexes. Chemistry 2009, 15, 5588–5597. [Google Scholar] [CrossRef]
- Kaluđerović, G.N.; Pérez-Quintanilla, D.; Sierra, I.; Prashar, S.; del Hierro, I.; Žižak, Ž.; Juranić, Z.D.; Fajardo, M.; Gómez-Ruiz, S. Study of the Influence of the Metal Complex on the Cytotoxic Activity of Titanocene-Functionalized Mesoporous Materials. J. Mater. Chem. 2010, 20, 806–814. [Google Scholar] [CrossRef]
- García-Peñas, A.; Gómez-Ruiz, S.; Pérez-Quintanilla, D.; Paschke, R.; Sierra, I.; Prashar, S.; del Hierro, I.; Kaluđerović, G.N. Study of the Cytotoxicity and Particle Action in Human Cancer Cells of Titanocene-Functionalized Materials with Potential Application against Tumors. J. Inorg. Biochem. 2012, 106, 100–110. [Google Scholar] [CrossRef]
- Ceballos-Torres, J.; Virag, P.; Cenariu, M.; Prashar, S.; Fajardo, M.; Fischer-Fodor, E.; Gómez-Ruiz, S. Anti-Cancer Applications of Titanocene-Functionalised Nanostructured Systems: An Insight into Cell Death Mechanisms. Chem.—A Eur. J. 2014, 20, 10811–10828. [Google Scholar] [CrossRef]
- Kaluđerović, G.N.; Pérez-Quintanilla, D.; Žižak, Ž.; Juranić, Z.D.; Gómez-Ruiz, S. Improvement of Cytotoxicity of Titanocene-Functionalized Mesoporous Materials by the Increase of the Titanium Content. Dalton Trans. 2010, 39, 2597–2608. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; García-Peñas, A.; Prashar, S.; Rodríguez-Diéguez, A.; Fischer-Fodor, E. Anticancer Applications of Nanostructured Silica-Based Materials Functionalized with Titanocene Derivatives: Induction of Cell Death Mechanism through TNFR1 Modulation. Materials 2018, 11, 224. [Google Scholar] [CrossRef]
- Díaz-García, D.; Cenariu, D.; Pérez, Y.; Cruz, P.; del Hierro, I.; Prashar, S.; Fischer-Fodor, E.; Gómez-Ruiz, S. Modulation of the Mechanism of Apoptosis in Cancer Cell Lines by Treatment with Silica-Based Nanostructured Materials Functionalized with Different Metallodrugs. Dalton Trans. 2018, 47, 12284–12299. [Google Scholar] [CrossRef] [PubMed]
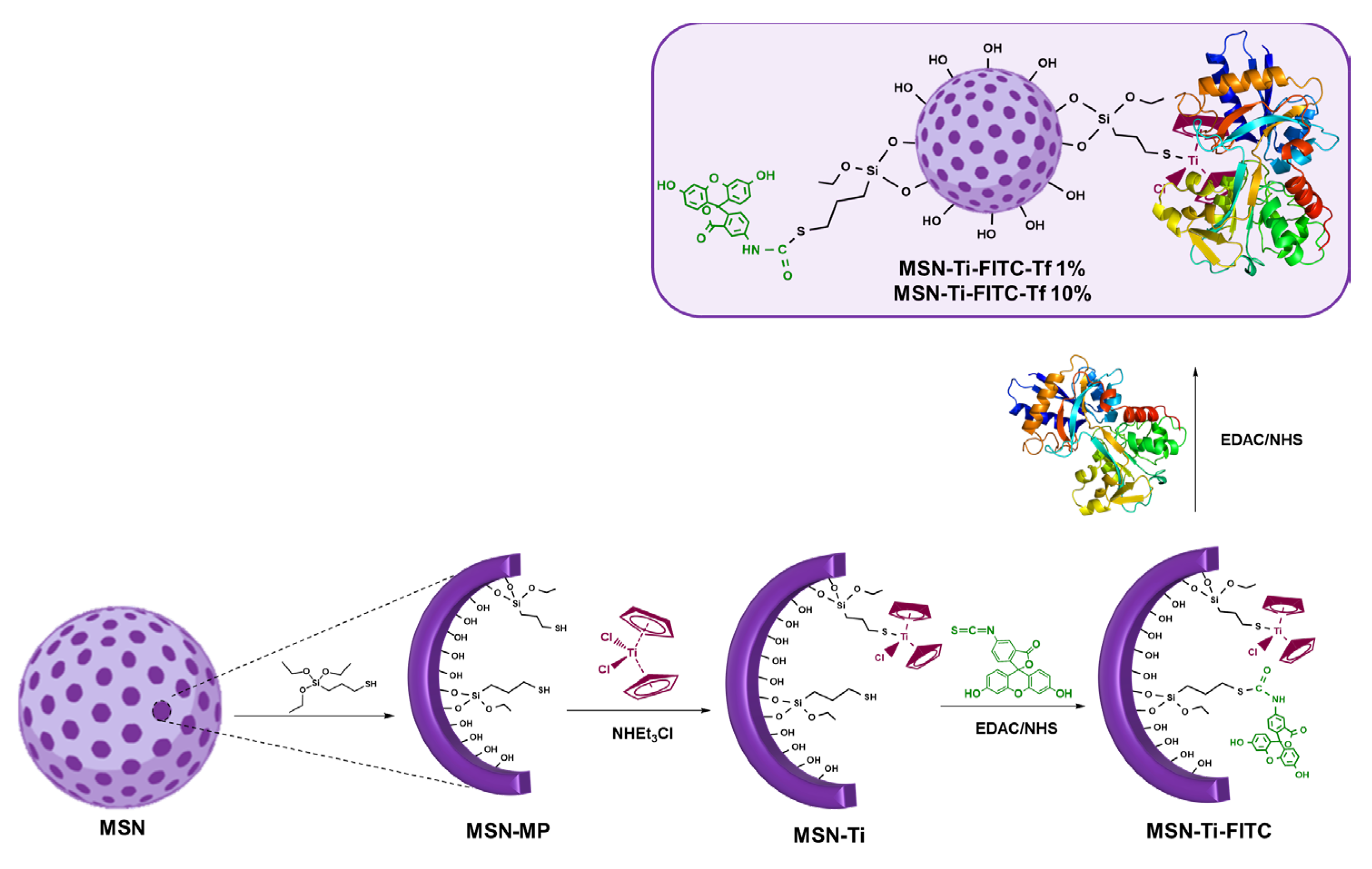
- Díaz-García, D.; Fischer-Fodor, E.; Vlad, C.I.; Méndez-Arriaga, J.M.; Prashar, S.; Gómez-Ruiz, S. Study of Cancer Cell Cytotoxicity, Internalization and Modulation of Growth Factors Induced by Transferrin-Conjugated Formulations of Metallodrug-Functionalized Mesoporous Silica Nanoparticles. Microporous Mesoporous Mater. 2021, 323, 111238. [Google Scholar] [CrossRef]
