Interleukin-13 (IL-13)—A Pleiotropic Cytokine Involved in Wound Healing and Fibrosis
Abstract
1. Introduction
2. IL-13 in Wound Healing Processes
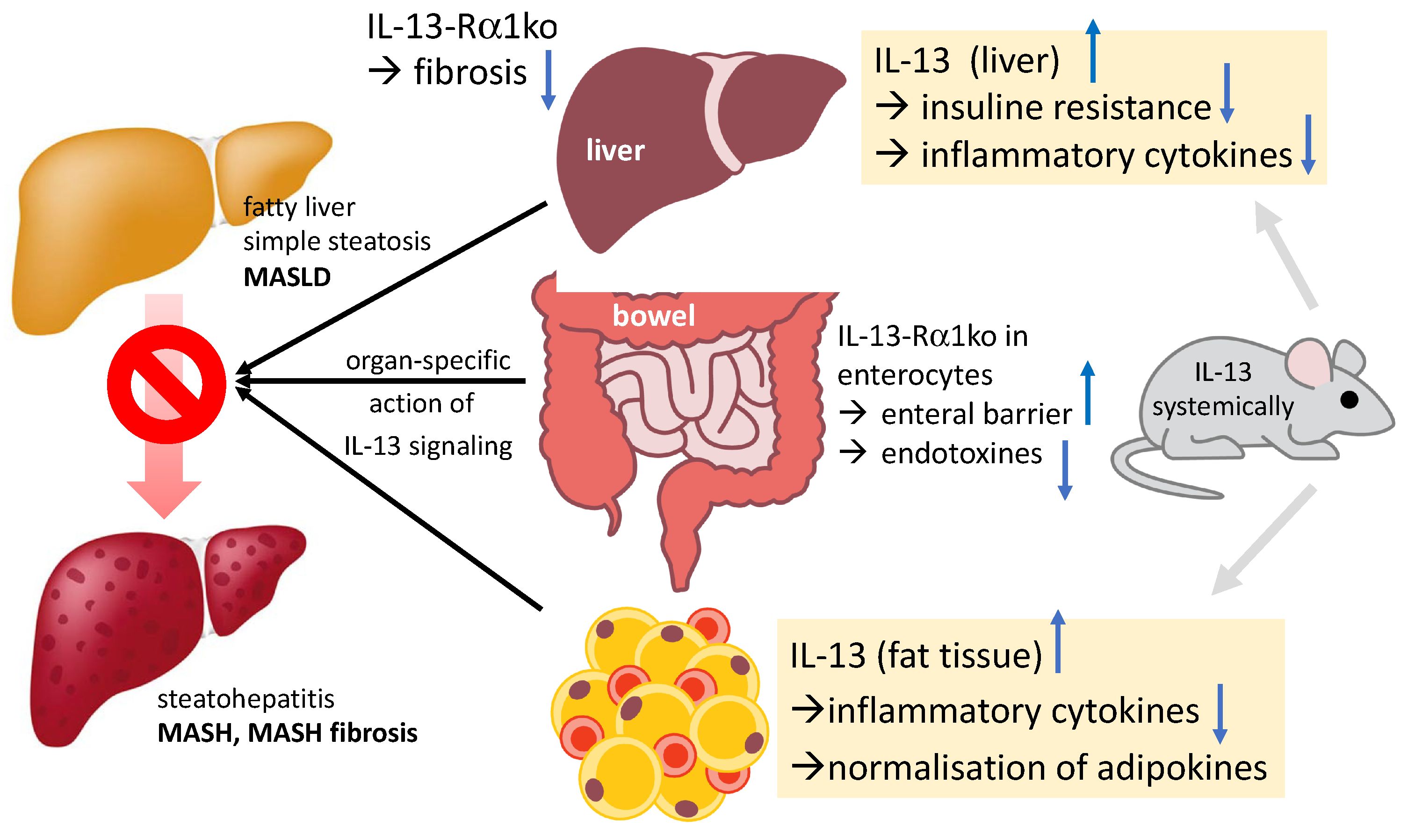
3. IL-13 and MASH (Metabolic-Dysfunction-Associated Steatohepatitis)
4. Different Cells React in Different Ways
5. Therapeutic Implications
6. Future Directions
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 101133. [Google Scholar] [CrossRef]
- Candia, R.; Ruiz, A.; Torres-Robles, R.; Chávez-Tapia, N.; Méndez-Sánchez, N.; Arrese, M. Risk of non-alcoholic fatty liver disease in patients with psoriasis: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 656–662. [Google Scholar] [CrossRef]
- Heitmann, J.; Frings, V.G.; Geier, A.; Goebeler, M.; Kerstan, A. Non-alcoholic fatty liver disease and psoriasis—Is there a shared proinflammatory network? J. Dtsch. Dermatol. Ges. 2021, 19, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Perez-Carreras, M.; Casis-Herce, B.; Rivera, R.; Fernandez, I.; Martinez-Montiel, P.; Villena, V. Non-alcoholic fatty liver disease in patients with intestinal, pulmonary or skin diseases: Inflammatory cross-talk that needs a multidisciplinary approach. World J. Gastroenterol. 2021, 27, 7113–7124. [Google Scholar] [CrossRef] [PubMed]
- Ertunc, M.E.; Hotamisligil, G.S. Lipid signaling and lipotoxicity in metaflammation: Indications for metabolic disease pathogenesis and treatment. J. Lipid Res. 2016, 57, 2099–2114. [Google Scholar] [CrossRef]
- Caputo, T.; Gilardi, F.; Desvergne, B. From chronic overnutrition to metaflammation and insulin resistance: Adipose tissue and liver contributions. FEBS Lett. 2017, 591, 3061–3088. [Google Scholar] [CrossRef]
- Pejnovic, N.; Jeftic, I.; Jovicic, N.; Arsenijevic, N.; Lukic, M.L. Galectin-3 and IL-33/ST2 axis roles and interplay in diet-induced steatohepatitis. World J. Gastroenterol. 2016, 22, 9706–9717. [Google Scholar] [CrossRef]
- Roeb, E.; Canbay, A.; Bantel, H.; Bojunga, J.; de Laffolie, J.; Demir, M.; Denzer, U.W.; Geier, A.; Hofmann, W.P.; Hudert, C.; et al. Aktualisierte S2k-Leitlinie nicht-alkoholische Fettlebererkrankung der Deutschen Gesellschaft für Gastroenterologie, Verdauungs-und Stoffwechselkrankheiten (DGVS)–April 2022–AWMF-Registernummer: 021–025. Z. Gastroenterol. 2022, 60, 1346–1421. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Paik, J.M.; Henry, L.; Yang, J.; Fernandes, G.; Stepanova, M.; Nader, F. The Growing Economic and Clinical Burden of Nonalcoholic Steatohepatitis (NASH) in the United States. J. Clin. Exp. Hepatol. 2023, 13, 454–467. [Google Scholar] [CrossRef]
- Younossi, Z.; Golabi, P.; Paik, J.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Roeb, E. Diagnostic and Therapy of Nonalcoholic Fatty Liver Disease: A Narrative Review. Visc. Med. 2022, 38, 126–132. [Google Scholar] [CrossRef]
