Miltefosine and Nifuratel Combination: A Promising Therapy for the Treatment of Leishmania donovani Visceral Leishmaniasis
Abstract
1. Introduction
2. Results
2.1. Effect of NFT/MTF Combinations In Vitro and Ex Vivo
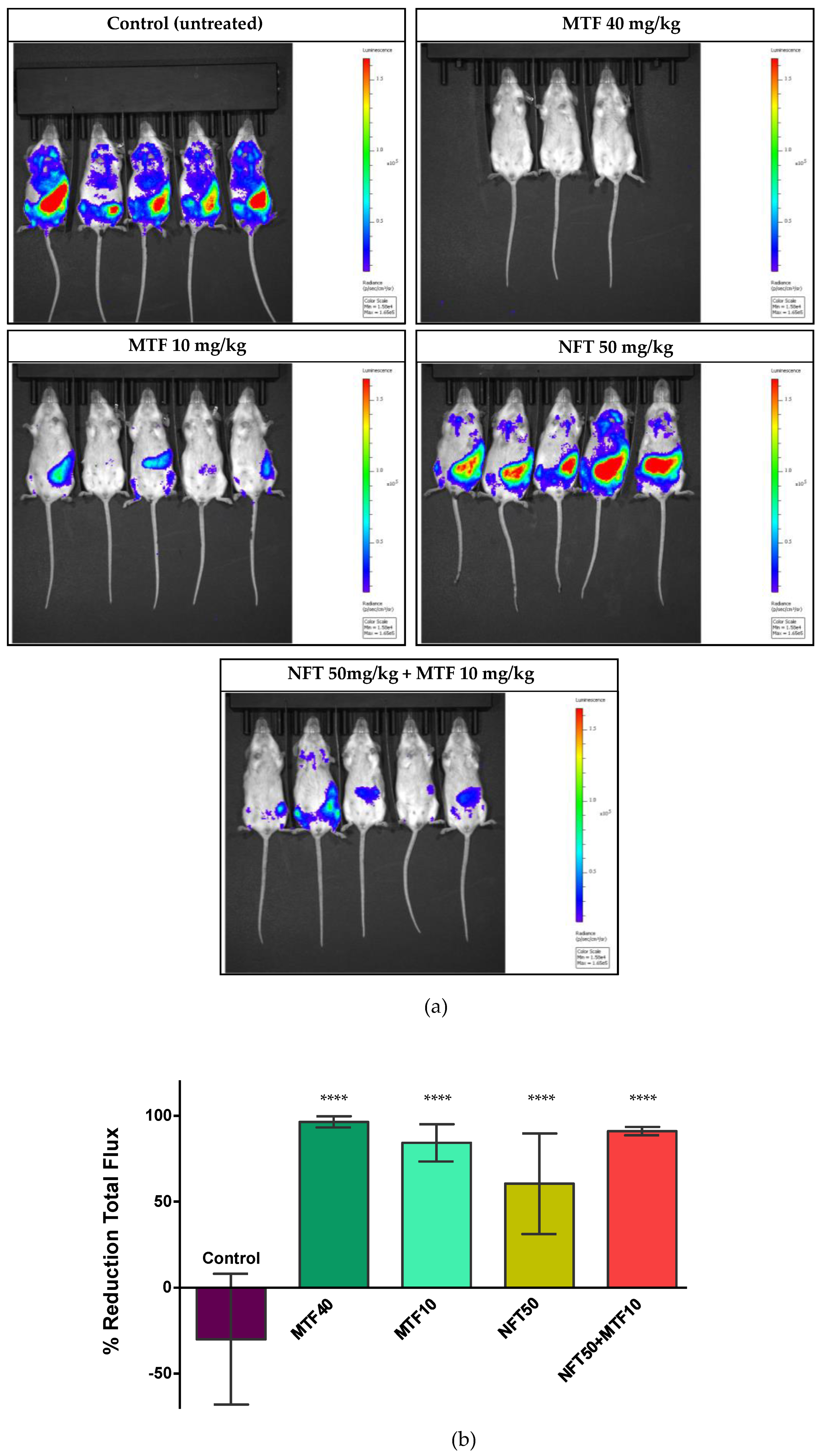
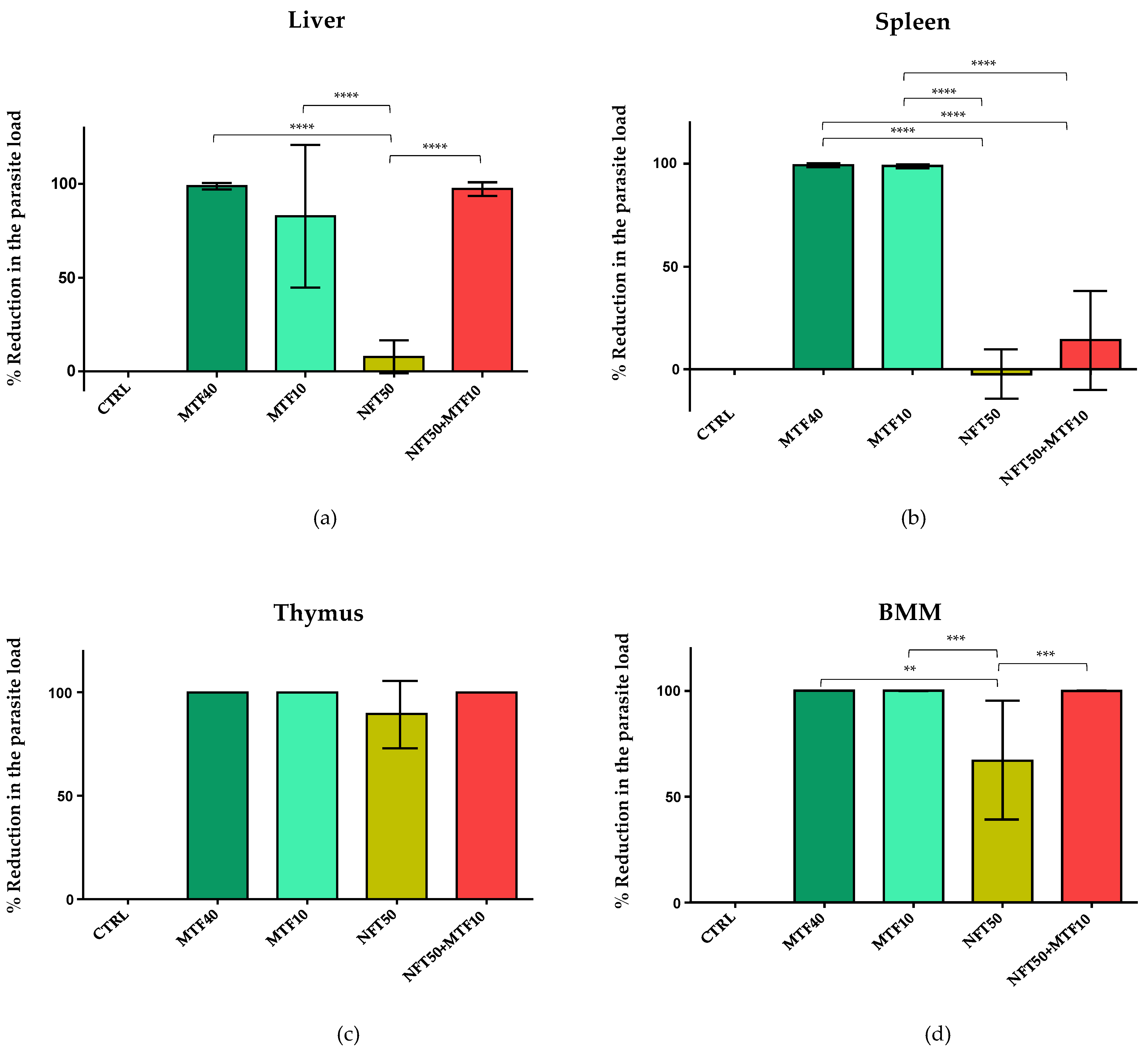
2.2. Effect of NFT/MTF Combination In Vivo
2.3. Inhibition of TR Activity
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Experimental Animals and Ethical Statement
4.3. Parasites
4.4. Experimental Infections and Set Up of Primary Cultures
4.5. Axenic and Intramacrophagic Amastigotes Viability Assays
4.6. Cell Cytotoxicity
4.7. In Vivo Efficacy of Drug Combinations against L. donovani VL Mouse Model
4.8. Trypanothione Reductase (TR) Assay
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Saudagar, P. Leishmaniasis: Where are we and where are we heading? Parasitol. Res. 2021, 120, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Van Griensven, J.; Diro, E. Visceral leishmaniasis. Infect. Dis. Clin. N. Am. 2012, 26, 309–322. [Google Scholar] [CrossRef] [PubMed]
- WHO. Control of the Leishmaniasis. Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases. Geneva, Switzerland: World Health Organization. 2010. Available online: https://apps.who.int/iris/bitstream/handle/10665/44412/WHO_TRS_949_eng.pdf?sequence=1&isAllowed=y (accessed on 9 January 2023).
- Van Griensven, J.; Diro, E. Visceral leishmaniasis, recent advances in diagnostic and treatment regimens. Infect. Dis. Clin. N. Am. 2019, 33, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Frézard, F.; Demicheli, C.; Ribeiro, R.R. Pentavalent antimonials: New perspectives for old drugs. Molecules 2009, 14, 2317–2336. [Google Scholar] [CrossRef]
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. An update on pharmacotherapy for leishmaniasis. Expert Opin. Pharmacother. 2015, 16, 237–252. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, A.; Agrawal, N.; Chakravarty, J. Effectiveness of single-dose liposomal amphotericin b in visceral leishmaniasis in Bihar. Am. J. Trop. Med. Hyg. 2019, 101, 795–798. [Google Scholar] [CrossRef]
- Hung, C.T.; Lam, F.C.; Perrier, D.G.; Souter, A. A stability study of amphotericin B in aqueous media using factorial design. Int. J. Pharm. 1988, 44, 117–123. [Google Scholar] [CrossRef]
- Sindermann, H.; Engel, J. Development of miltefosine as an oral treatment for leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2006, 100 (Suppl. S1), S17–S20. [Google Scholar] [CrossRef]
- Sundar, S.; Singh, A.; Rai, M.; Prajapati, V.K.; Singh, A.K.; Ostyn, B.; Boelaert, M.; Dujardin, J.C.; Chakravarty, J. Efficacy of miltefosine in the treatment of visceral leishmaniasis in India after a decade of use. Clin. Infect. Dis. 2012, 55, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Palić, S.; Beijnen, J.H.; Dorlo, T.P. An update on the clinical pharmacology of miltefosine in the treatment of leishmaniasis. Int. J. Antimicrob. Agents 2021, 22, 106459. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Mishra, J.; Gupta, A.K.; Singh, A.; Shankar, P.; Singh, S. Laboratory confirmed miltefosine resistant cases of visceral leishmaniasis from India. Parasites Vectors 2017, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Rijal, S.; Ostyn, B.; Uranw, S.; Rai, K.; Bhattarai, N.R.; Dorlo, T.P.C.; Beijnen, J.H.; Vanaerschot, M.; Decuypere, S.; Dhakal, S.S.; et al. Increasing failure of miltefosine in the treatment of Kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin. Infect. Dis. 2013, 56, 1530–1538. [Google Scholar] [CrossRef]
- Shaw, C.D.; Lonchamp, J.; Downing, T.; Imamura, H.; Freeman, T.M.; Cotton, J.A.; Sanders, M.; Blackburn, G.; Dujardin, J.C.; Rijal, S.; et al. In vitro selection of miltefosine resistance in promastigotes of Leishmania donovani from Nepal: Genomic and metabolomic characterization. Mol. Microbiol. 2016, 99, 1134–1148. [Google Scholar] [CrossRef]