- Crowe, A.J.; Smith, P.J.; Atassi, G. Investigations into the Antitumour Activity of Organotin Compounds. I. Diorganotin Dihalide and Di-Pseudohalide Complexes. Chem.-Biol. Interact. 1980, 32, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Esmail, S.A.A.; Shamsi, M.; Chen, T.; Al-Asbahy, W.M. Design, Synthesis and Characterization of Tin-Based Cancer Chemotherapy Drug Entity: In Vitro DNA Binding, Cleavage, Induction of Cancer Cell Apoptosis by Triggering DNA Damage-Mediated P53 Phosphorylation and Molecular Docking. Appl. Organomet. Chem. 2019, 33, e4651. [Google Scholar] [CrossRef]
- Khan, M.I.; Baloch, M.K.; Ashfaq, M.; Obaidullah. Synthesis, characterization and in vitro cytotoxic effects of new organotin(IV)-2-maleimidopropanoates. Appl. Organomet. Chem. 2006, 20, 463–470. [Google Scholar] [CrossRef]
- Pellerito, L.; Nagy, L. Organotin(IV)N+ Complexes Formed with Biologically Active Ligands: Equilibrium and Structural Studies, and Some Biological Aspects. Coord. Chem. Rev. 2002, 224, 111–150. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; Kaluđerović, G.N.; Prashar, S.; Hey-Hawkins, E.; Erić, A.; Žižak, Ž.; Juranić, Z.D. Study of the Cytotoxic Activity of Di and Triphenyltin(IV) Carboxylate Complexes. J. Inorg. Biochem. 2008, 102, 2087–2096. [Google Scholar] [CrossRef]
- Kaluđerović, G.N.; Kommera, H.; Hey-Hawkins, E.; Paschke, R.; Gómez-Ruiz, S. Synthesis and Biological Applications of Ionic Triphenyltin(IV) Chloride Carboxylate Complexes with Exceptionally High Cytotoxicity. Metallomics 2010, 2, 419–428. [Google Scholar] [CrossRef]
- Vafaee, M.; Amini, M.M.; Najafi, E.; Sadeghi, O.; Amani, V. Modified Nanoporous Silicas for Oral Delivery of the Water Insoluble Organotin Compound: Loading and Release of Methylphenyltin Dichloride as an Anti-Tumor Drug Model. J. Sol-Gel Sci. Technol. 2012, 64, 411–417. [Google Scholar] [CrossRef]
- Bensing, C.; Mojić, M.; Gómez-Ruiz, S.; Carralero, S.; Dojčinović, B.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. Evaluation of Functionalized Mesoporous Silica SBA-15 as a Carrier System for Ph3Sn(CH2)3OH against the A2780 Ovarian Carcinoma Cell Line. Dalton Trans. 2016, 45, 18984–18993. [Google Scholar] [CrossRef]
- Maksimović-Ivanić, D.; Bulatović, M.; Edeler, D.; Bensing, C.; Golić, I.; Korać, A.; Kaluđerović, G.N.; Mijatović, S. The Interaction between SBA-15 Derivative Loaded with Ph3Sn(CH2)6OH and Human Melanoma A375 Cell Line: Uptake and Stem Phenotype Loss. J. Biol. Inorg. Chem. 2019, 24, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Edeler, D.; Drača, D.; Petković, V.; Natalio, F.; Maksimović-Ivanić, D.; Mijatović, S.; Schmidt, H.; Kaluđerović, G.N. Impact of the Mesoporous Silica SBA-15 Functionalization on the Mode of Action of Ph3Sn(CH2)6OH. Mater. Sci. Eng. C 2019, 100, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Bulatović, M.Z.; Maksimović-Ivanić, D.; Bensing, C.; Gómez-Ruiz, S.; Steinborn, D.; Schmidt, H.; Mojić, M.; Korać, A.; Golić, I.; Pérez-Quintanilla, D.; et al. Organotin(IV)-Loaded Mesoporous Silica as a Biocompatible Strategy in Cancer Treatment. Angew. Chem. Int. Ed. Engl. 2014, 53, 5982–5987. [Google Scholar] [CrossRef] [PubMed]
- Bensing, C.; Mojić, M.; Bulatović, M.; Edeler, D.; Pérez-Quintanilla, D.; Gómez-Ruiz, S.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. Effect of Chain Length on the Cytotoxic Activity of (Alkyl-ω-Ol)Triphenyltin(IV) Loaded into SBA-15 Nanostructured Silica and In Vivo Study of SBA-15~Cl|Ph3Sn(CH2)8OH. Biomater. Adv. 2022, 140, 213054. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, D.; Sommerova, L.; Martisova, A.; Skoupilova, H.; Prashar, S.; Vaculovic, T.; Kanicky, V.; del Hierro, I.; Hrstka, R.; Gómez-Ruiz, S. Mesoporous Silica Nanoparticles Functionalized with a Dialkoxide Diorganotin(IV) Compound: In Search of More Selective Systems against Cancer Cells. Microporous Mesoporous Mater. 2020, 300, 110154. [Google Scholar] [CrossRef]
- Díaz-García, D.; Montalbán-Hernández, K.; Mena-Palomo, I.; Achimas-Cadariu, P.; Rodríguez-Diéguez, A.; López-Collazo, E.; Prashar, S.; Ovejero Paredes, K.; Filice, M.; Fischer-Fodor, E.; et al. Role of Folic Acid in the Therapeutic Action of Nanostructured Porous Silica Functionalized with Organotin(IV) Compounds against Different Cancer Cell Lines. Pharmaceutics 2020, 12, 512. [Google Scholar] [CrossRef]
- Choudante, P.C.; Nethi, S.K.; Díaz-García, D.; Prashar, S.; Misra, S.; Gómez-Ruiz, S.; Patra, C.R. Tin-Loaded Mesoporous Silica Nanoparticles: Antineoplastic Properties and Genotoxicity Assessment. Biomater. Adv. 2022, 137, 212819. [Google Scholar] [CrossRef]
- Ovejero Paredes, K.; Díaz-García, D.; García-Almodóvar, V.; Lozano Chamizo, L.; Marciello, M.; Díaz-Sánchez, M.; Prashar, S.; Gómez-Ruiz, S.; Filice, M. Multifunctional Silica-Based Nanoparticles with Controlled Release of Organotin Metallodrug for Targeted Theranosis of Breast Cancer. Cancers 2020, 12, 187. [Google Scholar] [CrossRef]
- Ovejero-Paredes, K.; Díaz-García, D.; Mena-Palomo, I.; Marciello, M.; Lozano-Chamizo, L.; Morato, Y.L.; Prashar, S.; Gómez-Ruiz, S.; Filice, M. Synthesis of a Theranostic Platform Based on Fibrous Silica Nanoparticles for the Enhanced Treatment of Triple-Negative Breast Cancer Promoted by a Combination of Chemotherapeutic Agents. Biomater. Adv. 2022, 137, 212823. [Google Scholar] [CrossRef]