- Geier, A.; Rau, M.; Pathil-Warth, A.; von der Ohe, M.; Schattenberg, J.; Dikopoulos, N.; Stein, K.; Serfert, Y.; Berg, T.; Buggisch, P.; et al. Clinical characteristics of patients with non-alcoholic fatty liver disease (NAFLD) in Germany—First data from the German NAFLD-Registry. Z. Gastroenterol. 2023, 61, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Roeb, E. Non-alcoholic fatty liver diseases: Current challenges and future directions. Ann. Transl. Med. 2021, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Mark, H.E.; Allen, A.M.; Arab, J.P.; Carrieri, P.; Noureddin, M.; Alazawi, W.; Alkhouri, N.; Alqahtani, S.A.; Anstee, Q.M.; et al. A global action agenda for turning the tide on fatty liver disease. Hepatology 2023. [Google Scholar] [CrossRef] [PubMed]
- Koning, H.; Neijens, H.J.; Baert, M.R.; Oranje, A.P.; Savelkoul, H.F. T cell subsets and cytokines in allergic and non-allergic children. I. Analysis of IL-4, IFN-gamma and IL-13 mRNA expression and protein production. Cytokine 1997, 9, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, Z.J.; Shenoy, A.; Chen, A.; Heller, N.M.; Spangler, J.B. Engineering the IL-4/IL-13 axis for targeted immune modulation. Immunol. Rev. 2023. [Google Scholar] [CrossRef] [PubMed]
- Borriello, F.; Longo, M.; Spinelli, R.; Pecoraro, A.; Granata, F.; Staiano, R.I.; Loffredo, S.; Spadaro, G.; Beguinot, F.; Schroeder, J.; et al. IL-3 synergises with basophil-derived IL-4 and IL-13 to promote the alternative activation of human monocytes. Eur. J. Immunol. 2015, 45, 2042–2051. [Google Scholar] [CrossRef]
- Brøgger, P.; Blom, L.H.; Simonsen, S.; Thyssen, J.P.; Skov, L. Antagonism of the interleukin 4 receptor α promotes T(H) 1-signalling among T cells from patients with atopic dermatitis after stimulation. Scand. J. Immunol. 2020, 91, e12835. [Google Scholar] [CrossRef]
- Fichtner-Feigl, S.; Strober, W.; Kawakami, K.; Puri, R.K.; Kitani, A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat. Med. 2006, 12, 99–106. [Google Scholar] [CrossRef]
- Marone, G.; Granata, F.; Pucino, V.; Pecoraro, A.; Heffler, E.; Loffredo, S.; Scadding, G.W.; Varricchi, G. The Intriguing Role of Interleukin 13 in the Pathophysiology of Asthma. Front. Pharmacol. 2019, 10, 1387. [Google Scholar] [CrossRef]
- Allgire, E.; Ahlbrand, R.A.; Nawreen, N.; Ajmani, A.; Hoover, C.; McAlees, J.W.; Lewkowich, I.P.; Sah, I.P. Altered fear behavior in aeroallergen house dust mite exposed C57Bl/6 mice: A model of Th2-skewed airway inflammation. Neuroscience 2023, in press. [CrossRef] [PubMed]
- Bullens, D.M.; Kasran, A.; Thielemans, K.; Bakkus, M.; Ceuppens, J.L. CD40L-induced IL-12 production is further enhanced by the Th2 cytokines IL-4 and IL-13. Scand. J. Immunol. 2001, 53, 455–463. [Google Scholar] [CrossRef]
