Multi-Faceted Role of Luteolin in Cancer Metastasis: EMT, Angiogenesis, ECM Degradation and Apoptosis
Abstract
1. Introduction
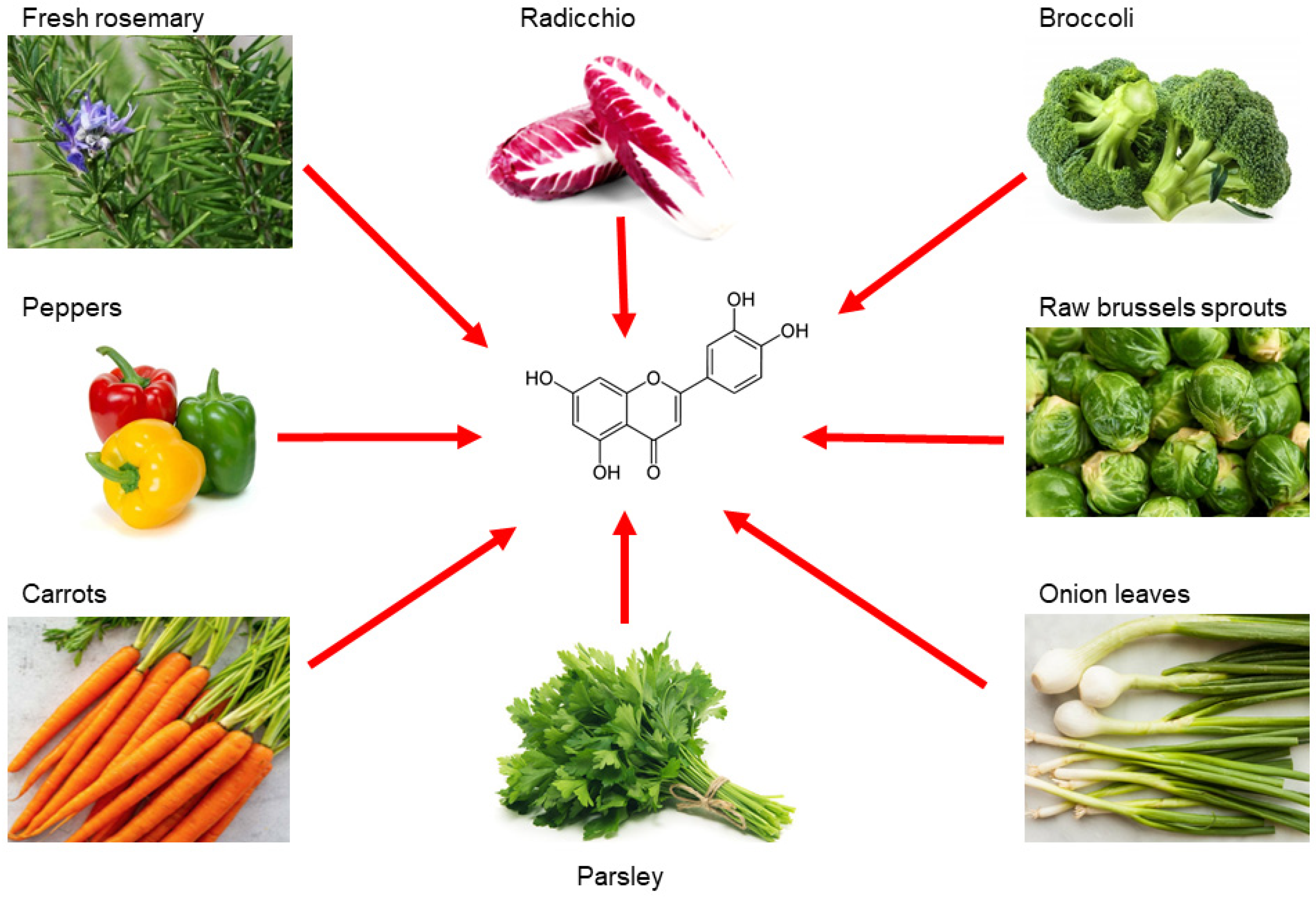
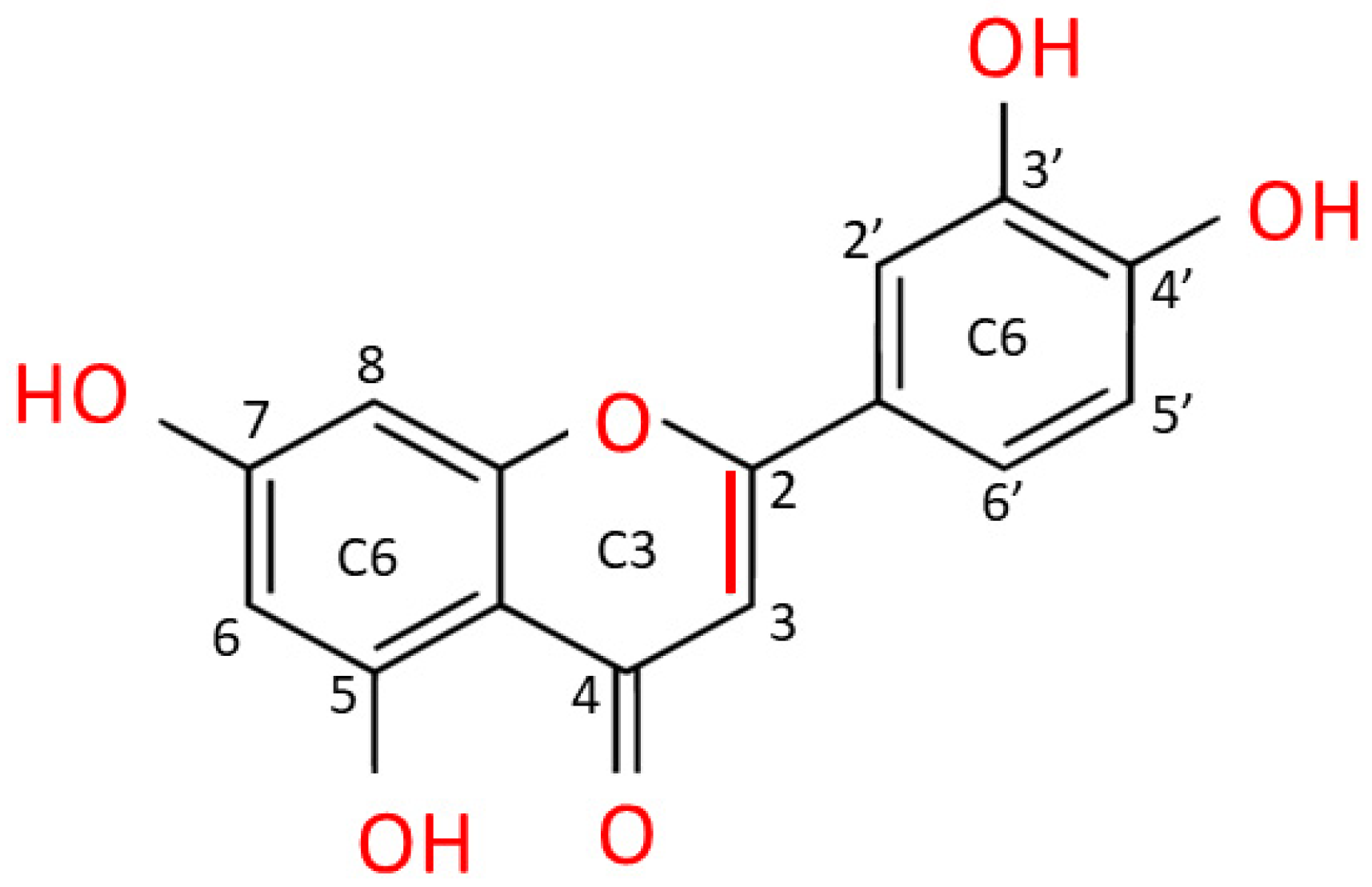
2. Luteolin
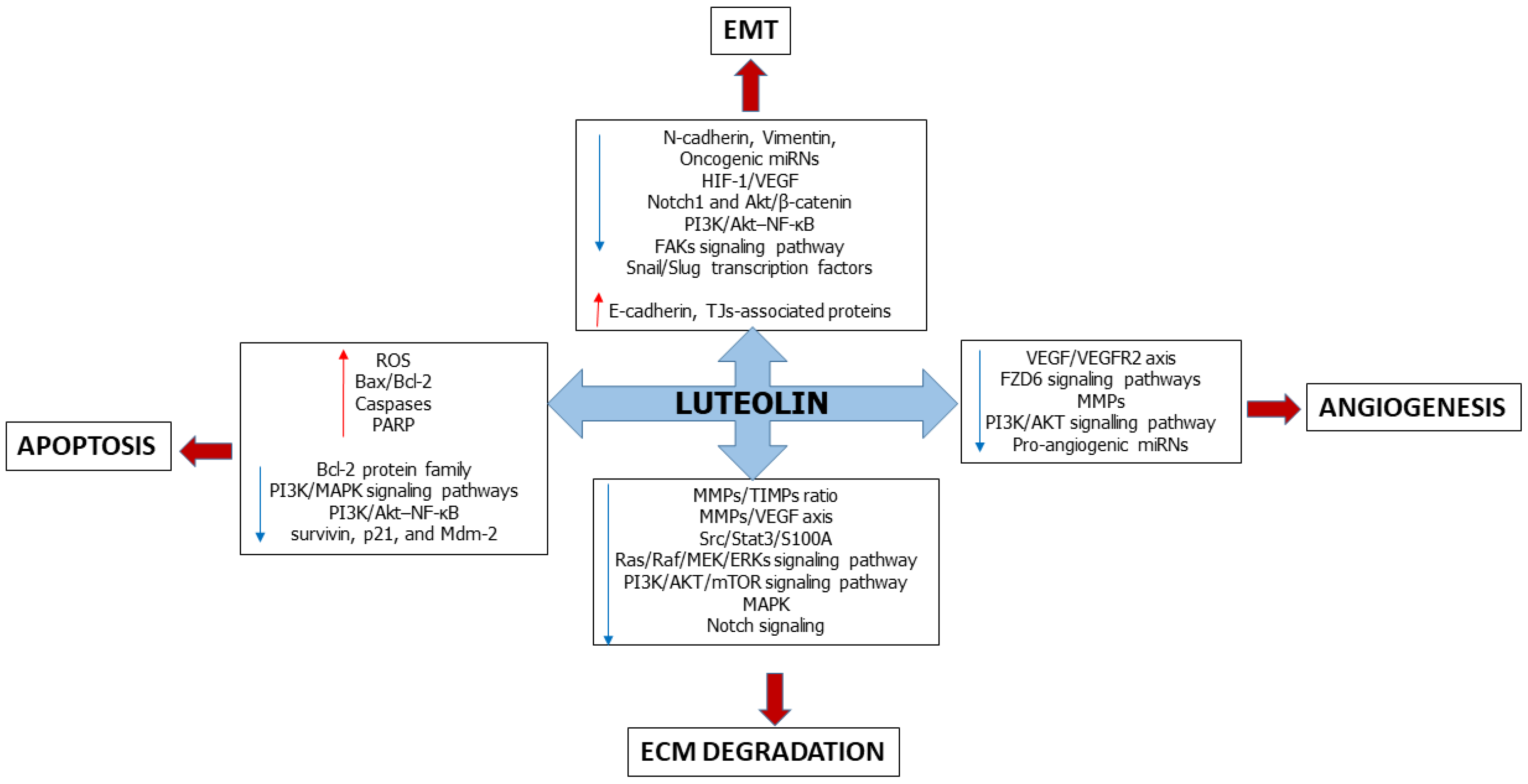
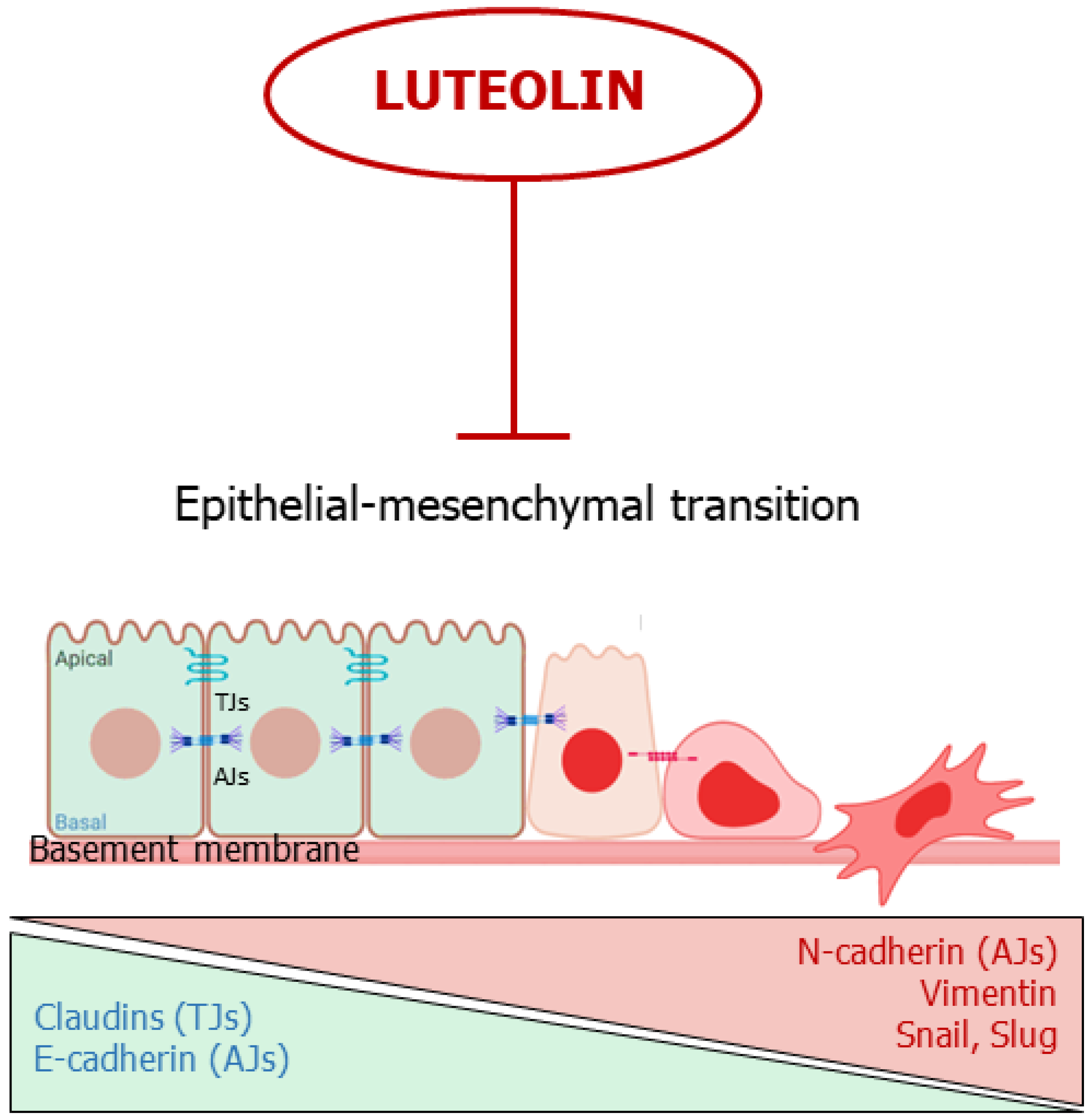
3. Luteolin Affects the Epithelial-Mesenchymal Transition
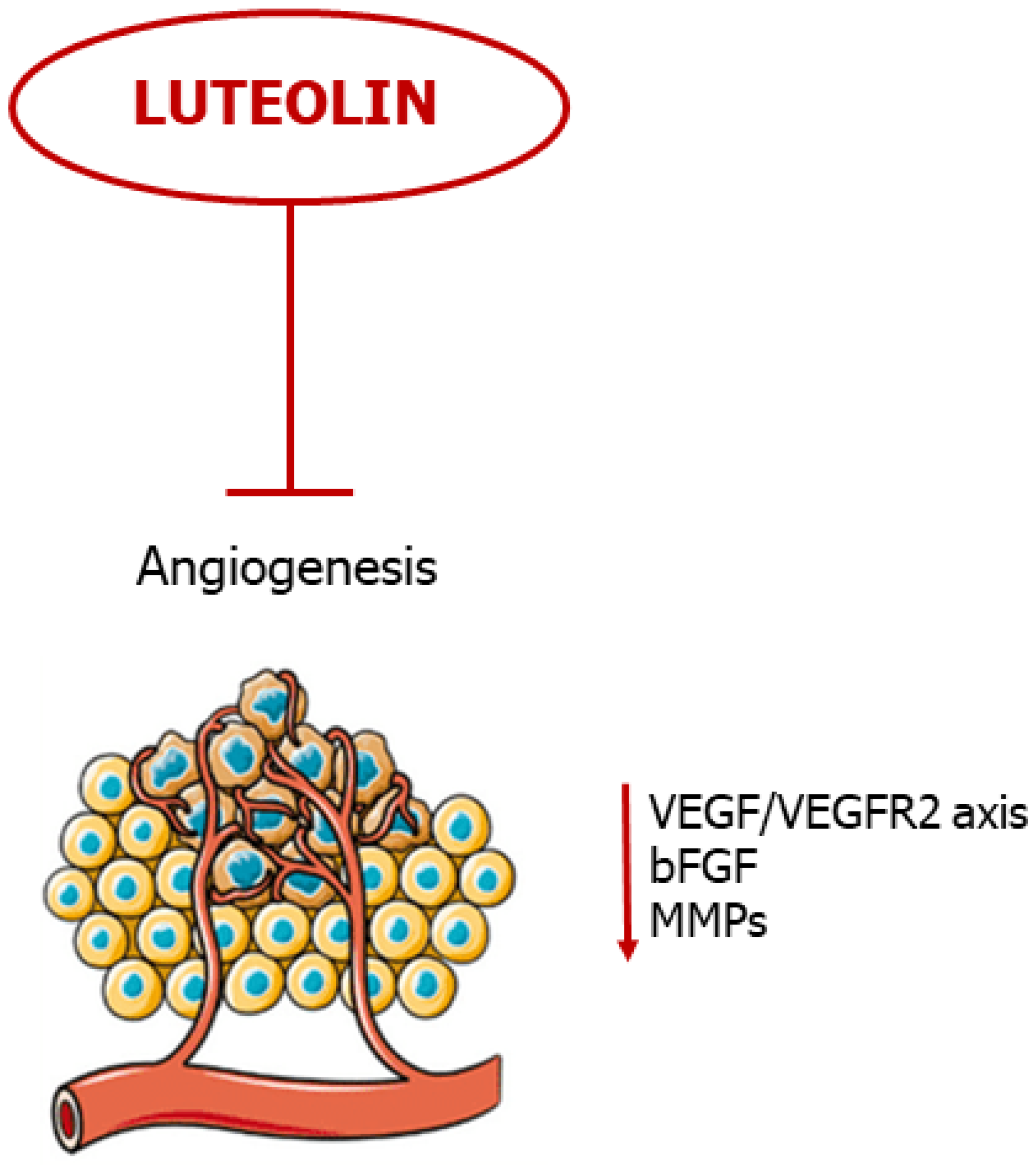
4. Luteolin Suppresses Angiogenesis
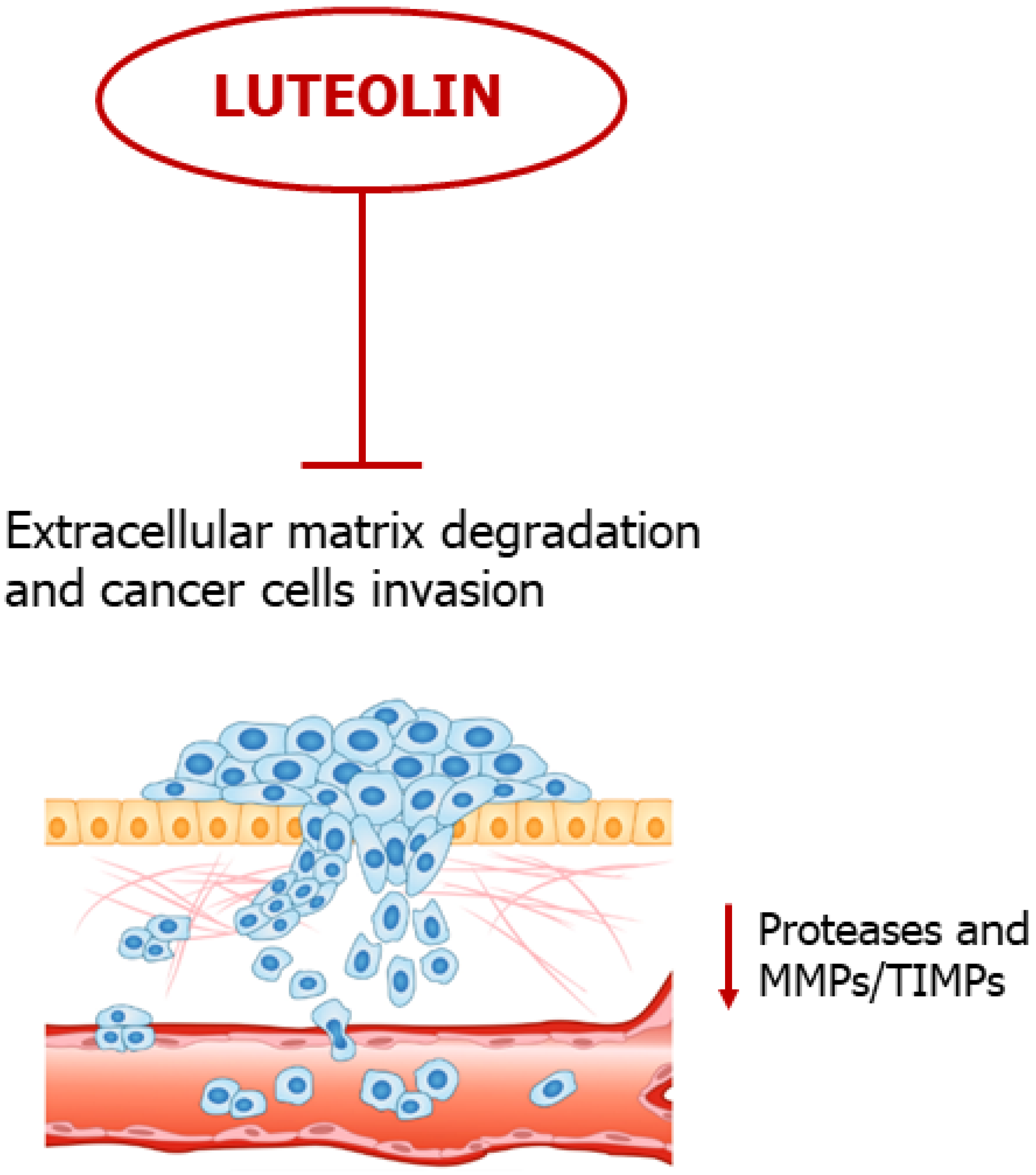
5. Luteolin Slows down Extracellular Matrix Degradation
6. Luteolin Induces Apoptosis
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | Protein-kinase B |
| Bax | BCL2 associated X, apoptosis regulator |
| Bcl-2 | B-cell lymphoma 2 |
| bFGF | Basic-fibroblast growth factor |
| CAM | Chorioallantoic membrane |
| Caspases | Cysteine-aspartate proteases |
| CC | Colon cancer |
| C-Myc | bHLH transcription factor |
| CXCR4 | C-X-C motif chemokine receptor-4 |
| DNA | Deoxyribonucleic Acid |
| EGFR | Epidermal growth factor receptor |
| ECM | Extracellular matrix |
| EMT | Epithelial-mesenchymal transition |
| ERK1/2 | Extracellular-regulated kinase1/2 |
| FAKs | Focal adhesion kinases |
| FZD6 | Frizzled family receptor 6 |
| GC | Gastric cancer |
| GSK-3β | Glycogen synthase kinase 3-beta |
| HIF1/2 | Hypoxia-inducible factor 1/2 |
| hTERT | human telomerase reverse transcriptase |
| HUVECs | Human umbilical vein endothelial cells |
| IL-6 | Interleukin 6 |
| JNK | Jun N-terminal kinase |
| MAPK | Mitogen-activated protein kinase |
| MEK | MAP kinase-ERK kinase |
| miRs | MicroRNA |
| MMPs | Metalloproteases |
| mRNA | Messenger ribonucleic acid. |
| mTOR | Mammalian target of rapamycin |
| NF-κB | Nuclear factor-κappaB |
| Notch | Signal transducer and activator of transcription |
| NSCLC | Non-small cell lung carcinoma |
| PARP | Poly-ADP ribose polymerase |
| PI3K | Phosphatidylinositol-3-kinase |
| Raf | Rapidly accelerated fibrosarcoma |
| Ras | Small guanosine triphosphatases |
| Snail | Snail homolog 1/2 of drosophila |
| ROS | Reactive oxygen species |
| RPS19 | Ribosomial protein S19 |
| Src | Proto-oncogene tyrosine-protein kinase |
| STAT3 | Signal transducer and activator of transcription-3 |
| TGF-β | Transforming growth factor-beta |
| TIMPs | Tissue inhibitors of metalloproteases |
| TNBC | Triple-negative breast cancer |
| TNF | Tumor necrosis factor |
| TWIST | Twist family bHLH transcription factor |
| VEGF | Vascular endothelial growth factor |
| VEGFR2 | Vascular endothelial growth factor receptor-2 |
| ZEB | Zinc finger E-box binding homeobox |
| ZO-1 | Zonula Occludens-1 |
References
- WHO. 2020. Available online: http://gco.iarc.fr/today/fact-sheets-cancers (accessed on 5 December 2020).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.-J.; Chen, C.; Zhao, Y.; Wang, P.C. Circumventing Tumor Resistance to Chemotherapy by Nanotechnology. Methods Mol. Biol. 2009, 596, 467–488. [Google Scholar]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef]
- Singh, V.; Khan, N.J.G. Vector engineering, strategies and targets in cancer gene therapy. Cancer Gene Ther. 2022, 29, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L. Tumor dormancy and disease recurrence. Cancer Metastasis Rev. 2023, 42, 9–12. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Jiang, W.G.; Sanders, A.J.; Katoh, M.; Ungefroren, H.; Gieseler, F.; Prince, M.; Thompson, S.K.; Zollo, M.; Spano, D.; Dhawan, P.; et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin. Cancer Biol. 2015, 35, S244–S275. [Google Scholar] [CrossRef]
- Barber, F.D. Adverse Events of Oncologic Immunotherapy and Their Management. Asia-Pac. J. Oncol. Nurs. 2019, 6, 212–226. [Google Scholar] [CrossRef]
- Magee, D.E.; Hird, A.E.; Klaassen, Z.; Sridhar, S.S.; Nam, R.K.; Wallis, C.J.D.; Kulkarni, G.S. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: A systematic review and meta-analysis of randomized clinical trial. Ann. Oncol. 2020, 31, 50–60. [Google Scholar] [CrossRef]
- Galateanu, B.; Pușcașu, A.I.; Tircol, S.A.; Tanase, B.C.; Hudita, A.; Negrei, C.; Burcea-Dragomiroiu, G.-T.-A.; Negreanu, L.; Vacaroiu, I.A.; Ginghină, O. Allergy in Cancer Care: Antineoplastic Therapy-Induced Hypersensitivity Reactions. Int. J. Mol. Sci. 2023, 24, 3886. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M.; Bavbek, S.; Alvarez-Cuesta, E.; Berna Dursun, A.; Bonadonna, P.; Castells, M.; Cernadas, J.; Chiriac, A.; Sahar, H.; Madrigal-Burgaleta, R. Hypersensitivity Reactions to Chemotherapy: An EAACI Position Paper. Allergy 2022, 77, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Roselló, S.; Blasco, I.; Fabregat, L.G.; Cervantes, A.; Jordan, K. Management of Infusion Reactions to Systemic Anticancer Therapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2017, 28, iv100–iv118. [Google Scholar] [CrossRef] [PubMed]
