In Vitro and In Vivo Biological Assessments of 3D-Bioprinted Scaffolds for Dental Applications
Abstract
:1. Introduction
2. In Vitro Assessments
2.1. Bone
2.1.1. Cellular Behavior Activities
2.1.2. Differentiation Activities (Alkaline Phosphatase)
2.1.3. Expression of the Markers
2.2. Periodontal Tissues
2.2.1. Cell Behavior Activities
2.2.2. Expression of the Markers
2.3. Dentin and Pulp
2.3.1. Cellular Behavior Activities
2.3.2. Expression of the Markers
3. In Vivo Assessments
3.1. Bone
3.2. Periodontal Tissue
3.3. Dentin
4. Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skeldon, G.; Lucendo-Villarin, B.; Shu, W. Three-dimensional bioprinting of stem-cell derived tissues for human regenerative medicine. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170224. [Google Scholar] [CrossRef]
- Latimer, J.M.; Maekawa, S.; Yao, Y.; Wu, D.T.; Chen, M.; Giannobile, W.V. Regenerative Medicine Technologies to Treat Dental, Oral, and Craniofacial Defects. Front. Bioeng. Biotechnol. 2021, 9, 704048. [Google Scholar] [CrossRef]
- Atala, A.; Forgacs, G. Three-Dimensional Bioprinting in Regenerative Medicine: Reality, Hype, and Future. Stem Cells Transl. Med. 2019, 8, 744–745. [Google Scholar] [CrossRef] [PubMed]
- Mohd, N.; Razali, M.; Ghazali, M.J.; Abu Kasim, N.H. Current Advances of Three-Dimensional Bioprinting Application in Dentistry: A Scoping Review. Materials 2022, 15, 6398. [Google Scholar] [CrossRef] [PubMed]
- Hung, B.P.; Naved, B.A.; Nyberg, E.L.; Dias, M.; Holmes, C.A.; Elisseeff, J.H.; Dorafshar, A.H.; Grayson, W.L. Three-Dimensional Printing of Bone Extracellular Matrix for Craniofacial Regeneration. ACS Biomater. Sci. Eng. 2016, 2, 1806–1816. [Google Scholar] [CrossRef]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Mohd, N.; Razali, M.; Ghazali, M.J.; Abu Kasim, N.H. 3D-Printed Hydroxyapatite and Tricalcium Phosphates-Based Scaffolds for Alveolar Bone Regeneration in Animal Models: A Scoping Review. Materials 2022, 15, 2621. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Zhang, J.; Zhang, M.; An, Y.; Chen, F.; Wu, Z.F. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials 2010, 31, 7892–7927. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Galler, K.M.; Krastl, G.; Simon, S.; Van Gorp, G.; Meschi, N.; Vahedi, B.; Lambrechts, P. European Society of Endodontology position statement: Revitalization procedures. Int. Endod. J. 2016, 49, 717–723. [Google Scholar] [CrossRef]
- Theus, A.S.; Ning, L.; Hwang, B.; Gil, C.; Chen, S.; Wombwell, A.; Mehta, R.; Serpooshan, V. Bioprintability: Physiomechanical and Biological Requirements of Materials for 3D Bioprinting Processes. Polymers 2020, 12, 2262. [Google Scholar] [CrossRef]
- Xu, T.; Zhao, W.; Zhu, J.M.; Albanna, M.Z.; Yoo, J.J.; Atala, A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials 2013, 34, 130–139. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Ahlfeld, T.; Lode, A.; Richter, R.F.; Pradel, W.; Franke, A.; Rauner, M.; Stadlinger, B.; Lauer, G.; Gelinsky, M.; Korn, P. Toward Biofabrication of Resorbable Implants Consisting of a Calcium Phosphate Cement and Fibrin-A Characterization In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 1218. [Google Scholar] [CrossRef] [PubMed]
- Chamo, D.; Msallem, B.; Sharma, N.; Aghlmandi, S.; Kunz, C.; Thieringer, F.M. Accuracy assessment of molded, patient-specific polymethylmethacrylate craniofacial implants compared to their 3D printed originals. J. Clin. Med. 2020, 9, 832. [Google Scholar] [CrossRef] [PubMed]
- Zaszczyńska, A.; Moczulska-Heljak, M.; Gradys, A.; Sajkiewicz, P. Advances in 3D Printing for Tissue Engineering. Materials 2021, 14, 3149. [Google Scholar] [CrossRef]
- Jared, B.H.; Aguilo, M.A.; Beghini, L.L.; Boyce, B.L.; Clark, B.W.; Cook, A.; Kaehr, B.J.; Robbins, J. Additive manufacturing: Toward holistic design. Scr. Mater. 2017, 135, 141–147. [Google Scholar] [CrossRef]
- Thurzo, A.; Gálfiová, P.; Nováková, Z.V.; Polák, Š.; Varga, I.; Strunga, M.; Urban, R.; Surovková, J.; Leško, Ľ.; Hajdúchová, Z.; et al. Fabrication and In Vitro Characterization of Novel Hydroxyapatite Scaffolds 3D Printed Using Polyvinyl Alcohol as a Thermoplastic Binder. Int. J. Mol. Sci. 2022, 23, 14870. [Google Scholar] [CrossRef]
- Vrana, N.E.; Gupta, S.; Mitra, K.; Rizvanov, A.A.; Solovyeva, V.V.; Antmen, E.; Salehi, M.; Ehterami, A.; Pourchet, L.; Barthes, J.; et al. From 3D printing to 3D bioprinting: The material properties of polymeric material and its derived bioink for achieving tissue specific architectures. Cell Tissue Bank. 2022, 23, 417–440. [Google Scholar] [CrossRef]