- Beklen, A. Effects of IL-13 on TGF-β and MMP-1 in periodontitis. Biotech. Histochem. 2017, 92, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zheng, T.; Homer, R.J.; Kim, Y.K.; Chen, N.Y.; Cohn, L.; Hamid, Q.; Elias, J.A. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 2004, 304, 1678–1682. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, A.; Hogaboam, C.M.; Lukacs, N.W.; Lincoln, P.M.; Evanoff, H.L.; Strieter, R.M.; Kunkel, S.L. Expression and contribution of endogenous IL-13 in an experimental model of sepsis. J. Immunol. 2000, 164, 2738–2744. [Google Scholar] [CrossRef]
- Raabe, J.; Kaiser, K.M.; ToVinh, M.; Finnemann, C.; Lutz, P.; Hoffmeister, C.; Bischoff, J.; Goeser, F.; Kaczmarek, D.J.; Glowka, T.R.; et al. Identification and characterisation of a hepatic IL-13 producing ILC3-like population potentially involved in liver fibrosis. Hepatology 2023. [Google Scholar] [CrossRef]
- Trigona, W.L.; Hirano, A.; Brown, W.C.; Estes, D.M. Immunoregulatory roles of interleukin-13 in cattle. J. Interferon Cytokine Res. 1999, 19, 1317–1324. [Google Scholar] [CrossRef]
- Mentink-Kane, M.M.; Wynn, T.A. Opposing roles for IL-13 and IL-13 receptor alpha 2 in health and disease. Immunol. Rev. 2004, 202, 191–202. [Google Scholar] [CrossRef]
- Abd Allah, M.H.; Zaalouk, T.K.; Abo-Sheishaa, G.A.; Shalash, I.R.; Bayoumy, A.S. Role of IL-17A in enhancing liver fibrosis induced by TGF-β1 and IL-13 in Schistosoma mansoni infected mice. Egypt J. Immunol. 2022, 29, 174–183. [Google Scholar] [CrossRef]
- Zhang, M.; Duffen, J.L.; Nocka, K.H.; Kasaian, M.T. IL-13 Controls IL-33 Activity through Modulation of ST2. J. Immunol. 2021, 207, 3070–3080. [Google Scholar] [CrossRef]
- Yuan, X.; Waterworth, D.; Perry, J.R.; Lim, N.; Song, K.; Chambers, J.C.; Zhang, W.; Vollenweider, P.; Stirnadel, H.; Johnson, T.; et al. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am. J. Hum. Genet. 2008, 83, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Maggi, L.; Camelo, G.M.A.; Rocha, I.C.; Pereira Alves, W.; Moreira, J.M.P.; Almeida Pereira, T.; Tafuri, W.L.; Rabelo, É.M.L.; Correa, A., Jr.; Ecco, R.; et al. Role of the IL-33/ST2 Activation Pathway in the Development of the Hepatic Fibrosis Induced by Schistosoma mansoni Granulomas in Mice. Int. J. Mol. Sci. 2023, 24, 237. [Google Scholar] [CrossRef] [PubMed]
- Darkhal, P.; Gao, M.; Ma, Y.; Liu, D. Blocking high-fat diet-induced obesity, insulin resistance and fatty liver by overexpression of Il-13 gene in mice. Int. J. Obes. 2015, 39, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Hahn, L.; Helmrich, N.; Herebian, D.; Mayatepek, E.; Drebber, U.; Domann, E.; Olejniczak, S.; Weigel, M.; Hain, T.; Rath, T.; et al. IL-13 as Target to Reduce Cholestasis and Dysbiosis in Abcb4 Knockout Mice. Cells 2020, 9, 1949. [Google Scholar] [CrossRef]
- Ahdieh, M.; Vandenbos, T.; Youakim, A. Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-gamma. Am. J. Physiol. Cell Physiol. 2001, 281, C2029–C2038. [Google Scholar] [CrossRef]
- Reiman, R.M.; Thompson, R.W.; Feng, C.G.; Hari, D.; Knight, R.; Cheever, A.W.; Rosenberg, H.F.; Wynn, T.A. Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect. Immun. 2006, 74, 1471–1479. [Google Scholar] [CrossRef]
