Abstract
Alcoholic liver damage is caused by long-term drinking, and it further develops into alcoholic liver diseases. In this study, we prepared a probiotic fermentation product of Grifola frondosa total active components (PFGF) by fermentation with Lactobacillus acidophilus, Lactobacillus rhamnosus, and Pediococcus acidilactici. After fermentation, the total sugar and protein content in the PFGF significantly decreased, while the lactic acid level and antioxidant activity of the PFGF increased. Afterward, we investigated the alleviating effect of PFGF on alcoholic liver injury in alcohol-fed mice. The results showed that the PFGF intervention reduced the necrosis of the liver cells, attenuated the inflammation of the liver and intestines, restored the liver function, increased the antioxidant factors of the liver, and maintained the cecum tissue barrier. Additionally, the results of the 16S rRNA sequencing analysis indicated that the PFGF intervention increased the relative abundance of beneficial bacteria, such as Lactobacillus, Ruminococcaceae, Parabacteroids, Parasutterella, and Alistipes, to attenuate intestinal inflammation. These results demonstrate that PFGF can potentially alleviate alcoholic liver damage by restoring the intestinal barrier and regulating the intestinal microflora.
1. Introduction
Alcoholic liver damage (ALD) is the damage to liver function, and it is caused by drinking. ALD further develops into alcoholic liver diseases such as alcoholic fatty liver, inflammatory hepatitis, hepatic cirrhosis, and hepatic fibrosis [1]. The Global Status Report on Alcohol and Health (2018) published by the World Health Organization indicates that 3 million deaths occur every year as a result of harmful alcohol use [2]. Oxidative stress is the major cause of ALD. Oxidative stress is caused by reactive oxygen species (ROS) from hepatic alcohol metabolizers. Overwhelming oxidative stress causes cellular damage and produces hepatotoxic substances such as malondialdehyde [3]. Oxidative stress can also reduce the content of antioxidant factors such as superoxide dismutase and glutathione [4]. Chronic alcohol consumption damages the intestinal barrier, which leads to intestinal bacterial dysbiosis and increases the systemic levels of bacterial products. Harmful substances such as lipopolysaccharide transfer from the intestines to the liver and cause liver inflammation [5]. Currently, stopping alcohol consumption is the most helpful method of alleviating ALD, as considerable side effects are associated with the use of chemical drugs [6]. Thus, the search for less toxic treatments is a priority.
More recently, some studies have demonstrated an association between the intestinal microbiota and ALD [7]. The effect of alcohol on the intestinal microbiota is called dysbiosis [8]. Alcohol can increase the abundance of endotoxin-producing microbiota and decrease the abundance of short-chain fatty acids (SCFAs)-producing microbiota and anti-inflammatory microbiota [9,10]. Negative alterations in the intestinal microbiota increase the liver damage through the gut–liver axis [11]. In general, probiotics refer to live microorganisms that, when they are administered in adequate amounts, confer health benefits to the host. Probiotics can boost the immunological system, protect the gut, and positively regulate the intestinal microbiota [12]. In addition, probiotic fermentation can improve the functional characteristics of food. Fermented dairy foods have been consumed for many years as traditional probiotic fermented foods, and they have proven benefits [13]. At present, many studies of nondairy probiotic fermented foods have also been conducted. Zhang et al. found that blueberry juice fermented by Lactobacillus plantarum had increased antioxidant activity [14]. Hu et al. found that fermented carrot juice could attenuate type 2 diabetes by mediating the intestinal microbiota in rats [15].
Lactobacillus has a long history use as fermentation-engineering bacteria because they are beneficial in the prevention of intestinal diseases and the regulation of intestinal flora [16]. The strains that are commonly studied worldwide include: Lactobacillus acidophilus, Lactobacillus rhamnosus, and Pediococcus acidilactici, etc. [17]. Lactobacillus rhamnosus is one of the most widely studied probiotic strains, and it can attenuate inflammation, improve hydrogen-peroxide-induced damage, reduce the viability of pathogenic bacteria, regulate the intestinal microbiota, and regain intestinal barrier function, etc. [18]. Forsyth et al. found that gavage with Lactobacillus rhamnosus GG reduced the severity of alcohol-induced gut hyperpermeability and alcohol-induced tissue and systemic oxidative stress to ameliorate alcohol-induced gut leakiness and liver injury [19]. Lactobacillus acidophilus also has antibacterial and anti-inflammatory activities [20]. Lv et al. found that Lactobacillus acidophilus could alleviate D-GalN-induced liver injury in rats through downregulating the expression of the infection- and inflammation-related pathways [21]. Pediococcus acidilactici is a safe probiotic that is widely used in the food industry [22]. Zhang et al. found that Pediococcus acidilactici FZU106 could significantly reduce the accumulation of excessive lipid in the liver caused by high-fat diet feeding in mice [23]. Bikheet et al. found that Pediococcus acidilactici-fermented milk could treat the liver fibrosis caused by CCl4 [24].
Grifola frondosa is recognized as a functional food, and it has been extensively studied for its antitumor, immunoregulation, anti-inflammation, and hepatoprotective effects [25]. Grifola frondosa has ameliorating effects on liver damage. Chen et al. found that Grifola frondosa polysaccharides could inhibit liver lipid peroxidation in rats [26]. In our previous studies, we have found that 95% and 55% Grifola frondosa ethanol extract and Grifola frondosa heteropolysaccharide could improve glucose and lipid metabolism disorders by attenuating tissue inflammation, reducing the occurrence of liver lesions, and regulating the intestinal microbiota [27,28,29]. Therefore, Grifola frondosa has anti-inflammation and hepatoprotective abilities and improves the structure of the intestinal microbiota, so it shows potential for improving ALD. However, a few studies have been conducted on the ameliorating effects of Grifola frondosa on ALD. In this study, we describe the total active components of a probiotic fermentation of Grifola frondosa, and we determined the ability of the total active components of Grifola frondosa and its fermentation products to improve ALD.
2. Results and Discussion
2.1. Composition Analysis and Antioxidant Activities In Vitro
Data about the total sugar content (TS), reducing sugar content (RS), protein content (PR), total flavonoid content (TF), total polyphenol content (TP), and lactic acid content (LC) are shown in Table 1. Compared with the composition of the unfermented Grifola frondosa total active components (GF) sample, the TS, RS, and PR in the fermentation products of Grifola frondosa total active components (PFGF) group were significantly lower, TF and TP were not significantly different, while the LC was significantly higher. The results of the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity assay, the Fe2+ chelating activity assay (FCA), and the reducing power assay (RPA) are also shown in Table 1. The PFGF showed significantly higher antioxidant activities than GF did.