- Cui, X.; Boland, T.; D’Lima, D.D.; Lotz, M.K. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat. Drug Deliv. Formul. 2012, 6, 149–155. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, Y.J.; Shim, J.H.; Park, J.H.; Cho, D.W. Development of a 3D cell printed structure as an alternative to autologs cartilage for auricular reconstruction. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2019, 18, 100479. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.C.; Reid, R.R. 3-D bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Zhang, R. Recent trends and challenges in complex organ manufacturing. Tissue Eng. Part B Rev. 2010, 16, 189–197. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Martin, J.A.; Ozbolat, I.T. Evaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channels. J. Biomech. Eng. 2013, 135, 91011. [Google Scholar] [CrossRef]
- Askari, M.; Afzali Naniz, M.; Kouhi, M.; Saberi, A.; Zolfagharian, A.; Bodaghi, M. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: A comprehensive review with focus on advanced fabrication techniques. Biomater. Sci. 2021, 9, 535–573. [Google Scholar] [CrossRef]
- Boland, T.; Xu, T.; Damon, B.; Cui, X. Application of inkjet printing to tissue engineering. Biotechnol. J. 2006, 1, 910–917. [Google Scholar] [CrossRef]
- Xu, T.; Jin, J.; Gregory, C.; Hickman, J.J.; Boland, T. Inkjet printing of viable mammalian cells. Biomaterials 2005, 26, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Remy, M.; Bordenave, L.; Amedee, J.; et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Perez, V.; Qu, J.; Tsin, A.; Xu, B.; Li, J. A State-of-the-Art Review of Laser-Assisted Bioprinting and its Future Research Trends. ChemBioEng Rev. 2021, 8, 517–534. [Google Scholar] [CrossRef]
- Wang, M.; He, J.; Liu, Y.; Li, M.; Li, D.; Jin, Z. The trend towards in vivo bioprinting. Int. J. Bioprint. 2015, 1, 12. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef]
- Busra, M.F.M.; Lokanathan, Y. Recent Development in the Fabrication of Collagen Scaffolds for Tissue Engineering Applications: A Review. Curr. Pharm. Biotechnol. 2019, 20, 992–1003. [Google Scholar] [CrossRef]
- Zorlutuna, P.; Vrana, N.E.; Khademhosseini, A. The expanding world of tissue engineering: The building blocks and new applications of tissue engineered constructs. IEEE Rev. Biomed. Eng. 2013, 6, 47–62. [Google Scholar] [CrossRef]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef]
- Kuss, M.A.; Harms, R.; Wu, S.; Wang, Y.; Untrauer, J.B.; Carlson, M.A.; Duan, B. Short-term hypoxic preconditioning promotes prevascularization in 3D bioprinted bone constructs with stromal vascular fraction derived cells. RSC Adv. 2017, 7, 29312–29320. [Google Scholar] [CrossRef]
- Chimene, D.; Miller, L.; Cross, L.M.; Jaiswal, M.K.; Singh, I.; Gaharwar, A.K. Nanoengineered Osteoinductive Bioink for 3D Bioprinting Bone Tissue. ACS Appl. Mater. Interfaces 2020, 12, 15976–15988. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.; Ferreira, J.A.; Malda, J.; Bhaduri, S.B.; Bottino, M.C. Extracellular Matrix/Amorphous Magnesium Phosphate Bioink for 3D Bioprinting of Craniomaxillofacial Bone Tissue. ACS Appl. Mater. Interfaces 2020, 12, 23752–23763. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Gillispie, G.J.; Copus, J.S.; Zhang, W.; Atala, A.; Yoo, J.J.; Yelick, P.C.; Lee, S.J. The effect of BMP-mimetic peptide tethering bioinks on the differentiation of dental pulp stem cells (DPSCs) in 3D bioprinted dental constructs. Biofabrication 2020, 12, 035029. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, M.; Liu, Y.; Shi, C.; Wang, Y.; Liu, T.; Huang, Y.; Zhong, P.; Dai, J.; Liu, X. The performance of 3D bioscaffolding based on a human periodontal ligament stem cell printing technique. J. Biomed. Mater. Res. A 2021, 109, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Moncal, K.K.; Gudapati, H.; Godzik, K.P.; Heo, D.N.; Kang, Y.; Rizk, E.; Ravnic, D.J.; Wee, H.; Pepley, D.F.; Ozbolat, V.; et al. Intra-Operative Bioprinting of Hard, Soft, and Hard/Soft Composite Tissues for Craniomaxillofacial Reconstruction. Adv. Funct. Mater. 2021, 31, 2010858. [Google Scholar] [CrossRef]
- Moncal, K.K.; Tigli Aydın, R.S.; Godzik, K.P.; Acri, T.M.; Heo, D.N.; Rizk, E.; Wee, H.; Lewis, G.S.; Salem, A.K.; Ozbolat, I.T. Controlled Co-delivery of pPDGF-B and pBMP-2 from intraoperatively bioprinted bone constructs improves the repair of calvarial defects in rats. Biomaterials 2022, 281, 121333. [Google Scholar] [CrossRef]
- Keriquel, V.; Oliveira, H.; Rémy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amédée, J.; Guillemot, F.; et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 2017, 7, 1778. [Google Scholar] [CrossRef]
- Kérourédan, O.; Hakobyan, D.; Rémy, M.; Ziane, S.; Dusserre, N.; Fricain, J.C.; Delmond, S.; Thébaud, N.B.; Devillard, R. In situ prevascularization designed by laser-assisted bioprinting: Effect on bone regeneration. Biofabrication 2019, 11, 045002. [Google Scholar] [CrossRef]
- Touya, N.; Devun, M.; Handschin, C.; Casenave, S.; Ahmed Omar, N.; Gaubert, A.; Dusserre, N.; De Oliveira, H.; Kérourédan, O.; Devillard, R. In vitroandin vivocharacterization of a novel tricalcium silicate-based ink for bone regeneration using laser-assisted bioprinting. Biofabrication 2022, 14, 024104. [Google Scholar] [CrossRef]