- Vicentino, A.R.R.; Carneiro, V.C.; Allonso, D.; Guilherme, R.F.; Benjamim, C.F.; Dos Santos, H.A.M.; Xavier, F.; Pyrrho, A.D.S.; Gomes, J.A.S.; Fonseca, M.C.; et al. Emerging Role of HMGB1 in the Pathogenesis of Schistosomiasis Liver Fibrosis. Front. Immunol. 2018, 9, 1979. [Google Scholar] [CrossRef]
- Mata-Santos, H.A.; Dutra, F.F.; Rocha, C.C.; Lino, F.G.; Xavier, F.R.; Chinalia, L.A.; Hossy, B.H.; Castelo-Branco, M.T.; Teodoro, A.J.; Paiva, C.N.; et al. Silymarin reduces profibrogenic cytokines and reverses hepatic fibrosis in chronic murine schistosomiasis. Antimicrob. Agents Chemother. 2014, 58, 2076–2083. [Google Scholar] [CrossRef]
- Yombo, D.J.K.; Odayar, V.; Gupta, N.; Jegga, A.G.; Madala, S.K. The Protective Effects of IL-31RA Deficiency During Bleomycin-Induced Pulmonary Fibrosis. Front. Immunol. 2021, 12, 645717. [Google Scholar] [CrossRef]
- Paiva, L.A.; Brand, C.; Bandeira-Melo, C.; Bozza, P.T.; El-Cheikh, M.C.; Silva, P.M.; Borojevic, R.; Perez, S.A. Hepatic myofibroblasts derived from Schistosoma mansoni-infected mice are a source of IL-5 and eotaxin: Controls of eosinophil populations in vitro. Parasit Vectors 2015, 8, 577. [Google Scholar] [CrossRef][Green Version]
- de Oliveira, V.G.; Rodrigues, V.F.; Moreira, J.M.P.; Rodrigues, J.L.; Maggi, L.; Resende, S.D.; Negrão-Corrêa, D. Eosinophils participate in modulation of liver immune response and tissue damage induced by Schistosoma mansoni infection in mice. Cytokine 2022, 149, 155701. [Google Scholar] [CrossRef] [PubMed]
- Hart, K.M.; Fabre, T.; Sciurba, J.C.; Gieseck, R.L., 3rd; Borthwick, L.A.; Vannella, K.M.; Acciani, T.H.; de Queiroz Prado, R.; Thompson, R.W.; White, S.; et al. Type 2 immunity is protective in metabolic disease but exacerbates NAFLD collaboratively with TGF-β. Sci. Transl. Med. 2017, 9, eaal3694. [Google Scholar] [CrossRef] [PubMed]
- Spits, H.; Di Santo, J.P. The expanding family of innate lymphoid cells: Regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 2011, 12, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tang, T.; Wei, X.; Yang, S.; Tian, Z. Type 1 innate lymphoid cells contribute to the pathogenesis of chronic hepatitis B. Innate immunity 2015, 21, 665–673. [Google Scholar] [CrossRef]
- Hofmann, U.; Knorr, S.; Vogel, B.; Weirather, J.; Frey, A.; Ertl, G.; Frantz, S. Interleukin-13 deficiency aggravates healing and remodeling in male mice after experimental myocardial infarction. Circ. Heart Fail. 2014, 7, 822–830. [Google Scholar] [CrossRef]
- Yang, S.J.; Allahverdian, S.; Saunders, A.D.R.; Liu, E.; Dorscheid, D.R. IL-13 signaling through IL-13 receptor α2 mediates airway epithelial wound repair. FASEB J. 2019, 33, 3746–3757. [Google Scholar] [CrossRef]
- Nguyen, J.K.; Austin, E.; Huang, A.; Mamalis, A.; Jagdeo, J. The IL-4/IL-13 axis in skin fibrosis and scarring: Mechanistic concepts and therapeutic targets. Arch. Dermatol. Res. 2020, 312, 81–92. [Google Scholar] [CrossRef]
