The Role of Increased Expression of Sirtuin 6 in the Prevention of Premature Aging Pathomechanisms
Abstract
1. Introduction
2. Structure
Activation and Reactivation of Gene Expression
3. Aging
4. Metabolic Activity
5. Inflammation
6. Role in the Wound Healing Process
7. Role of Physical Activity
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haigis, M.C.; Sinclair, D.A. Mammalian Sirtuins: Biological Insights and Disease Relevance. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 253–295. [Google Scholar] [CrossRef]
- Carafa, V.; Rotili, D.; Forgione, M.; Cuomo, F.; Serretiello, E.; Hailu, G.S.; Jarho, E.; Lahtela-Kakkonen, M.; Mai, A.; Altucci, L. Sirtuin Functions and Modulation: From Chemistry to the Clinic. Clin. Epigenetics 2016, 8, 61. [Google Scholar] [CrossRef]
- Frydzińska, Z.; Owczarek, A.; Winiarska, K. Sirtuiny i Ich Rola w Regulacji Metabolizmu. Postep. Biochem. 2019, 65, 31–40. [Google Scholar] [CrossRef]
- Jing, H.; Lin, H. Sirtuins in Epigenetic Regulation. Chem. Rev. 2015, 115, 2350–2375. [Google Scholar] [CrossRef]
- Sinclair, D.A.; Guarente, L. Extrachromosomal RDNA Circles-A Cause of Aging in Yeast. Cell 1997, 91, 1033–1042. [Google Scholar] [CrossRef]
- Sharma, A.; Diecke, S.; Zhang, W.Y.; Lan, F.; He, C.; Mordwinkin, N.M.; Chua, K.F.; Wu, J.C. The Role of SIRT6 Protein in Aging and Reprogramming of Human Induced Pluripotent Stem Cells. J. Biol. Chem. 2013, 288, 18439–18447. [Google Scholar] [CrossRef]
- Madsen, A.S.; Andersen, C.; Daoud, M.; Anderson, K.A.; Laursen, J.S.; Chakladar, S.; Huynh, F.K.; Colaço, A.R.; Backos, D.S.; Fristrup, P.; et al. Investigating the Sensitivity of NAD+-Dependent Sirtuin Deacylation Activities to NADH. J. Biol. Chem. 2016, 291, 7128–7141. [Google Scholar] [CrossRef]
- Kanfi, Y.; Naiman, S.; Amir, G.; Peshti, V.; Zinman, G.; Nahum, L.; Bar-Joseph, Z.; Cohen, H.Y. The Sirtuin SIRT6 Regulates Lifespan in Male Mice. Nature 2012, 483, 218–221. [Google Scholar] [CrossRef]
- Vitiello, M.; Zullo, A.; Servillo, L.; Mancini, F.P.; Borriello, A.; Giovane, A.; Della Ragione, F.; D’Onofrio, N.; Balestrieri, M.L. Multiple Pathways of SIRT6 at the Crossroads in the Control of Longevity, Cancer, and Cardiovascular Diseases. Ageing Res. Rev. 2017, 35, 301–311. [Google Scholar] [CrossRef]
- Ran, L.K.; Chen, Y.; Zhang, Z.Z.; Tao, N.N.; Ren, J.H.; Zhou, L.; Tang, H.; Chen, X.; Chen, K.; Li, W.Y.; et al. SIRT6 Overexpression Potentiates Apoptosis Evasion in Hepatocellular Carcinoma via BCL2-Associated X Protein-Dependent Apoptotic Pathway. Clin. Cancer Res. 2016, 22, 3372–3382. [Google Scholar] [CrossRef]
- Dominy, J.E.; Lee, Y.; Jedrychowski, M.P.; Chim, H.; Jurczak, M.J.; Camporez, J.P.; Ruan, H.B.; Feldman, J.; Pierce, K.; Mostoslavsky, R.; et al. The Deacetylase Sirt6 Activates the Acetyltransferase GCN5 and Suppresses Hepatic Gluconeogenesis. Mol. Cell 2012, 48, 900–913. [Google Scholar] [CrossRef]
- Elbarbary, R.A.; Lucas, B.A.; Maquat, L.E. Retrotransposons as Regulators of Gene Expression. Science 2016, 351, 6274. [Google Scholar] [CrossRef]