- Iiizumi, M.; Liu, W.; Pai, S.K.; Furuta, E.; Watabe, K. Drug development against metastasis-related genes and their pathways: A rationale for cancer therapy. Biochim. Biophys. Acta 2008, 1786, 87–104. [Google Scholar] [CrossRef]
- Gonzalez-Valdivieso, J.; Girotti, A.; Schneider, J.; Arias, F.J. Advanced nanomedicine and cancer: Challenges and opportunities in clinical translation. Int. J. Pharm. 2021, 599, 120438. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Spencer, D.S.; Amey Puranik, S.N.A.P. Intelligent nanoparticles for advanced drug delivery in cancer treatment. Curr. Opin. Chem. Eng. 2015, 7, 84–92. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Ichikawa, H.; Garodia, P.; Weerasinghe, P.; Sethi, G.; Bhatt, I.D.; Pandey, M.K.; Shishodia, S.; Nair, M.G. From traditional Ayurvedic medicine to modern medicine: Identification of therapeutic targets for suppression of inflammation and cancer. Expert Opin. Ther. Targets 2006, 10, 87–118. [Google Scholar] [CrossRef]
- Ali, M.; Benfante, V.; Stefano, A.; Yezzi, A.; Di Raimondo, D.; Tuttolomondo, A.; Comelli, A. Anti-Arthritic and Anti-Cancer Activities of Polyphenols: A Review of the Most Recent In Vitro Assays. Life 2023, 13, 361. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, H.; Keikhaei, B.M.S. A review of molecular mechanisms involved in anticancer and antiangiogenic effects of natural polyphenolic compounds. Phytother. Res. 2019, 33, 2002–2014. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.T. Mechanism of metastasis suppression by luteolin in breast cancer. Breast Cancer Targets Ther. 2018, 10, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Rath, P.; Chauhan, A.; Sak, K.; Aggarwal, D.; Choudhary, R.; Sharma, U.; Vashishth, K.; Sharma, S.; Kumar, M.; et al. Luteolin, a Potent Anticancer Compound: From Chemistry to Cellular Interactions and Synergetic Perspectives. Cancers 2022, 14, 5373. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Kuang, G.; Wan, J.; Zhang, X.; Li, H.; Gong, X.; Li, H. Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expression. Oncol. Rep. 2017, 37, 895–902. [Google Scholar] [CrossRef]
- Gao, G.; Ge, R.; Li, Y.; Liu, S. Luteolin exhibits anti-breast cancer property through up-regulating miR-203. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3265–3271. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Zhu, G.Y.; Lu, Y.; Yang, A.; Chen, D.; Huang, H.J.; Peng, S.X.; Chen, L.W.; Li, Y.W. Luteolin suppresses epithelial-mesenchymal transition and migration of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Biomed. Pharmacother. 2020, 129, 110462. [Google Scholar] [CrossRef]
- Cook, M.T.; Liang, Y.; Besch-Williford, C.; Goyette, S.; Mafuvadze, B.; Hyder, S.M. Luteolin inhibits progestin-dependent angiogenesis, stem cell-like characteristics, and growth of human breast cancer xenografts. Springerplus 2015, 4, 444. [Google Scholar] [CrossRef]
- Cook, M.T.; Liang, Y.; Besch-Williford, C.; Hyder, S.M. Luteolin inhibits lung metastasis, cell migration, and viability of triple-negative breast cancer cells. Breast Cancer Dove Med. Press 2016, 9, 9–19. [Google Scholar] [CrossRef]
- Sun, D.W.; Zhang, H.D.; Mao, L.; Mao, C.F.; Chen, W.; Cui, M.; Ma, R.; Cao, H.X.; Jing, C.W.; Wang, Z.; et al. Luteolin Inhibits Breast Cancer Development and Progression In Vitro and In Vivo by Suppressing Notch Signaling and Regulating MiRNAs. Cell Physiol. Biochem. 2015, 37, 1693–1711. [Google Scholar] [CrossRef]
- Wu, H.T.; Lin, J.; Liu, Y.E.; Chen, H.F.; Hsu, K.W.; Lin, S.H.; Peng, K.Y.; Lin, K.J.; Hsieh, C.C.; Chen, D.R. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomedicine 2021, 81, 153437. [Google Scholar] [CrossRef]
- Huang, L.; Jin, K.; Lan, H. Luteolin inhibits cell cycle progression and induces apoptosis of breast cancer cells through downregulation of human telomerase reverse transcriptase. Oncol. Lett. 2019, 17, 3842–3850. [Google Scholar] [CrossRef] [PubMed]