- Bomb, K.; Pradhan, L.; Zhang, Q.; Jarai, B.M.; Bhattacharjee, A.; Burris, D.L.; Kloxin, A.M.; Fromen, C.A. Destructive fibrotic teamwork: How both microenvironment stiffness and profibrotic interleukin 13 impair alveolar macrophage phenotype and function. Biomater. Sci. 2022, 10, 5689–5706. [Google Scholar] [CrossRef]
- Sengoku, Y.; Higashi, M.; Nagayabu, K.; Takayama, S.; Fumino, S.; Aoi, S.; Furukawa, T.; Tajiri, T. IL13 and periostin in active fibrogenic areas of the extrahepatic bile ducts in biliary atresia patients. Pediatr. Surg. Int. 2022, 38, 1847–1853. [Google Scholar] [CrossRef]
- Heller, F.; Florian, P.; Bojarski, C.; Richter, J.; Christ, M.; Hillenbrand, B.; Mankertz, J.; Gitter, A.H.; Bürgel, N.; Fromm, M.; et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 2005, 129, 550–564. [Google Scholar] [CrossRef]
- Heller, F.; Fromm, A.; Gitter, A.H.; Mankertz, J.; Schulzke, J.D. Epithelial apoptosis is a prominent feature of the epithelial barrier disturbance in intestinal inflammation: Effect of pro-inflammatory interleukin-13 on epithelial cell function. Mucosal Immunol. 2008, 1 (Suppl. S1), S58–S61. [Google Scholar] [CrossRef]
- Krug, S.M.; Bojarski, C.; Fromm, A.; Lee, I.M.; Dames, P.; Richter, J.F.; Turner, J.R.; Fromm, M.; Schulzke, J.D. Tricellulin is regulated via interleukin-13-receptor α2, affects macromolecule uptake, and is decreased in ulcerative colitis. Mucosal Immunol. 2018, 11, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Chen, Q.; Zhao, J.; Rafaels, N.; Mathias, P.; Liang, H.; Potee, J.; Campbell, M.; Zhang, B.; Gao, L.; et al. An IL-13 promoter polymorphism associated with liver fibrosis in patients with Schistosoma japonicum. PLoS ONE 2015, 10, e0135360. [Google Scholar] [CrossRef] [PubMed]
- Mewamba, E.M.; Nyangiri, O.A.; Noyes, H.A.; Egesa, M.; Matovu, E.; Simo, G. The Genetics of Human Schistosomiasis Infection Intensity and Liver Disease: A Review. Front. Immunol. 2021, 12, 613468. [Google Scholar] [CrossRef] [PubMed]
- Aemero, M.; Boissier, J.; Climent, D.; Moné, H.; Mouahid, G.; Berhe, N.; Erko, B. Genetic diversity, multiplicity of infection and population structure of Schistosoma mansoni isolates from human hosts in Ethiopia. BMC Genet. 2015, 16, 137. [Google Scholar] [CrossRef]
- Watson, C.K.; Schloesser, D.; Fundel-Clemens, K.; Lerner, C.; Gabler, S.; Baskaran, P.; Wohnhaas, C.T.; Dichtl, S.; Huber, H.J.; Ask, K.; et al. Antifibrotic Drug Nintedanib Inhibits CSF1R to Promote IL-4-associated Tissue Repair Macrophages. Am. J. Respir. Cell Mol. Biol. 2023, 68, 366–380. [Google Scholar] [CrossRef]
- Lee, P.J.; Zhang, X.; Shan, P.; Ma, B.; Lee, C.G.; Homer, R.J.; Zhu, Z.; Rincon, M.; Mossman, B.T.; Elias, J.A. ERK1/2 mitogen-activated protein kinase selectively mediates IL-13-induced lung inflammation and remodeling in vivo. J. Clin. Investig. 2006, 116, 163–173. [Google Scholar] [CrossRef]