- Imai, S.i.; Guarente, L. NAD+ and Sirtuins in Aging and Disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef]
- Tennen, R.I.; Chua, K.F. Chromatin Regulation and Genome Maintenance by Mammalian SIRT6. Trends Biochem. Sci. 2011, 36, 39–46. [Google Scholar] [CrossRef]
- Mahlknecht, U.; Ho, A.; Volter-Mahlknecht, S. Chromosomal Organization and Fluorescence in Situhybridization of the Human Sirtuin 6 Gene. Int. J. Oncol. 2006, 28, 447–456. [Google Scholar]
- Gertler, A.A.; Cohen, H.Y. SIRT6, a Protein with Many Faces. Biogerontology 2013, 14, 629–639. [Google Scholar] [CrossRef]
- Bielawski, A.; Nalepa, I. Sirtuins—Intriguing multi-tasking "keepers" of life processes. Wszechświat 2019, 20, 4–6. [Google Scholar]
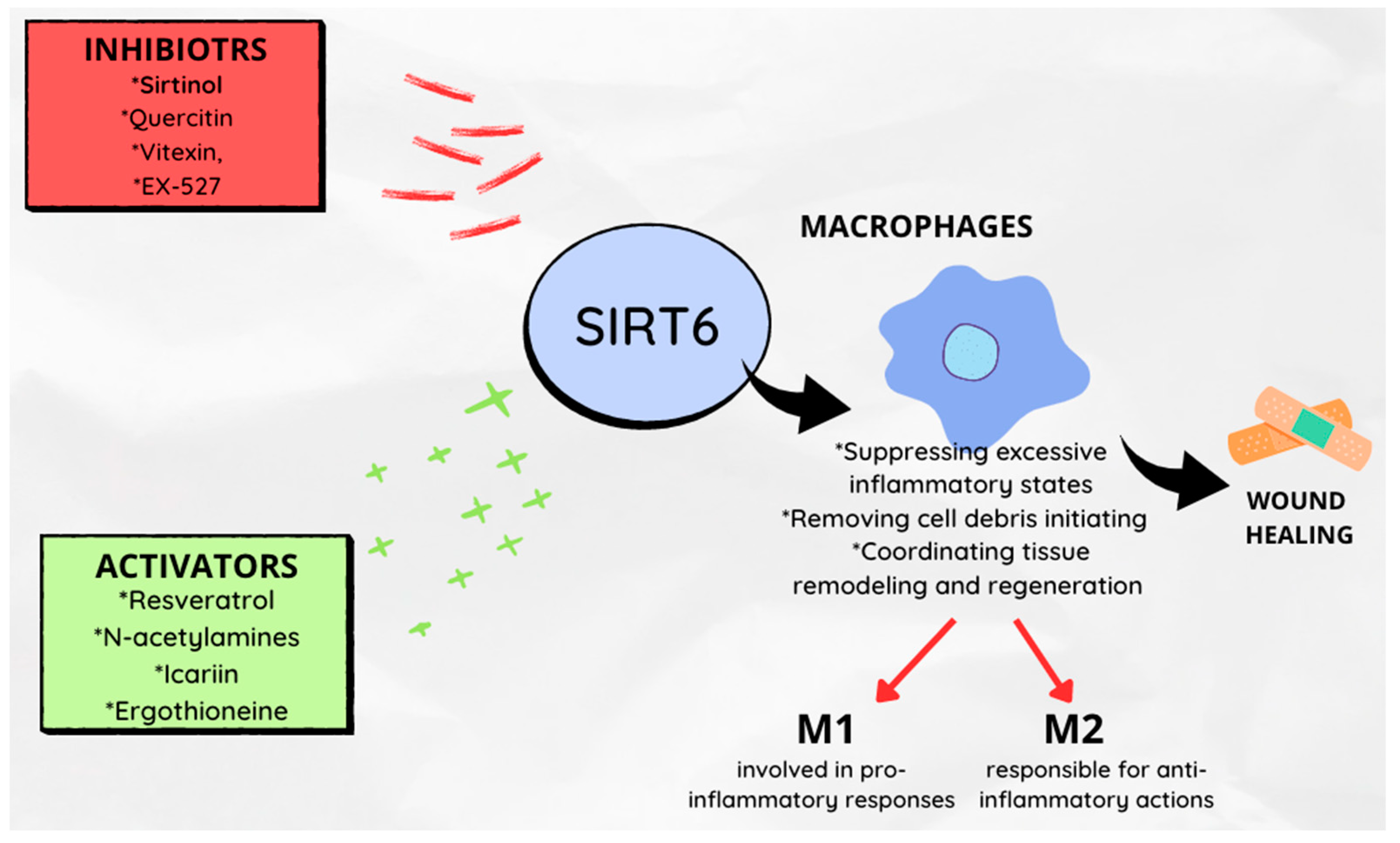
- Koo, J.H.; Jang, H.Y.; Lee, Y.; Moon, Y.J.; Bae, E.J.; Yun, S.K.; Park, B.H. Myeloid Cell-Specific Sirtuin 6 Deficiency Delays Wound Healing in Mice by Modulating Inflammation and Macrophage Phenotypes. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 Polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Chang, A.R.; Ferrer, C.M.; Mostoslavsky, R. SIRT6, a Mammalian Deacylase with Multitasking Abilities. Physiol. Rev. 2020, 100, 145–169. [Google Scholar] [CrossRef]
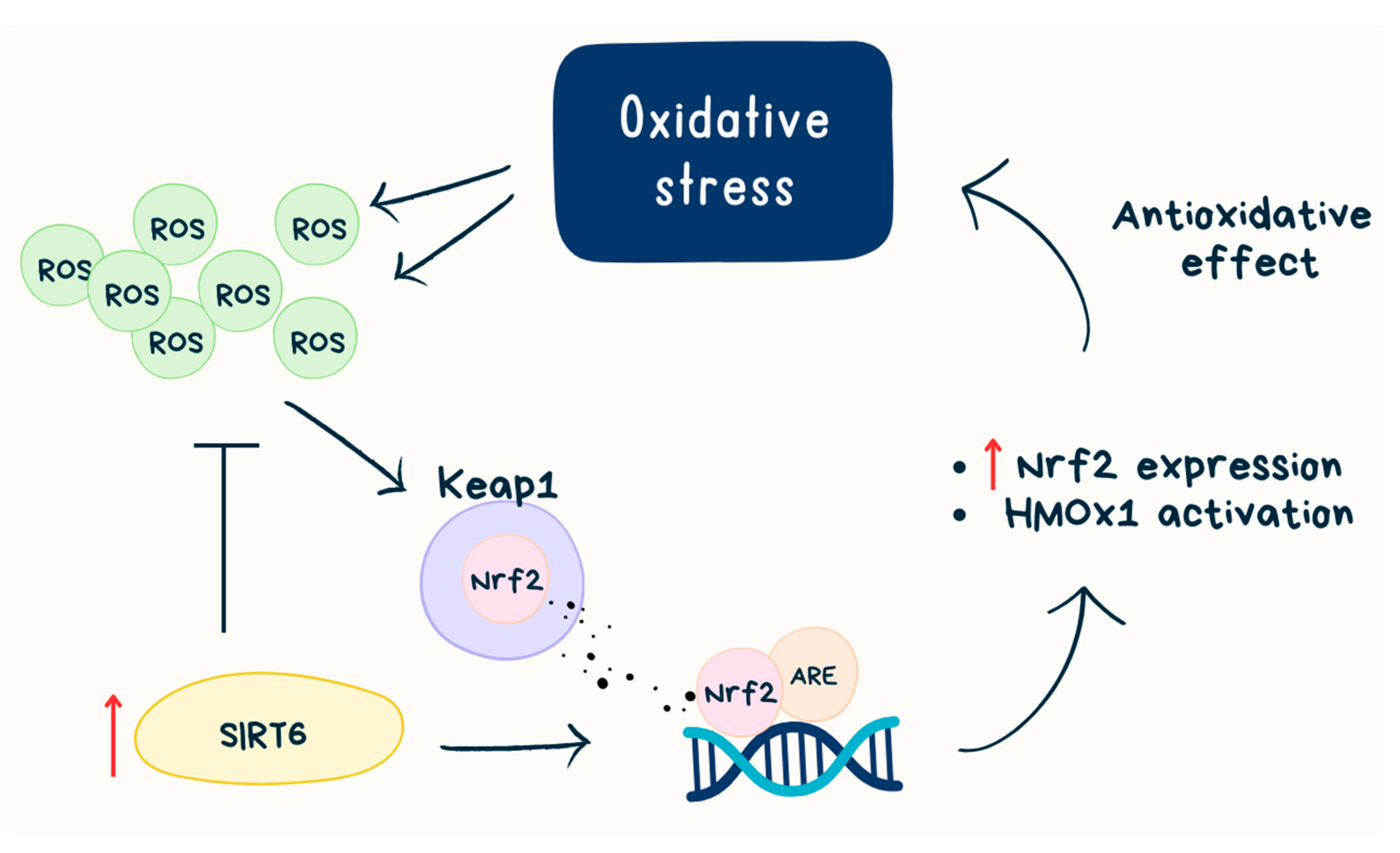
- Pan, H.; Guan, D.; Liu, X.; Li, J.; Wang, L.; Wu, J.; Zhou, J.; Zhang, W.; Ren, R.; Zhang, W.; et al. SIRT6 Safeguards Human Mesenchymal Stem Cells from Oxidative Stress by Coactivating NRF2. Cell Res. 2016, 26, 190–205. [Google Scholar] [CrossRef]
- Thandavarayan, R.A.; Garikipati, V.N.S.; Joladarashi, D.; Suresh Babu, S.; Jeyabal, P.; Verma, S.K.; Mackie, A.R.; Khan, M.; Arumugam, S.; Watanabe, K.; et al. Sirtuin-6 Deficiency Exacerbates Diabetes-Induced Impairment of Wound Healing. Exp. Dermatol. 2015, 24, 773–778. [Google Scholar] [CrossRef]
- Itoh, K.; Tong, K.I.; Yamamoto, M. Molecular Mechanism Activating Nrf2-Keap1 Pathway in Regulation of Adaptive Response to Electrophiles. Free. Radic. Biol. Med. 2004, 36, 1208–1213. [Google Scholar] [CrossRef]
- Kanwal, A.; Pillai, V.B.; Gupta, M.P. The Nuclear and Mitochondrial Sirtuins, Sirt6 and Sirt3, Regulate each others’ Activity and Protect the Heart from Developing Obesity-Mediated Diabeticcardiomyopathy. J. Fed. Am. Soc. Exp. Biol. 2020, 34, 14057. [Google Scholar]
- Dziechciaż, M.; Filip, R. Biological Psychological and Social Determinants of Old Age: Bio-Psycho-Social Aspects of Human Aging. Ann. Agric. Environ. Med. 2014, 21, 835–838. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194. [Google Scholar] [CrossRef]
- Franco, A.C.; Aveleira, C.; Cavadas, C. Skin Senescence: Mechanisms and Impact on Whole-Body Aging. Trends Mol. Med. 2022, 28, 97–109. [Google Scholar] [CrossRef]
- Mao, Z.; Hine, C.; Tian, X.; Van Meter, M.; Au, M.; Vaidya, A.; Seluanov, A.; Gorbunova, V. SIRT6 Promotes DNA Repair under Stress by Activating PARP1. Science 2011, 332, 1443–1446. [Google Scholar] [CrossRef]