- Hanif, M.; Hartinger, C.G. Anticancer Metallodrugs: Where Is the next Cisplatin? Future Med. Chem. 2018, 10, 615–617. [Google Scholar] [CrossRef]
- Levina, A.; Chetcuti, A.R.M.; Lay, P.A. Controversial Role of Transferrin in the Transport of Ruthenium Anticancer Drugs. Biomolecules 2022, 12, 1319. [Google Scholar] [CrossRef]
- Meier-Menches, S.M.; Gerner, C.; Berger, W.; Hartinger, C.G.; Keppler, B.K. Structure–Activity Relationships for Ruthenium and Osmium Anticancer Agents—Towards Clinical Development. Chem. Soc. Rev. 2018, 47, 909–928. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Wang, Z.; Zhang, P.; Yin, C.; Wang, J.; Sun, Y.; Chen, Y.; Wang, W.; Sun, B.; Fan, C. Ruthenium-Loaded Mesoporous Silica as Tumor Microenvironment-Response Nano-Fenton Reactors for Precise Cancer Therapy. J. Nanobiotechnol. 2021, 19, 98. [Google Scholar] [CrossRef]
- Mladenović, M.; Morgan, I.; Ilić, N.; Saoud, M.; Pergal, M.V.; Kaluđerović, G.N.; Knežević, N.Ž. PH-Responsive Release of Ruthenium Metallotherapeutics from Mesoporous Silica-Based Nanocarriers. Pharmaceutics 2021, 13, 460. [Google Scholar] [CrossRef]
- Rojas, S.; Carmona, F.J.; Barea, E.; Maldonado, C.R. Inorganic Mesoporous Silicas as Vehicles of Two Novel Anthracene-Based Ruthenium Metalloarenes. J. Inorg. Biochem. 2017, 166, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ellahioui, Y.; Patra, M.; Mari, C.; Kaabi, R.; Karges, J.; Gasser, G.; Gómez-Ruiz, S. Mesoporous Silica Nanoparticles Functionalised with a Photoactive Ruthenium(II) Complex: Exploring the Formulation of a Metal-Based Photodynamic Therapy Photosensitiser. Dalton Trans. 2019, 48, 5940–5951. [Google Scholar] [CrossRef] [PubMed]
- Karges, J.; Díaz-García, D.; Prashar, S.; Gómez-Ruiz, S.; Gasser, G. Ru(II) Polypyridine Complex-Functionalized Mesoporous Silica Nanoparticles as Photosensitizers for Cancer Targeted Photodynamic Therapy. ACS Appl. Bio Mater. 2021, 4, 4394–4405. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Ho, Q.P.; Morand, J.; García, A.; Ortega, E.; Erthal, L.C.S.; Ruiz-Hernandez, E.; Santana, M.D.; Ruiz, J.; Vallet-Regí, M.; et al. Amino-Functionalized Mesoporous Silica Nanoparticle-Encapsulated Octahedral Organoruthenium Complex as an Efficient Platform for Combatting Cancer. Inorg. Chem. 2020, 59, 10275–10284. [Google Scholar] [CrossRef]
- Chitambar, C.R. Gallium and Its Competing Roles with Iron in Biological Systems. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2016, 1863, 2044–2053. [Google Scholar] [CrossRef]