Table 1.
Composition and antioxidant activities of GF and PFGF; * indicates statistically significant differences (p < 0.05).
Grifola frondosa is rich in polysaccharides and proteins. In our previous studies, we found that Grifola frondosa extracts are also rich in flavonoids and polyphenols. These compositions are the fermentation substrates for probiotics, and they are the source of the antioxidant activity of Grifola frondosa [27]. Lactobacillus and Pediococcus acidilactici can use carbohydrates as an energy source to produce organic acids such as lactic acid [30,31]. During GF fermentation, the probiotics used the sugars and proteins in the fermentation system and produced a large amount of lactic acid.
Lactic acid is one of the important metabolites of Lactobacillus. Lactic acid can inhibit the growth of pathogenic bacteria by reducing the intestinal pH [32]. Wang et al. found that lactic acid has an antibacterial ability and can completely inhibit the growth of Salmonella enteritidis, E. coli, and L. monocytogenes cells [33]. This proved that lactic acid can maintain the homeostasis of intestinal flora by inhibiting the growth of harmful bacteria, thereby attenuating intestinal inflammation.
The antioxidant activity of GF also increased after fermentation, which is consistent with previous findings [14,34]. Lactobacillus can metabolize reducing substances, such as glutathione (GSH), which can play an antioxidant role [35]. In addition, the preservation of flavonoids and polyphenols and the production of bioactive polysaccharides and peptides with antioxidant activity that were produced by the enzymatic hydrolysis of probiotics also increased the antioxidant capacity of PFGF [36].
2.2. Effects of PFGF on Body Weight and Liver Index
The results of the body weight, liver weight, and liver index are shown in Figure 1A–D. After the alcohol gavage, the weight of the mice significantly decreased. After 6 weeks of intragastrical administration, compared with the model (EtOH) group, the probiotic (P) and GF groups showed no significant recovery in their body weight, whereas that of the PFGF group significantly increased. Compared with the EtOH group, the mice in the P, GF, and PFGF groups showed significantly decreased liver weights and liver indexes.
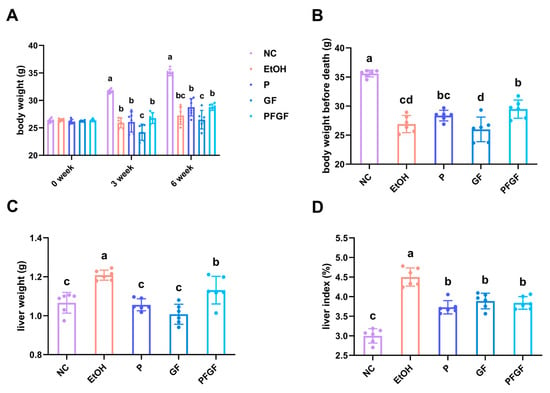
Figure 1.
Effects of P, GF, and PFGF on body weight (A), body weight before death (B), liver weight (C), and liver index (D) in alcohol-fed mice. Data are expressed as mean ± SD (n = 6). Different superscript letters indicate statistically significant differences between groups (p < 0.05).
Body weight is an important parameter indicating the physical state of the mice, and it can reflect the intestinal absorption of nutrients [2,37]. The liver index reflects the degree of liver damage [38]. The recovery of body weight indicated that PFGF could better restore the function of intestinal absorption, which might be related to the direct intake of lactic acid with PFGF [39]. The decline in the liver index indicated that P, GF and PFGF could decrease the amount of liver damage.
2.3. Effects of PFGF on Biochemical Parameters of Serum and Liver
The data about the serum total cholesterol (TC), triglyceride (TG), endotoxin (LPS), aspartate aminotransferase (AST), alanine aminotransferase (ALT), liver superoxide dismutase (SOD), GSH, LPS, and malondialdehyde (MDA) are shown in Figure 2A–I. After 6 weeks of intragastrical administration, compared with the M group, all of the biochemical parameters of the P, GF, and PFGF groups improved. Compared with the GF group, the TC of the PFGF group significantly decreased. Compared with that of the P group, the SOD of the PFGF group was also significantly increased.
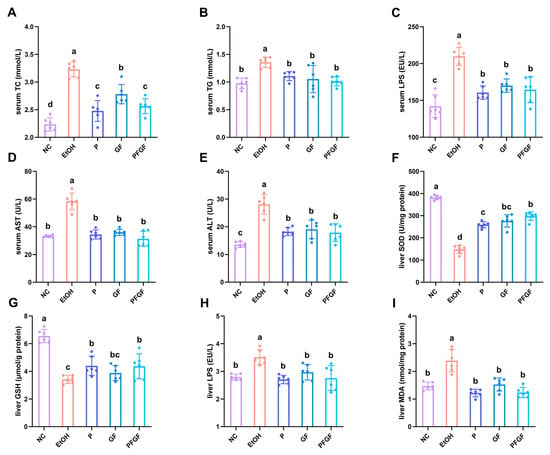
Figure 2.
Effects of P, GF, and PFGF on serum TC (A), TG (B), LPS (C), AST (D), and ALT (E), and liver SOD (F), GSH (G), LPS (H), and MDA (I) in alcohol-fed mice. Data are expressed as mean ± SD (n = 6). Different superscript letters indicate statistically significant differences between groups (p < 0.05).
The TC and TG in the serum reflect the liver’s function. The AST and ALT in the serum reflect the levels of hepatocyte necrosis [40]. LPS and MDA are major factors in inflammation and liver damage, whereas SOD and GSH are antioxidative factors that protect the liver against the excessively increased levels of LPS and MDA [41,42].
The amelioration of the biochemical parameters in this study indicated that P, GF, and PFGF had a recovery effect on the liver damage, of which PFGF produced the best effect. The metabolites of P in the intestinal tract, GF and PFGF, show a favorable antioxidant activity, which can reduce the alcohol-induced intestinal inflammation [43]. Both the probiotics and Grifola frondosa have a positive intestinal microbiota regulation ability [23,44,45]. They have the ability to attenuate the intestinal inflammation caused by the alcohol-induced flora disorder. The amelioration of intestinal inflammation decreases intestinal permeability, preventing harmful bacterial products from entering the blood system by crossing the intestinal barrier, recovering the liver from injury, and further improving the liver’s function.
2.4. Effects of PFGF on Liver and Cecal Tissue
The histopathology results of the liver and cecal tissue are shown in Figure 3. The clear staining of the liver cells of the normal control (NC) group showed that they were orderly. There were many binucleate cells, but there were no necrotic cells. The EtOH group showed obvious inflammatory infiltration in the liver tissue. The cells showed notable ulceration and necrosis. We observed almost no binuclear cells. With intraperitoneal administration, we observed no notable ulceration or necrosis of the liver cells in the P, GF and PFGF groups; the liver tissue remained in good condition.