- Cardus, A.; Uryga, A.K.; Walters, G.; Erusalimsky, J.D. SIRT6 Protects Human Endothelial Cells from DNA Damage, Telomere Dysfunction, and Senescence. Cardiovasc. Res. 2013, 97, 571–579. [Google Scholar] [CrossRef]
- Mostoslavsky, R.; Chua, K.F.; Lombard, D.B.; Pang, W.W.; Fischer, M.R.; Gellon, L.; Liu, P.; Mostoslavsky, G.; Franco, S.; Murphy, M.M.; et al. Genomic Instability and Aging-like Phenotype in the Absence of Mammalian SIRT6. Cell 2006, 124, 315–329. [Google Scholar] [CrossRef]
- Tower, J. Sex-Specific Gene Expression and Life Span Regulation. Trends Endocrinol. Metab. 2017, 28, 735–747. [Google Scholar] [CrossRef]
- Bétry, C.; Meugnier, E.; Pflieger, M.; Grenet, G.; Hercberg, S.; Galan, P.; Kesse-Guyot, E.; Vidal, H.; Laville, M. High Expression of CPT1b in Skeletal Muscle in Metabolically Healthy Older Subjects. Diabetes Metab. 2019, 45, 152–159. [Google Scholar] [CrossRef]
- Smirnov, D.; Eremenko, E.; Stein, D.; Kaluski, S.; Jasinska, W.; Cosentino, C.; Martinez-Pastor, B.; Brotman, Y.; Mostoslavsky, R.; Khrameeva, E.; et al. SIRT6 Is a Key Regulator of Mitochondrial Function in the Brain. Cell Death Dis. 2023, 14, 35. [Google Scholar] [CrossRef]
- Shay, J.W. Telomeres and Aging. Curr. Opin. Cell Biol. 2018, 52, 1–7. [Google Scholar] [CrossRef]
- Michishita, E.; McCord, R.A.; Berber, E.; Kioi, M.; Padilla-Nash, H.; Damian, M.; Cheung, P.; Kusumoto, R.; Kawahara, T.L.A.; Barrett, J.C.; et al. SIRT6 Is a Histone H3 Lysine 9 Deacetylase That Modulates Telomeric Chromatin. Nature 2008, 452, 492–496. [Google Scholar] [CrossRef]
- Kugel, S.; Mostoslavsky, R. Chromatin and beyond: The Multitasking Roles for SIRT6. Trends Biochem. Sci. 2014, 39, 72–81. [Google Scholar] [CrossRef]
- Balestrieri, M.L.; Rizzo, M.R.; Barbieri, M.; Paolisso, P.; D’onofrio, N.; Giovane, A.; Siniscalchi, M.; Minicucci, F.; Sardu, C.; D’andrea, D.; et al. Sirtuin 6 Expression and Inflammatory 7 Activity in Diabetic Atherosclerotic Plaques: Effects of Incretin Treatment. Diabetes 2015, 64, 1395–1406. [Google Scholar] [CrossRef]
- Van Meter, M.; Kashyap, M.; Rezazadeh, S.; Geneva, A.J.; Morello, T.D.; Seluanov, A.; Gorbunova, V. SIRT6 Represses LINE1 Retrotransposons by Ribosylating KAP1 but This Repression Fails with Stress and Age. Nat. Commun. 2014, 5, 5011. [Google Scholar] [CrossRef]
- Gruber, F.; Kremslehner, C.; Eckhart, L.; Tschachler, E. Cell Aging and Cellular Senescence in Skin Aging—Recent Advances in Fibroblast and Keratinocyte Biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef]
- Kanfi, Y.; Peshti, V.; Gil, R.; Naiman, S.; Nahum, L.; Levin, E.; Kronfeld-Schor, N.; Cohen, H.Y. SIRT6 Protects against Pathological Damage Caused by Diet-Induced Obesity. Aging Cell 2010, 9, 162–173. [Google Scholar] [CrossRef]
- Zhong, L.; D’Urso, A.; Toiber, D.; Sebastian, C.; Henry, R.E.; Vadysirisack, D.D.; Guimaraes, A.; Marinelli, B.; Wikstrom, J.D.; Nir, T.; et al. The Histone Deacetylase Sirt6 Regulates Glucose Homeostasis via Hif1α. Cell 2010, 140, 280–293. [Google Scholar] [CrossRef]