- Ellahioui, Y.; Prashar, S.; Gómez-Ruiz, S. Anticancer Applications and Recent Investigations of Metallodrugs Based on Gallium, Tin and Titanium. Inorganics 2017, 5, 4. [Google Scholar] [CrossRef]
- Mohan Viswanathan, T.; Krishnakumar, V.; Senthilkumar, D.; Chitradevi, K.; Vijayabhaskar, R.; Rajesh Kannan, V.; Senthil Kumar, N.; Sundar, K.; Kunjiappan, S.; Babkiewicz, E.; et al. Combinatorial Delivery of Gallium (III) Nitrate and Curcumin Complex-Loaded Hollow Mesoporous Silica Nanoparticles for Breast Cancer Treatment. Nanomaterials 2022, 12, 1472. [Google Scholar] [CrossRef] [PubMed]
- Malekmohammadi, S.; Hadadzadeh, H.; Amirghofran, Z. Preparation of Folic Acid-Conjugated Dendritic Mesoporous Silica Nanoparticles for PH-Controlled Release and Targeted Delivery of a Cyclometallated Gold(III) Complex as an Antitumor Agent. J. Mol. Liq. 2018, 265, 797–806. [Google Scholar] [CrossRef]
- Sargazi, S.; Laraib, U.; Er, S.; Rahdar, A.; Hassanisaadi, M.; Zafar, M.N.; Díez-Pascual, A.M.; Bilal, M. Application of Green Gold Nanoparticles in Cancer Therapy and Diagnosis. Nanomaterials 2022, 12, 1102. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.N.; Alina, T.B.; Curry, S.D.; Ganguly, S.; Cha, J.N.; Goodwin, A.P. Silica-Coated Gold Nanorods with Hydrophobic Modification Show Both Enhanced Two-Photon Fluorescence and Ultrasound Drug Release. J. Mater. Chem. B 2022, 10, 9789–9793. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhao, Y.; Sun, L.; Zou, X. Fabrication of Mesoporous Silica-Covered Gold Nanostars for Chemophototherapy. J. Ind. Eng. Chem. 2022, 114, 115–125. [Google Scholar] [CrossRef]
- Hernández Montoto, A.; Montes, R.; Samadi, A.; Gorbe, M.; Terrés, J.M.; Cao-Milán, R.; Aznar, E.; Ibañez, J.; Masot, R.; Marcos, M.D.; et al. Gold Nanostars Coated with Mesoporous Silica Are Effective and Nontoxic Photothermal Agents Capable of Gate Keeping and Laser-Induced Drug Release. ACS Appl. Mater. Interfaces 2018, 10, 27644–27656. [Google Scholar] [CrossRef] [PubMed]
- Hernández Montoto, A.; Llopis-Lorente, A.; Gorbe, M.; Terrés, J.M.; Cao-Milán, R.; Díaz de Greñu, B.; Alfonso, M.; Ibañez, J.; Marcos, M.D.; Orzáez, M.; et al. Janus Gold Nanostars–Mesoporous Silica Nanoparticles for NIR-Light-Triggered Drug Delivery. Chem.—A Eur. J. 2019, 25, 8471–8478. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an Antimicrobial Agent: Recent Advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef]
- Barras, F.; Aussel, L.; Ezraty, B. Silver and Antibiotic, New Facts to an Old Story. Antibiotics 2018, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Yu, Y.; Lu, R. Mesoporous Silica Nanoparticles as Carrier to Overcome Bacterial Drug Resistant Barriers. Int. J. Pharm. 2022, 631, 122529. [Google Scholar] [CrossRef] [PubMed]