- Ma, Y.; Ji, Y.; Zhong, T.; Wan, W.; Yang, Q.; Li, A.; Zhang, X.; Lin, M. Bioprinting-Based PDLSC-ECM Screening for in vivo Repair of Alveolar Bone Defect Using Cell-Laden, Injectable and Photocrosslinkable Hydrogels. ACS Biomater. Sci. Eng. 2017, 3, 3534–3545. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.L.; Yun, S.; Cao, H.L.; Ahn, G.; Shim, J.H.; Woo, S.H.; Choung, P.H. Bioprinting on 3D Printed Titanium Scaffolds for Periodontal Ligament Regeneration. Cells 2021, 10, 1337. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chiu, Y.C.; Lee, A.K.; Lin, Y.A.; Lin, P.Y.; Shie, M.Y. Biofabrication of Gingival Fibroblast Cell-Laden Collagen/Strontium-Doped Calcium Silicate 3D-Printed Bi-Layered Scaffold for Osteoporotic Periodontal Regeneration. Biomedicines 2021, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ji, Y.; Huang, G.; Ling, K.; Zhang, X.; Xu, F. Bioprinting 3D cell-laden hydrogel microarray for screening human periodontal ligament stem cell response to extracellular matrix. Biofabrication 2015, 7, 044105. [Google Scholar] [CrossRef] [PubMed]
- Athirasala, A.; Tahayeri, A.; Thrivikraman, G.; França, C.M.; Monteiro, N.; Tran, V.; Ferracane, J.; Bertassoni, L.E. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication 2018, 10, 024101. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Kim, D.S.; Jang, H.; Kim, H.R.; Kang, H.W. Bioprinting of three-dimensional dentin-pulp complex with local differentiation of human dental pulp stem cells. J. Tissue Eng. 2019, 10, 2041731419845849. [Google Scholar] [CrossRef]
- Lin, Y.T.; Hsu, T.T.; Liu, Y.W.; Kao, C.T.; Huang, T.H. Bidirectional Differentiation of Human-Derived Stem Cells Induced by Biomimetic Calcium Silicate-Reinforced Gelatin Methacrylate Bioink for Odontogenic Regeneration. Biomedicines 2021, 9, 929. [Google Scholar] [CrossRef]
- Han, J.; Jeong, W.; Kim, M.K.; Nam, S.H.; Park, E.K.; Kang, H.W. Demineralized Dentin Matrix Particle-Based Bio-Ink for Patient-Specific Shaped 3D Dental Tissue Regeneration. Polymers 2021, 13, 1294. [Google Scholar] [CrossRef]
- Dutta, S.D.; Bin, J.; Ganguly, K.; Patel, D.K.; Lim, K.T. Electromagnetic field-assisted cell-laden 3D printed poloxamer-407 hydrogel for enhanced osteogenesis. RSC Adv. 2021, 11, 20342–20354. [Google Scholar] [CrossRef]
- Kim, D.; Lee, H.; Lee, G.H.; Hoang, T.H.; Kim, H.R.; Kim, G.H. Fabrication of bone-derived decellularized extracellular matrix/ceramic-based biocomposites and their osteo/odontogenic differentiation ability for dentin regeneration. Bioeng. Transl. Med. 2022, 7, e10317. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Zhang, S.; Kreimendahl, F.; Köpf, M.; Fischer, H.; Vogt, M.; Blaeser, A.; Apel, C.; Esteves-Oliveira, M. Hand-held bioprinting for de novo vascular formation applicable to dental pulp regeneration. Connect Tissue Res. 2020, 61, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, E.L.; Farris, A.L.; Hung, B.P.; Dias, M.; Garcia, J.R.; Dorafshar, A.H.; Grayson, W.L. 3D-Printing Technologies for Craniofacial Rehabilitation, Reconstruction, and Regeneration. Ann. Biomed. Eng. 2017, 45, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Damien, C.J.; Parsons, J.R. Bone graft and bone graft substitutes: A review of current technology and applications. J. Appl. Biomater. 1991, 2, 187–208. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36 (Suppl. S3), S20–S27. [Google Scholar] [CrossRef] [PubMed]
- Hikita, A.; Chung, U.I.; Hoshi, K.; Takato, T. Bone Regenerative Medicine in Oral and Maxillofacial Region Using a Three-Dimensional Printer. Tissue Eng. Part A 2017, 23, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Nkenke, E.; Weisbach, V.; Winckler, E.; Kessler, P.; Schultze-Mosgau, S.; Wiltfang, J.; Neukam, F.W. Morbidity of harvesting of bone grafts from the iliac crest for preprosthetic augmentation procedures: A prospective study. Int. J. Oral Maxillofac. Surg. 2004, 33, 157–163. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef]
- Haque, N.; Rahman, M.T.; Abu Kasim, N.H.; Alabsi, A.M. Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. Sci. World J. 2013, 2013, 632972. [Google Scholar] [CrossRef]
- Han, S.; Kim, C.M.; Jin, S.; Kim, T.Y. Study of the process-induced cell damage in forced extrusion bioprinting. Biofabrication 2021, 13, 035048. [Google Scholar] [CrossRef]
- Xu, H.Q.; Liu, J.C.; Zhang, Z.Y.; Xu, C.X. A review on cell damage, viability, and functionality during 3D bioprinting. Mil. Med. Res. 2022, 9, 70. [Google Scholar] [CrossRef]
- Elangovan, S.; D’Mello, S.R.; Hong, L.; Ross, R.D.; Allamargot, C.; Dawson, D.V.; Stanford, C.M.; Johnson, G.K.; Sumner, D.R.; Salem, A.K. The enhancement of bone regeneration by gene activated matrix encoding for platelet derived growth factor. Biomaterials 2014, 35, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, N.; Lee, B.; Hong, D.; Enick, P.N.; Roy, A.; Kumta, P.N. Synthesis, Osteoblast, and Osteoclast Viability of Amorphous and Crystalline Tri-Magnesium Phosphate. ACS Biomater. Sci. Eng. 2015, 1, 52–63. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.R.; Elangovan, S.; Hong, L.; Ross, R.D.; Sumner, D.R.; Salem, A.K. A Pilot Study Evaluating Combinatorial and Simultaneous Delivery of Polyethylenimine-Plasmid DNA Complexes Encoding for VEGF and PDGF for Bone Regeneration in Calvarial Bone Defects. Curr. Pharm. Biotechnol. 2015, 16, 655–660. [Google Scholar] [CrossRef]