- Magura, J.; Roshila Moodley, I.M. The effect of hesperidin and luteolin isolated from Eriocephalus africanus on apoptosis, cell cycle and miRNA expression in MCF-7. J. Biomol. Struct. Dyn. 2022, 40, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Chang, C.Y.; Lee, K.R.; Lin, H.J.; Chen, T.H.; Wan, L. Flavones inhibit breast cancer proliferation through the Akt/FOXO3a signaling pathway. BMC Cancer 2015, 15, 958. [Google Scholar] [CrossRef] [PubMed]
- Zang, M.D.; Hu, L.; Fan, Z.Y.; Wang, H.X.; Zhu, Z.L.; Cao, S.; Wu, X.Y.; Li, J.F.; Su, L.P.; Li, C.; et al. Luteolin suppresses gastric cancer progression by reversing epithelial-mesenchymal transition via suppression of the Notch signaling pathway. J. Transl. Med. 2017, 15, 52. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, T.; Wang, J.; Mao, Z.; Duan, B.; Long, Y.; Xue, F.; Liu, D.; Liu, S.G.Z. Luteolin exerts an anticancer effect on gastric cancer cells through multiple signaling pathways and regulating miRNAs. J. Cancer 2018, 9, 3669–3675. [Google Scholar] [CrossRef]
- Zang, M.; Hu, L.; Zhang, B.; Zhu, Z.; Li, J.; Zhu, Z.; Yan, M.; Liu, B. Luteolin suppresses angiogenesis and vasculogenic mimicry formation through inhibiting Notch1-VEGF signaling in gastric cancer. Biochem. Biophys. Res. Commun. 2017, 490, 913–919. [Google Scholar] [CrossRef]
- Lu, J.; Li, G.; He, K.; Jiang, W.; Xu, C.; Li, Z.; Wang, H.; Wang, W.; Wang, H.; Teng, X.; et al. Luteolin exerts a marked antitumor effect in cMet-overexpressing patient-derived tumor xenograft models of gastric cancer. J. Transl. Med. 2015, 13, 42. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Li, X.; Aisa, H.A. Luteolin induces apoptosis in vitro through suppressing the MAPK and PI3K signaling pathways in gastric cancer. Oncol. Lett. 2017, 14, 1993–2000. [Google Scholar] [CrossRef]
- Wu, H.; Huang, M.; Liu, Y.; Shu, Y.; Liu, P. Luteolin induces apoptosis by up-regulating miR-34a in human gastric cancer cells. Technol. Cancer Res. Treat. 2015, 14, 747–755. [Google Scholar] [CrossRef]
- Chen, K.C.; Chen, C.Y.; Lin, C.R.; Yang, T.Y.; Chen, T.H.; Wu, L.C.; Wu, C.C. Luteolin attenuates TGF-β1-induced epithelial-mesenchymal transition of lung cancer cells by interfering in the PI3K/Akt-NF-κB-Snail pathway. Life Sci. 2013, 93, 924–933. [Google Scholar] [CrossRef]
- Ruan, J.; Zhang, L.; Yan, L.; Liu, Y.; Yue, Z.; Chen, L.; Wang, A.Y.; Chen, W.; Zheng, S.; Wang, S.; et al. Inhibition of hypoxia-induced epithelial mesenchymal transition by luteolin in non-small cell lung cancer cells. Mol. Med. Rep. 2012, 6, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Cai, X.; Zheng, X.; Zhu, X.; Feng, J.; Wang, X. Luteolin inhibits viability, migration, angiogenesis and invasion of non-small cell lung cancer vascular endothelial cells via miR-133a-3p/purine rich element binding protein B-mediated MAPK and PI3K/Akt signaling pathways. Tissue Cell 2022, 75, 101740. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Oxidative stress in apoptosis and cancer: An update. Arch. Toxicol. 2012, 86, 1649–1665. [Google Scholar] [CrossRef]
- Ju, W.; Wang, X.; Shi, H.; Chen, W.; Belinsky, S.A.L.Y. A critical role of luteolin induced reactive oxygen species in blockage of tumor necrosis factor activated nuclear factor-kappab pathway and sensitization of apoptosis in lung cancer cells. Mol. Pharmacol. 2007, 71, 1381–1388. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Li, M.H.; Qin, Y.M.; Jiang, H.Y.; Zhang, X.; Wu, M.H. Luteolin inhibits tumorigenesis and induces apoptosis of non-small cell lung cancer cells via regulation of microRNA-34a-5p. Int. J. Mol. Sci. 2018, 19, 447. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Dai, S.; Dai, J.; Xiao, Y.; Bai, Y.; Chen, B.; Zhou, M. Luteolin decreases invasiveness, deactivates STAT3 signaling, and reverses interleukin-6 induced epithelial-mesenchymal transition and matrix metalloproteinase secretion of pancreatic cancer cells. OncoTargets Ther. 2015, 8, 2989–3001. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Lu, W.; Ye, T.; Lu, M.; Wang, J.; Huo, J.; Qian, S.; Wang, X.; Cao, P. The molecular mechanism of luteolin-induced apoptosis is potentially related to inhibition of angiogenesis in human pancreatic carcinoma cells. Oncol. Rep. 2012, 28, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.T.; Huang, Y.T.; Hwang, J.J.; Lee, P.P.; Nair, M.P.; Kandaswam, C.L.M. Blockade of the epidermal growth factor receptor tyrosine kinase activity by quercetin and luteolin leads to growth inhibition and apoptosis of pancreatic tumor cells. Anticancer Res. 2002, 22, 1615–1627. [Google Scholar]