- Liao, K.Y.; Chen, C.J.; Hsieh, S.K.; Pan, P.H.; Chen, W.Y. Interleukin-13 ameliorates postischemic hepatic gluconeogenesis and hyperglycemia in rat model of stroke. Metab. Brain Dis. 2020, 35, 1201–1210. [Google Scholar] [CrossRef]
- Stanya, K.J.; Jacobi, D.; Liu, S.; Bhargava, P.; Dai, L.; Gangl, M.R.; Inouye, K.; Barlow, J.L.; Ji, Y.; Mizgerd, J.P.; et al. Direct control of hepatic glucose production by interleukin-13 in mice. J. Clin. Investig. 2013, 123, 261–271. [Google Scholar] [CrossRef]
- Pello, O.M.; De Pizzol, M.; Mirolo, M.; Soucek, L.; Zammataro, L.; Amabile, A.; Doni, A.; Nebuloni, M.; Swigart, L.B.; Evan, G.I.; et al. Role of c-MYC in alternative activation of human macrophages and tumor-associated macrophage biology. Blood 2012, 119, 411–421. [Google Scholar] [CrossRef]
- Martinez-Nunez, R.T.; Louafi, F.; Sanchez-Elsner, T. The interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptor alpha1 (IL13Ralpha1). J. Biol. Chem. 2011, 286, 1786–1794. [Google Scholar] [CrossRef]
- Matsui, S.; Okabayashi, K.; Tsuruta, M.; Shigeta, K.; Seishima, R.; Ishida, T.; Kondo, T.; Suzuki, Y.; Hasegawa, H.; Shimoda, M.; et al. Interleukin-13 and its signaling pathway is associated with obesity-related colorectal tumorigenesis. Cancer Sci. 2019, 110, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.W.; Ting, Y.W.; Yong, Y.K.; Tan, H.Y.; Barathan, M.; Riazalhosseini, B.; Bee, C.J.; Tee, K.K.; Larsson, M.; Velu, V.; et al. Chronic inflammation involves CCL11 and IL-13 to facilitate the development of liver cirrhosis and fibrosis in chronic hepatitis B virus infection. Scand. J. Clin. Lab. Investig. 2021, 81, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.P.; Fan, J.G.; Wu, R.; Gao, X.Q.; Zhang, L.; Wang, H.; Farrell, G.C. Prevalence and risk factors of hepatic steatosis and its impact on liver injury in Chinese patients with chronic hepatitis B infection. J. Gastroenterol. Hepatol. 2008, 23, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Witayavanitkul, N.; Werawatganon, D.; Chayanupatkul, M.; Klaikeaw, N.; Siriviriyakul, P. Genistein and exercise treatment reduced NASH related HDAC3, IL-13 and MMP-12 expressions in ovariectomized rats fed with high fat high fructose diet. J. Tradit. Complement. Med. 2021, 11, 503–512. [Google Scholar] [CrossRef]
- El-Derany, M.O. Polymorphisms in Interleukin 13 Signaling and Interacting Genes Predict Advanced Fibrosis and Hepatocellular Carcinoma Development in Non-Alcoholic Steatohepatitis. Biology 2020, 9, 75. [Google Scholar] [CrossRef]
- Walford, H.H.; Doherty, T.A. STAT6 and lung inflammation. Jakstat 2013, 2, e25301. [Google Scholar] [CrossRef]
- Shimamura, T.; Fujisawa, T.; Husain, S.R.; Kioi, M.; Nakajima, A.; Puri, R.K. Novel role of IL-13 in fibrosis induced by nonalcoholic steatohepatitis and its amelioration by IL-13R-directed cytotoxin in a rat model. J. Immunol. 2008, 181, 4656–4665. [Google Scholar] [CrossRef]