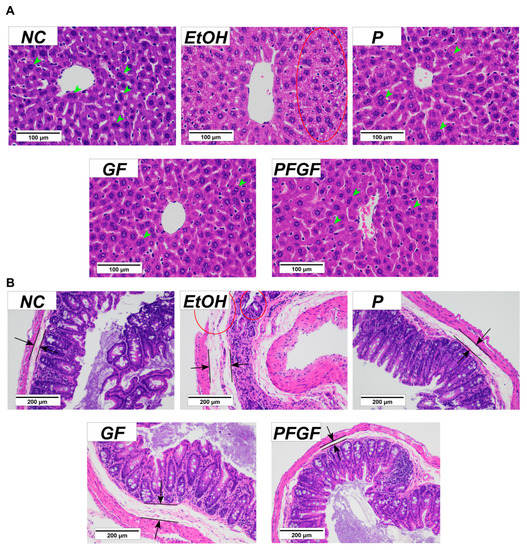
Figure 3.
Results of histopathological analysis of liver (A) and cecal (B) tissues in different groups with hematoxylin and eosin staining at 400× and 200× magnification, respectively. Histopathologic characteristics are marked in the figure. Binucleate cells are marked by green arrows, lipid layer is marked by black arrows and tissue damage is marked by red circle.
The cecal tissue of the NC group was clearly stained. The cecal villi were intact and neatly arranged, and the cecal wall was intact, with a thin lipid layer. The cecal tissue of the EtOH group showed substantial inflammation. The cecal villi were broken, the cecal wall was damaged, and the lipid layer was thickened. With intraperitoneal administration, the integrity and orderliness of the intestinal villi in the P, GF and PFGF groups were maintained, as was the integrity of the intestinal wall. A thin lipid layer was maintained in both the P and PFGF groups, but the GF group showed a thickening of the lipid layer.
The histopathology of the cecal tissue can reflect the states of the intestinal barrier and inflammation in the intestine. In our previous study, the compounds added with GF improved the jejunum tissue and reduced the level of IL-6 in type 2 diabetes mice, which indicates that GF can alleviate intestinal inflammation [45]. The results of the cecal tissue sections showed that the P, GF, and PFGF treatments had protective effects on the intestinal barrier. These protective effects were also reflected in the liver tissue sections. However, the cecal tissue in the GF group also showed inflammation, which suggested that P and PFGF could better protect the intestinal barrier.
2.5. Effects of PFGF on Composition of Intestinal Microbiota
The results of the 16S rRNA gene sequencing and the values of the relative abundance of Firmicutes and Bacteroides are shown in Figure 4A–C. The relative abundance of intestinal microbiota at phylum and genus are shown in Tables S1 and S2. At the phylum level (Figure 4A), the intestinal microbiota was mainly composed of Firmicutes, Bacteroidetes, Proteobacteria, and Actinbacteria. Compared with those of the M group, the relative abundance of Firmicutes in the GF and PFGF groups was significantly lower, but the relative abundance of Bacteroides in the NC, P, GF, and PFGF group were significantly higher. At the genus level (Figure 4B), the intestinal microbiota was mainly composed of Faecalibaculum, Lactobacillus, Dubosiella, Bacteroides, and Alloprevotella. The relative abundance of Lactobacillus in the P and PFGF groups was significantly higher than that in the other groups. Figure 4C shows that the Firmicutes and Bacteroides value of the EtOH group was significantly higher than that of the NC group. After the gavage treatment, the values for the P, GF, and PFGF group significantly decreased.
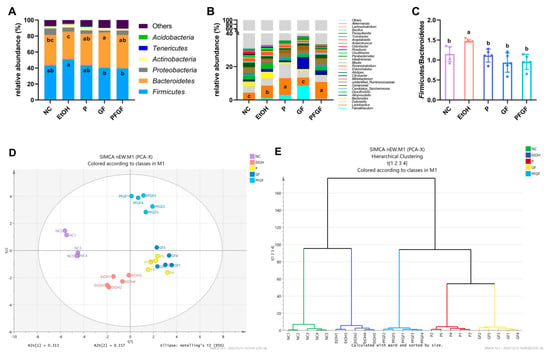
Figure 4.
Components of intestinal microbiota at phylum (A) and genus (B) levels; value of relative abundance of Firmicutes/Bacteroides (C); PCA score (D); hierarchical clustering; (E) plots of intestinal microbiota at genus level in different groups. Different superscript letters indicate statistically significant differences between groups (p < 0.05).
Lactobacillus acidophilus, Lactobacillus rhamnosus, and Pediococcus acidilactici all belong to Lactobacillus and Firmicutes. The increase in the relative abundance of Lactobacillus in the P and PFGF groups indicated that the probiotics had successfully colonized the mouse intestines. The intestinal microbiota can reflect the health status of the intestine; the Firmicutes/Bacteroides value can especially reflect intestinal inflammation and the intestinal permeability [46].
The PCA score plot and hierarchical clustering plot are shown in Figure 4D,F, which shows a substantial separation between the NC, EtOH, PFGF, P, and GF groups. The NC group is clustered on the negative half of the axis of the first principal component (PC1), while the EtOH group is clustered on the negative half of the axis of PC2, the P and GF groups are clustered on the positive half of the axis of PC1, and the PFGF group is clustered on the positive half of the axis of PC2. The hierarchical clustering plot shows that the samples of each group were clustered in the same class, and they were significantly different from the other groups.
PCA and hierarchical clustering are often used to analyze the similarities and differences between groups of intestinal microbiotas [47]. In the PCA score plot, compared with the NC group, the EtOH group significantly shifted to the negative axis of PC2, indicating that alcohol intake affected the host’s intestinal microbiota. The P, GF, and PFGF groups were distant from the NC and EtOH groups, indicating that P, GF, and PFGF all had the ability to regulate the intestinal microbiota. Similar results are also reflected in the hierarchical clustering plot.
2.6. Correlations of Intestinal Microbiota with Biochemical Parameters
The taxa with an LDA score threshold of >3.0 are shown in Figure 5A as a bar chart. Alistipes, Odoribacter, and Angelakisella, etc., were the characteristic microbes in the NC group. Bacteroides, Desulfovibrio, and Blautia, etc., were the characteristic microbes in the EtOH group. Lactobacillus was the characteristic microbe in the P group. Faecalibaculum, Alloprevotella, and Bifidobacterium were the characteristic microbes in the GF group. Unidentified_Ruminococcaceae and Parabacteroides, etc., were the characteristic microbes in the PFGF group.