- Li, C.; Wang, Q.; Shen, S.; Wei, X.; Li, G. HIF-1α/VEGF signaling-mediated epithelial-mesenchymal transition and angiogenesis is critically involved in anti-metastasis effect of luteolin in melanoma cells. Phyther. Res. 2019, 33, 798–807. [Google Scholar] [CrossRef]
- Ruan, J.S.; Liu, Y.P.; Zhang, L.; Yan, L.G.; Fan, F.T.; Shen, C.S.; Wang, A.Y.; Zheng, S.Z.; Wang, S.M.; Lu, Y. Luteolin reduces the invasive potential of malignant melanoma cells by targeting β3 integrin and the epithelial-mesenchymal transition. Acta Pharmacol. Sin. 2012, 33, 1325–1331. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wu, S.; Li, X.; Chen, M.; Liao, H.F. Luteolin Suppresses Three Angiogenesis Modes and Cell Interaction in Uveal Melanoma in Vitro. Curr. Eye Res. 2022, 47, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zheng, T.; Hou, Z.; Lv, C.; Xue, A.; Han, T.; Han, B.; Sun, X.; Wei, Y. Luteolin, an aryl hydrocarbon receptor ligand, suppresses tumor metastasis in vitro and in vivo. Oncol. Rep. 2020, 44, 2231–2240. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.L.; Chen, Y.F.; Liao, H.F. Effect of luteolin on apoptosis and vascular endothelial growth factor in human choroidal melanoma cells. Int. J. Ophthalmol. 2021, 14, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.L.; Chen, Y.F.; Wu, W.Q.; Lai, Y.; Jin, Q.; Qiu, W.L.; Yu, D.L.; Li, Y.Z.; Liao, H.F. Luteolin inhibits the proliferation, adhesion, migration and invasion of choroidal melanoma cells in vitro. Exp. Eye Res. 2021, 210, 108643. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Son, Y.O.; Budhraja, A.; Wang, X.; Ding, S.; Wang, L.; Hitron, A.; Lee, J.C.; Kim, D.; Divya, S.P.; et al. Luteolin inhibits human prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. PLoS ONE 2012, 7, e52279. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Wei, M.; Zhang, J.J.; Zhou, Y.; Wang, Y.L.; Su, Y.; Lin, S.C.; Gan, Z.H.; Sun, Y.N.; Min, D.L. Luteolin inhibited proliferation and induced apoptosis of prostate cancer cells through miR-301. OncoTargets Ther. 2016, 9, 3085–3094. [Google Scholar] [CrossRef]
- Dai, Y.; Zheng, H.; Liu, Z.; Wang, Y.; Hu, W. The flavonoid luteolin suppresses infantile hemangioma by targeting FZD6 in the Wnt pathway. Investig. New Drugs 2021, 39, 775–784. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Jia, Y.; Ding, H.; Zhang, L.; Pan, H. Luteolin reduces migration of human glioblastoma cell lines via inhibition of the p-IGF-1R/PI3K/AKT/mTOR signaling pathway. Oncol. Lett. 2017, 14, 3545–3551. [Google Scholar] [CrossRef]
- Anson, D.M.; Wilcox, R.M.; Huseman, E.D.; Stump, T.A.; Paris, R.L.; Darkwah, B.O.; Lin, S.; Adegoke, A.O.; Gryka, R.J.; Jean-Louis, D.S.A.S. Luteolin Decreases Epidermal Growth Factor Receptor-Mediated Cell Proliferation and Induces Apoptosis in Glioblastoma Cell Lines. Basic Clin. Pharmacol. Toxicol. 2018, 123, 678–686. [Google Scholar] [CrossRef]
- Kittiratphatthana, N.; Kukongviriyapan, V.; Prawan, A.; Senggunprai, L. Luteolin induces cholangiocarcinoma cell apoptosis through the mitochondrial-dependent pathway mediated by reactive oxygen species. J. Pharm. Pharmacol. 2016, 68, 1184–1192. [Google Scholar] [CrossRef]
- Lim, D.Y.; Jeong, Y.; Tyner, A.L.; Park, J.H. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G66–G75. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Dharmalingam, P.; Sadagopan, S.K.; Ramar, M.; Munusamy, A.; Ganapasam, S. Luteolin induces growth arrest in colon cancer cells through involvement of Wnt/β-catenin/GSK-3β signaling. J. Environ. Pathol. Toxicol. Oncol. 2013, 32, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Jung, S.K.; Byun, S.; Son, J.E.; Oh, M.H.; Lee, J.; Kang, M.J.; Heo, Y.S.; Lee, K.W.; Lee, H.J. Raf and PI3K are the molecular targets for the anti-metastatic effect of luteolin. Phyther. Res. 2013, 27, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Rao, C.; Zheng, G.; Wang, S. Luteolin suppresses colorectal cancer cell metastasis via regulation of the miR-384/pleiotrophin axis. Oncol. Rep. 2019, 42, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Chen, Y.R.; Chow, J.M.; Chien, M.H.; Yang, S.F.; Wen, Y.C.; Lee, W.J.; Tseng, T.H. Stimulation of Fas/FasL-mediated apoptosis by luteolin through enhancement of histone H3 acetylation and c-Jun activation in HL-60 leukemia cells. Mol. Carcinog. 2018, 57, 866–877. [Google Scholar] [CrossRef]