- Bartold, P.M.; McCulloch, C.A.; Narayanan, A.S.; Pitaru, S. Tissue engineering: A new paradigm for periodontal regeneration based on molecular and cell biology. Periodontology 2000, 24, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Zander, H.A. The attachment between tooth and gingival tissues after periodic root planing and soft tissue curettage. J. Periodontol. 1979, 50, 462–466. [Google Scholar] [CrossRef]
- Nibali, L.; Sultan, D.; Arena, C.; Pelekos, G.; Lin, G.H.; Tonetti, M. Periodontal infrabony defects: Systematic review of healing by defect morphology following regenerative surgery. J. Clin. Periodontol. 2021, 48, 100–113. [Google Scholar] [CrossRef]
- Xu, H.; Casillas, J.; Xu, C. Effects of printing conditions on cell distribution within microspheres during inkjet-based bioprinting. AIP Adv. 2019, 9, 095055. [Google Scholar] [CrossRef]
- Sawhney, A.S.; Pathak, C.P.; Hubbell, J.A. Interfacial photopolymerization of poly(ethylene glycol)-based hydrogels upon alginate-poly(l-lysine) microcapsules for enhanced biocompatibility. Biomaterials 1993, 14, 1008–1016. [Google Scholar] [CrossRef]
- Lin, F.S.; Lee, J.J.; Lee, A.K.; Ho, C.C.; Liu, Y.T.; Shie, M.Y. Calcium Silicate-Activated Gelatin Methacrylate Hydrogel for Accelerating Human Dermal Fibroblast Proliferation and Differentiation. Polymers 2020, 13, 70. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Alvarez-Pérez, M.A.; Narayanan, S.; Zeichner-David, M.; Rodríguez Carmona, B.; Arzate, H. Molecular cloning, expression and immunolocalization of a novel human cementum-derived protein (CP-23). Bone 2006, 38, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Hoz, L.; Romo, E.; Zeichner-David, M.; Sanz, M.; Nuñez, J.; Gaitán, L.; Mercado, G.; Arzate, H. Cementum protein 1 (CEMP1) induces differentiation by human periodontal ligament cells under three-dimensional culture conditions. Cell Biol. Int. 2012, 36, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Shen, Z.; Zhan, P.; Yang, J.; Huang, Q.; Huang, S.; Chen, L.; Lin, Z. Functional Dental Pulp Regeneration: Basic Research and Clinical Translation. Int. J. Mol. Sci. 2021, 22, 8991. [Google Scholar] [CrossRef]
- Samiei, M.; Aghazadeh, Z.; Abdolahinia, E.D.; Vahdati, A.; Daneshvar, S.; Noghani, A. The effect of electromagnetic fields on survival and proliferation rate of dental pulp stem cells. Acta Odontol. Scand. 2020, 78, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Albanna, M.; Binder, K.W.; Murphy, S.V.; Kim, J.; Qasem, S.A.; Zhao, W.; Tan, J.; El-Amin, I.B.; Dice, D.D.; Marco, J.; et al. In Situ Bioprinting of Autologous Skin Cells Accelerates Wound Healing of Extensive Excisional Full-Thickness Wounds. Sci. Rep. 2019, 9, 1856. [Google Scholar] [CrossRef] [PubMed]
- Keriquel, V.; Guillemot, F.; Arnault, I.; Guillotin, B.; Miraux, S.; Amédée, J.; Fricain, J.C.; Catros, S. In vivo bioprinting for computer- and robotic-assisted medical intervention: Preliminary study in mice. Biofabrication 2010, 2, 014101. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.I.; Baharin, B.; Lau, S.F.; Ibrahim, N.; Mohd, N.; Ahmad Fauzi, A.; Muhammad, N.; Fernandez, N.M. The Influence of Ficus deltoidea in Preserving Alveolar Bone in Ovariectomized Rats. Vet. Med. Int. 2020, 2020, 8862489. [Google Scholar] [CrossRef]
- Rodriguez-Salvador, M.; Ruiz-Cantu, L. Revealing emerging science and technology research for dentistry applications of 3D bioprinting. Int. J. Bioprint. 2019, 5, 170. [Google Scholar] [CrossRef]
- Lepowsky, E.; Muradoglu, M.; Tasoglu, S. Towards preserving post-printing cell viability and improving the resolution: Past, present, and future of 3D bioprinting theory. Bioprinting 2018, 11, e00034. [Google Scholar] [CrossRef]
- Lee, C.H.; Hajibandeh, J.; Suzuki, T.; Fan, A.; Shang, P.; Mao, J.J. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng. Part A 2014, 20, 1342–1351. [Google Scholar] [CrossRef]
- Graham, A.D.; Olof, S.N.; Burke, M.J.; Armstrong, J.P.K.; Mikhailova, E.A.; Nicholson, J.G.; Box, S.J.; Szele, F.G.; Perriman, A.W.; Bayley, H. High-Resolution Patterned Cellular Constructs by Droplet-Based 3D Printing. Sci. Rep. 2017, 7, 7004. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Menger, M.D. Prevascularization in tissue engineering: Current concepts and future directions. Biotechnol. Adv. 2016, 34, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Shamsuddin, S.A.; Ramli, R.; Razali, M.; Baharin, B.; Sulaiman, S.; Hwei Ng, M.; Low, C.K.; Jabar, M.N.A.; Nordin, R.; Yahaya, N. Guided bone regeneration using autologous plasma, bone marrow cells and β-TCP/HA granules for experimental alveolar ridge reconstruction in Macaca fascicularis. J. Biomater. Tissue Eng. 2017, 7, 111–118. [Google Scholar] [CrossRef]