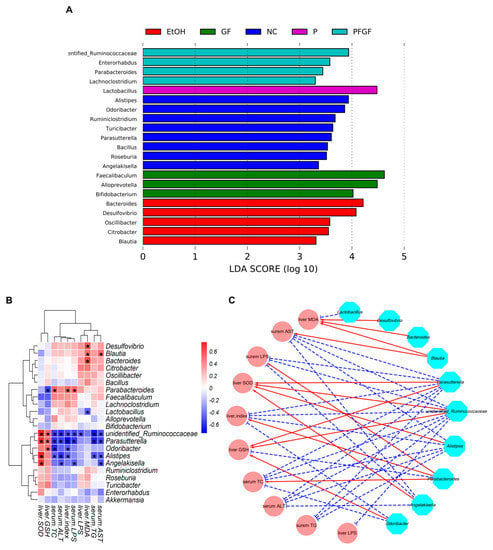
Figure 5.
LEfSe analysis of intestinal microbiota at genus-level among NC, EtOH, P, GF, and PFGF groups (A). Heatmap of correlations between significant different intestinal microbiota and biochemical parameters (B); * indicates Spearman’s rank correlation coefficient > 0.5 or < −0.5. Visualization of correlation network. Cyan nodes: intestinal microbial genera; pink nodes: parameters; red lines: Spearman’s rank correlation coefficient > 0.5, adjusted p < 0.01; blue lines: Spearman’s rank correlation coefficient < −0.5, adjusted p < 0.01 (C).
A heatmap of the correlations between the significantly different intestinal microbiota and biochemical parameters and a visualization of the correlation network are shown in Figure 5B,C. The correlation heatmap shows that Lactobacillus, unidentified_Ruminococcaceae, Parabacteroides, Odoribacter, Alistipes, and Angelakisella were positively correlated with the serum TC, TG, AST, ALT, and LPS and liver MDA, LPS, and index, and they were negatively correlated with the liver GSH and SOD. The opposite results were reflected in Bacteroides, Desulfovibrio, and Blautia. Squares indicating significant correlations (|r| > 0.5 and ρ < 0.01) are marked with an asterisk, and their correlations are shown in the visualization of the correlation network. These intestinal microbiotas that were significantly associated with biochemical indicators were defined as key intestinal microbiota, and their relative abundances are shown in Figure S1A–J.
In Section 1, we mentioned many benefits of Lactobacillus. Except for the opposite correlation between the relative abundance of Lactobacillus and liver LPS, we found no significant correlation between the relative abundance of Lactobacillus and the biochemical indicators in this study. This may have been caused by the relative abundance of Lactobacillus in the EtOH group which was also significantly increased compared with that in the NC group. Bull et al. also found that chronic alcohol feeding resulted in an increase in the relative abundance of Lactobacillus [48]. Recently, a study showed that not all Lactobacillus have positive effects [49]. Chronic alcohol feeding may increase the relative abundance of harmful Lactobacillus.
Ruminococcaceae is one of the hallmark bacteria of intestinal inflammation, which is reduced in colitis patients [50]. In this study, alcohol intake significantly reduced the relative abundance of Ruminococcaceae, but the PFGF treatment restored its relative abundance to normal levels. Shang et al. [51] found that the abundance of Ruminococcaceae in the intestinal microbiota negatively correlated with liver cirrhosis, nonalcoholic fatty liver, and increased intestinal permeability. We obtained similar results in our study. Lee et al. [52] also reported that colonization of Ruminococcus faecis and Ruminococcus bromii significantly improved the liver injury in nonalcoholic fatty liver mice.
Parabacteroides, as one of the core flora of human intestinal flora, has the ability to participate in carbohydrate metabolism and secrete short-chain fatty acids [53]. Parabacteroides was the characteristic microbe of the PFGF group, but in this study, we found a negative correlation between the relative abundance of Parabacteroides and the parameters of the liver’s function. This may have occurred because the relative abundance of Parabacteroides in the EtOH group was significantly higher than that in the NC, P, and GF groups. Li et al. and Liu et al. also found that the relative abundance of Parabacteroides in the intestinal flora of mice increased after alcohol feeding [54,55]. Their findings all showed that an increase in the relative abundance of Parabacteroides could improve the biochemical parameters of chronically alcohol-exposed mice.
Parasutterella is a core component of the human and mouse intestinal microbiota. Ju et al. found that Parasutterella can produce succinate, which can recover colitis [56,57]. Huang et al. found that Parasutterella plays a positive role in the production of short-chain fatty acids and the reduction in inflammation [49]. In this study, P, GF, and PFGF could significantly increase the relative abundance of Parasutterella, and we found a strong relationship between the abundance of Parasutterella and the biochemical parameters. Mu et al. found that the relative abundance of Parasutterella negatively correlated with TC and TG, which is consistent with our findings [58].
Alistipes and Odoribacter were common beneficial intestinal bacteria in our previous studies. Wang et al. and Pan et al. found that the relative abundance of Alistipes positively correlated with SOD and GSH, and it was negatively correlated with TC, TG, AST, and ALT [27,29]. Odoribacter is short-chain fatty-acids-producing bacteria, and it is thought to promote blood lipid metabolism [59,60]. Desulfovibrio is an intestinally harmful bacteria. They are a class of anaerobic bacteria that can reduce sulfate to producing H2S. Endogenic H2S can poison intestinal epithelial cells, causing intestinal leakage, abdominal pain, and chronic inflammation, etc. [61]. In this study, the PFGF restored the relative abundance of Alistipes and reduced the relative abundance of Desulfovibrio.
These results indicated that PFGF can regulate the microbial population structure, restore or increase the relative abundance of beneficial bacteria, and reduce the relative abundance of harmful bacteria, thus alleviating the effects of alcoholic liver damage.
3. Materials and Methods
3.1. Extraction of Active Components
We improved upon our previously reported method of extracting total active ingredients from Grifola frondosa (provided by the China National Engineering Research Center of JUNCAO Technology, Fujian Agriculture and Forestry University). In short, we extracted Grifola frondosa at different concentrations of alcohol under an ultrasound (45 kHz, 300 W) for one hour. We collected the supernatant, and we collected and dried the residue for the next extraction. After extraction with 95% (v/v) ethanol (10:1, v/w, 45 °C), 55% (v/v) ethanol (10:1, v/w, 45 °C), and deionized water (30:1, v/w, 75 °C), we collected the residue and added deionized water (10:1, v/w, 55 °C). We then adjusted the pH to 7.5 with NaHCO3, and we used papain (purchased from Solarbio Technology Co., Ltd., Beijing, China) to obtain the hydrolyzed protein. We collected the supernatant after 4 h of enzymatic hydrolysis. Finally, we filtered, concentrated, and freeze-dried all of the supernatants to obtain the Grifola frondosa total active components.