- López-Lázaro, M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Xu, H.; Linn, B.; Zhang, Y.; Ren, J. A review on the antioxidative and prooxidative properties of luteolin. React. Oxyg. Species 2019, 7, 136–147. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.A.; Rahaman, A.; Muhammad Aadil, R.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, e12974. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Singh, S.K.; Gulati, M.; Chellappan, D.K.; Zacconi, F.; De Rubis, G.; Gupta, G.; Sharifi-Rad, J.; Cho, W.C.; et al. Luteolin: A flavonoid with a multifaceted anticancer potential. Cancer Cell Int. 2022, 22, 386. [Google Scholar] [CrossRef]
- Zhou, P.; Li, L.P.; Luo, S.Q.; Jiang, H.D.; Zeng, S. Intestinal absorption of luteolin from peanut hull extract is more efficient than that from individual pure luteolin. J. Agric. Food Chem. 2008, 56, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Gao, C.; Tian, X. Pharmacokinetics, tissue distribution and excretion of luteolin and its major metabolites in rats: Metabolites predominate in blood, tissues and are mainly excreted via bile. J. Funct. Foods. 2017, 35, 332–340. [Google Scholar] [CrossRef]
- Hayasaka, N.; Shimizu, N.; Komoda, T.; Mohri, S.; Tsushida, T.; Eitsuka, T.; Miyazawa, T.; Nakagawa, K. Absorption and Metabolism of Luteolin in Rats and Humans in Relation to in Vitro Anti-inflammatory Effects. J. Agric. Food Chem. 2018, 66, 11320–11329. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Muthusamy, B.P.; Saeteurn, K.Y. Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr. Opin. Cell Biol. 2014, 31, 56–66. [Google Scholar] [CrossRef]
- Gonzalez, D.M.; Medici, D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci. Signal 2014, 7, re8. [Google Scholar] [CrossRef]
- Singh, M.; Yelle, N.; Venugopal, C.; Singh, S.K. EMT: Mechanisms and therapeutic implications. Pharmacol. Ther. 2018, 182, 80–94. [Google Scholar] [CrossRef]
- Scheel, C.; Onder, T.; Karnoub, A.; Weinberg, R.A. Adaptation versus selection: The origins of metastatic behavior. Cancer Res. 2007, 67, 11476–11480. [Google Scholar] [CrossRef]
- Deshmukh, A.P.; Vasaikar, S.V.; Tomczak, K.; Tripathi, S.; den Hollander, P.; Arslan, E.; Chakraborty, P.; Soundararajan, R.; Jolly, M.K.; Rai, K.; et al. Identification of EMT signaling cross-talk and gene regulatory networks by single-cell RNA sequencing. Proc. Natl. Acad. Sci. USA 2021, 118, e2102050118. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Zhu, Y.; Yuan, C.; Wang, D.; Zhang, W.; Qi, B.; Qiu, J.; Song, X.; Ye, J.; et al. The Hippo transducer TAZ promotes epithelial to mesenchymal transition and cancer stem cell maintenance in oral cancer. Mol. Oncol. 2015, 9, 1091–1105. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- Hussain, Y.; Cui, J.H.; Khan, H.; Aschner, M.; Batiha, G.E.; Jeandet, P. Luteolin and cancer metastasis suppression: Focus on the role of epithelial to mesenchymal transition. Med. Oncol. 2021, 38, 66. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.N. Small RNAs: Classification, biogenesis, and function. Mol. Cells 2005, 19, 1–15. [Google Scholar]
- Pan, G.; Liu, Y.; Shang, L.; Zhou, F.; Yang, S. EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun. (Lond.) 2021, 41, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Dutta, A. MicroRNAs in cancer. Annu. Rev. Pathol. 2009, 4, 199–227. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.J.; Hsu, W.H.; Lee, K.H.; Chen, K.C.; Lin, C.W.; Lee, Y.A.; Ko, T.P.; Lee, L.T.; Lee, M.T.; Chang, M.S.; et al. Dietary Flavonoids Luteolin and Quercetin Inhibit Migration and Invasion of Squamous Carcinoma through Reduction of Src/Stat3/S100A7 Signaling. Antioxidants 2019, 8, 557. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Hsu, W.H.; Ho, J.Y.; Lin, C.W.; Chu, C.Y.; Kandaswami, C.C.; Lee, M.T.; Cheng, C.H. Flavonoids Luteolin and Quercetin Inhibit RPS19 and contributes to metastasis of cancer cells through c-Myc reduction. J. Food Drug Anal. 2018, 26, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Liekens, S.; De Clercq, E.; Neyts, J. Angiogenesis: Regulators and clinical applications. Biochem. Pharmacol. 2001, 61, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.J.R. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr. Drug Targets 2010, 11, 1000–1017. [Google Scholar] [CrossRef]
- Wang, Z.; Dabrosin, C.; Yin, X.; Fuster, M.M.; Arreola, A.; Rathmell, W.K.; Generali, D.; Nagaraju, G.P.; El-Rayes, B.; Ribatti, D.; et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin. Cancer Biol. 2015, 35, S224–S243. [Google Scholar] [CrossRef]