- Musa, A.; Khalil, E.; Hailu, A.; Olobo, J.; Balasegaram, M.; Omollo, R.; Edwards, T.; Rashid, J.; Mbui, J.; Musa, B.; et al. Sodium stibogluconate (SSG) & paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: A randomised controlled trial. PLoS Negl. Trop. Dis. 2012, 6, e1674. [Google Scholar]
- Omollo, R.; Alexander, N.; Edwards, T.; Khalil, E.A.; Younis, B.M.; Abuzaid, A.A.; Wasunna, M.; Njoroge, N.; Kinoti, D.; Kirigi, G.; et al. Safety and efficacy of miltefosine alone and in combination with sodium stibogluconate and liposomal amphotericin B for the treatment of primary visceral leishmaniasis in East Africa: Study protocol for a randomized controlled trial. Trials 2011, 12, 166. [Google Scholar] [CrossRef]
- Chakravarty, J.; Sundar, S. Current and emerging medications for the treatment of leishmaniasis. Expert Opin. Pharmacother. 2019, 20, 1251–1265. [Google Scholar] [CrossRef]
- Reguera, R.M.; Pérez-Pertejo, Y.; Gutiérrez-Corbo, C.; Domínguez-Asenjo, B.; Ordóñez, C.; García-Estrada, C.; Martínez-Valladares, M.; Balaña-Fouce, R. Current and promising novel drug candidates against visceral leishmaniasis. Pure Appl. Chem. 2019, 91, 1385–1404. [Google Scholar] [CrossRef]
- Reguera, R.M.; Calvo-Álvarez, E.; Alvarez-Velilla, R.; Balaña-Fouce, R. Target-based vs. phenotypic screenings in Leishmania drug discovery: A marriage of convenience or a dialogue of the deaf? Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 355–357. [Google Scholar] [CrossRef]
- Balaña-Fouce, R.; Pérez Pertejo, M.Y.; Domínguez-Asenjo, B.; Gutiérrez-Corbo, C.; Reguera, R.M. Walking a tightrope: Drug discovery in visceral leishmaniasis. Drug Discov. Today 2019, 24, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Roatt, B.M.; de Oliveira-Cardoso, J.M.; De Brito, R.C.F.; Coura-Vital, W.; de Oliveira Aguiar-Soares, R.D.; Reis, A.B. Recent advances and new strategies on leishmaniasis treatment. Appl. Microbiol. Biotechnol. 2020, 104, 8965–8977. [Google Scholar] [CrossRef] [PubMed]
- Charlton, R.L.; Rossi-Bergmann, B.; Denny, P.W.; Steel, P.G. Repurposing as a strategy for the discovery of new anti-leishmanials: The-state-of-the-art. Parasitology 2018, 145, 219–236. [Google Scholar] [CrossRef]
- Calvo-Álvarez, E.; Stamatakis, K.; Punzón, C.; Álvarez-Velilla, R.; Tejería, A.; Escudero-Martínez, J.M.; Pérez-Pertejo, Y.; Fresno, M.; Balaña-Fouce, R.; Reguera, R.M. Infrared fluorescent imaging as a potent tool for in vitro, ex vivo and in vivo models of visceral leishmaniasis. PLoS Negl. Trop. Dis. 2015, 9, e0003666. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Asenjo, B.; Gutiérrez-Corbo, C.; Álvarez-Bardón, M.; Pérez-Pertejo, Y.; Balaña-Fouce, R.; Reguera, R.M. Ex vivo phenotypic screening of two small repurposing drug collections identifies nifuratel as a potential new treatment against visceral and cutaneous leishmaniasis. ACS Infect. Dis. 2021, 7, 2390–2401. [Google Scholar] [CrossRef]
- Fowler, W.; Hussain, M. Nifuratel (Magmilor) in trichomonal vaginitis. Br. J. Vener. Dis. 1968, 44, 331–333. [Google Scholar] [CrossRef]
- Churcher, G.M.; Evans, A.J. Inhibition of Neisseria gonorrhoeae by nifuratel. Br. J. Vener. Dis. 1969, 45, 149–150. [Google Scholar] [CrossRef]
- Patterson, S.; Wyllie, S. Nitro drugs for the treatment of trypanosomatid diseases: Past, present, and future prospects. Trends Parasitol. 2014, 30, 289–298. [Google Scholar] [CrossRef]
- Wyllie, S.; Roberts, A.J.; Norval, S.; Patterson, S.; Foth, B.J.; Berriman, M.; Read, K.D.; Fairlamb, A.H. Activation of bicyclic nitro-drugs by a novel nitroreductase (NTR2) in Leishmania. PLoS Pathog. 2016, 12, e1005971. [Google Scholar] [CrossRef]
- Beig, M.; Oellien, F.; Garoff, L.; Noack, S.; Krauth-Siegel, R.L.; Selzer, P.M. Trypanothione reductase: A target protein for a combined in vitro and in silico screening approach. PLoS Negl. Trop. Dis. 2015, 9, e0003773. [Google Scholar] [CrossRef]