3.2. Probiotic Strain and Inoculum Preparation
Lactobacillus rhamnosus (CICC number 20255, which we purchased from China Center of Industrial Culture Collection, Beijing, China) and Pediococcus acidilactici (CICC number 10146, purchased from China Center of Industrial Culture Collection, China) were recovered and inoculated into an MRS medium (Merck & Company, Inc., Darmstadt, Germany). We recovered Lactobacillus acidophilus (Provided by College of Food Science, Fujian Agriculture and Forestry University, Fuzhou, China), which we inoculated into Bengal red medium (Merck & Company, Inc., Darmstadt, Germany), and cultured the sample at 37 °C for 24 h, and then centrifuged it at 800× g for 10 min at 4 °C. We collected and resuspended the precipitate with normal saline. Finally, we mixed all of the probiotics at 1:1:1 by mass and resuspended them. We calculated the bacterial concentration by the plate-counting method, and we diluted the probiotics with normal saline to 1 × 109 CFU/mL for later use.
3.3. Preparation of Probiotic Fermentation Products
The fermentation system included 0.4 g of glucose, 99 mL of deionized water, and 10 g of pasteurized Grifola frondosa total active components. We adjusted the pH of the fermentation system to 6.5 with NaHCO3. Then, we added 1 mL of Lactobacillus rhamnosus, Pediococcus acidilactici, and Lactobacillus acidophilus bacterial solutions. After fermentation at 37 °C for 48 h and after we reached a total bacterial concentration > 1 × 109 CFU/mL, we collected the suspension to obtain the fermentation products of Grifola frondosa total active components (PFGF). The unfermented Grifola frondosa total active components (GF) served as the control.
3.4. Composition Determination
We measured the total sugar content (TS), reducing sugar content (RS), protein content (PR), total flavonoid content (TF), total polyphenol content (TP), and lactic acid content (LC) in GF and PFGF. We used the phenol–sulfuric acid method to determine the TS [62]. We used the dinitrosalicylic acid method was used to determine the RS [63]. We determined the PR according to a BCA kit (Lablead Biotechnology Co., Ltd., Beijing, China). We determined the TF and TP by referring to the method of Toro-Uribe et al. [64]. We determined the lactic acid content according to an LD kit (Nanjing Jiancheng Bioengineering Co., Ltd., Nanjing, China). We purchased the reagents used from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China).
3.5. Determination of Antioxidant Activities In Vitro
For the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity assay and the Fe2+ chelating activity assay (FCA) of GF and PFGF, we referred to the method of Nilgün Ozdemir et al. [65]. For the reducing power assay (RPA), we referred to the method of Cao et al. [66]. We purchased the reagents used from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China).
3.6. Animal Model, Administration, and Sample Collection
We purchased 50 male ICR mice from Wu’s Animal Center (Fuzhou, China), which we housed in a chamber (23 ± 2 °C) with a 12 h dark/light cycle and free access to food and water. After 1 week of acclimatization, we randomly divided the mice into 5 groups. We intragastrically administered all of the mice in the morning, and we administered the probiotic (P, n = 10) group, the unfermented Grifola frondosa total active components (GF, n = 10) group, and the fermentation products of Grifola frondosa total active components (PFGF, n = 10) group by gavage with probiotics (100 mg/kg), GF (100 mL/kg) and PFGF (100 mL/kg), respectively. We administered the normal control (NC, n = 10) group and model (EtOH, n = 10) group by gavage with equal volumes of normal saline instead. We administered the second gavage to all of the mice in the afternoon, and we administered the EtOH, P, GF, and PFGF groups by gavage with 52% liquor alcohol (10 mL/kg, 36% liquor alcohol in the first week). The NC group was administered by gavage with an equal volume of normal saline instead. We measured their body weight every 2 weeks. After 6 weeks of gavage, we sacrificed all of the mice by cervical dislocation after weighing, and we retained the serum, liver, cecum, and intestinal contents, which we stored at −80 °C until further use.
3.7. Biochemical Parameters of Serum and Liver
We weighed the mouse livers, and we calculated liver index (the ratio of liver weight to body weight). We determined the contents of superoxide dismutase (SOD), glutathione (GSH), endotoxin (LPS), and malondialdehyde (MDA) using a lipopolysaccharide ELISA kit, a superoxide dismutase assay kit, a malondialdehyde assay kit, and a reduced glutathione assay kit, respectively. We centrifuged the serum and removed the supernatant. We determined the contents of total cholesterol (TC), triglyceride (TG), LSP, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) using an aspartate aminotransferase assay kit, an alanine aminotransferase assay kit, a lipopolysaccharide ELISA kit, a total cholesterol assay kit, and a triglyceride assay kit, respectively. We purchased all of the kits from Nanjing Jiancheng Bioengineering Co., Ltd. (Nanjing, China).
3.8. Preparation of Liver and Cecal Tissue Sections
We processed liver and cecal samples according to the method of Huang et al. [47], which we then stained with hematoxylin and eosin. We observed the liver and cecal tissue sections under an optical microscope (Nikon, Tokyo, Japan), and we performed the histopathological evaluation according to Roth et al. [67].
3.9. High-Throughput Sequencing Analysis of Intestinal Microbiota
We extracted genomic DNA from intestinal contents according to the method of Ge et al. [68]. We amplified the V3−V4 region of the bacterial 16S rRNA gene using universal primers 338F and 806R. We produced the 16S rRNA gene sequencing libraries of bacteria with a TruSeq DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, CA, USA) [69]. Novogene Co., Ltd. (Beijing, China) provided the Illumina NovaSeq PE250 platform for us to complete high-throughput sequencing.
We drew the rank abundance curves using the “ggalt” and “BiodiversityR” packages in R software (ver. 4.1.0). We conducted a principal component analysis (PCA) of the intestinal microbiota at the genus level with SIMCA-14.1. We used the Huttenhower Lab Galaxy server (http://huttenhower.sph.harvard.edu/lefse/, accessed on 3 August 2022) to analyze variation in the different experimental groups with the linear discriminant analysis (LDA) effect size (LEfSe) algorithm (threshold > 3). We calculated the relationship between the relative abundance of the biochemical parameters and intestinal microbiota by Spearman’s rank correlation analysis, and we visualized the results using the “psych” and “pheatmap” packages in R software (ver. 4.1.0). We plotted the correlation network with Cytoscape software (ver. 3.7.1).