- Kim, H.S.; Xiao, C.; Wang, R.H.; Lahusen, T.; Xu, X.; Vassilopoulos, A.; Vazquez-Ortiz, G.; Jeong, W.I.; Park, O.; Ki, S.H.; et al. Hepatic-Specific Disruption of SIRT6 in Mice Results in Fatty Liver Formation Due to Enhanced Glycolysis and Triglyceride Synthesis. Cell Metab. 2010, 12, 224–236. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Pirinen, E.; Auwerx, J. Sirtuins as Regulators of Metabolism and Healthspan. Nat. Rev. Mol. Cell Biol. 2012, 13, 225–238. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, G.; Tao, R.; Wu, P.; Kono, T.; Li, K.; Ding, W.X.; Tong, X.; Tersey, S.A.; Harris, R.A.; et al. Sirtuin 6 Regulates Glucose-Stimulated Insulin Secretion in Mouse Pancreatic Beta Cells. Diabetologia 2016, 59, 151–160. [Google Scholar] [CrossRef]
- Kuang, J.; Zhang, Y.; Liu, Q.; Shen, J.; Pu, S.; Cheng, S.; Chen, L.; Li, H.; Wu, T.; Li, R.; et al. Fat-Specific Sirt6 Ablation Sensitizes Mice to High-Fat Diet-Induced Obesity and Insulin Resistance by Inhibiting Lipolysis. Diabetes 2017, 66, 1159–1171. [Google Scholar] [CrossRef]
- Tang, Q.; Gao, Y.; Liu, Q.; Yang, X.; Wu, T.; Huang, C.; Huang, Y.; Zhang, J.; Zhang, Z.; Li, R.; et al. Sirt6 in Pro-Opiomelanocortin Neurons Controls Energy Metabolism by Modulating Leptin Signaling. Mol. Metab. 2020, 37, 100994. [Google Scholar] [CrossRef]
- Kanfi, Y.; Shalman, R.; Peshti, V.; Pilosof, S.N.; Gozlan, Y.M.; Pearson, K.J.; Lerrer, B.; Moazed, D.; Marine, J.C.; de Cabo, R.; et al. Regulation of SIRT6 Protein Levels by Nutrient Availability. FEBS Lett. 2008, 582, 543–548. [Google Scholar] [CrossRef]
- Mccay, C.M.; Crowell, M.F.; Maynard, L.A. The Effect of Retarded Growth upon the Length of Life Span and upon the Ultimate Body Size. 1935. Nutrition 1989, 5, 155–171. [Google Scholar]
- Pallauf, K.; Giller, K.; Huebbe, P.; Rimbach, G. Nutrition and Healthy Ageing: Calorie Restriction or Polyphenol-Rich “MediterrAsian” Diet? Oxid. Med. Cell Longev. 2013, 2013, 707421. [Google Scholar] [CrossRef]
- Menendez, J.A.; Joven, J.; Aragonès, G.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Borrás-Linares, I.; Camps, J.; Corominas-Faja, B.; Cufí, S.; Fernández-Arroyo, S.; et al. Xenohormetic and Anti-Aging Activity of Secoiridoid Polyphenols Present in Extra Virgin Olive Oil: A New Family of Gerosuppressant Agents. Cell Cycle 2013, 12, 555–578. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L.; et al. Small Molecule Activators of Sirtuins Extend Saccharomyces Cerevisiae Lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Puertas, B.; Fernández, M.I.; Palma, M.; Cantos-Villar, E. Induction of Stilbenes in Grapes by UV-C: Comparison of Different Subspecies of Vitis. Innov. Food Sci. Emerg. Technol. 2010, 11, 231–238. [Google Scholar] [CrossRef]
- Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. Human Nutrition and Metabolism Dietary Intakes of Flavonols, Flavones and Isoflavones by Japanese Women and the Inverse Correlation between Quercetin Intake and Plasma LDL Cholesterol Concentration 1. J. Nutr. 2000, 130, 2243–2250. [Google Scholar] [CrossRef]
