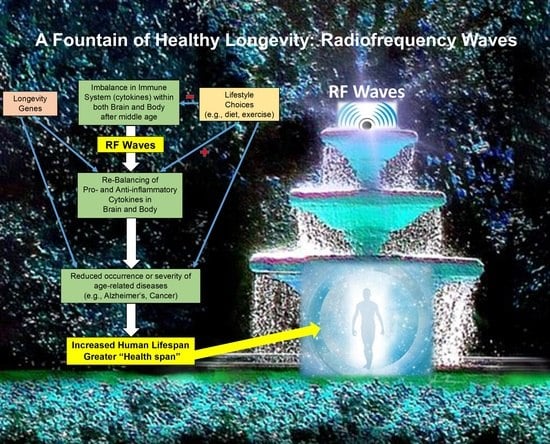
Transcranial Electromagnetic Wave Treatment: A Fountain of Healthy Longevity?
Abstract
1. Introduction
- (1)
- Trying to get rid of senescent cells in the body to limit inflammation [1];
- (2)
- Experimenting with supplements for diseases of aging, such as Nicotinamide (NAD) precursors [2];
- (3)
- Building databases of human genotypes in an effort to develop genetic interventions (ClinicalTrial.gov NCT00707694; accessed on 17 April 2023);
- (4)
- (1)
- Methformin: Methformin has been used for many years to treat type 2 diabetes. In addition to its anti-hyperglycemic effect, it appears able to indirectly suppress pro-inflammatory pathways. Methformin has a long record of clinical trials purporting to reduce cancer, cognitive impairment, and mortality [5], apparently indirectly through its antihyperglycemic and insulin-sensitizing effects [6]. A completed clinical trial (Methformin in Longevity Study; MILES) administered Methformin or placebo for 6 weeks to normal aged subjects, resulting in reduced gene expression in muscle and fat tissue [7]. A second clinical trial (Targeting Aging with Methformin; TAME), not yet started, is being planned to administer Methformin or placebo for 6 years to normal individuals aged 65–80 years old, with endpoints of age-related disease occurrence and biomarkers of aging [6].
- (2)
- Nicotinamide (NAD) Precursors: As a naturally-occurring metabolite, NAD is involved in redox reactions and cellular energy production. Since it becomes reduced in many tissues during aging [8,9], precursors such as nicotinamide riboside and nicotinamide mononucleotide are being evaluated pre-clinically.
- (3)
- Rapamycin: Rapamycin is an FDA-approved immunosuppressive drug used primarily to prevent organ allograft rejection. A small controlled clinical trial administering Rapamycin to healthy aged subjects for eight weeks did not induce any changes in cognitive performance, immune endpoints, or self-perceived health [10].
- (4)
- Dasatinib+Quercetin, Fisetin: Senescent cells are cells that undergo age-related changes and/or have stopped dividing, after which they begin producing an array of pro-inflammatory cytokines and growth factors (called the Senescence-Associated Secretory Phenotype; SASP). These pro-inflammatory secretions from senescent cells are thought to contribute to the increased inflammation (“inflamm-aging”) in body and brain during aging [11]. Senolytics such as Dasatinib+Quercetin (DQ) and Fisetin search out and attempt to destroy such senescent cells to reduce inflammation [1]. Among the clinical trials of Dasatinib+Quercetin and/or Fisetin that have been planned or are on-going are those involving kidney disease, osteoporosis, osteoarthritic, and early AD [12,13]. Regarding early AD, a pilot clinical trial of five early AD subjects being given DQ for 3 months was recently completed, which reported no beneficial cognitive effects and no changes in AD markers in plasma or brain post-treatment [14]. Although the number of clinical trials involving senolytics is increasing, there have been no clinical trials to our knowledge showing that any senolytic drug can delay, stop, or reverse the main characteristics of an age-related disease or enhance lifespan.