3.10. Statistical Analysis
We analyzed all of the data with SPSS statistics 17.0, and we drew figures using GraphPad Prism 9. All of the data are shown as the mean ± standard deviation (SD). We used Student’s t-test to determine statistical significance between two groups. We considered p < 0.05 statistically significant. We used one-way analysis of variance (ANOVA) with Tukey’s correction to determine the statistical significance between multiple groups. We considered p < 0.05 statistically significant.
4. Conclusions
We revealed that P, GF, and PFGF can alleviate alcoholic liver damage in chronically alcohol-fed mice. In addition, PFGF produced the best alleviation effect. PFGF could restore the intestinal barrier function by attenuating intestinal inflammation, thus reducing the amount of harmful substances entering the liver through the gut–liver axis, alleviating liver damage, and improving the liver’s function. Moreover, PFGF could restore the homeostasis of intestinal flora and increase the relative abundance of beneficial bacteria such as Lactobacillus, Ruminococcaceae, Parabacteroids, Parasutterella, and Alistipes, etc., to attenuate intestinal inflammation. However, the molecular mechanism of PFGF in improving alcoholic liver disease and the interaction of intestinal flora on alcoholic liver disease need further study and verification. Overall, this study provides a scientific basis for the processing and functional improvement caused by Grifola frondosa.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24021406/s1. Table S1: The relative abundance of intestinal microbiota at phylum.; Table S2: The relative abundance of intestinal microbiota at genus. Figure S1: The relative abundance of key intestinal microbiota.
Author Contributions
Conceptualization, X.-Y.H., Y.-X.Z. and B.L.; methodology, X.-Q.J. and F.-R.Z.; software, X.-Y.H.; validation, X.-Y.H., Y.-J.L. and Z.-R.H.; formal analysis, Y.-Y.Q.; investigation, Y.-X.Z.; resources, B.L.; data curation, X.-Y.H., F.Z. and B.L.; writing—original draft preparation, X.-Y.H.; writing—review and editing, X.-Y.H., Z.-R.H. and F.Z.; visualization, X.-Y.H.; supervision, F.Z.; project administration, B.L.; funding acquisition, B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fujian Province Science and Technology Major Special Projects (2021NZ0101), Interdisciplinary Integration to Promote the High-quality Development Projects of Juncao Science and Industry (XKJC-712021030), and Science and Technology Planning Project of Fujian Province (2021L3007).
Institutional Review Board Statement
The animal experimental protocols were conducted by the regulations of the Welfare Committee of the College of Food Science, Fujian Agriculture and Forestry University, China (Approval number: JC-2021-020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Thank Yin Fei for her support and help with the writing process.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Axley, P.D.; Richardson, C.T.; Singal, A.K. Epidemiology of Alcohol Consumption and Societal Burden of Alcoholism and Alcoholic Liver Disease. Clin. Liver Dis. 2019, 23, 39–50. [Google Scholar] [CrossRef]
- Xiao, T.; Luo, Z.; Guo, Z.; Wang, X.; Ding, M.; Wang, W.; Shen, X.; Zhao, Y. Multiple Roles of Black Raspberry Anthocyanins Protecting against Alcoholic Liver Disease. Molecules 2021, 26, 2313. [Google Scholar] [CrossRef]
- Hsu, J.; Lin, H.; Hsu, C.; Chen, B.; Chen, J. Aqueous Extract of Pepino (Solanum muriactum Ait) Leaves Ameliorate Lipid Accumulation and Oxidative Stress in Alcoholic Fatty Liver Disease. Nutrients 2018, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Han, K. Relationships among alcoholic liver disease, antioxidants, and antioxidant enzymes. World J. Gastroenterol. 2016, 22, 37. [Google Scholar] [CrossRef]
- Yang, A.; Inamine, T.; Hochrath, K.; Chen, P.; Wang, L.; Llorente, C.; Bluemel, S.; Hartmann, P.; Xu, J.; Koyama, Y.; et al. Intestinal fungi contribute to development of alcoholic liver disease. J. Clin. Investig. 2017, 127, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef]
- Day, A.W.; Kumamoto, C.A. Gut Microbiome Dysbiosis in Alcoholism: Consequences for Health and Recovery. Front. Cell. Infect. Microbiol. 2022, 12, 198. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.P.; Suk, K.T.; Kim, D.J. Significance of gut microbiota in alcoholic and non-alcoholic fatty liver diseases. World J. Gastroenterol. 2021, 27, 6161–6179. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Kakiyama, G.; Zhao, D.; Takei, H.; Fagan, A.; Hylemon, P.; Zhou, H.; Pandak, W.M.; Nittono, H.; Fiehn, O.; et al. Continued Alcohol Misuse in Human Cirrhosis is Associated with an Impaired Gut-Liver Axis. Alcoholism 2017, 41, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.W.; Fouts, D.E.; Brandl, J.; Starkel, P.; Torralba, M.; Schott, E.; Tsukamoto, H.; Nelson, K.E.; Brenner, D.A.; Schnabl, B. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology 2011, 53, 96–105. [Google Scholar] [CrossRef]
- Bajaj, J.S. Alcohol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Caroline, N.A.; Carine, N.A.; Rafael, C.R.M.; Anderson, S.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016, 58, 96–114. [Google Scholar] [CrossRef]
- Ranadheera, C.; Vidanarachchi, J.; Rocha, R.; Cruz, A.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Wei, Z.; Yin, B.; Man, C.; Jiang, Y. Enhancement of functional characteristics of blueberry juice fermented by Lactobacillus plantarum. LWT 2021, 139, 110590. [Google Scholar] [CrossRef]
- Hu, R.; Zeng, F.; Wu, L.; Wan, X.; Chen, Y.; Zhang, J.; Liu, B. Fermented carrot juice attenuates type 2 diabetes by mediating gut microbiota in rats. Food Funct. 2019, 10, 2935–2946. [Google Scholar] [CrossRef] [PubMed]
- Molin, G. Probiotics in foods not containing milk or milk constituents, with special reference to Lactobacillus plantarum 299v1. Am. J. Clin. Nutr. 2001, 73, 380s–385s. [Google Scholar] [CrossRef] [PubMed]