- Leenaars, C.H.C.; Kouwenaar, C.; Stafleu, F.R.; Bleich, A.; Ritskes-Hoitinga, M.; De Vries, R.B.M.; Meijboom, F.L.B. Animal to human translation: A systematic scoping review of reported concordance rates. J. Transl. Med. 2019, 17, 223. [Google Scholar] [CrossRef] [PubMed]
- Pound, P.; Ritskes-Hoitinga, M. Is it possible to overcome issues of external validity in preclinical animal research? Why most animal models are bound to fail. J. Transl. Med. 2018, 16, 304. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, C.A. The history of tissue engineering. J. Cell. Mol. Med. 2006, 10, 569–576. [Google Scholar] [CrossRef] [PubMed]
- de Vries, R.B.; Oerlemans, A.; Trommelmans, L.; Dierickx, K.; Gordijn, B. Ethical aspects of tissue engineering: A review. Tissue Eng. Part B Rev. 2008, 14, 367–375. [Google Scholar] [CrossRef]
- Hyun, I. The bioethics of stem cell research and therapy. J. Clin. Investig. 2010, 120, 71–75. [Google Scholar] [CrossRef]
- Tong, A.; Pham, Q.L.; Abatemarco, P.; Mathew, A.; Gupta, D.; Iyer, S.; Voronov, R. Review of Low-Cost 3D Bioprinters: State of the Market and Observed Future Trends. SLAS Technol. 2021, 26, 333–366. [Google Scholar] [CrossRef]
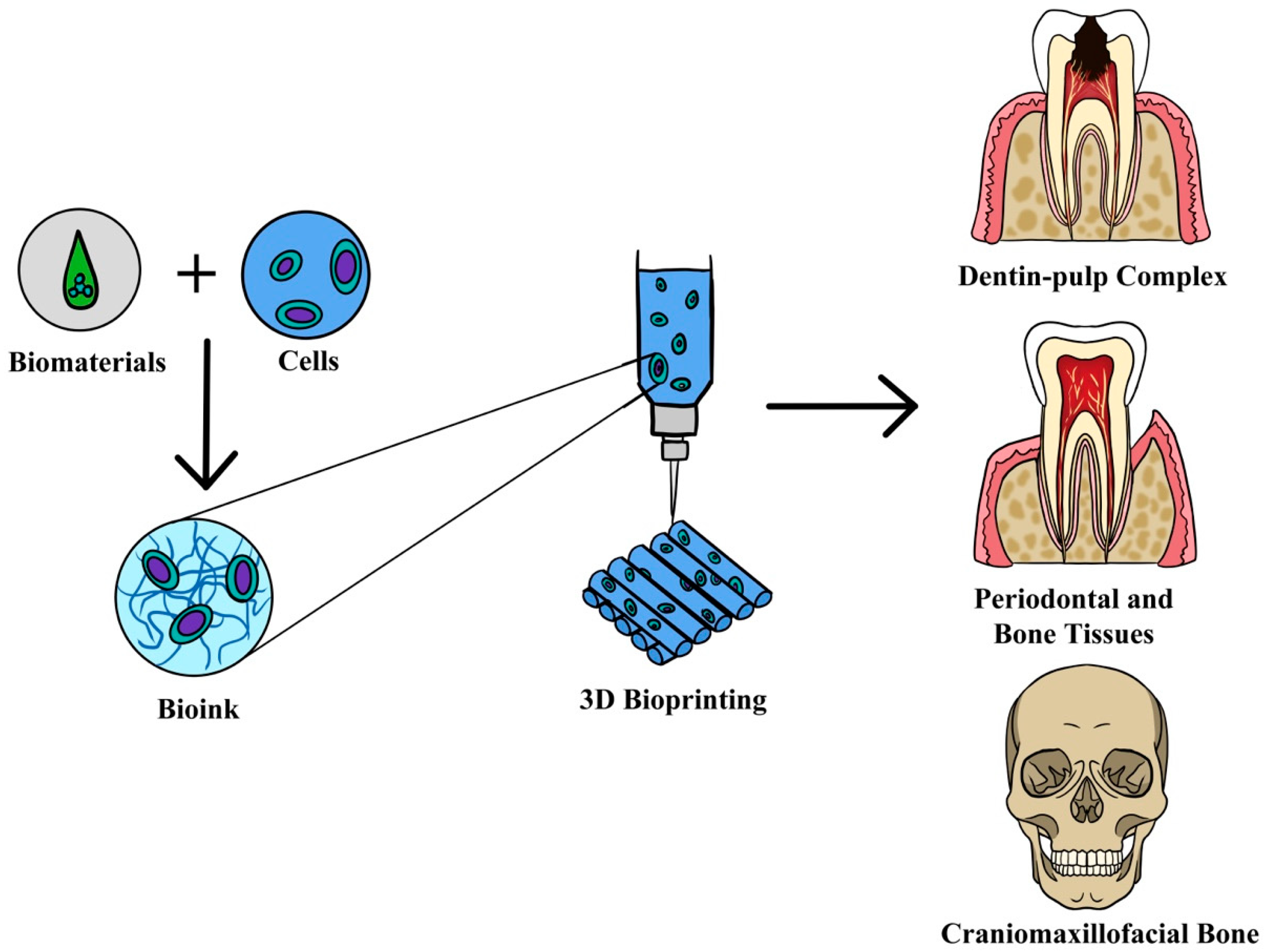
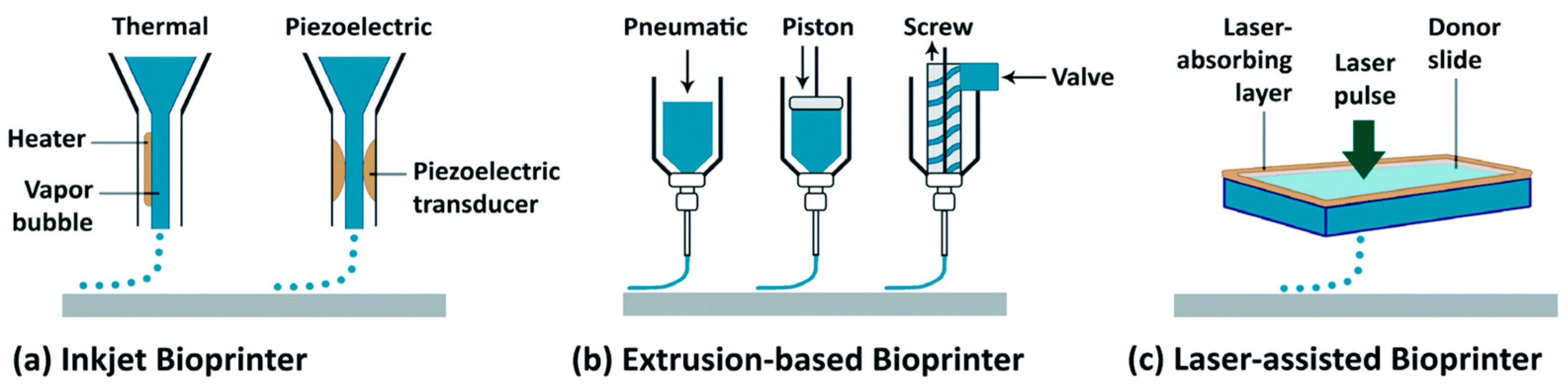
| Tissue Type | Bioprinting Technique | 3D Bioprinter | Cell-Laden Bioink | Cell Types | Study Design | Author |
|---|---|---|---|---|---|---|
| Bone | Extrusion | Integrated tissue–organ printing system | Gelatin + fibrinogen + HA + glycerol | hAFSCs | In vitro and in vivo | Kang et al., 2016 [40] |
| Extrusion | 3D Bioplotter (EnvisionTEC GmbH, Gladbeck, Germany) | MeHA + GelMA + HA | Porcine stromal vascular fraction from adipose tissue | In vitro and in vivo | Kuss et al., 2017 [41] | |
| Extrusion | Modified ANET A8 3D printer, Shenzhen, China | GelMA + kCA + nSi(NICE bioink) | Human primary bone-marrow-derived MSCs | In vitro | Chimene et al., 2020 [42] | |
| Extrusion | 3DDiscovery, regenHU, Villaz-St-Pierre, Switzerland | ECM + AMP | DPSCs | In vitro | Dubey et al., 2020 [43] | |
| Extrusion | Integrated tissue–organ printing system | Gelatin + GelMA + HA + glycerol | DPSCs | In vitro | Park et al., 2020 [44] | |
| Extrusion | 3D Bioplotter (EnvisionTEC GmbH, Gladbeck, Germany) | Alginate + gelatin + nHAp | hPDLSCs | In vitro | Tian et al., 2021 [45] | |
| Extrusion | In-house developed MultiArm Bioprinter, Iowa City, IA, USA | Collagen + chitosan + β-glycerophosphate + nHAp | Rat BMSCs | In vitro | Moncal et al., 2021 [46] | |
| Extrusion | In-house developed MultiArm Bioprinter, Iowa City, IA, USA | Collagen + chitosan + β-glycerophosphate + nHAp | Rat BMSCs | In vitro | Moncal et al., 2022 [47] | |
| LAB | LAB workstation (U1026, Inserm, Bordeaux, France) | Collagen type 1 + nHAp | Mouse bone marrow stromal precursor D1 cell line | In vitro and in vivo | Keriquel et al., 2017 [48] | |
| LAB | LAB workstation (U1026, Inserm, Bordeaux, France) | Collagen type 1 | SCAPs and HUVECs | In vivo | Kérourédan et al., 2019 [49] | |
| LAB | LAB workstation (U1026, Inserm, Bordeaux, France) | Collagen type 1 + TCP (BioRoot RCS®, Septodont, France) | SCAPs | In vitro and in vivo | Touya et al., 2022 [50] | |
| Inkjet | Customer-designed pressure-assisted valve-based bioprinting system | GelMA + PEGDA | Rat PDLSCs | In vitro | Ma et al., 2017 [51] | |
| Periodontal | Extrusion | 3DX Printer, T and R Biofab Co., Ltd., Siheung, Korea | Collagen | hPDLSCs | In vitro and in vivo | Lee et al., 2021 [52] |
| Extrusion | BioScaffolder 3.1, GeSiM, Groβerkmannsdorf, Germany | Collagen | Human gingiva fibroblasts | In vitro and in vivo | Wang et al., 2021 [53] | |
| Inkjet | Customer-designed pressure-assisted valve-based bioprinting system | GelMA + PEGDA | hPDLSCs | In vitro | Ma et al., 2015 [54] | |
| Dentin & Pulp | Extrusion | Hyrel 3D, Norcross, GA, USA | Alginate + dentin matrix | SCAPs | In vitro | Athirasala et al., 2018 [55] |
| Extrusion | Integrated tissue–organ printing system | Gelatin + fibrinogen + HA + glycerol | DPSCs | In vitro | Han et al., 2019 [56] | |
| Dentin | Extrusion | BioX, CELLINK, Gothenburg, Sweden | Calcium silicate + GelMA | DPSCs | In vitro | Lin et al., 2021 [57] |
| Extrusion | Homemade 3D bioprinter, Ulsan, Korea | Demineralized dentin matrix particles + fibrinogen + gelatin | DPSCs | In vitro | Han et al., 2021 [58] | |
| Extrusion | CELLINK BIO-X 3D printer, Gothenburg, Sweden | Poloxamer-407 | SCAPs | In vitro | Dutta et al., 2021 [59] | |
| Extrusion | DTR3-2210 T-SG; DASA Robot, Bucheon, Korea | Collagen type 1 or dECMs + β-TCP | DPSCs | In vitro and in vivo | Kim et al., 2022 [60] | |
| Pulp | Inkjet | Hand-held bioprinter (DropGun, BlackDrop Biodrucker GmbH, Aachen, Germany) | Collagen type 1 + agarose | DPSCs & HUVECs | In vitro | Duarte Campos et al., 2020 [61] |
| Tissue Type | Bioprinting Technique | Bioink | Assessments | Outcomes | Cell Densities | Cell Viability | Author |
|---|---|---|---|---|---|---|---|