- Yoshidome, H.; Kato, A.; Miyazaki, M.; Edwards, M.J.; Lentsch, A.B. IL-13 activates STAT6 and inhibits liver injury induced by ischemia/reperfusion. Am. J. Pathol. 1999, 155, 1059–1064. [Google Scholar] [CrossRef]
- Russi, A.E.; Shivakumar, P.; Luo, Z.; Bezerra, J. Plasticity between ILC2 subsets and amphiregulin expression regulate epithelial repair in biliary atresia. Hepatology 2023. [Google Scholar] [CrossRef]
- Fujimoto, M.; Yokoyama, M.; Kiuchi, M.; Hosokawa, H.; Nakayama, A.; Hashimoto, N.; Sakuma, I.; Nagano, H.; Yamagata, K.; Kudo, F.; et al. Liver group 2 innate lymphoid cells regulate blood glucose levels through IL-13 signaling and suppression of gluconeogenesis. Nat. Commun. 2022, 13, 5408. [Google Scholar] [CrossRef] [PubMed]
- Low, L.D.; Lu, L.; Chan, C.Y.; Chen, J.; Yang, H.H.; Yu, H.; Lee, C.G.L.; Ng, K.H.; Yap, H.K. IL-13-driven alterations in hepatic cholesterol handling contributes to hypercholesterolemia in a rat model of minimal change disease. Clin. Sci. 2020, 134, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Gieseck, R.L., 3rd; Ramalingam, T.R.; Hart, K.M.; Vannella, K.M.; Cantu, D.A.; Lu, W.Y.; Ferreira-González, S.; Forbes, S.J.; Vallier, L.; Wynn, T.A. Interleukin-13 Activates Distinct Cellular Pathways Leading to Ductular Reaction, Steatosis, and Fibrosis. Immunity 2016, 45, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, Z.; Huang, J. Interleukin-13 contributes to the occurrence of oral submucosal fibrosis. J. Cell Mol. Med. 2023, 27, 1797–1805. [Google Scholar] [CrossRef]
- Arndt, L.; Lindhorst, A.; Neugebauer, J.; Hoffmann, A.; Hobusch, C.; Alexaki, V.I.; Ghosh, A.; Blüher, M.; Wolfrum, C.; Glaß, M.; et al. The Role of IL-13 and IL-4 in Adipose Tissue Fibrosis. Int. J. Mol. Sci. 2023, 24, 5672. [Google Scholar] [CrossRef]
- Chao, H.; Zheng, L.; Hsu, P.; He, J.; Wu, R.; Xu, S.; Zeng, R.; Zhou, Y.; Ma, H.; Liu, H.; et al. IL-13RA2 downregulation in fibroblasts promotes keloid fibrosis via JAK/STAT6 activation. JCI Insight 2023, 8, e157091. [Google Scholar] [CrossRef]
- Li, J.; Razumilava, N.; Gores, G.J.; Walters, S.; Mizuochi, T.; Mourya, R.; Bessho, K.; Wang, Y.H.; Glaser, S.S.; Shivakumar, P.; et al. Biliary repair and carcinogenesis are mediated by IL-33-dependent cholangiocyte proliferation. J. Clin. Investig. 2014, 124, 3241–3251. [Google Scholar] [CrossRef]
- Weng, H.L.; Liu, Y.; Chen, J.L.; Huang, T.; Xu, L.J.; Godoy, P.; Hu, J.H.; Zhou, C.; Stickel, F.; Marx, A.; et al. The etiology of liver damage imparts cytokines transforming growth factor beta1 or interleukin-13 as driving forces in fibrogenesis. Hepatology 2009, 50, 230–243. [Google Scholar] [CrossRef]
- Weng, S.Y.; Wang, X.; Vijayan, S.; Tang, Y.; Kim, Y.O.; Padberg, K.; Regen, T.; Molokanova, O.; Chen, T.; Bopp, T.; et al. IL-4 Receptor Alpha Signaling through Macrophages Differentially Regulates Liver Fibrosis Progression and Reversal. EBioMedicine 2018, 29, 92–103. [Google Scholar] [CrossRef]