- Van Griensven, J.; Balasegaram, M.; Meheus, F.; Alvar, J.; Lynen, L.; Boelaert, M. Combination therapy for visceral leishmaniasis. Lancet Infect. Dis. 2010, 10, 184–194. [Google Scholar] [CrossRef]
- Sun, W.; Sanderson, P.E.; Zheng, W. Drug combination therapy increases successful drug repositioning. Drug Discov. Today 2016, 21, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, S.; Van den Kerkhof, M.; Mabille, D.; Cos, P.; Delputte, P.; Maes, L.; Caljon, G. Combined treatment of miltefosine and paromomycin delays the onset of experimental drug resistance in Leishmania infantum. PLoS Negl. Trop. Dis. 2017, 11, e0005620. [Google Scholar] [CrossRef]
- Musa, A.M.; Mbui, J.; Mohammed, R.; Olobo, J.; Ritmeijer, K.; Alcoba, G.; Muthoni Ouattara, G.; Egondi, T.; Nakanwagi, P.; Omollo, T.; et al. Paromomycin and miltefosine combination as an alternative to treat patients with visceral leishmaniasis in eastern Africa: A randomized, controlled, multicountry trial. Clin. Infect. Dis. 2022, ciac643. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug com-bination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Álvarez-Velilla, R.; Gutiérrez-Corbo, M.D.C.; Punzón, C.; Pérez-Pertejo, M.Y.; Balaña-Fouce, R.; Fresno, M.; Reguera, R.M. A chronic bioluminescent model of experimental visceral leishmaniasis for accelerating drug discovery. PLoS Negl. Trop. Dis. 2019, 13, e0007133. [Google Scholar] [CrossRef]
- Titus, R.G.; Marchand, M.; Boon, T.; Louis, J. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985, 7, 545–555. [Google Scholar] [CrossRef]
- Domínguez-Asenjo, B.; Gutiérrez-Corbo, C.; Pérez-Pertejo, Y.; Iborra, S.; Balaña-Fouce, R.; Reguera, R.M. Bioluminescent imaging identifies thymus, as overlooked colonized organ, in a chronic model of Leishmania donovani mouse visceral leishmaniasis. ACS Infect. Dis. 2021, 7, 871–883. [Google Scholar] [CrossRef]
- Paulino, M.; Iribarne, F.; Dubin, M.; Aguilera-Morales, S.; Tapia, O.; Stoppani, A.O. The chemotherapy of chagas’ disease: An overview. Mini Rev. Med. Chem. 2005, 5, 499–519. [Google Scholar] [CrossRef]
- Arias, D.G.; Herrera, F.E.; Garay, A.S.; Rodrigues, D.; Forastieri, P.S.; Luna, L.E.; Bürgi, M.D.; Prieto, C.; Iglesias, A.A.; Cravero, R.M.; et al. Rational design of nitrofuran derivatives: Synthesis and valuation as inhibitors of Trypanosoma cruzi trypanothione reductase. Eur. J. Med. Chem. 2017, 125, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Ponte-Sucre, A.; Gamarro, F.; Dujardin, J.C.; Barrett, M.P.; López-Vélez, R.; García-Hernández, R.; Pountain, A.W.; Mwenechanya, R.; Papadopoulou, B. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl. Trop. Dis. 2017, 11, e000605. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B. Combination therapy strategies for the treatment of malaria. Molecules 2019, 24, 3601. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Perno, C.F.; Mallon, P.W.; Behrens, G.; Corbeau, P.; Routy, J.P.; Darcis, G. Two-drug vs. three-drug combinations for HIV-1: Do we have enough data to make the switch? HIV Med. 2019, 20 (Suppl. S4), 2–12. [Google Scholar] [CrossRef]
- Larkins-Ford, J.; Degefu, Y.N.; Van, N.; Sokolov, A.; Aldridge, B.B. Design principles to assemble drug combinations for effective tuberculosis therapy using interpretable pairwise drug response measurements. Cell Rep. Med. 2022, 3, 100737. [Google Scholar] [CrossRef]
- Rebello, K.M.; Andrade-Neto, V.V.; Gomes, C.R.B.; de Souza, M.V.N.; Branquinha, M.H.; Santos, A.L.S.; Torres-Santos, E.C.; d’Avila-Levy, C.M. Miltefosine-Lopinavir combination therapy against Leishmania infantum infection: In vitro and in vivo approaches. Front. Cell Infect. Microbiol. 2019, 9, 229. [Google Scholar] [CrossRef]
- Valdivieso, E.; Mejías, F.; Carrillo, E.; Sánchez, C.; Moreno, J. Potentiation of the leishmanicidal activity of nelfinavir in combination with miltefosine or amphotericin B. Int. J. Antimicrob. Agents 2018, 52, 682–687. [Google Scholar] [CrossRef]