2. Following the Pathway of Centenarians to Healthy Longevity
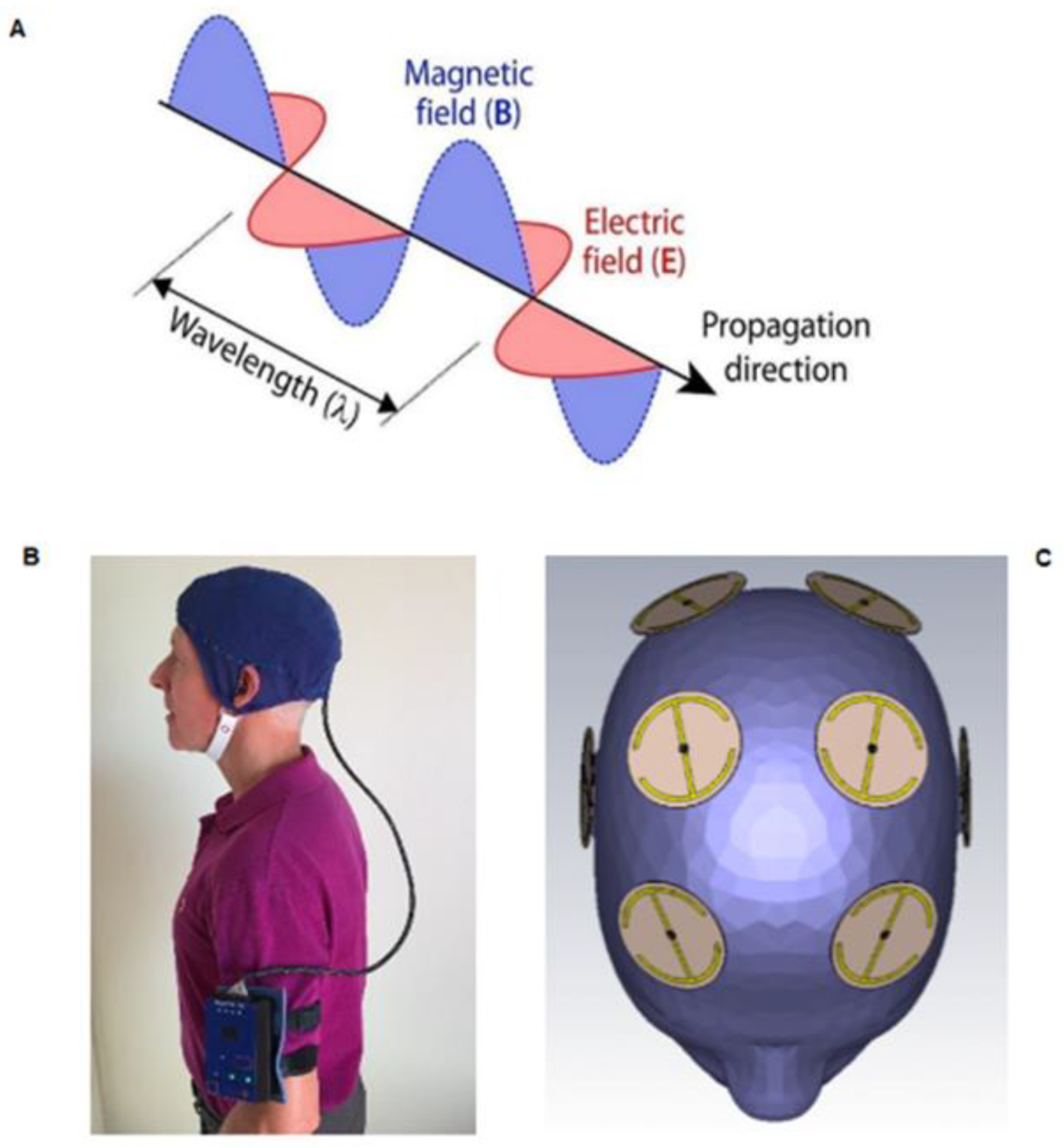
3. Transcranial Electromagnetic Wave Treatment: Effects on Longevity, Cognition, AD Markers, Brain Imaging, and Mechanisms of Action
- (1)
- A 15–20% increase in cell survival occurred in N2a/APPswe cell cultures given twice-daily EMF treatment compared to controls (Cao, being submitted).
- (2)
- With life-long EMF treatment, twice as many rats survived to two years of age vs. control rats, with survival probabilities of 0.50 and 0.28, respectively [48].