- Amara, A.A.; Shibl, A. Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharm. J. 2015, 23, 107–114. [Google Scholar] [CrossRef]
- Segers, M.E.; Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG-host interactions. Microb. Cell Fact. 2014, 13 (Suppl. 1), S7. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Farhadi, A.; Jakate, S.M.; Tang, Y.; Shaikh, M.; Keshavarzian, A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol 2009, 43, 163–172. [Google Scholar] [CrossRef]
- Hiramatsu, Y.; Satho, T.; Irie, K.; Shiimura, S.; Okuno, T.; Sharmin, T.; Uyeda, S.; Fukumitsu, Y.; Nakashima, Y.; Miake, F.; et al. Differences in TLR9-dependent inhibitory effects of H2O2-induced IL-8 secretion and NF-kappa B/I kappa B-alpha system activation by genomic DNA from five Lactobacillus species. Microbes Infect. 2013, 15, 96–104. [Google Scholar] [CrossRef]
- Lv, L.; Yao, C.; Yan, R.; Jiang, H.; Wang, Q.; Wang, K.; Ren, S.; Jiang, S.; Xia, J.; Li, S.; et al. Lactobacillus acidophilus LA14 Alleviates Liver Injury. mSystems 2021, 6, e38421. [Google Scholar] [CrossRef]
- Barbosa, J.; Borges, S.; Teixeira, P. Pediococcus acidilactici as a potential probiotic to be used in food industry. Int. J. Food Sci. Technol. 2015, 50, 1151–1157. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, W.; Chen, G.; Qian, M.; Han, J.; Lv, X.; Chen, L.; Rao, P.; Ai, L.; Ni, L. Pediococcus acidilactici FZU106 alleviates high-fat diet-induced lipid metabolism disorder in association with the modulation of intestinal microbiota in hyperlipidemic rats. Curr. Res. Food Sci. 2022, 5, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Bikheet, M.M.; Mahmoud, M.E.; Yassien, E.E.; Hassan, H.M. Effect of lactic acid bacteria isolated from some fermented dairy products on carbon tetrachloride-induced hepatotoxicity and nephrotoxicity of albino rats. Environ. Sci. Pollut. Res. 2022, 29, 11790–11800. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X.; Zhao, Z. Polysaccharides in Grifola frondosa mushroom and their health promoting properties: A review. Int. J. Biol. Macromol. 2017, 101, 910–921. [Google Scholar] [CrossRef]
- Chen, G.; Ma, X.; Liu, S.; Liao, Y.; Zhao, G. Isolation, purification and antioxidant activities of polysaccharides from Grifola frondosa. Carbohydr. Polym. 2012, 89, 61–66. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, F.; Liu, Y.; Pan, Y.; Xu, J.; Ge, X.; Zheng, H.; Pang, J.; Liu, B.; Huang, Y. Coumarin-rich Grifola frondosa ethanol extract alleviate lipid metabolism disorders and modulates intestinal flora compositions of high-fat diet rats. J. Funct. Food. 2021, 85, 104649. [Google Scholar] [CrossRef]
- Li, X.; Zeng, F.; Huang, Y.; Liu, B. The Positive Effects of Grifola frondosa Heteropolysaccharide on NAFLD and Regulation of the Gut Microbiota. Int. J. Mol. Sci. 2019, 20, 5302. [Google Scholar] [CrossRef]
- Pan, Y.Y.; Zeng, F.; Guo, W.L.; Li, T.T.; Jia, R.B.; Huang, Z.R.; Lv, X.C.; Zhang, J.; Liu, B. Effect of Grifola frondosa 95% ethanol extract on lipid metabolism and gut microbiota composition in high-fat diet-fed rats. Food Funct. 2018, 9, 6268–6278. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, S.; Lu, J.; Zhang, C.; Pang, X.; Lv, J. Screening for Cholesterol-Lowering Probiotics from Lactic Acid Bacteria Isolated from Corn Silage Based on Three Hypothesized Pathways. Int. J. Mol. Sci. 2019, 20, 2073. [Google Scholar] [CrossRef]
- Qiu, Z.; Fang, C.; Gao, Q.; Bao, J. A short-chain dehydrogenase plays a key role in cellulosic D-lactic acid fermentability of Pediococcus acidilactici. Bioresour. Technol. 2020, 297, 122473. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chen, W.; Chen, H.; Chen, W.; Zhong, Q. Combination of Lactobacillus plantarum and Saccharomyces cerevisiae DV10 as Starter Culture to Produce Mango Slurry: Microbiological, Chemical Parameters and Antioxidant Activity. Molecules 2019, 24, 4349. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Wouters, D.; Bernaert, N.; Anno, N.; Van Droogenbroeck, B.; De Loose, M.; Van Bockstaele, E.; De Vuyst, L. Application’ and validation of autochthonous lactic acid bacteria starter cultures for controlled leek fermentations and their influence on the antioxidant properties of leek. Int. J. Food Microbiol. 2013, 165, 121–133. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Zhang, L.; Zhang, X.; Huang, L.; Li, D.; Niu, C.; Yang, Z.; Wang, Q. Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food Chem. 2012, 135, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef]
- Zhang, P.; Jiao, H.; Wang, C.; Lin, Y.; You, S. Chlorogenic Acid Ameliorates Colitis and Alters Colonic Microbiota in a Mouse Model of Dextran Sulfate Sodium-Induced Colitis. Front. Physiol. 2019, 10, 325. [Google Scholar] [CrossRef]
- Kumstel, S.; Vasudevan, P.; Palme, R.; Zhang, X.; Wendt, E.H.U.; David, R.; Vollmar, B.; Zechner, D. Benefits of non-invasive methods compared to telemetry for distress analysis in a murine model of pancreatic cancer. J. Adv. Res. 2020, 21, 35–47. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef]
- Wang, R.; Yang, Z.; Zhang, J.; Mu, J.; Zhou, X.; Zhao, X. Liver Injury Induced by Carbon Tetrachloride in Mice Is Prevented by the Antioxidant Capacity of Anji White Tea Polyphenols. Antioxidants 2019, 8, 64. [Google Scholar] [CrossRef]
- Thakur, V. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: Role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-α production. J. Leukoc. Biol. 2006, 79, 1348–1356. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Hu, X.; Liu, Z.; Chen, J.; Lu, Y.; Liu, J.; Liao, S.; Zhang, Y.; Liang, R.; et al. The hepatoprotective role of reduced glutathione and its underlying mechanism in oxaliplatin-induced acute liver injury. Oncol. Lett. 2018, 15, 2266–2272. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, A.; Zielińska, M.; Storr, M.; Fichna, J. Beneficial Effects of Probiotics, Prebiotics, Synbiotics, and Psychobiotics in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liang, S.; Zhang, Y.; Deng, Y.; He, Y.; Chen, Y.; Liu, C.; Lin, C.; Yang, Q. Oral Administration of Compound Probiotics Ameliorates HFD-Induced Gut Microbe Dysbiosis and Chronic Metabolic Inflammation via the G Protein-Coupled Receptor 43 in Non-alcoholic Fatty Liver Disease Rats. Probiotics Antimicrob. Proteins 2019, 11, 175–185. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, C.; Zhu, Y.; Jiang, X.; Qiu, Y.; Yin, F.; Xiong, W.; Liu, B.; Huang, Y. Spirulina compounds show hypoglycemic activity and intestinal flora regulation in type 2 diabetes mellitus mice. Algal Res. 2022, 66, 102791. [Google Scholar] [CrossRef]