- Kar, A.; Charan Raja, M.R.; Jayaraman, A.; Srinivasan, S.; Debnath, J.; Kar Mahapatra, S. Oral combination of eugenol oleate and miltefosine induce immune response during experimental visceral leishmaniasis through nitric oxide generation with advanced cytokine demand. Cytokine 2021, 146, 155623. [Google Scholar] [CrossRef]
- Tadele, M.; Abay, S.M.; Makonnen, E.; Hailu, A. Leishmania donovani growth inhibitors from pathogen box compounds of medicine for malaria venture. Drug Des. Dev. Ther. 2020, 14, 1307–1317. [Google Scholar] [CrossRef]
- Chavali, A.K.; Blazier, A.S.; Tlaxca, J.L.; Jensen, P.A.; Pearson, R.D.; Papin, J.A. Metabolic network analysis predicts efficacy of FDA-approved drugs targeting the causative agent of a neglected tropical disease. BMC Syst. Biol. 2012, 6, 27. [Google Scholar] [CrossRef]
- Andrade-Neto, V.V.; Rebello, K.M.; Pereira, T.M.; Torres-Santos, E.C. Effect of itraconazole-ezetimibe-Miltefosine ternary therapy in murine visceral leishmaniasis. Antimicrob. Agents Chemother. 2021, 65, e02676-20. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Podder, I.; Das, A.; Sil, A.; Das, N.K. Therapeutic Modalities in Post Kala-azar Dermal Leishmaniasis: A systematic review of the effectiveness and safety of the treatment options. Indian J. Dermatol. 2021, 66, 34–43. [Google Scholar] [PubMed]
- Loiseau, P.M.; Bories, C. Mechanisms of drug action and drug resistance in Leishmania as basis for therapeutic target identification and design of antileishmanial modulators. Curr. Top. Med. Chem. 2006, 6, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Wadhone, P.; Maiti, M.; Agarwal, R.; Kamat, V.; Martin, S.; Saha, B. Miltefosine promotes IFN-γ-dominated anti-leishmanial immune response. J. Immunol. 2009, 182, 7146–7154. [Google Scholar] [CrossRef] [PubMed]
- Wyllie, S.; Patterson, S.; Fairlamb, A.H. Assessing the essentiality of Leishmania donovani nitroreductase and its role in nitro drug activation. Antimicrob. Agents Chemother. 2013, 57, 901–906. [Google Scholar] [CrossRef]
- Dumas, C.; Ouellette, M.; Tovar, J.; Cunningham, M.L.; Fairlamb, A.H.; Tamar, S.; Olivier, M.; Papadopoulou, B. Disruption of the trypanothione reductase gene of Leishmania decreases its ability to survive oxidative stress in macrophages. EMBO J. 1997, 16, 2590–2598. [Google Scholar] [CrossRef]
- Henderson, G.B.; Ulrich, P.; Fairlamb, A.H.; Rosenberg, I.; Pereira, M.; Sela, M.; Cerami, A. “Subversive” substrates for the enzyme trypanothione disulfide reductase: Alternative approach to chemotherapy of Chagas disease. Proc. Natl. Acad. Sci. USA 1988, 85, 5374–5378. [Google Scholar] [CrossRef]
- Scuri, R.; Failla, L. Propietá biologiche dell’N(5-nitro-2-furfuriledene)-3-amino-5-metilmercaptometil-2-ossazolidinone. Farmaco 1964, 4, 301–311. [Google Scholar]
- Wu, Y.; Xiang, B.; Yang, J.; Fu, J.; Jiang, J. Determination of nifuratel in human plasma by HPLC and study on its pharmacokinetics. J. Chromatogr. Sci. 2005, 43, 179–182. [Google Scholar] [CrossRef]
- Jiménez-Antón, M.D.; García-Calvo, E.; Gutiérrez, C.; Escribano, M.D.; Kayali, N.; Luque-García, J.L.; Olías-Molero, A.I.; Corral, M.J.; Costi, M.P.; Torrado, J.J.; et al. Pharmacokinetics and disposition of miltefosine in healthy mice and hamsters experimentally infected with Leishmania infantum. Eur. J. Pharm. Sci. 2018, 121, 281–286. [Google Scholar] [CrossRef]
- Dorlo, T.P.; Balasegaram, M.; Beijnen, J.H.; de Vries, P.J. Miltefosine: A review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597. [Google Scholar] [CrossRef] [PubMed]
- Pijpers, J.; den Boer, M.L.; Essink, D.R.; Ritmeijer, K. The safety and efficacy of miltefosine in the long-term treatment of post-kala-azar dermal leishmaniasis in South Asia-A review and meta-analysis. PLoS Negl. Trop. Dis. 2019, 13, e0007173. [Google Scholar] [CrossRef] [PubMed]
- Miltefosine Pregnancy and Breastfeeding Warnings. Available online: https://www.drugs.com/pregnancy/miltefosine.html (accessed on 22 June 2022).