- Mateo, E.M.; Jiménez, M. Silver Nanoparticle-Based Therapy: Can It Be Useful to Combat Multi-Drug Resistant Bacteria? Antibiotics 2022, 11, 1205. [Google Scholar] [CrossRef]
- Flores, J.C.; Torres, V.; Popa, M.; Crespo, D.; Calderón-Moreno, J.M. Preparation of Core–Shell Nanospheres of Silica–Silver: SiO2@Ag. J. Non-Cryst. Solids 2008, 354, 5435–5439. [Google Scholar] [CrossRef]
- Misran, H.; Salim, M.A.; Ramesh, S. Effect of Ag Nanoparticles Seeding on the Properties of Silica Spheres. Ceram. Int. 2018, 44, 5901–5908. [Google Scholar] [CrossRef]
- Pavoski, G.; Kalikoski, R.; Souza, G.; Brum, L.F.W.; dos Santos, C.; Abo Markeb, A.; dos Santos, J.H.Z.; Font, X.; dell’Erba, I.; Galland, G.B. Synthesis of Polyethylene/Silica-Silver Nanocomposites with Antibacterial Properties by in Situ Polymerization. Eur. Polym. J. 2018, 106, 92–101. [Google Scholar] [CrossRef]
- Montalvo-Quirós, S.; Gómez-Graña, S.; Vallet-Regí, M.; Prados-Rosales, R.C.; González, B.; Luque-Garcia, J.L. Mesoporous Silica Nanoparticles Containing Silver as Novel Antimycobacterial Agents against Mycobacterium Tuberculosis. Colloids Surf. B Biointerfaces 2021, 197, 111405. [Google Scholar] [CrossRef]
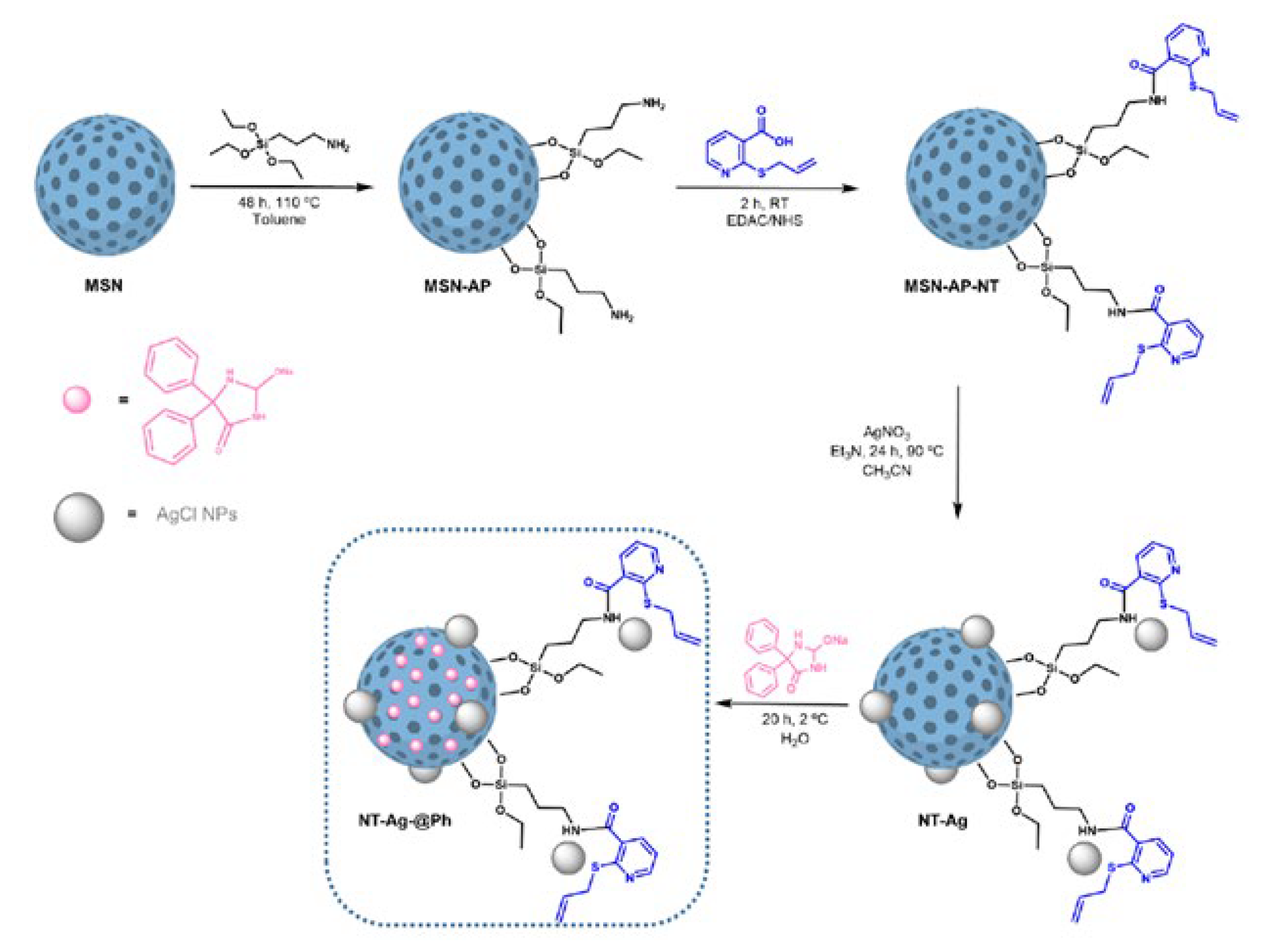
- Ugalde-Arbizu, M.; Aguilera-Correa, J.J.; Mediero, A.; Esteban, J.; Páez, P.L.; San Sebastian, E.; Gómez-Ruiz, S. Hybrid Nanosystems Based on Nicotinate-Functionalized Mesoporous Silica and Silver Chloride Nanoparticles Loaded with Phenytoin for Preventing Pseudomonas Aeruginosa Biofilm Development. Pharmaceuticals 2022, 15, 884. [Google Scholar] [CrossRef]
- Kuthati, Y.; Kankala, R.K.; Lin, S.-X.; Weng, C.-F.; Lee, C.-H. PH-Triggered Controllable Release of Silver–Indole-3 Acetic Acid Complexes from Mesoporous Silica Nanoparticles (IBN-4) for Effectively Killing Malignant Bacteria. Mol. Pharm. 2015, 12, 2289–2304. [Google Scholar] [CrossRef]
- Dennison, C. The Coordination Chemistry of Copper Uptake and Storage for Methane Oxidation. Chem. Eur. J. 2019, 25, 74–86. [Google Scholar] [CrossRef]
- Giachino, A.; Waldron, K.J. Copper Tolerance in Bacteria Requires the Activation of Multiple Accessory Pathways. Mol. Microbiol. 2020, 144, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.; Pazos, M.; Edoo, Z.; Hugonnet, J.-E.; Martorana, A.M.; Polissi, A.; VanNieuwenhze, M.S.; Arthur, M.; Vollmer, W. Copper Inhibits Peptidoglycan LD-Transpeptidases Suppressing β-Lactam Resistance Due to Bypass of Penicillin-Binding Proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10786–10791. [Google Scholar] [CrossRef] [PubMed]