- Xiong, F.; Wu, S.; Zhang, J.; Jakovlić, I.; Li, W.; Zou, H.; Li, M.; Wang, G. Dietary Bile Salt Types Influence the Composition of Biliary Bile Acids and Gut Microbiota in Grass Carp. Front. Microbiol. 2018, 9, 2209. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, L.; Zhu, F.; Liu, Y.; Xiao, J.; Chen, Z.; Lv, X.; Huang, Y.; Liu, B. Anti-Diabetic Effects of Ethanol Extract from Sanghuangporous vaninii in High-Fat/Sucrose Diet and Streptozotocin-Induced Diabetic Mice by Modulating Gut Microbiota. Foods 2022, 11, 974. [Google Scholar] [CrossRef]
- Bull-Otterson, L.; Feng, W.; Kirpich, I.; Wang, Y.; Qin, X.; Liu, Y.; Gobejishvili, L.; Joshi-Barve, S.; Ayvaz, T.; Petrosino, J.; et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS ONE 2013, 8, e53028. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, Q.; Chen, K.; Huang, Z.; Liu, Y.; Jia, R.; Liu, B. Sanghuangporus vaninii fruit body polysaccharide alleviates hyperglycemia and hyperlipidemia via modulating intestinal microflora in type 2 diabetic mice. Front. Nutr. 2022, 9, 1013466. [Google Scholar] [CrossRef]
- Sinha, S.R.; Haileselassie, Y.; Nguyen, L.P.; Tropini, C.; Wang, M.; Becker, L.S.; Sim, D.; Jarr, K.; Spear, E.T.; Singh, G.; et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microbe 2020, 27, 659–670. [Google Scholar] [CrossRef]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef]
- Vojinovic, D.; Radjabzadeh, D.; Kurilshikov, A.; Amin, N.; Wijmenga, C.; Franke, L.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Relationship between gut microbiota and circulating metabolites in population-based cohorts. Nat. Commun. 2019, 10, 5813. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef]
- Li, B.; Mao, Q.; Zhou, D.; Luo, M.; Gan, R.; Li, H.; Huang, S.; Saimaiti, A.; Shang, A.; Li, H. Effects of Tea against Alcoholic Fatty Liver Disease by Modulating Gut Microbiota in Chronic Alcohol-Exposed Mice. Foods 2021, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, M.; Fu, X.; Zhang, Z.; Zhu, L.; Zheng, X.; Liu, J. Astaxanthin Prevents Alcoholic Fatty Liver Disease by Modulating Mouse Gut Microbiota. Nutrients 2018, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Kong, J.Y.; Stothard, P.; Willing, B.P. Defining the role of Parasutterella, a previously uncharacterized member of the core gut microbiota. ISME J. 2019, 13, 1520–1534. [Google Scholar] [CrossRef] [PubMed]
- Osaka, T.; Moriyama, E.; Arai, S.; Date, Y.; Yagi, J.; Kikuchi, J.; Tsuneda, S. Meta-Analysis of Fecal Microbiota and Metabolites in Experimental Colitic Mice during the Inflammatory and Healing Phases. Nutrients 2017, 9, 1329. [Google Scholar] [CrossRef]
- Mu, H.; Zhou, Q.; Yang, R.; Zeng, J.; Li, X.; Zhang, R.; Tang, W.; Li, H.; Wang, S.; Shen, T.; et al. Naringin Attenuates High Fat Diet Induced Non-alcoholic Fatty Liver Disease and Gut Bacterial Dysbiosis in Mice. Front. Microbiol. 2020, 11, 585066. [Google Scholar] [CrossRef]
- Göker, M.; Gronow, S.; Zeytun, A.; Nolan, M.; Lucas, S.; Lapidus, A.; Hammon, N.; Deshpande, S.; Cheng, J.; Pitluck, S.; et al. Complete genome sequence of Odoribacter splanchnicus type strain (1651/6T). Stand. Genom. Sci. 2011, 4, 200–209. [Google Scholar] [CrossRef]
- Yun, K.E.; Kim, J.; Kim, M.; Park, E.; Kim, H.; Chang, Y.; Ryu, S.; Kim, H. Major Lipids, Apolipoproteins, and Alterations of Gut Microbiota. J. Clin. Med. 2020, 9, 1589. [Google Scholar] [CrossRef]
- Liu, J.; Chang, R.; Zhang, X.; Wang, Z.; Wen, J.; Zhou, T. Non-isoflavones Diet Incurred Metabolic Modifications Induced by Constipation in Rats via Targeting Gut Microbiota. Front. Microbiol. 2018, 9, 3002. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.; Lee, Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- Miller, L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Toro-Uribe, S.; Ibañez, E.; Decker, E.A.; Villamizar-Jaimes, A.R.; López-Giraldo, L.J. Food-Safe Process for High Recovery of Flavonoids from Cocoa Beans: Antioxidant and HPLC-DAD-ESI-MS/MS Analysis. Antioxidants 2020, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, N.; Pashazadeh, H.; Zannou, O.; Koca, I. Phytochemical content, and antioxidant activity, and volatile compounds associated with the aromatic property, of the vinegar produced from rosehip fruit (Rosa canina L.). LWT 2022, 154, 112716. [Google Scholar] [CrossRef]
- Cao, J.; Xia, X.; Dai, X.; Wang, Q.; Xiao, J. Chemical composition and bioactivities of flavonoids-rich extract from Davallia cylindrica Ching. Environ. Toxicol. Pharmacol. 2014, 37, 571–579. [Google Scholar] [CrossRef]
- Roth, N.C.; Qin, J. Histopathology of Alcohol-Related Liver Diseases. Clin. Liver Dis. 2019, 23, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; He, X.; Lin, Z.; Zhu, Y.; Jiang, X.; Zhao, L.; Zeng, F.; Chen, L.; Xu, W.; Liu, T.; et al. 6,8-(1,3-Diaminoguanidine) luteolin and its Cr complex show hypoglycemic activities and alter intestinal microbiota composition in type 2 diabetes mice. Food Funct. 2022, 13, 3572–3589. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, M.; Guo, W.; Li, T.; Liu, B.; Bai, W.; Ai, L.; Rao, P.; Ni, L.; Lv, X. Monascus purpureus-fermented common buckwheat protects against dyslipidemia and non-alcoholic fatty liver disease through the regulation of liver metabolome and intestinal microbiome. Food Res. Int. 2020, 136, 109511. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).