- Van Thiel, P.P.; Leenstra, T.; Kager, P.A.; de Vries, H.J.; van Vugt, M.; van der Meide, W.F.; Bart, A.; Zeegelaar, J.E.; van der Sluis, A.; Schallig, H.D.; et al. Miltefosine treatment of Leishmania major infection: An observational study involving Dutch military personnel returning from northern Afghanistan. Clin. Infect. Dis. 2010, 50, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Filonov, G.S.; Piatkevich, K.D.; Ting, L.M.; Zhang, J.; Kim, K.; Verkhusha, V.V. Bright and stable near-infrared fluorescent protein for in vivo imaging. Nat. Biotechnol. 2012, 29, 757–761. [Google Scholar] [CrossRef]
- De Muylder, G.; Ang, K.K.; Chen, S.; Arkin, M.R.; Engel, J.C.; McKerrow, J.H. A screen against Leishmania intracellular amastigotes: Comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl. Trop. Dis. 2011, 5, e1253. [Google Scholar] [CrossRef]
- Selas, A.; Fuertes, M.; Melcón-Fernández, E.; Pérez-Pertejo, Y.; Reguera, R.M.; Balaña-Fouce, R.; Knudsen, B.R.; Palacios, F.; Alonso, C. Hybrid quinolinyl phosphonates as heterocyclic carboxylate isosteres: Synthesis and biological evaluation against topoisomerase 1B (TOP1B). Pharmaceuticals 2021, 14, 784. [Google Scholar] [CrossRef]
- Tejería, A.; Pérez-Pertejo, Y.; Reguera, R.M.; Carbajo-Andrés, R.; Balaña-Fouce, R.; Alonso, C.; Martin-Encinas, E.; Selas, A.; Rubiales, G.; Palacios, F. Antileishmanial activity of new hybrid tetrahydroquinoline and quinoline derivatives with phosphorus substituents. Eur. J. Med. Chem. 2019, 162, 18–31. [Google Scholar] [CrossRef]
- Van den Bogaart, E.; Schoone, G.J.; England, P.; Faber, D.; Orrling, K.M.; Dujardin, J.C.; Sundar, S.; Schallig, H.D.; Adams, E.R. Simple colorimetric trypanothione reductase-based assay for high-throughput screening of drugs against Leishmania intracellular amastigotes. Antimicrob. Agents Chemother. 2014, 58, 527–535. [Google Scholar] [CrossRef]
- Lo Presti, M.S.; Bazán, P.C.; Strauss, M.; Báez, A.L.; Rivarola, H.W.; Paglini-Oliva, P.A. Trypanothione reductase inhibitors: Overview of the action of thioridazine in different stages of Chagas disease. Acta Trop. 2015, 145, 79–87. [Google Scholar] [CrossRef]
 ), NFT (
), NFT ( ), NFT/MTF 1/10 combination (
), NFT/MTF 1/10 combination ( ) and NFT/MTF 1/30 combination (
) and NFT/MTF 1/30 combination ( ) tested in axenic amastigotes (a) or in intramacrophagic amastigotes (b). For NFT, 1/2 serial dilutions were prepared, starting with 0.5 µM for axenic amastigotes and with 3 μM for intramacrophagic amastigotes, whereas for MTF, 1/3 and 2/3 serial dilutions were performed, starting with 5 µM for axenic amastigotes and with 30 μM for intramacrophagic amastigotes. The starting concentrations in the NFT/MTF 1/10 combination were 0.125 µM/1.25 µM, with 2/3 serial dilutions for axenic amastigotes, and 3 µM/30 µM, with 1/2 serial dilutions for intramacrophagic amastigotes. In the NFT/MTF 1/30, the starting concentrations were 0.0625 µM/1.875 µM, with 2/3 dilutions for axenic amastigotes, and 0.3 µM/30 µM, with 1/2 serial dilutions. Dose–response curves were adjusted with SigmaPlot software. Results represent the mean values ± SD of three independent experiments with four technical replicates each.
) tested in axenic amastigotes (a) or in intramacrophagic amastigotes (b). For NFT, 1/2 serial dilutions were prepared, starting with 0.5 µM for axenic amastigotes and with 3 μM for intramacrophagic amastigotes, whereas for MTF, 1/3 and 2/3 serial dilutions were performed, starting with 5 µM for axenic amastigotes and with 30 μM for intramacrophagic amastigotes. The starting concentrations in the NFT/MTF 1/10 combination were 0.125 µM/1.25 µM, with 2/3 serial dilutions for axenic amastigotes, and 3 µM/30 µM, with 1/2 serial dilutions for intramacrophagic amastigotes. In the NFT/MTF 1/30, the starting concentrations were 0.0625 µM/1.875 µM, with 2/3 dilutions for axenic amastigotes, and 0.3 µM/30 µM, with 1/2 serial dilutions. Dose–response curves were adjusted with SigmaPlot software. Results represent the mean values ± SD of three independent experiments with four technical replicates each.