- Macomber, L.; Rensing, C.; Imlay, J.A. Intracellular Copper Does Not Catalyze the Formation of Oxidative DNA Damage in Escherichia Coli. J. Bacteriol. 2007, 189, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Palza, H.; Delgado, K.; Curotto, N. Synthesis of Copper Nanostructures on Silica-Based Particles for Antimicrobial Organic Coatings. Appl. Surf. Sci. 2015, 357, 86–90. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, T.; Ding, G.; Punyapitak, D.; Zhu, J.; Guo, D.; Zhang, Z.; Li, J.; Cao, Y. Nanosilica Schiff-Base Copper(II) Complexes with Sustainable Antimicrobial Activity against Bacteria and Reduced Risk of Harm to Plant and Environment. ACS Sustain. Chem. Eng. 2017, 5, 502–509. [Google Scholar] [CrossRef]
- Tahmasbi, L.; Sedaghat, T.; Motamedi, H.; Kooti, M. Mesoporous Silica Nanoparticles Supported Copper(II) and Nickel(II) Schiff Base Complexes: Synthesis, Characterization, Antibacterial Activity and Enzyme Immobilization. J. Solid State Chem. 2018, 258, 517–525. [Google Scholar] [CrossRef]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. “Ghost” Silica Nanoparticles of “Host”-Inherited Antibacterial Action. ACS Appl. Mater. Interfaces 2019, 11, 38519–38530. [Google Scholar] [CrossRef]
- Díaz-García, D.; Ardiles, P.R.; Prashar, S.; Rodríguez-Diéguez, A.; Páez, P.L.; Gómez-Ruiz, S. Preparation and Study of the Antibacterial Applications and Oxidative Stress Induction of Copper Maleamate-Functionalized Mesoporous Silica Nanoparticles. Pharmaceutics 2019, 11, 30. [Google Scholar] [CrossRef]
- Díaz-García, D.; Ardiles, P.R.; Díaz-Sánchez, M.; Mena-Palomo, I.; Del Hierro, I.; Prashar, S.; Rodríguez-Diéguez, A.; Páez, P.L.; Gómez-Ruiz, S. Copper-Functionalized Nanostructured Silica-Based Systems: Study of the Antimicrobial Applications and ROS Generation against Gram Positive and Gram Negative Bacteria. J. Inorg. Biochem. 2020, 203, 110912. [Google Scholar] [CrossRef]
- Videira-Quintela, D.; Guillén, F.; Montalvo, G.; Martin, O. Silver, Copper, and Copper Hydroxy Salt Decorated Fumed Silica Hybrid Composites as Antibacterial Agents. Colloids Surf. B Biointerfaces 2020, 195, 111216. [Google Scholar] [CrossRef]
- Díaz-García, D.; Ferrer-Donato, Á.; Méndez-Arriaga, J.M.; Cabrera-Pinto, M.; Díaz-Sánchez, M.; Prashar, S.; Fernandez-Martos, C.M.; Gómez-Ruiz, S. Design of Mesoporous Silica Nanoparticles for the Treatment of Amyotrophic Lateral Sclerosis (ALS) with a Therapeutic Cocktail Based on Leptin and Pioglitazone. ACS Biomater. Sci. Eng. 2022, 8, 4838–4849. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Webster, T.J. Mesoporous Silica Based Nanostructures for Bone Tissue Regeneration. Front. Mater. 2021, 8, 692309. [Google Scholar] [CrossRef]