- Rasbach, K.A.; Schnellmann, R.G. Isoflavones Promote Mitochondrial Biogenesis. J. Pharmacol. Exp. Ther. 2008, 325, 536–543. [Google Scholar] [CrossRef]
- Song, T.; Barua, K.; Buseman, G.; Murphy, P. Soy Isoflavone Analysis: Quality Control and a New Internalstandard. Am. J. Clin. Nutr. 1998, 68, 1474–1479. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Chia, C.C.; Chen, P.J.; Huang, S.E.; Huang, S.C.; Kuo, P.L. Shallot and Licorice Constituent Isoliquiritigenin Arrests Cell Cycle Progression and Induces Apoptosis through the Induction of ATM/P53 and Initiation of the Mitochondrial System in Human Cervical Carcinoma HeLa Cells. Mol. Nutr. Food Res. 2009, 53, 826–835. [Google Scholar] [CrossRef]
- Jiang, H.; Khan, S.; Wang, Y.; Charron, G.; He, B.; Sebastian, C.; Du, J.; Kim, R.; Ge, E.; Mostoslavsky, R.; et al. SIRT6 Regulates TNF-α Secretion through Hydrolysis of Long-Chain Fatty Acyl Lysine. Nature 2013, 496, 110–113. [Google Scholar] [CrossRef]
- Mendes, K.L.; Lelis, D.d.F.; Santos, S.H.S. Nuclear Sirtuins and Inflammatory Signaling Pathways. Cytokine Growth Factor Rev. 2017, 38, 98–105. [Google Scholar] [CrossRef]
- Lappas, M. Anti-Inflammatory Properties of Sirtuin 6 in Human Umbilical Vein Endothelial Cells. Mediat. Inflamm. 2012, 2012, 597514. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Meng, L.; Cao, G.; Wu, Y. Sirtuin 6 Overexpression Relieves Sepsis-Induced Acute Kidney Injury by Promoting Autophagy. Cell Cycle 2019, 18, 425–436. [Google Scholar] [CrossRef]
- Chen, J.; Chen, S.; Cai, D.; Wang, Q.; Qin, J. The Role of Sirt6 in Osteoarthritis and Its Effect on Macrophage Polarization. Bioengineered 2022, 13, 9677–9689. [Google Scholar] [CrossRef]
- Guo, Z.; Li, P.; Ge, J.; Li, H. SIRT6 in Aging, Metabolism, Inflammation and Cardiovascular Diseases. Aging Dis. 2022, 13, 1787–1822. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-ΚB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct Target Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Kawahara, T.L.A.; Michishita, E.; Adler, A.S.; Damian, M.; Berber, E.; Lin, M.; McCord, R.A.; Ongaigui, K.C.L.; Boxer, L.D.; Chang, H.Y.; et al. SIRT6 Links Histone H3 Lysine 9 Deacetylation to NF-ΚB-Dependent Gene Expression and Organismal Life Span. Cell 2009, 136, 62–74. [Google Scholar] [CrossRef]
- Xia, W.; Xiao, J.; Tong, C.L.; Lu, J.; Tu, Y.; Li, S.; Ni, L.; Shi, Y.; Luo, P.; Zhang, X.; et al. Orientin Inhibits Inflammation in Chondrocytes and Attenuates Osteoarthritis through Nrf2/NF-ΚB and SIRT6/NF-ΚB Pathway. J. Orthop. Res. 2023, 1–13. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, X.; Yang, X.; Bai, X.; Lu, Y.; Zhu, L.; Cheng, W.; Shu, M.; Zhu, Y.; Du, X.; et al. Deacetylation of Caveolin-1 by Sirt6 Induces Autophagy and Retards High Glucose-Stimulated LDL Transcytosis and Atherosclerosis Formation. Metabolism 2022, 131, 155162. [Google Scholar] [CrossRef]