- (3)
- Glioma (brain cancer) patients exposed to TEMT had increased survival in comparison to glioma patients having no exposure [49].
- (4)
- A 30–40% reduction in hospitalizations due to Alzheimer’s Disease, all other types of dementia, and Parkinson’s Disease was observed for subjects having long-term TEMT exposure [50].
- (5)
- During a 2½-year period of TEMT in AD subjects averaging 71 years of age, no new incidence of any age-related diseases (e.g., neurological diseases, cancer, arthritis) occurred [51].
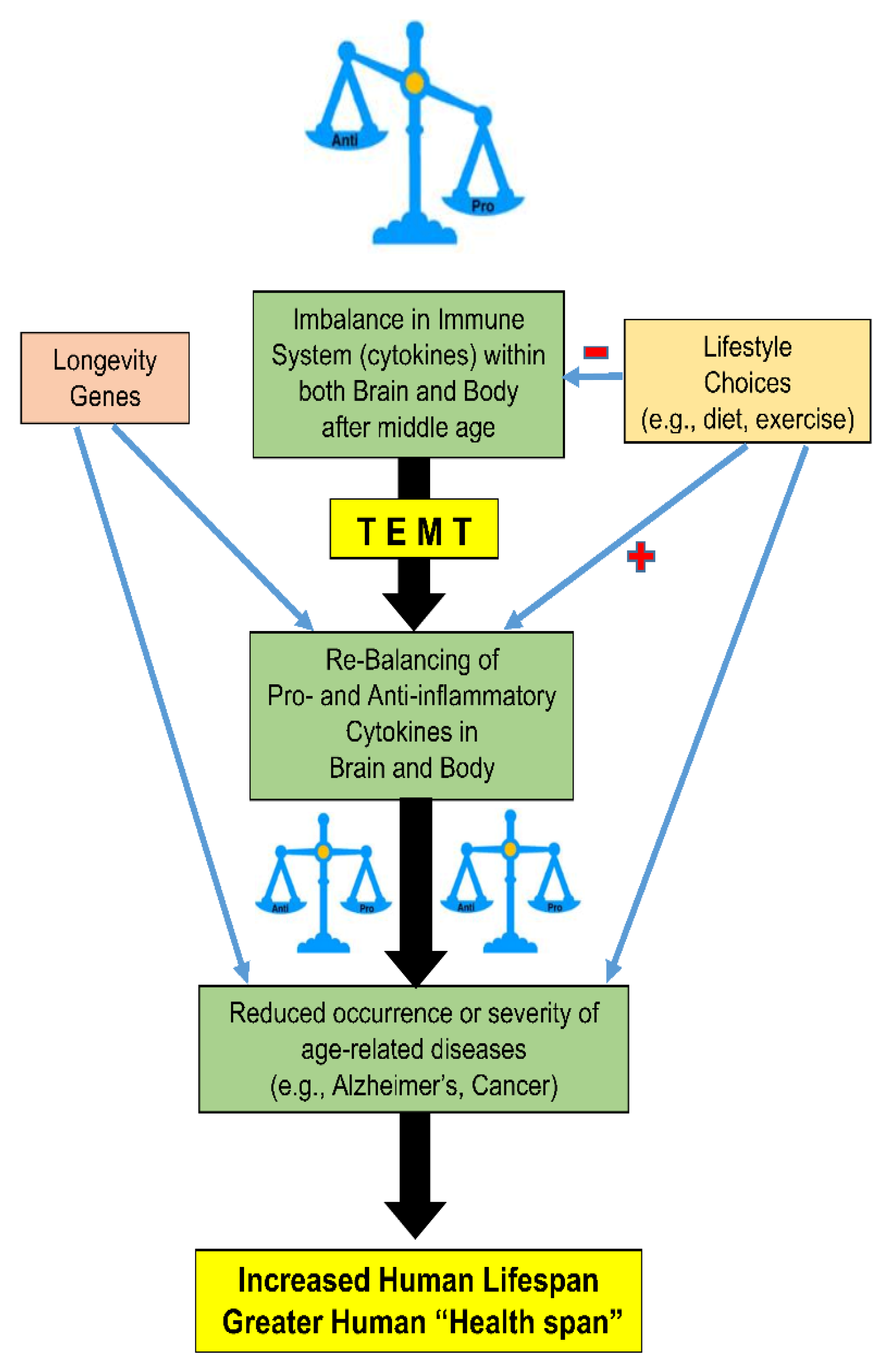