- Jeftic, I.; Jovicic, N.; Pantic, J.; Arsenijevic, N.; Lukic, M.L.; Pejnovic, N. Galectin-3 Ablation Enhances Liver Steatosis, but Attenuates Inflammation and IL-33-Dependent Fibrosis in Obesogenic Mouse Model of Nonalcoholic Steatohepatitis. Mol. Med. 2015, 21, 453–465. [Google Scholar] [CrossRef]
- Martínez-Reyes, C.P.; Gómez-Arauz, A.Y.; Torres-Castro, I.; Manjarrez-Reyna, A.N.; Palomera, L.F.; Olivos-García, A.; Mendoza-Tenorio, E.; Sánchez-Medina, G.A.; Islas-Andrade, S.; Melendez-Mier, G.; et al. Serum Levels of Interleukin-13 Increase in Subjects with Insulin Resistance but Do Not Correlate with Markers of Low-Grade Systemic Inflammation. J. Diabetes Res. 2018, 2018, 7209872. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, R.; Enjoji, M.; Nakamuta, M.; Ohta, S.; Kohjima, M.; Fukushima, M.; Kuniyoshi, M.; Arimura, E.; Morizono, S.; Kotoh, K.; et al. Effect of IL-4 and IL-13 on collagen production in cultured LI90 human hepatic stellate cells. Liver Int. 2005, 25, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Tralokinumab. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.

| Function of IL-13 | Cells | Organ | Literature |
|---|---|---|---|
| Suppressing gluconeogenesis in hepatocytes via STAT3 | Group 2 innate lymphoid cells (ILC2s) | Liver pancreas | Fujimoto, Nat Commun, 2022 [71] |
| Induction of hypercholesterolemia No induction of fibrosis | Hepatocytes Hepatocytes | Liver Liver | Low, Clin Sci, 2020 [72] Gieseck, Immunity, 2016 [73] |
| Polarization of M2-macrophages inducing white adipose tissue fibrosis | M2-macrophages Macrophages | Oral tissue Adipose tissue | Wang, J Cell Mol Med, 2023 [74] Arndt, Int J Mol Sci, 2023 [75] |
| Induction of keloid fibrosis via JAK/STAT6 activation Eosinophil recruitment, liver fibrosis | (Keloid) fibroblasts Liver fibroblasts | Skin Liver | Chao, JCI Insight, 2023 [76] Gieseck, Immunity, 2016 [73] |
| Induction of ductular reaction/cholestasis, cholangiocyte differentiation, biliary regeneration | Cholangiocytes Biliary cells | Liver | Gieseck, Immunity, 2016 [73] |
| Intestinal barrier disruption | Mucosal cells | Bowel | Heller, Gastroenterol, 2005 [50] Hahn, Cells, 2020 [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roeb, E. Interleukin-13 (IL-13)—A Pleiotropic Cytokine Involved in Wound Healing and Fibrosis. Int. J. Mol. Sci. 2023, 24, 12884. https://doi.org/10.3390/ijms241612884
Roeb E. Interleukin-13 (IL-13)—A Pleiotropic Cytokine Involved in Wound Healing and Fibrosis. International Journal of Molecular Sciences. 2023; 24(16):12884. https://doi.org/10.3390/ijms241612884
Chicago/Turabian StyleRoeb, Elke. 2023. "Interleukin-13 (IL-13)—A Pleiotropic Cytokine Involved in Wound Healing and Fibrosis" International Journal of Molecular Sciences 24, no. 16: 12884. https://doi.org/10.3390/ijms241612884
APA StyleRoeb, E. (2023). Interleukin-13 (IL-13)—A Pleiotropic Cytokine Involved in Wound Healing and Fibrosis. International Journal of Molecular Sciences, 24(16), 12884. https://doi.org/10.3390/ijms241612884