- Li, B.; Xin, Z.; Gao, S.; Li, Y.; Guo, S.; Fu, Y.; Xu, R.; Wang, D.; Cheng, J.; Liu, L.; et al. SIRT6-Regulated Macrophage Efferocytosis Epigenetically Controls Inflammation Resolution of Diabetic Periodontitis. Theranostics 2023, 13, 231–249. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Q.; Wang, H.; Gao, Y.; Nie, K.; Tang, Y.; Su, H.; Hu, M.; Gong, J.; Fang, K.; et al. Diosgenin Protects against Podocyte Injury in Early Phase of Diabetic Nephropathy through Regulating SIRT6. Phytomedicine 2022, 104, 154276. [Google Scholar] [CrossRef]
- Bae, E.J.; Park, B.-H. Multiple Roles of Sirtuin 6 in Adipose Tissue Inflammation. Diabetes Metab. J. 2023, 47, 164–172. [Google Scholar] [CrossRef]
- Gao, S.; Yang, Q.; Peng, Y.; Kong, W.; Liu, Z.; Li, Z.; Chen, J.; Bao, M.; Li, X.; Zhang, Y.; et al. SIRT6 Regulates Obesity-Induced Oxidative Stress via ENDOG/SOD2 Signaling in the Heart. Cell Biol. Toxicol. 2022. [Google Scholar] [CrossRef]
- Ji, M.L.; Jiang, H.; Li, Z.; Geng, R.; Hu, J.Z.; Lin, Y.C.; Lu, J. Sirt6 Attenuates Chondrocyte Senescence and Osteoarthritis Progression. Nat. Commun. 2022, 13, 7658. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Nair, M.G. Macrophages in Wound Healing: Activation and Plasticity. Immunol. Cell Biol. 2019, 97, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Delavary, B.M.; van der Veer, W.M.; van Egmond, M.; Niessen, F.B.; Beelen, R.H.J. Macrophages in Skin Injury and Repair. Immunobiology 2011, 216, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, Y.; Zhu, L.; Yang, K.; Liang, K.; Tan, J.; Yu, B. SIRT6 Promotes Angiogenesis and Hemorrhage of Carotid Plaque via Regulating HIF-1α and Reactive Oxygen Species. Cell Death Dis. 2021, 12, 77. [Google Scholar] [CrossRef]
- Zhang, P.; He, L.; Zhang, J.; Mei, X.; Zhang, Y.; Tian, H.; Chen, Z. Preparation of Novel Berberine Nano-Colloids for Improving Wound Healing of Diabetic Rats by Acting Sirt1/NF-ΚB Pathway. Colloids Surf. B Biointerfaces 2020, 187, 110647. [Google Scholar] [CrossRef]
- Jiang, X.; Yao, Z.; Wang, K.; Lou, L.; Xue, K.; Chen, J.; Zhang, G.; Zhang, Y.; Du, J.; Lin, C.; et al. MDL-800, the SIRT6 Activator, Suppresses Inflammation via the NF- κ B Pathway and Promotes Angiogenesis to Accelerate Cutaneous Wound Healing in Mice. Oxid. Med. Cell Longev. 2022, 2022, 1619651. [Google Scholar] [CrossRef]
- Spallotta, F.; Cencioni, C.; Straino, S.; Nanni, S.; Rosati, J.; Artuso, S.; Manni, I.; Colussi, C.; Piaggio, G.; Martelli, F.; et al. A Nitric Oxide-Dependent Cross-Talk between Class i and III Histone Deacetylases Accelerates Skin Repair. J. Biol. Chem. 2013, 288, 11004–11012. [Google Scholar] [CrossRef]
- O’Connor, M.; Wang, J.V.; Saedi, N. Whole- and Partial-Body Cryotherapy in Aesthetic Dermatology: Evaluating a Trendy Treatment. J. Cosmet. Dermatol. 2019, 18, 1435–1437. [Google Scholar] [CrossRef]
- Huang, X.; Sun, J.; Chen, G.; Niu, C.; Wang, Y.; Zhao, C.; Sun, J.; Huang, H.; Huang, S.; Liang, Y.; et al. Resveratrol Promotes Diabetic Wound Healing via SIRT1-FoxO1-c-Myc Signaling Pathway-Mediated Angiogenesis. Front. Pharmacol. 2019, 10, 421. [Google Scholar] [CrossRef]