| Bone | Extrusion | Gelatin + fibrinogen + HA + glycerol | 1. Cell viability 2. Cell proliferation 3. Osteogenic differentiation | 1. Printing process did not adversely affect cell viability at day 1 of culture 2. Cell proliferation increased over 15-day period 3. Calcium deposition in the hAFSCs-laden hydrogel in 3D bone structures | 5 × 106 cells/mL | 91 ± 2% | Kang et al., 2016 [40] |
| Extrusion | MeHA + GelMA + HA | 1. Cell viability 2. Alkaline phosphatase activity 3. Gene expression | 1. SVFC in bioprinted constructs showed high cell viability in both normoxic and hypoxic environments at day 7; however, long-term hypoxia (more than 14 days) impaired cell viability and vascularization 2. No significant difference in ALP activity between normoxia and hypoxia groups (after 21 days) in 3D bioprinted bone constructs using SVFC-laden hydrogels and PCL/HAp 3. Short-term hypoxia promoted vascularization of SVFC by significantly upregulating VEGFA and HIF1A expression in SVFC-laden hydrogels culture in GM/EGM | 4 × 106 cells/mL | - | Kuss et al., 2017 [41] | |
| Extrusion | GelMA + kCA + nSi (NICE bioink) | 1. Cell-assisted matrix remodeling (histological) 2. Calcium content 3. Gene expression | 1. Cells deposit cartilage/osteoid-like matrix of glycosaminoglycans, proteoglycans and collagen followed by mineralization of the surrounding matrix 2. Calcium content increased steadily from day 0 to day 60 3. Upregulated gene expression of SMADs 1/4/5/7, SOX9, TGF-β, osteonectin (SPARC), cadherin-11 | - | - | Chimene et al., 2020 [42] | |
| Extrusion | ECM + AMP | 1. Cell viability 2. Osteogenic differentiation 3. Gene expression | 1. Cell-laden bioink with and without AMP showed viable cells ~90% up to day 5 2. Cell-laden bioprinted constructs with AMP showed high level of ALP activity 3. ECM/AMP bioink increased OPN and COL1A1 osteogenic gene expression at day 14 | 1 × 106 cells/mL | >90% | Dubey et al., 2020 [43] | |
| Extrusion | Gelatin + GelMA + HA + glycerol | 1. Cell viability 2. Cell proliferation 3. Osteogenic differentiation 4. Gene expression | 1. hDPSCs viability was >90% for bioprinted GelMA and BMP-GelMA constructs at all time points 2. hDPSCs maintained the proliferation capability in both constructs 3. BMP mimicking peptide can promote osteogenic expression 4. Increase in the expression of RUNX2 at 2 weeks in BMP-GelMA compared to GelMA group, both cultured in normal growth medium. COL1A1 and OCN expression increased in all groups after 4 weeks. No significant difference of expression level of DSPP in all medium conditions | - | >90% | Park et al., 2020 [44] | |
| Extrusion | Alginate + gelatin + nHAp | 1. Cell viability 2. Cell adhesion 3. Cell proliferation 4. ALP activity | 1. hPDLSCs were viable in alginate + gel + nHAp and alginate only bioscaffolds 2. Cell adhesion of alginate + gel + nHAp bioscaffold was better than that of alginate only 3. Cell proliferation activity rate of alginate + gel + nHAp bioscaffold was higher than that of alginate only at days 2, 4 and 6 4. ALP activity of alginate + gel + nHAp was higher than that of alginate only bioscaffold after 14 days | - | - | Tian et al., 2021 [45] | |
| Extrusion | Collagen + chitosan + β-glycerophosphate + nHAp | 1. Cell viability 2. Cell proliferation 3. Gene expression | 1. Cell viability after printing increased to >95% in a week 2. rBMSCs significantly proliferated between day 4 and day 7 3. ALP, OPN and OCN were upregulated and showed favorable osteogenic properties | 5 × 106 cells/mL | >95% | Moncal et al., 2021 [46] | |
| Extrusion | Collagen + chitosan + β-glycerophosphate + nHAp | 1. Cell viability 2. Cell proliferation 3. Calcium deposition 4. Protein expression 5. Gene expression | 1. Cell viability after printing increased to >95% in a week 2. Bioink + PDGF groups resulted in increase in cell proliferation rate compared to the BMP-2 group 3. Bioink + pPDGF-B + CS-NPs (pBMP-2) had a significant increase in calcium ion deposition in week 3 compared to other groups 4. Bioink + CS-NPs (pBMP-2) group had the highest BMP-2 production on day 4 compared to other groups 5. All osteogenic regulator genes, RUNX2, ALP, BMP-2 and OCN indicated that Bioink + pPDGF-B + CS-NPs(pBMP-2) promoted the most accelerated osteogenic differentiation | 8 × 105 cells/mL | >95% | Moncal et al., 2022 [47] | |
| LAB | Collagen type 1 + nHAp | 1. Cell proliferation 2. Metabolic activity | 1. Cells proliferated and fill the voids at day 2 and day 4 2. Cells showed an increase in metabolic activity from day 1 to day 8 | 120 × 106 cells/mL | - | Keriquel et al., 2017 [48] | |
| LAB | Collagen type 1 + TCP (BioRoot RCS®, Septodont, France) | 1. ALP activity 2. Osteogenic differentiation 3. Cell migration | 1. Cells in osteogenic medium expressed higher ALP activity compared to other conditions at day 14 2. The use of mineralized ink (MI) was not able to meet the level of osteogenic differentiation with a dedicated medium 3. Cell migration speed was found to be enhanced by the presence of MI | 2 × 103 cells/mL | - | Touya et al., 2022 [50] | |