 ), NFT (
), NFT ( ), NFT/MTF 1/10 combination (
), NFT/MTF 1/10 combination ( ) and NFT/MTF 1/30 combination (
) and NFT/MTF 1/30 combination ( ) tested in axenic amastigotes (a) or in intramacrophagic amastigotes (b). For NFT, 1/2 serial dilutions were prepared, starting with 0.5 µM for axenic amastigotes and with 3 μM for intramacrophagic amastigotes, whereas for MTF, 1/3 and 2/3 serial dilutions were performed, starting with 5 µM for axenic amastigotes and with 30 μM for intramacrophagic amastigotes. The starting concentrations in the NFT/MTF 1/10 combination were 0.125 µM/1.25 µM, with 2/3 serial dilutions for axenic amastigotes, and 3 µM/30 µM, with 1/2 serial dilutions for intramacrophagic amastigotes. In the NFT/MTF 1/30, the starting concentrations were 0.0625 µM/1.875 µM, with 2/3 dilutions for axenic amastigotes, and 0.3 µM/30 µM, with 1/2 serial dilutions. Dose–response curves were adjusted with SigmaPlot software. Results represent the mean values ± SD of three independent experiments with four technical replicates each.
) tested in axenic amastigotes (a) or in intramacrophagic amastigotes (b). For NFT, 1/2 serial dilutions were prepared, starting with 0.5 µM for axenic amastigotes and with 3 μM for intramacrophagic amastigotes, whereas for MTF, 1/3 and 2/3 serial dilutions were performed, starting with 5 µM for axenic amastigotes and with 30 μM for intramacrophagic amastigotes. The starting concentrations in the NFT/MTF 1/10 combination were 0.125 µM/1.25 µM, with 2/3 serial dilutions for axenic amastigotes, and 3 µM/30 µM, with 1/2 serial dilutions for intramacrophagic amastigotes. In the NFT/MTF 1/30, the