- Rahnasto-Rilla, M.; Tyni, J.; Huovinen, M.; Jarho, E.; Kulikowicz, T.; Ravichandran, S.; Bohr, V.A.; Ferrucci, L.; Lahtela-Kakkonen, M.; Moaddel, R. Natural Polyphenols as Sirtuin 6 Modulators. Sci. Rep. 2018, 8, 4163. [Google Scholar] [CrossRef]
- Rahnasto-Rilla, M.; Kokkola, T.; Jarho, E.; Lahtela-Kakkonen, M.; Moaddel, R. N-Acylethanolamines Bind to SIRT6. ChemBioChem 2016, 17, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, T.; Wu, J.; Kalionis, B.; Zhang, C.; Yuan, D.; Huang, J.; Cai, W.; Fang, H.; Xia, S. Icariin Intervenes in Cardiac Inflammaging through Upregulation of Sirt6 Enzyme Activity and Inhibition of the NF-Kappa B Pathway. Biomed. Res. Int. 2015, 2015, 895976. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Servillo, L.; Giovane, A.; Casale, R.; Vitiello, M.; Marfella, R.; Paolisso, G.; Balestrieri, M.L. Ergothioneine Oxidation in the Protection against High-Glucose Induced Endothelial Senescence: Involvement of SIRT1 and SIRT6. Free. Radic. Biol. Med. 2016, 96, 211–222. [Google Scholar] [CrossRef]
- Yasuda, M.; Wilson, D.R.; Fugmann, S.D.; Moaddel, R. Synthesis and Characterization of SIRT6 Protein Coated Magnetic Beads: Identification of a Novel Inhibitor of SIRT6 Deacetylase from Medicinal Plant Extracts. Anal. Chem. 2011, 83, 7400–7407. [Google Scholar] [CrossRef]
- Kokkonen, P.; Rahnasto-Rilla, M.; Kiviranta, P.H.; Huhtiniemi, T.; Laitinen, T.; Poso, A.; Jarho, E.; Lahtela-Kakkonen, M. Peptides and Pseudopeptides as SIRT6 Deacetylation Inhibitors. ACS Med. Chem. Lett. 2012, 3, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Kokkonen, P.; Rahnasto-Rilla, M.; Mellini, P.; Jarho, E.; Lahtela-Kakkonen, M.; Kokkola, T. Studying SIRT6 Regulation Using H3K56 Based Substrate and Small Molecules. Eur. J. Pharm. Sci. 2014, 63, 71–76. [Google Scholar] [CrossRef] [PubMed]
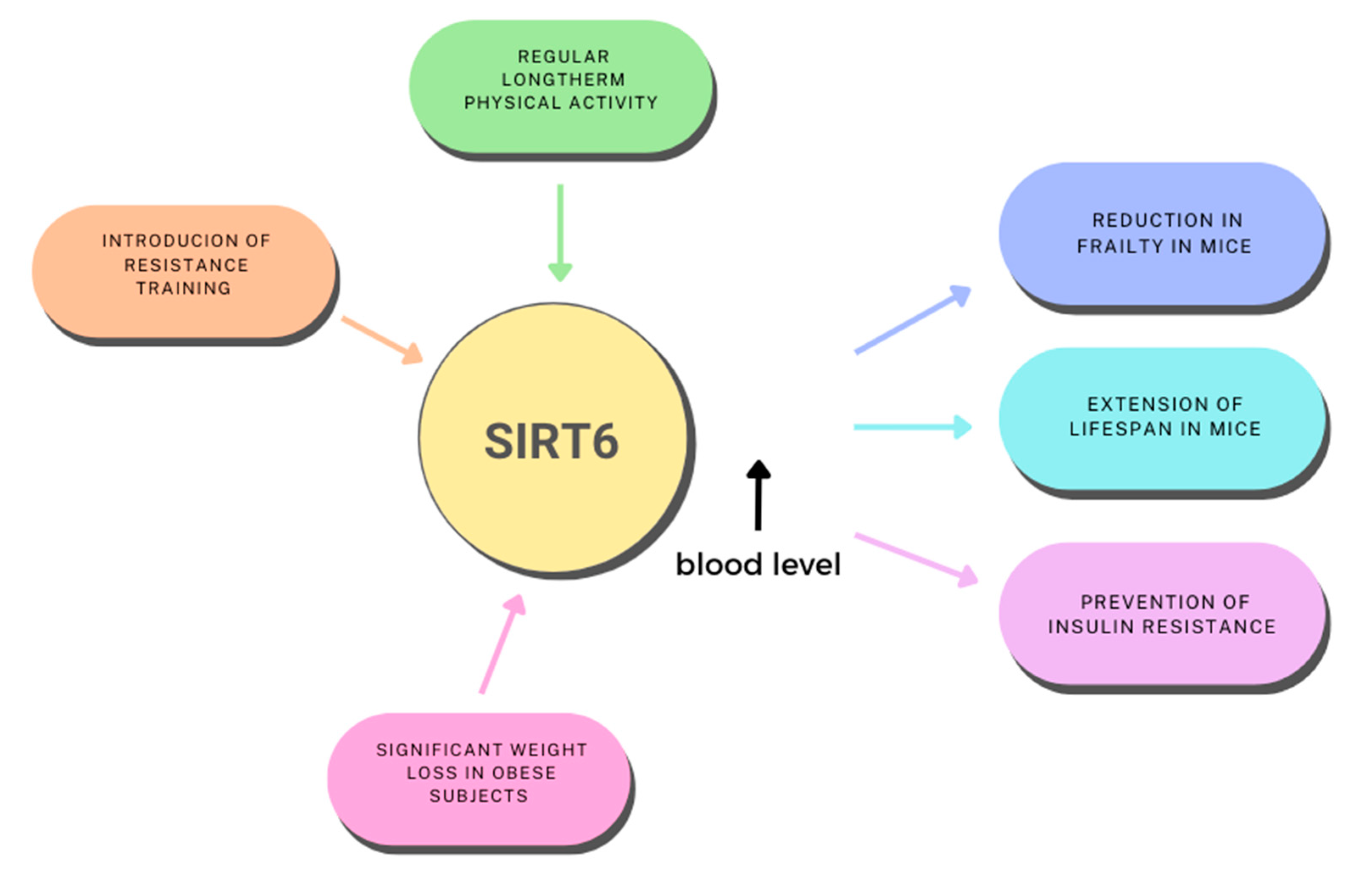
- Hooshmand-Moghadam, B.; Eskandari, M.; Golestani, F.; Rezae, S.; Mahmoudi, N.; Gaeini, A.A. The Effect of 12-Week Resistance Exercise Training on Serum Levels of Cellular Aging Process Parameters in Elderly Men. Exp. Gerontol. 2020, 141, 111090. [Google Scholar] [CrossRef]
- Moschen, A.R.; Wieser, V.; Gerner, R.R.; Bichler, A.; Enrich, B.; Moser, P.; Ebenbichler, C.F.; Kaser, S.; Tilg, H. Adipose Tissue and Liver Expression of SIRT1, 3, and 6 Increase after Extensive Weight Loss in Morbid Obesity. J. Hepatol. 2013, 59, 1315–1322. [Google Scholar] [CrossRef]
- Roichman, A.; Elhanati, S.; Aon, M.A.; Abramovich, I.; Di Francesco, A.; Shahar, Y.; Avivi, M.Y.; Shurgi, M.; Rubinstein, A.; Wiesner, Y.; et al. Restoration of Energy Homeostasis by SIRT6 Extends Healthy Lifespan. Nat. Commun. 2021, 12, 3208. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Xu, Z.; Wan, J.; Hua, T.; Sun, Q. Exercise Ameliorates Insulin Resistance and Improves Sirt6-Mediated Insulin Signaling Transduction in Liver of Obese Rats. Can. J. Physiol. Pharmacol. 2021, 99, 506–511. [Google Scholar] [CrossRef]