| Inkjet | GelMA + PEGDA | 1. Cell viability 2. Cell proliferation 3. Cell spreading 4. ALP activity 5. Gene expression | 1. PDLSCs viability was ~90% in the composite hydrogels with GelMA to PEGDA volume proportion of 2:3 to 4:1 2. GelMA/PEGDA proportion of 4:1 hydrogel showed significant PDLSCs proliferation after 7 days 3. PDLSCs spreading was enhanced as the volume proportion of GelMA to PEGDA increased 4. ALP activity of PDLSCs increased as the volume proportion of GelMA to PEGDA increased at day 7 and day 10 5. OCN and OPN expression of PDLSCs were increased when the volume proportion of GelMA/PEGDA increased from 1:4 to 4:1 | 1 × 106 cells/mL | ~90% | Ma et al., 2017 [51] | |
| Periodontal | Extrusion | Collagen | 1. Cell viability 2. Cell proliferation | 1. Cell viability was lower in cell printing group compared to cell seeding group on day 1 with no significant difference 2. Cell proliferation in cell printing group showed good extent on day 7 | 1 × 107 cells/mL | - | Lee et al., 2021 [52] |
| Extrusion | Collagen | 1. Cell viability 2. Cell proliferation 3. Quantification of growth factors 4. Protein expression | 1. No dead cells in Col-based bioink at week 0, 1, 2, 4, 6 and 8 2. Proliferation levels were higher in bi-layer scaffold (Col/SrCS) compared to one-layer Col bioink at days 3, 7 and 14 3. Bi-layer group had higher secretions of FGF-2, BMP-2 and VEGF from human gingival fibroblasts at all time points 4. Increased secretion of osteogenic-related proteins ALP, BSP and OC from the bi-layer scaffold (Col/SrCS) at days 7 and 14 | 5 × 105 cells/mL | - | Wang et al., 2021 [53] | |
| Inkjet | GelMA + PEGDA | 1. Cell viability 2. Cell spreading 3. Cell proliferation | 1. PDLSCs viability was 82.4 ± 4.7% after 72 h for a pressure range of 40–60 kPa 2. Spreading area of PDLSCs reduced dramatically with a decrease in GelMA and increase in PEG volume proportion 3. Viable cells decreased with decreasing proportion of GelMA on day 3 and day 5 | 1 × 106 cells/mL | 82.4 ± 4.7% | Ma et al., 2015 [54] | |
| Dentin & Pulp | Extrusion | Alginate + dentin matrix | 1. Cell viability 2. Protein expression 3. Gene expression | 1. Cells encapsulated in Alg-Dent hydrogels had higher cell viability >90% after 5 days 2. Increased expression of ALP at the protein levels in cell-laden bioink 3. Increased in ALP and RUNX2 gene expression in cell-laden bioink after 10 days | 0.8 × 106 cells/mL | >90% | Athirasala et al., 2018 [55] |
| Extrusion | Gelatin + fibrinogen + HA + glycerol | 1. Cell viability 2. Cell proliferation 3. Gene expression | 1. Viability of hDPSCs was >90% in all groups at day 4 2. hDPSCs proliferation rate decreased with increasing fibrinogen concentration 3. Expression of DMP-1 and DSPP increased with fibrinogen concentration | 3 × 106 cells/mL | >90% | Han et al., 2019 [56] | |
| Dentin | Extrusion | Calcium silicate + GelMA | 1. Cell viability 2. Cell proliferation 3. Calcium deposition 4. Protein expression | 1. hDPSCs viability increased when CS concentration increased in CS/GelMA bioink 2. CS/GelMA bioink enhanced the proliferation rate of hDPSCs on day 7 as the concentration of CS increased 3. Calcium deposition increased in CS10 group at day 7 and day 14 4. The expressions of ALP, DMP-1 and OC were enhanced from the release of silicon ions in CS/GelMA bioink | 5 × 106 cells/mL | - | Lin et al., 2021 [57] |
| Extrusion | Demineralized dentin matrix particles (DDMp) + fibrinogen + gelatin | 1. Cell viability 2. Cell proliferation 3. Osteogenic differentiation 4. Gene expression | 1. Viability of DPSCs > 95% in all concentrations of DDMp bioinks and fibrinogen-gelatin mixture at day 7 2. DPSCs proliferation rate decreased as the DDMp concentration increased at day 7 3. Higher mineralization in DDMp bioink group compared to fibrogen–gelatin mixture after culturing with differentiation medium for 15 days 4. Expression levels of DSPP and DMP-1 were higher in DDMp bioink | 3 × 106 cells/mL | >95% | Han et al., 2021 [58] | |
| Extrusion | Poloxamer-407 | 1. Cell viability 2. Cell morphology 3. Cell migration 4. Gene expression | 1. SCAPs viability increased in 5 V-1 Hz (0.62 mT) EMF exposure after 3 days of culture 2. The entire 3D matrix was covered by cells in 5 V EMF-treated groups after 3 days of culture 3. The number of migrated cells increased in EMF-treated and PAI-1 + EMF-treated samples 4. Higher expression of ALP, DSPP, DMP-1 and Col-1 in 5 V EMF treatment | 2.5 × 104 cells/mL | - | Dutta et al., 2021 [59] | |
| Extrusion | Collagen type 1 or dECMs + β-TCP | 1. Cell viability 2. Cell proliferation 3. Gene expression | 1. hDPSCs viability in collagen/β-TCP (CTS-20) and dECM/β-TCP (dECM-20) were approximately 97% after 1 day 2. Cell proliferation in dECM-20 bioink was higher than CTS-20 3. Significant increase in osteogenic gene expression of OPN, OCN, BGN and odontogenic gene expression of DSPP and DMP-1 in dECM-20 | 1 × 107 cells/mL | >95% | Kim et al., 2022 [60] | |