starting concentrations were 0.0625 µM/1.875 µM, with 2/3 dilutions for axenic amastigotes, and 0.3 µM/30 µM, with 1/2 serial dilutions. Dose–response curves were adjusted with SigmaPlot software. Results represent the mean values ± SD of three independent experiments with four technical replicates each.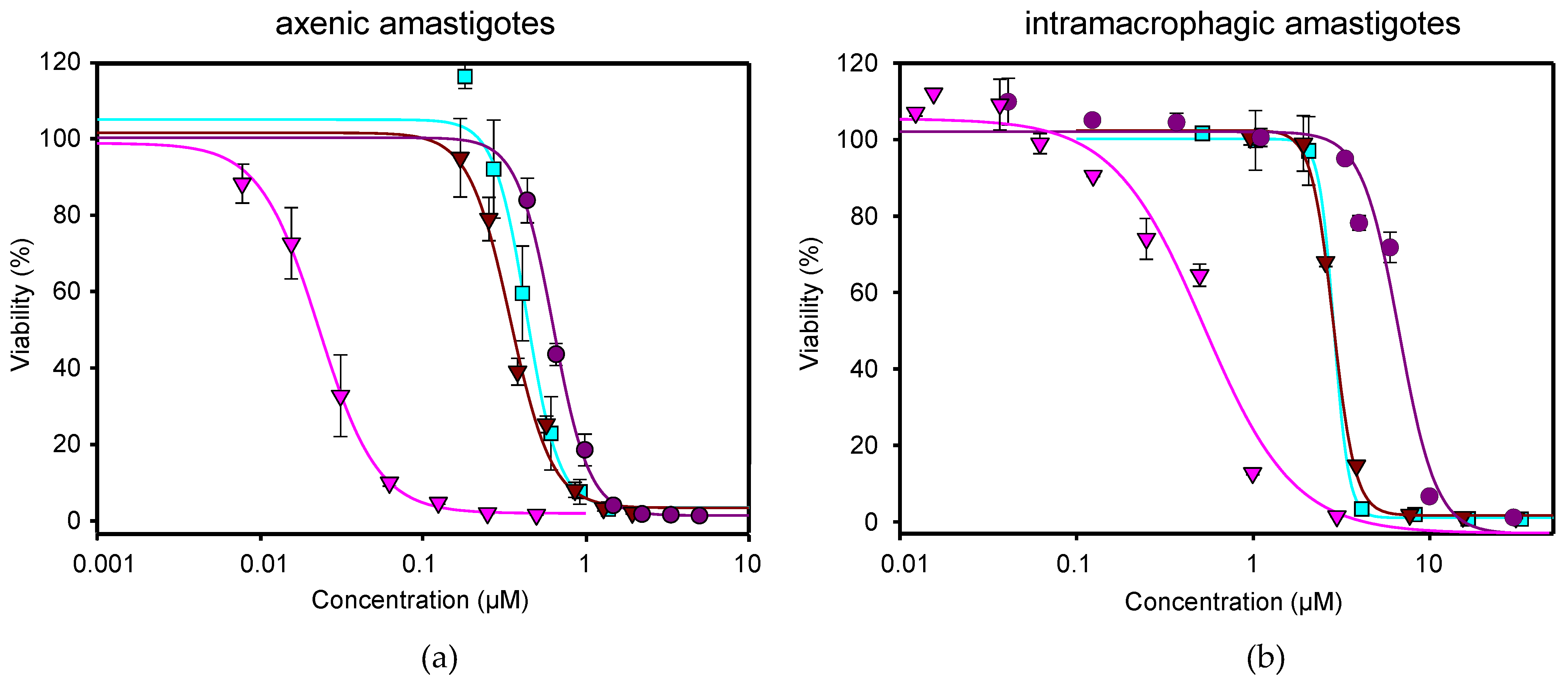
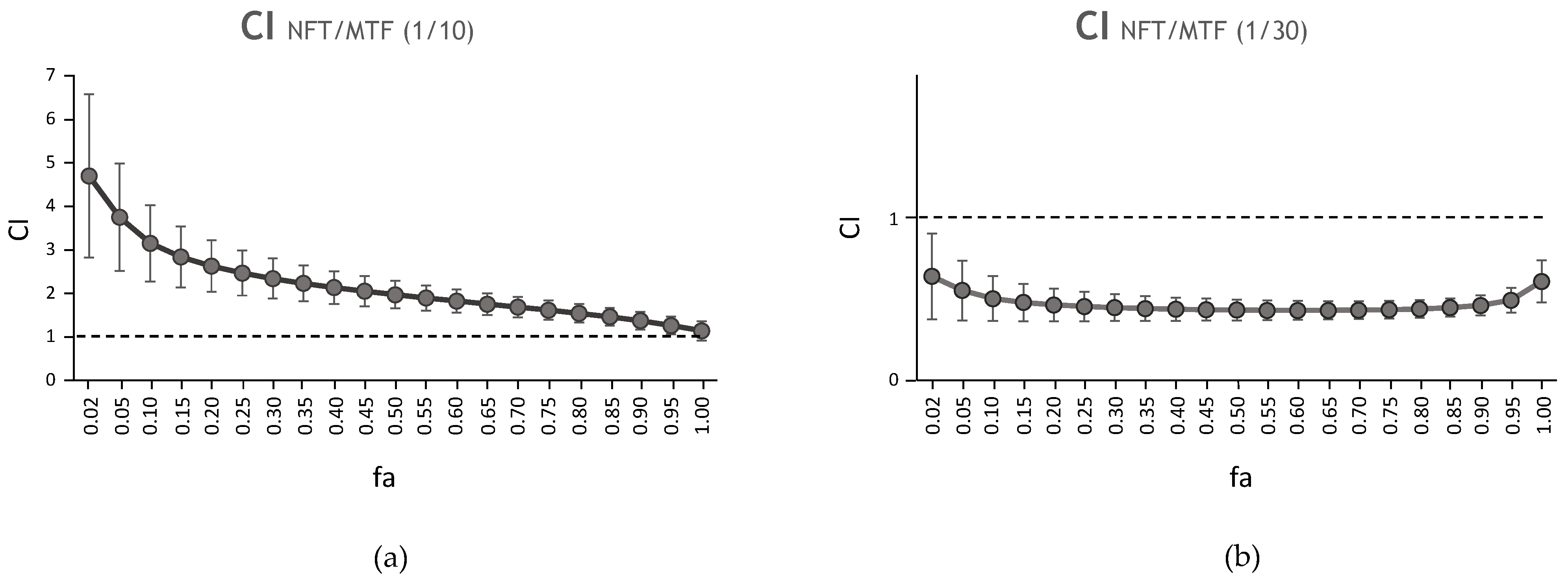
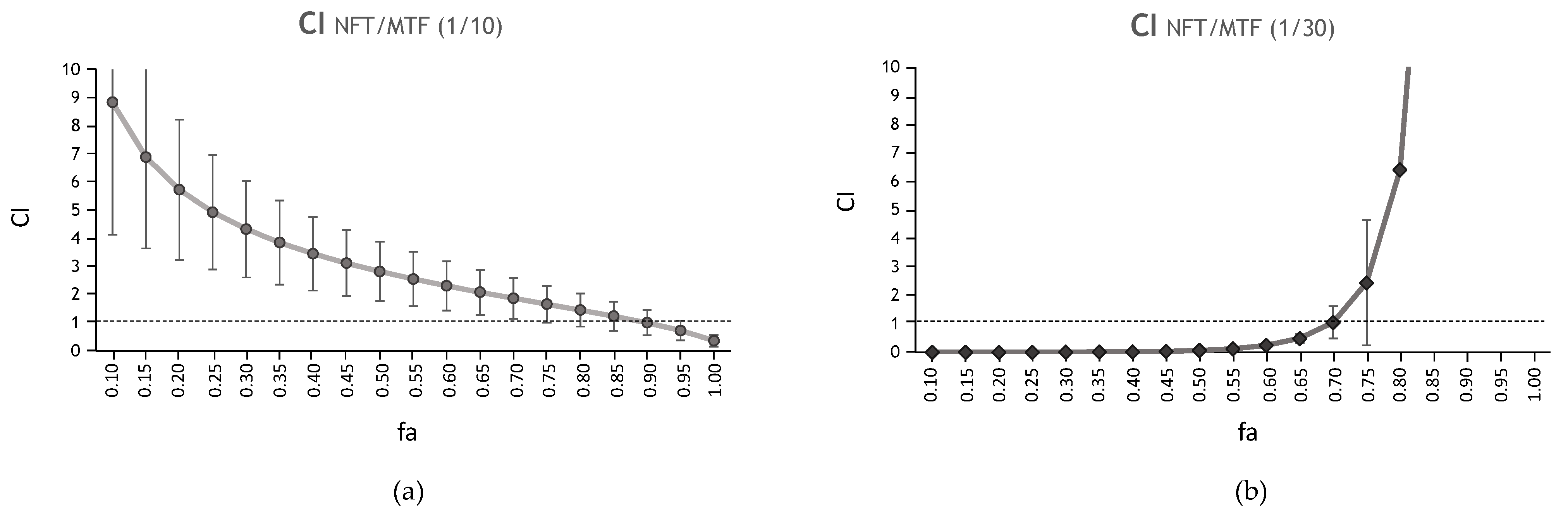
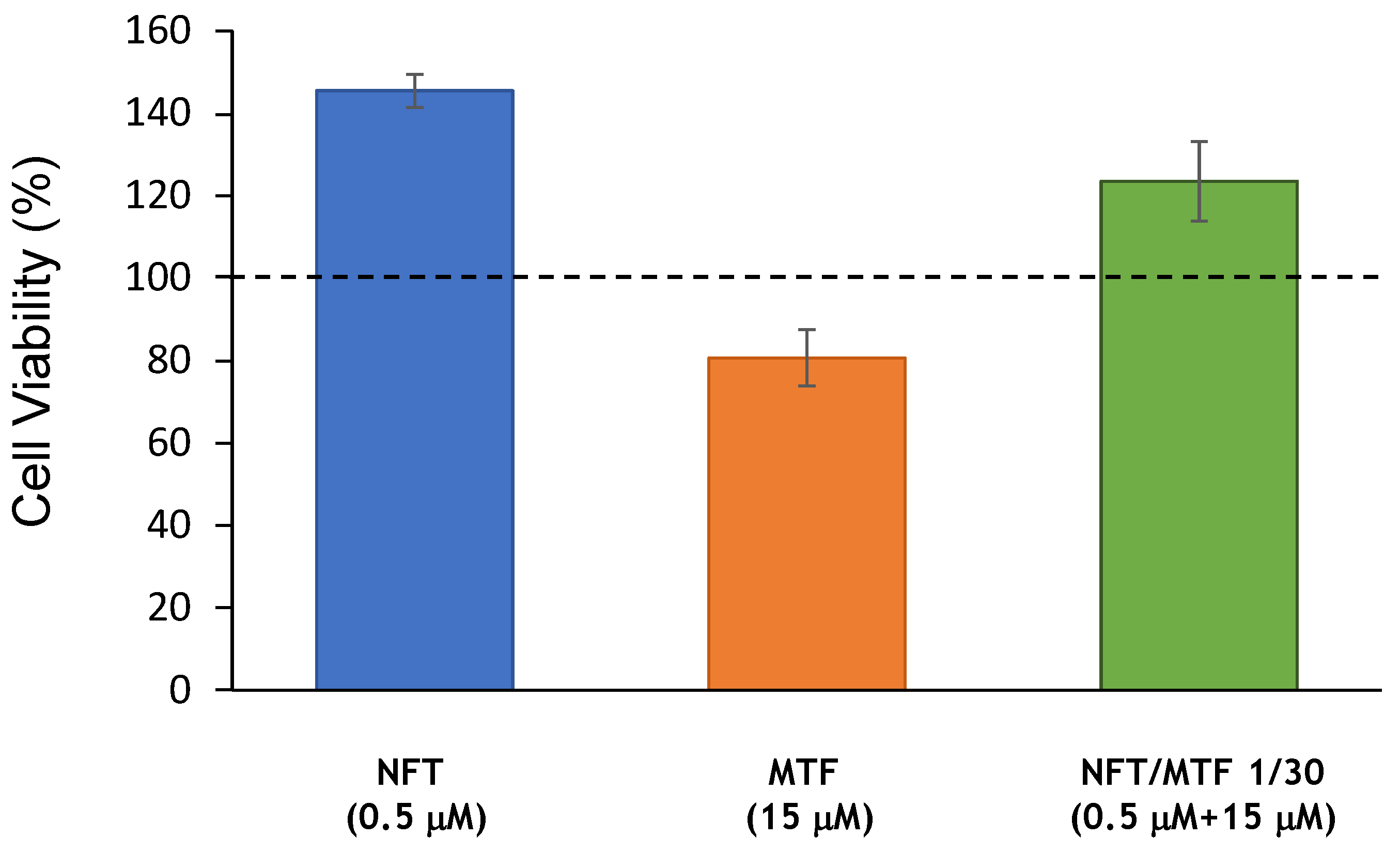

| CI Values at Following Effect Levels | ||||||||
|---|---|---|---|---|---|---|---|---|
| Drug/s | EC50 | Dm | m | r | 25% | 50% | 75% | 100% |
| NFT | 0.02 ± 0.00 | 0.03 | 1.61 | 0.97 | *N/A | *N/A | *N/A | *N/A |
| MTF | 0.63 ± 0.01 | 1.74 | 5.29 | 0.96 | *N/A | *N/A | *N/A | *N/A |
| NFT/MTF 1/10 | N/A | 0.05 | 2.72 | 0.99 | 2.46 | 1.96 | 1.61 | 1.36 |
| NFT/MTF 1/30 | N/A | 0.01 | 2.40 | 0.97 | 0.57 | 0.52 | 0.49 | 0.50 |
| CI Values at Following Effect Levels | ||||||||
|---|---|---|---|---|---|---|---|---|
| Drug/s | EC50 | Dm | m | r | 25% | 50% | 75% | 100% |
| NFT | 0.53 ± 0.05 | 0.09 | 0.94 | 0.92 | *N/A | *N/A | *N/A | *N/A |
| MTF | 5.60 ± 0.38 | 2.19 | 1.20 | 0.95 | *N/A | *N/A | *N/A | *N/A |
| NFT/MTF 1/10 | N/A | 0.36 | 3.34 | 0.94 | 4.93 | 2.82 | 1.65 | 1.00 |
| NFT/MTF 1/30 | N/A | 0.002 | 0.23 | 0.84 | 0.002 | 0.06 | 2.43 | 98.06 |
| DRI Values at Following Effect Levels | ||||
|---|---|---|---|---|
| Drug/s | 25% | 50% | 75% | 90% |
| NFT MTF | NFT MTF | NFT MTF | NFT MTF | |
| NFT/MTF 1/10 | 0.45 4.54 | 0.59 3.73 | 0.78 3.07 | 1.03 2.52 |
| NFT/MTF 1/30 | 2.27 7.68 | 2.84 5.99 | 3.56 4.66 | 4.46 3.63 |
| DRI Values at Following Effect Levels | ||||