- Song, M.Y.; Han, C.Y.; Moon, Y.J.; Lee, J.H.; Bae, E.J.; Park, B.H. Sirt6 Reprograms Myofibers to Oxidative Type through CREB-Dependent Sox6 Suppression. Nat. Commun. 2022, 13, 1808. [Google Scholar] [CrossRef] [PubMed]
- Chilton, W.L.; Marques, F.Z.; West, J.; Kannourakis, G.; Berzins, S.P.; O’Brien, B.J.; Charchar, F.J. Acute Exercise Leads to Regulation of Telomere-Associated Genes and Microrna Expression in Immune Cells. PLoS ONE 2014, 9, e92088. [Google Scholar] [CrossRef] [PubMed]

| SIRT6 and Metabolic Activity | |
|---|---|
| Obesity | SIRT6 plays a protective role against the metabolic consequences of diet-induced obesity, which suggests a potentially beneficial effect of SIRT6 activation on age-related metabolic diseases [40]. In obese patients, the expression of SIRT6 is reduced. It suggests that SIRT6 is an attractive therapeutic target for treating obesity and obesity-related metabolic disorders [45]. |
| Fat metabolism | SIRT6 plays a critical role in fat metabolism, and may therefore be a potential target in the treatment of liver diseases characterized by lipid accumulation [42]. |
| Carbohydrate metabolism | Activation of hepatic by SIRT6 may be therapeutically useful for treating insulin-resistant diabetes [11]. SIRT6 may be useful to improve insulin secretion in diabetes [44]. |
| Energy balance | SIRT6 is an important molecular regulator for POMC neurons to promote negative energy balance [46]. |
| Other | SIRT6 appears to function as a corepressor of the Hif1α—a critical regulator of nutrient stress responses [41]. Expression of SIRT6 increased upon nutrient deprivation in cultured cells. The increase in SIRT6 levels is due to the stabilization of the SIRT6 protein, and not via an increase in SIRT6 transcription [47]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzidek, A.; Czerwińska-Ledwig, O.; Żychowska, M.; Pilch, W.; Piotrowska, A. The Role of Increased Expression of Sirtuin 6 in the Prevention of Premature Aging Pathomechanisms. Int. J. Mol. Sci. 2023, 24, 9655. https://doi.org/10.3390/ijms24119655
Dzidek A, Czerwińska-Ledwig O, Żychowska M, Pilch W, Piotrowska A. The Role of Increased Expression of Sirtuin 6 in the Prevention of Premature Aging Pathomechanisms. International Journal of Molecular Sciences. 2023; 24(11):9655. https://doi.org/10.3390/ijms24119655
Chicago/Turabian StyleDzidek, Adrianna, Olga Czerwińska-Ledwig, Małgorzata Żychowska, Wanda Pilch, and Anna Piotrowska. 2023. "The Role of Increased Expression of Sirtuin 6 in the Prevention of Premature Aging Pathomechanisms" International Journal of Molecular Sciences 24, no. 11: 9655. https://doi.org/10.3390/ijms24119655
APA StyleDzidek, A., Czerwińska-Ledwig, O., Żychowska, M., Pilch, W., & Piotrowska, A. (2023). The Role of Increased Expression of Sirtuin 6 in the Prevention of Premature Aging Pathomechanisms. International Journal of Molecular Sciences, 24(11), 9655. https://doi.org/10.3390/ijms24119655