| Pulp | Inkjet | Collagen type 1 + agarose | 1. Vasculogenesis | 1. Vascular tube formation in all tested hydrogels | 3 × 106 cells/mL | - | Duarte Campos et al., 2020 [61] |
| Tissue Type | Bioprinting Technique | Bioink | Animal Model | Defect Area | In Vivo Testing | Outcomes | Author |
|---|---|---|---|---|---|---|---|
| Bone | Extrusion | Gelatin + fibrinogen + HA + glycerol | Sprague Dawley rats 250–300 g | Calvarium 8 mm diameter, 1.2 mm depth | 1. Histology 2. Immuno-histology | 1. Bioprinted materials showed newly vascularized bone tissues with no necrosis at implanted sites 2. Large blood vessel formation within newly formed bone tissues | Kang et al., 2016 [40] |
| Extrusion | MeHA + GelMA + HA | Female athymic nude mice 8 weeks old | Dorsal sub-cutaneous | 1. Histology 2. Immuno-histology 3. Microvessel density and area distribution | 1. Dense populated cells with obvious microvascularity throughout the bioprinted constructs 2. Integration of formed lumens with existing host vasculature 3. Lumen sizes were larger, and broader vessel area distribution in constructs conditioned with hypoxic environment compared to normoxia group | Kuss et al., 2017 [41] | |
| LAB | Collagen type 1 + nHAp | Female Balb/c mice 12 weeks old 19–20 g | Calvarium 3.3 mm diameter | 1. Micro-CT 2. Histology | 1. Increase in BV/TV at 2 months after printing in nHAp-collagen-D1 cells with disk geometry 2. Substantial and well-distributed new bone formation throughout the defect at 1 month and formation of mature bone at the center of the defect at 2 months in nHAp-collagen-D1 cells with disk geometry | Keriquel et al., 2017 [48] | |
| LAB | Collagen type 1 | Female NSG mice 10 weeks old 25–26 g | Calvarium 3.3 mm diameter | 1. Fluorescence imaging 2. Micro-CT 3. Histology | 1. Vascular network were well interconnected in printed pattern when compared to randomly seeded cells which had weak network organization into the defect 2. Increased percentage bone formation (BV/TV) in printed HUVECs in calvarial defects at 2 months 3. Printed HUVECs increased the vessel density in bone defects at 2 months | Kérourédan et al., 2019 [49] | |
| LAB | Collagen type 1 + TCP (BioRoot RCS®, Septodont, France) | Female NSG mice 8 weeks old | Calvarium 3.3 mm diameter | 1. Micro-CT 2. Histology | 1. Mineralized Ink (MI) was found not to be effective in improving bone repair and there was no difference between the two patterns after 2 months 2. No difference in vessel density between defects filled with MI, control and pipette deposit | Touya et al., 2022 [50] | |
| Periodontal | Extrusion | Collagen | Male athymic rats 9 weeks old | Calvarium 8 mm diameter, 1.2 mm depth | 1. Histology 2. Immunohisto-chemistry | 1. Fibrous connective tissue was apparent in the cell printing group which was not observed in the seeding group. Periodontal-like tissue was oriented parallel to the porous titanium implant surface in the cell printing group 2. HLA, periostin, vWF and CEMP1 were expressed in the connective tissues produced in the cell printing groups | Lee et al., 2021 [52] |
| Extrusion | Collagen | Female New Zealand white rabbits 2 kg | Calvarium 7 mm diameter, 8 mm depth | 1. Micro-CT 2. Histology | 1. hGF-laden bi-layered scaffolds had higher Tb.Th and BV/TV ratio after 12 weeks of implantation 2. hGF-laden bi-layered scaffolds were wrapped by new bone tissues compared to SrCS scaffold which had new bone growth at the periphery of the scaffold | Wang et al., 2021 [53] | |
| Dentin | Extrusion | Collagen type 1 or dECMs + β-TCP | Athymic nude mice | Dorsal sub-cutaneous | 1. Histology 2. Immuno-histochemistry | 1. Increase in blood vessel formation in the implanted dECM-20 scaffold 2. Strong signal of OPN and OCN in dECM-20 DSPP and DMP-1 were strongly expressed in the dECM-20 at 8 weeks | Kim et al., 2022 [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohd, N.; Razali, M.; Fauzi, M.B.; Abu Kasim, N.H. In Vitro and In Vivo Biological Assessments of 3D-Bioprinted Scaffolds for Dental Applications. Int. J. Mol. Sci. 2023, 24, 12881. https://doi.org/10.3390/ijms241612881
Mohd N, Razali M, Fauzi MB, Abu Kasim NH. In Vitro and In Vivo Biological Assessments of 3D-Bioprinted Scaffolds for Dental Applications. International Journal of Molecular Sciences. 2023; 24(16):12881. https://doi.org/10.3390/ijms241612881
Chicago/Turabian StyleMohd, Nurulhuda, Masfueh Razali, Mh Busra Fauzi, and Noor Hayaty Abu Kasim. 2023. "In Vitro and In Vivo Biological Assessments of 3D-Bioprinted Scaffolds for Dental Applications" International Journal of Molecular Sciences 24, no. 16: 12881. https://doi.org/10.3390/ijms241612881
APA StyleMohd, N., Razali, M., Fauzi, M. B., & Abu Kasim, N. H. (2023). In Vitro and In Vivo Biological Assessments of 3D-Bioprinted Scaffolds for Dental Applications. International Journal of Molecular Sciences, 24(16), 12881. https://doi.org/10.3390/ijms241612881