|---|---|---|---|---|
| Drug/s | 25% | 50% | 75% | 90% |
| NFT MTF | NFT MTF | NFT MTF | NFT MTF | |
| NFT/MTF 1/10 | 0.49 0.35 | 0.72 0.70 | 1.05 1.42 | 1.54 2.87 |
| NFT/MTF 1/30 | 1253.97 1301.74 | 36.52 29.53 | 1.06 0.67 | 0.03 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melcon-Fernandez, E.; Galli, G.; García-Estrada, C.; Balaña-Fouce, R.; Reguera, R.M.; Pérez-Pertejo, Y. Miltefosine and Nifuratel Combination: A Promising Therapy for the Treatment of Leishmania donovani Visceral Leishmaniasis. Int. J. Mol. Sci. 2023, 24, 1635. https://doi.org/10.3390/ijms24021635
Melcon-Fernandez E, Galli G, García-Estrada C, Balaña-Fouce R, Reguera RM, Pérez-Pertejo Y. Miltefosine and Nifuratel Combination: A Promising Therapy for the Treatment of Leishmania donovani Visceral Leishmaniasis. International Journal of Molecular Sciences. 2023; 24(2):1635. https://doi.org/10.3390/ijms24021635
Chicago/Turabian StyleMelcon-Fernandez, Estela, Giulio Galli, Carlos García-Estrada, Rafael Balaña-Fouce, Rosa M. Reguera, and Yolanda Pérez-Pertejo. 2023. "Miltefosine and Nifuratel Combination: A Promising Therapy for the Treatment of Leishmania donovani Visceral Leishmaniasis" International Journal of Molecular Sciences 24, no. 2: 1635. https://doi.org/10.3390/ijms24021635
APA StyleMelcon-Fernandez, E., Galli, G., García-Estrada, C., Balaña-Fouce, R., Reguera, R. M., & Pérez-Pertejo, Y. (2023). Miltefosine and Nifuratel Combination: A Promising Therapy for the Treatment of Leishmania donovani Visceral Leishmaniasis. International Journal of Molecular Sciences, 24(2), 1635. https://doi.org/10.3390/ijms24021635