- Mukherjee, A.; Madamsetty, V.S.; Paul, M.K.; Mukherjee, S. Recent Advancements of Nanomedicine towards Antiangiogenic Therapy in Cancer. Int. J. Mol. Sci. 2020, 21, 455. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Poveda, B.; Torres-Vargas, J.A.; Ocaña, M.D.C.; García-Caballero, M.; Medina, M.Á.; Quesada, A.R. The Mediterranean Diet, a Rich Source of Angiopreventive Compounds in Cancer. Nutrients 2019, 11, 2036. [Google Scholar] [CrossRef] [PubMed]
- Tosetti, F.; Ferrari, N.; De Flora, S.; Albini, A. Angioprevention’: Angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J. 2002, 16, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Bagli, E.; Stefaniotou, M.; Morbidelli, L.; Ziche, M.; Psillas, K.; Murphy, C.; Fotsis, T. Luteolin inhibits vascular endothelial growth factor-induced angiogenesis; inhibition of endothelial cell survival and proliferation by targeting phosphatidylinositol 3’-kinase activity. Cancer Res. 2004, 64, 7936–7946. [Google Scholar] [CrossRef]
- Fang, B.; Chen, X.; Wu, M.; Kong, H.; Chu, G.; Zhou, Z.; Zhang, C.; Chen, B. Luteolin inhibits angiogenesis of the M2-like TAMs via the downregulation of hypoxia inducible factor-1α and the STAT3 signalling pathway under hypoxia. Mol. Med. Rep. 2018, 18, 2914–2922. [Google Scholar] [CrossRef]
- Basset, P.; Okada, A.; Chenard, M.P.; Kannan, R.; Stoll, I.; Anglard, P.; Bellocq, J.P.; Rio, M.C. Matrix metalloproteinases as stromal effectors of human carcinoma progression: Therapeutic implications. Matrix Biol. 1997, 15, 535–541. [Google Scholar] [CrossRef]
- John, A.; Tuszynski, G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol. Oncol. Res. 2001, 7, 14–23. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Fuchs, Y.; Steller, H. Programmed cell death in animal development and disease. Cell 2011, 147, 742–758. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.H.A. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature 2012, 491, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Piao, M.J.; Ryu, Y.S.; Hyun, Y.J.; Park, J.E.; Shilnikova, K.; Zhen, A.X.; Kang, H.K.; Koh, Y.S.; Jeong, Y.J.; et al. Luteolin induces apoptotic cell death via antioxidant activity in human colon cancer cells. Int. J. Oncol. 2017, 51, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Pramodh, S.; Rais, N.; Haque, S.; Shafarin, J.; Bajbouj, K.; Hamad, M.; Hussain, A. Luteolin inhibits proliferation, triggers apoptosis and modulates Akt/mTOR and MAP kinase pathways in HeLa cells. Oncol. Lett. 2021, 21, 192. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.T.N. miRNA interplay: Mechanisms and consequences in cancer. Dis. Model. Mech. 2021, 14, dmm047662. [Google Scholar] [CrossRef]
- Osaki, M.; Oshimura, M.I.H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis 2004, 9, 667–676. [Google Scholar] [CrossRef]
- Fresno Vara, J.A.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.G.-B.M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef]
- Luo, M.F.L.-W. Redundant kinase activation and resistance of EGFR-tyrosine kinase inhibitors. Am. J. Cancer Res. 2014, 4, 608–628. [Google Scholar]
- Wee, P.W.Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar] [CrossRef]
- Quatrale, A.E.; Porcelli, L.; Silvestris, N.; Colucci, G.; Angelo, A.A.A. EGFR tyrosine kinases inhibitors in cancer treatment: In vitro and in vivo evidence. Front. Biosci. Landmark Ed. 2011, 16, 1962–1972. [Google Scholar] [CrossRef] [PubMed]
- Taliou, A.; Zintzaras, E.; Lykouras, L.; Francis, K. An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effectson behavior in children with autism spectrum disorders. Clin. Ther. 2013, 35, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Di Stadio, A.; D’Ascanio, L.; Vaira, L.A.; Cantone, E.; De Luca, P.; Cingolani, C.; Motta, G.; De Riu, G.; Vitelli, F.; Spriano, G.; et al. Ultramicronized Palmitoylethanolamide and Luteolin. Supplement Combined with Olfactory Training to Treat Post-COVID-19 Olfactory Impairment: A Multi-Center Double-Blinded Randomized Placebo- Controlled Clinical Trial. Curr. Neuropharmacol. 2022, 20, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- De Luca, P.; Camaioni, A.; Marra, P.; Salzano, G.; Carriere, G.; Ricciardi, L.; Pucci, R.; Montemurro, N.; Brenner, M.J.; Di Stadio, A. Effect of Ultra-Micronized Palmitoylethanolamide and Luteolin on Olfaction and Memory in Patients with Long COVID: Results of a Longitudinal Study. Cells 2022, 11, 2552. [Google Scholar] [CrossRef] [PubMed]