- Eren, T.; Baysal, G.; Doğan, F. Biocidal Activity of Bone Cements Containing Curcumin and Pegylated Quaternary Polyethylenimine. J. Polym. Environ. 2020, 28, 2469–2480. [Google Scholar] [CrossRef]




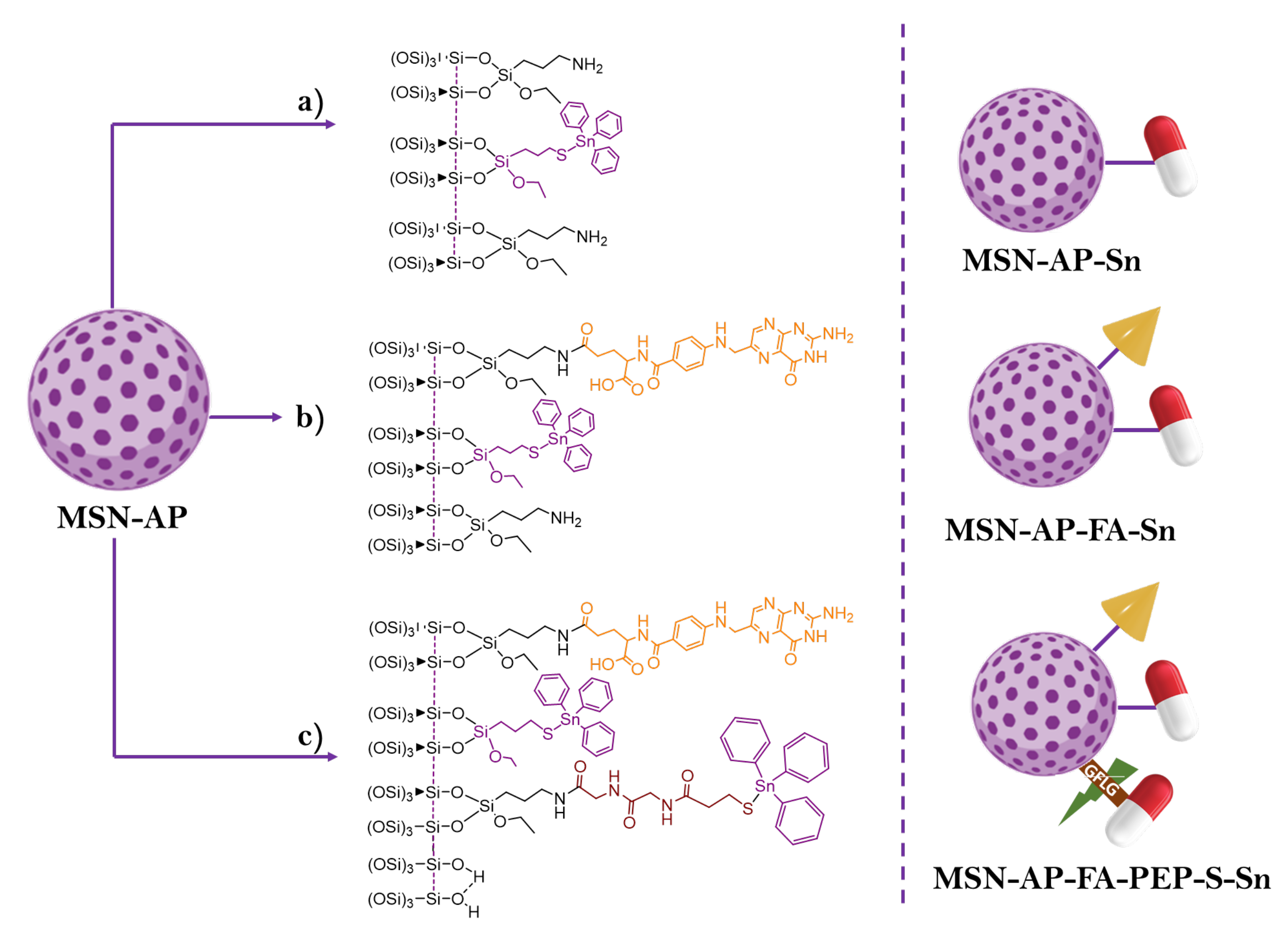
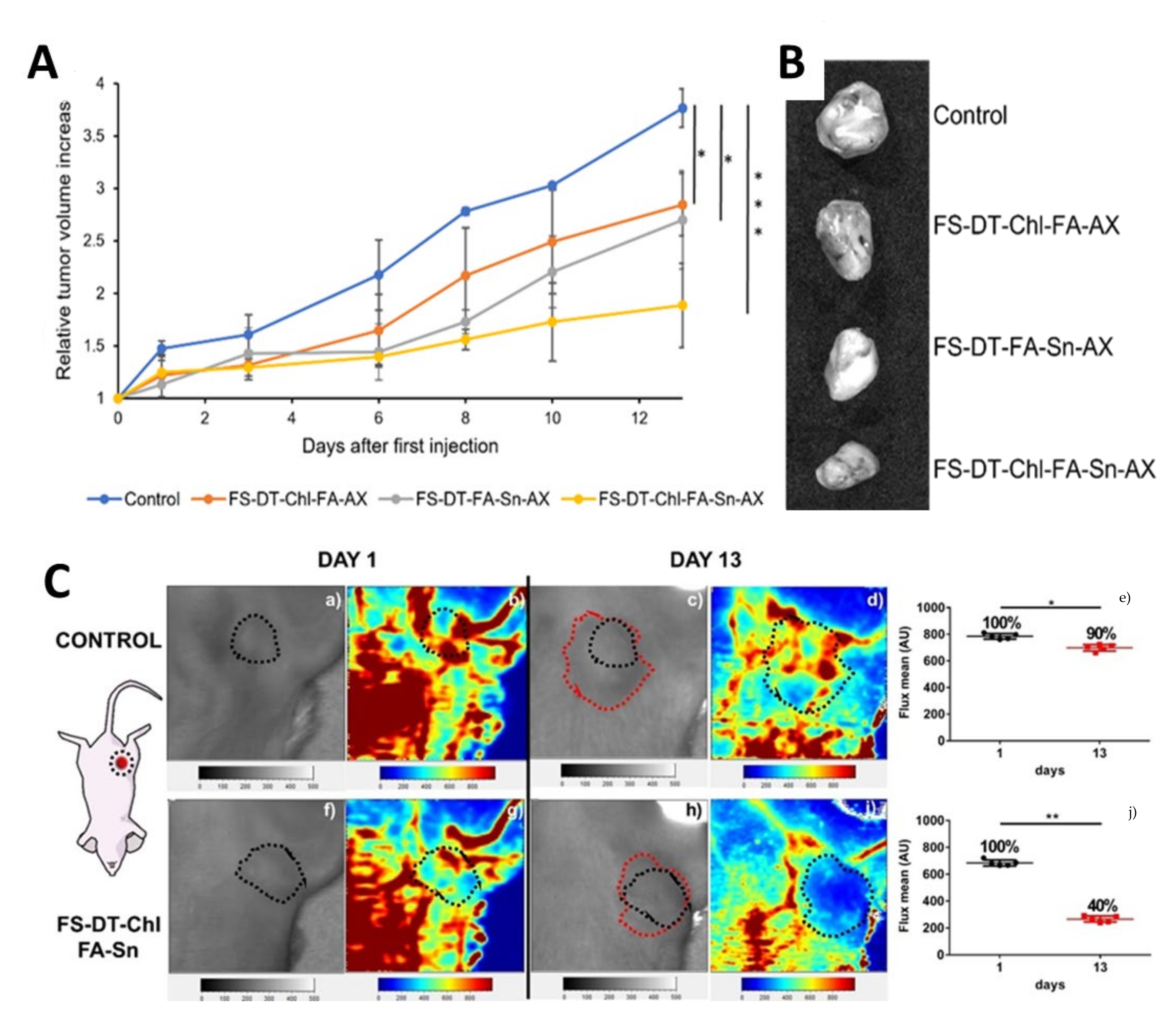
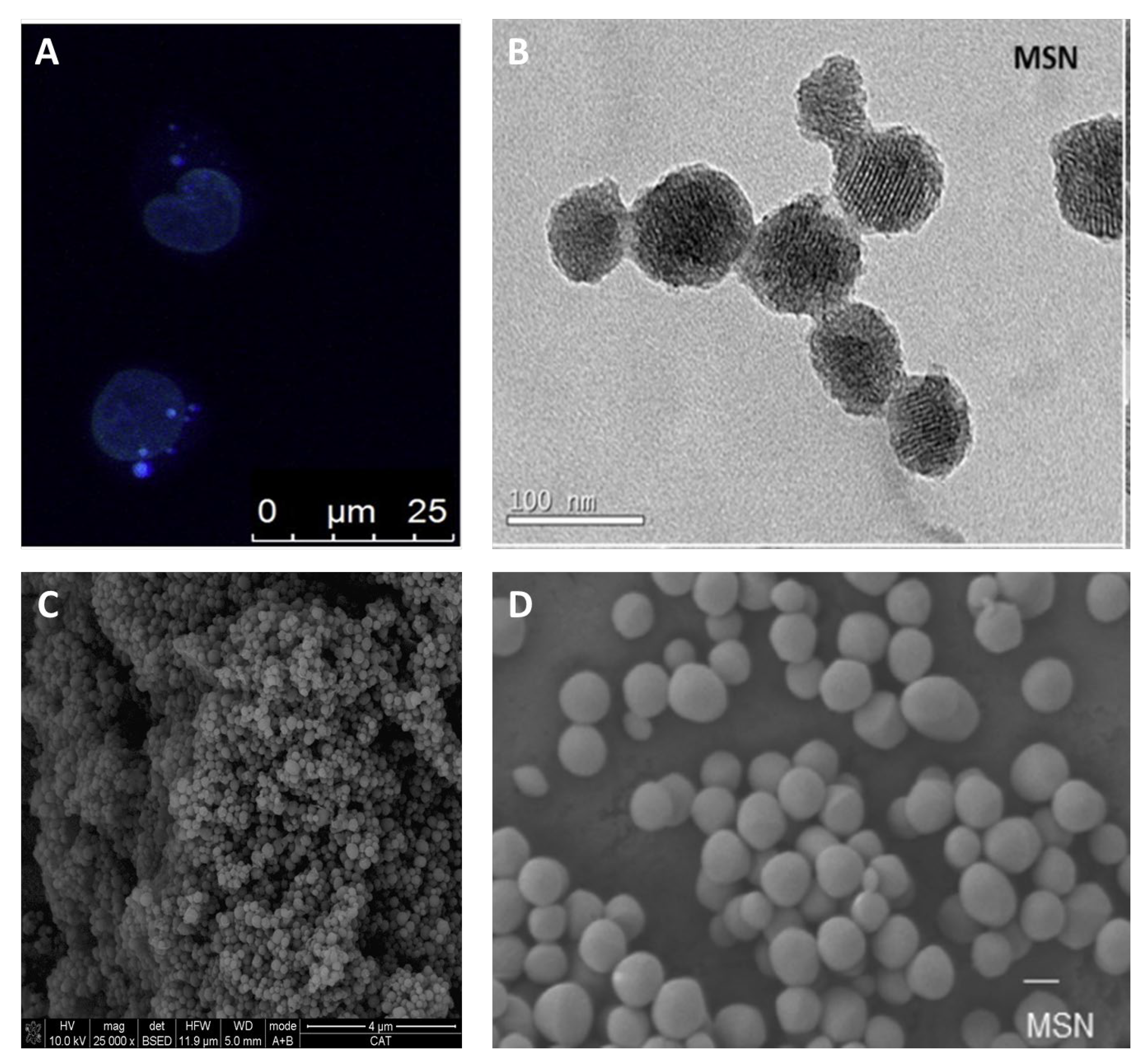
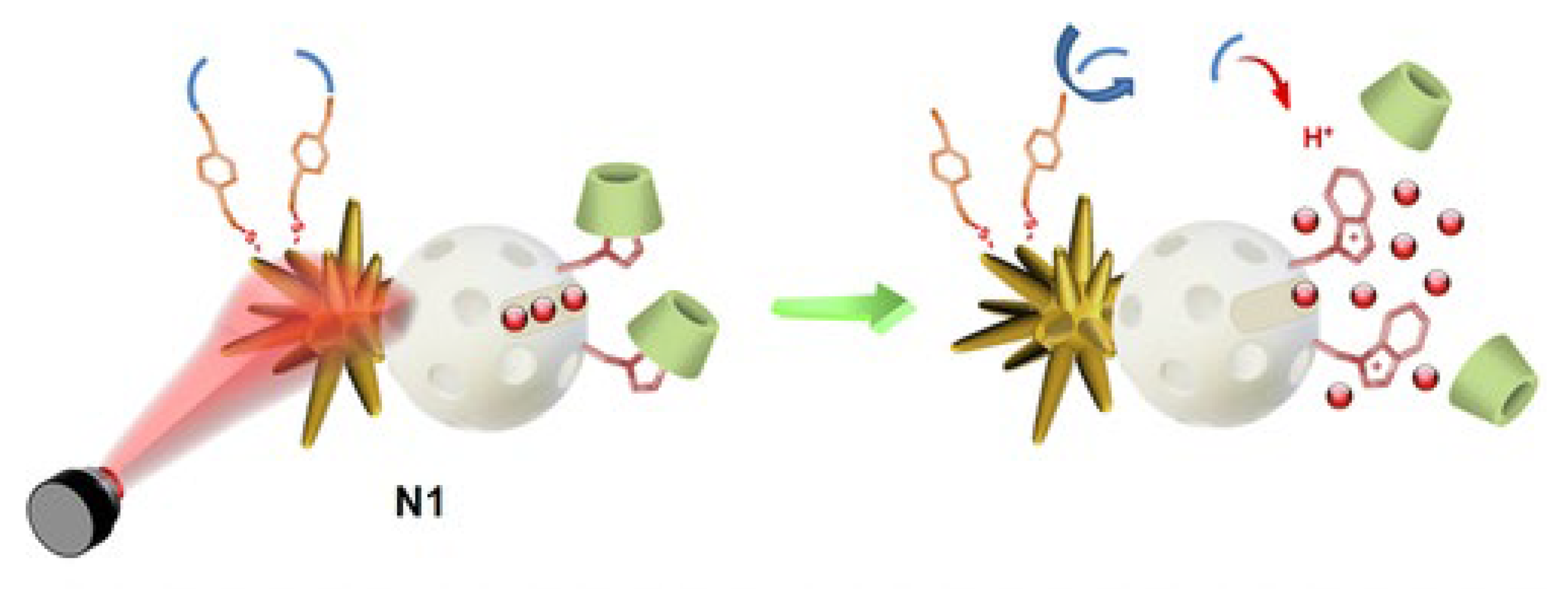
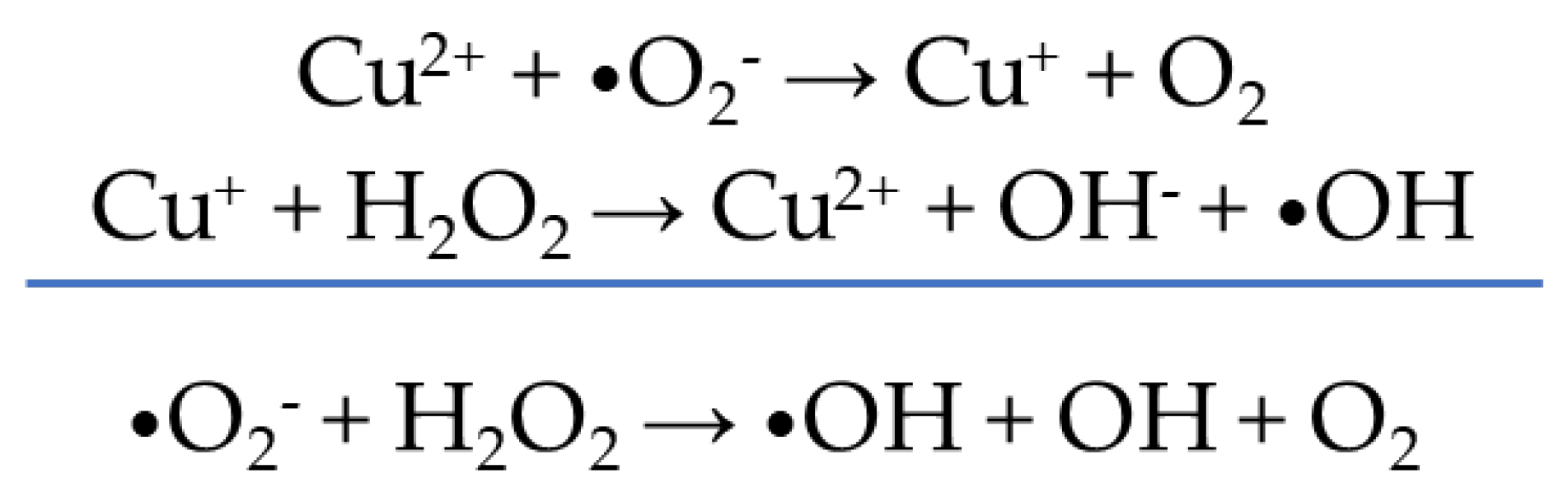
| Metal | Application | Reference |
|---|---|---|
| Pt | Anticancer (phototherapy) | [19] |
| Sn | Anticancer (pancreatic carcinoma, erythroleukemia, glioblastoma) | [20] |
| Ti | Anticancer (Adenocarcinoma and cervix cancer) | [21] |
| Ru | Anticancer (prostate, breast cancer, cervix and liver cancer) | [22] |
| Ga | Anticancer (anaplastic thyroid cancer, head and neck tumor, lung carcinoma, ovarian cancer, colon carcinoma) | [23] |
| Au | Anticancer (colon carcinoma) | [24] |
| Ag | Antimicrobial (S. aureus, E. coli, P. aeruginosa and Mycobacteria strains M. bovis and M. tuberculosis) and anticancer (breast cancer and hepatocellular carcinoma) | [25] |
| Cu | Antibacterial (S. aureus, E. faecalis, E. coli and P. aeruginosa), antifungal (C. albicans, C. glabrata, A. fumigatus and A. alternata) and anticancer (breast and colorectal cancer) | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-García, D.; Prashar, S.; Gómez-Ruiz, S. Biological Use of Nanostructured Silica-Based Materials Functionalized with Metallodrugs: The Spanish Perspective. Int. J. Mol. Sci. 2023, 24, 2332. https://doi.org/10.3390/ijms24032332
Díaz-García D, Prashar S, Gómez-Ruiz S. Biological Use of Nanostructured Silica-Based Materials Functionalized with Metallodrugs: The Spanish Perspective. International Journal of Molecular Sciences. 2023; 24(3):2332. https://doi.org/10.3390/ijms24032332
Chicago/Turabian StyleDíaz-García, Diana, Sanjiv Prashar, and Santiago Gómez-Ruiz. 2023. "Biological Use of Nanostructured Silica-Based Materials Functionalized with Metallodrugs: The Spanish Perspective" International Journal of Molecular Sciences 24, no. 3: 2332. https://doi.org/10.3390/ijms24032332
APA StyleDíaz-García, D., Prashar, S., & Gómez-Ruiz, S. (2023). Biological Use of Nanostructured Silica-Based Materials Functionalized with Metallodrugs: The Spanish Perspective. International Journal of Molecular Sciences, 24(3), 2332. https://doi.org/10.3390/ijms24032332