- Versace, V.; Ortelli, P.; Dezi, S.; Ferrazzoli, D.; Alibardi, A.; Bonini, I.; Engl, M.; Maestri, R.; Assogna, M.; Ajello, V.; et al. Co-ultramicronized palmitoylethanolamide/luteolin normalizes GABAB-ergic activity and cortical plasticity in long COVID-19 syndrome. Clin. Neurophysiol. 2023, 145, 81–88. [Google Scholar] [CrossRef]
- Terzo, S.; Amato, A.; Magán-Fernández, A.; Castellino, G.; Calvi, P.; Chianetta, R.; Giglio, R.V.; Patti, A.M.; Nikolic, D.; Firenze, A.; et al. Nutraceutical Containing Chlorogenic Acid and Luteolin Improves CardiometabolicParameters in Subjects with Pre-Obesity: A 6-Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2023, 15, 462. [Google Scholar] [CrossRef]
- Tawornchat, P.; Pattarakankul, T.; Palaga, T.; Varol Intasanta, S.W. Polymerized Luteolin Nanoparticles: Synthesis, Structure Elucidation, and Anti-Inflammatory Activity. ACS Omega 2021, 6, 2846–2855. [Google Scholar] [CrossRef]
- Shinde, P.; Agraval, H.; Singh, A.; Umesh, C.S.; Yadav, U.K. Synthesis of luteolin loaded zein nanoparticles for targeted cancer therapy improving bioavailability and efficacy. J. Drug Deliv. Sci. Technol. 2019, 52, 369–378. [Google Scholar] [CrossRef]
- Wu, G.; Li, J.; Yue, J.; Zhang, S.Y.K. Liposome encapsulated luteolin showed enhanced antitumor efficacy to colorectal carcinoma. Mol. Med. Rep. 2018, 17, 2456–2464. [Google Scholar] [CrossRef]
- Sinha, A.P.K.S. Enhanced Induction of Apoptosis in HaCaT Cells by Luteolin Encapsulated in PEGylated Liposomes-Role of Caspase-3/Caspase-14. Appl. Biochem. Biotechnol. 2019, 188, 147–164. [Google Scholar] [CrossRef]

| Tumor Entity | EMT | Angiogenesis | ECM Degradation | Apoptosis |
|---|---|---|---|---|
| Breast Cancer | Reversal of EMT by suppressing β-catenin signaling; inhibition of cancer cell invasion and metastatic potential [25]. Inhibition of the pro-invasive Ras/Raf/MEK/ERK signaling; increase of miR-203 expression [26]. Increase of E-cadherin expression and reduction of protein levels of fibronectin, N-cadherin and vimentin; decrease of transcriptional activity of YAP/TAZ [27]. | Blockade of VEGF secretion within breast cancer cells responsive to natural and synthetic progestins, both in vitro and in a xenograft model [28]. Repression of VEGF secretion by TNBC cells and suppression of their metastatic potential in vitro and in vivo [29]. Repression of Notch signaling and its downstream targets Notch-1, Hes-1, VEGF and gelatinases by regulating the level of oncogenic miRs [30]. | Modulation of the biogenesis of specific miRs, inhibition of gelatinases secretion and VEGF/Notch signaling pathway [30]. Epigenetically downregulation of gelatinases expression and activation of AKT/mTOR signaling pathway [31]. | Reduction of telomerase expression by targeting NF-κB/c-Myc; increase of Bax/Bcl-2 ratio and caspase-3 [32]. By modulating miR-21 and miR-16, upregulation of Bax/Bcl-2 ratio; triggering of both the intrinsic and the extrinsic pathways of apoptosis [33]. Repression of PI3K/Akt pathway; induction of FOXO3a expression and increase of p21 and p27; induction of PARP cleavage and release of cytochrome c [34]. |
| Gastric Carcinoma | Reversal of EMT by inhibiting Akt/β-catenin and Notch signaling pathways [35]. Decreased migration and invasion by regulating Notch1/ PI3K/ AKT/ mTOR/ERK/STAT3 and P38 signaling pathways; regulation of several oncogenic miRs expression in vitro and in vivo [36]. | Suppression of VEGF secretion by acting on Notch1 expression and inhibition of the formation of tube-like structures of HUVECs seeded in a Matrigel layer [37]. | , Reduction of gelatinases expression via inhibition of cMet/Akt/ERK signaling [38]. | Suppression of PI3K/MAPK signaling with increase of Bax/Bcl-2 ratio and cytochrome c release [39]. Decrease of Bcl-2 expression through upregulation of miR-34a [40]. |
| Lung Cancer | Reversal of TGF-β1 induced-EMT by slowing down the activation of PI3K/Akt/IκBa/NF-κB/Snail pathway [41]. Inhibition of hypoxia-induced EMT by blocking integrin β1 expression and FAK-signaling pathway [42]. | Repression of VEGF and gelatinases by upregulating miR-133a-p69, and by regulating MAPK/PI3K/Akt signaling pathways [43]. | Downregulation of the pro-metastatic markers CXCR4, gelatinases in vitro and in vivo [44]. | Induction of ROS accumulation via suppression of SOD activity; suppression of NF-kB potentiating JNK to sensitize cancer cells to TNF [45]. Upregulation of miR-34a-5p via targeting MDM4 oncogene, increase of p53 and p21expression; increase of Bax/Bcl-2 ratio, followed by activation of caspase-3 and -9 [46]. |
| Pancreatic cancer | Deactivation of STAT3 signaling with consequent reversal of Il-6-induced EMT [47]. | Decrease of VEGF secretion and VEGF mRNA expression via NF-κB inhibition [48]. | - | Attenuation of EGFR signaling pathway and induction of PARP degradation followed by DNA fragmentation [49]. |
| Melanoma | Upregulation of E-cadherin/N-cadherin ratio through inhibition of HIF-1α/VEGF axis [50]. Enhancement of E-cadherin expression via inhibition of β3 integrin/FAK signal pathway in vitro and in vivo [51]. | Inhibition of different pathways of tumor neovascularization, including angiogenesis, vasculogenesis and vasculogenic mimicry, by suppressing PI3K/AKT signaling pathway [52]. | Downregulation of the pro-metastatic markers, gelatinases and CXCR4 [53]. | - |
| Choroidal melanoma | - | Reduction of VEGF secretion, in a concentration-dependent manner, followed by induction of cell death [54]. | Decrease of gelatinase secretion in vitro via inhibition of PI3K/Akt signaling pathway [55]. | Increase of Bax/Bcl-2 ratio [52]. |
| Prostate Cancer | - | Inhibition of VEGFR2/AKT/ERK/ mTOR/P70S6K signaling pathway and of neovascularization in ex vivo chicken chorioallantoic membrane (CAM) assay, and in a Matrigel plug assay, as well as in a xenograft model [56]. | - | Downregulation of miR-301 that promotes the expression of pro-death DNA-binding effector domain-containing protein 2 (DEDD2) [57]. |
| Haemangioma | - | Suppression of VEGF-A expression with consequential inhibition of microvessel density and vasculogenesis in vivo targeting FZD6 signaling pathway [58]. | - | - |
| Glioblastoma | - | - | Inactivation of the p-IGF-1R/PI3K/AKT/mTOR signaling pathway and alteration of the gelatinase/TIMPs ratio [59]. | Stimulation of PARP cleavage, DNA degradation and caspases activation [60]. |
| Colon cancer | - | - | - | Activation of antioxidant enzymes and MAPK signaling; by unbalancing ROS, acting on cytochrome c release and caspase-9 and -3 activation [61]. Activation of caspases 3, 7, 9 and PARP cleavage; downregulation of p21, survivin, Mcl-1, Bcl-x(L) and Mdm-2 [62]. By involving Wnt/β-catenin/GSK-3β signaling, increase of Bax/Bcl-2 ratio and activation of caspase-3 [63]. |
| Colorectal Cancer | - | - | Inactivation of gelatinases by suppressing Raf/PI3K signaling pathways [64]. Downregulation of MMP-2, -9, -3 and -16 expression coupled with an enhancement of miR-384 biogenesis and with suppression of PTN [65]. | - |
| Cholangiocarcinoma | - | - | - | Inhibition of Nrf2 with consequent downregulation of the antioxidant genes γ-glutamylcysteine ligase and heme oxygenase-1, and increase of mitochondrial membrane potential dissipation and caspases -3 and 9 activation [61]. |
| Cervical cancer | - | - | - | Disruption of pro-apoptotic/anti-apoptotic genes equilibrium interfering with the RAS-RAF/MAPK/AKT/ PI3K signaling pathway; triggering collapse of the mitochondrial membrane and DNA fragmentation [33]. |
| Leukemia | - | - | - | Induction of histone H3 hyper-acetylation by activating the ERK /JNKs pathways; increase of Fas and FasL expression culminating in caspases-8/-3 activation [66]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocchetti, M.T.; Bellanti, F.; Zadorozhna, M.; Fiocco, D.; Mangieri, D. Multi-Faceted Role of Luteolin in Cancer Metastasis: EMT, Angiogenesis, ECM Degradation and Apoptosis. Int. J. Mol. Sci. 2023, 24, 8824. https://doi.org/10.3390/ijms24108824
Rocchetti MT, Bellanti F, Zadorozhna M, Fiocco D, Mangieri D. Multi-Faceted Role of Luteolin in Cancer Metastasis: EMT, Angiogenesis, ECM Degradation and Apoptosis. International Journal of Molecular Sciences. 2023; 24(10):8824. https://doi.org/10.3390/ijms24108824
Chicago/Turabian StyleRocchetti, Maria Teresa, Francesco Bellanti, Mariia Zadorozhna, Daniela Fiocco, and Domenica Mangieri. 2023. "Multi-Faceted Role of Luteolin in Cancer Metastasis: EMT, Angiogenesis, ECM Degradation and Apoptosis" International Journal of Molecular Sciences 24, no. 10: 8824. https://doi.org/10.3390/ijms24108824
APA StyleRocchetti, M. T., Bellanti, F., Zadorozhna, M., Fiocco, D., & Mangieri, D. (2023). Multi-Faceted Role of Luteolin in Cancer Metastasis: EMT, Angiogenesis, ECM Degradation and Apoptosis. International Journal of Molecular Sciences, 24(10), 8824. https://doi.org/10.3390/ijms24108824