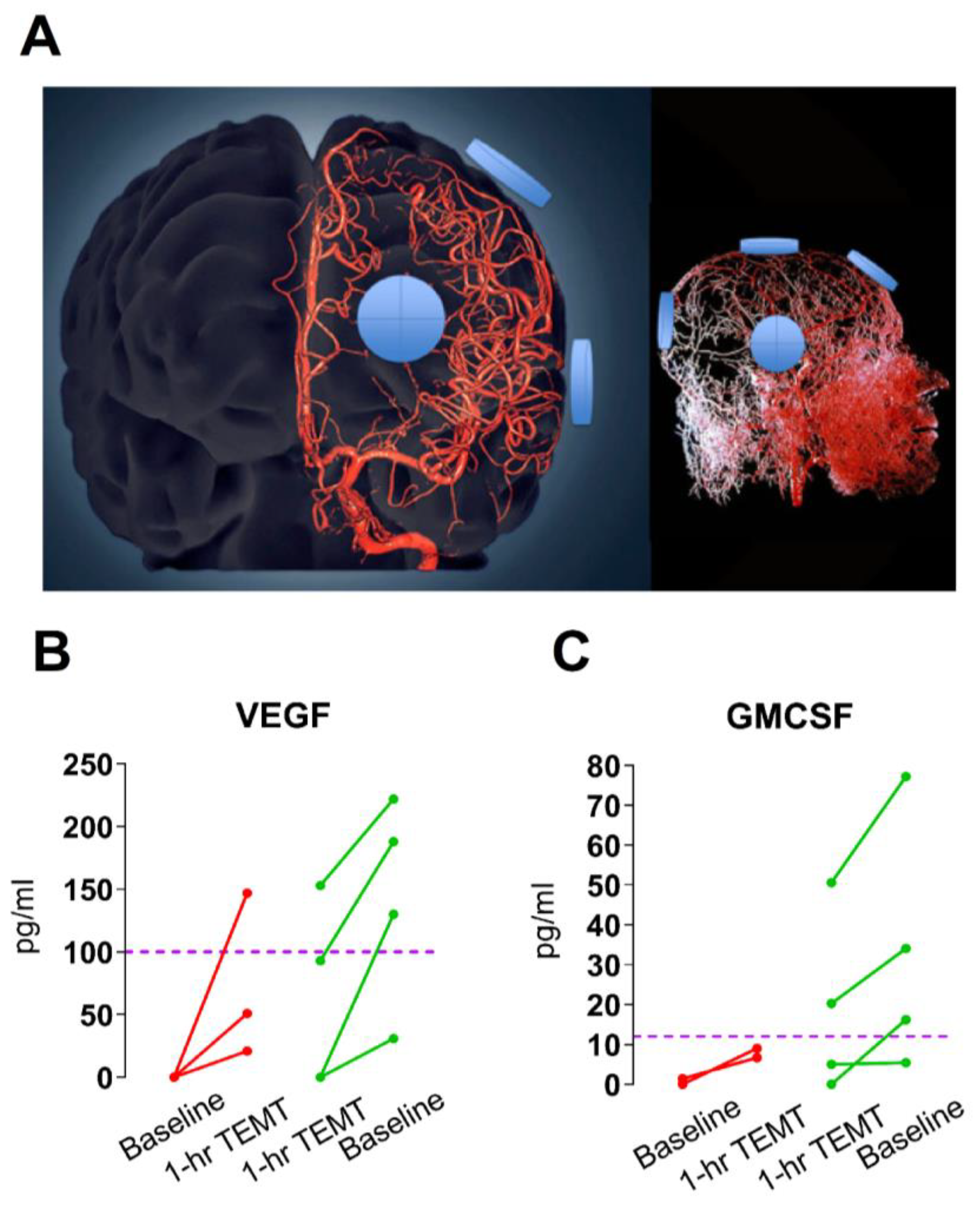
4. TEMT Rebalances the Peripheral (Blood) Immune System in AD Subjects to Reduce Inflammation
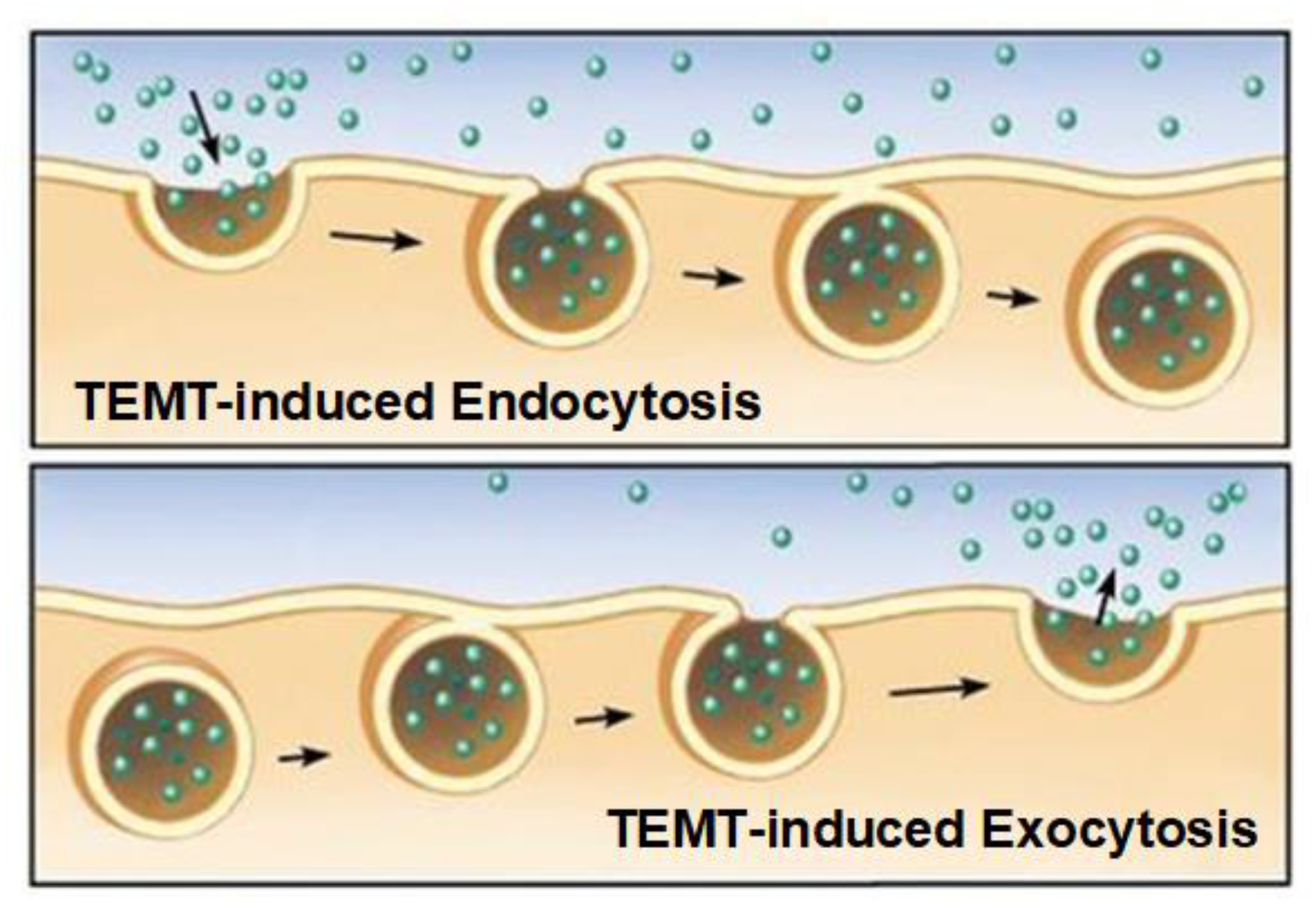
5. Mechanism of TEMT-Induced Rebalancing of Human Peripheral (Blood) Cytokines
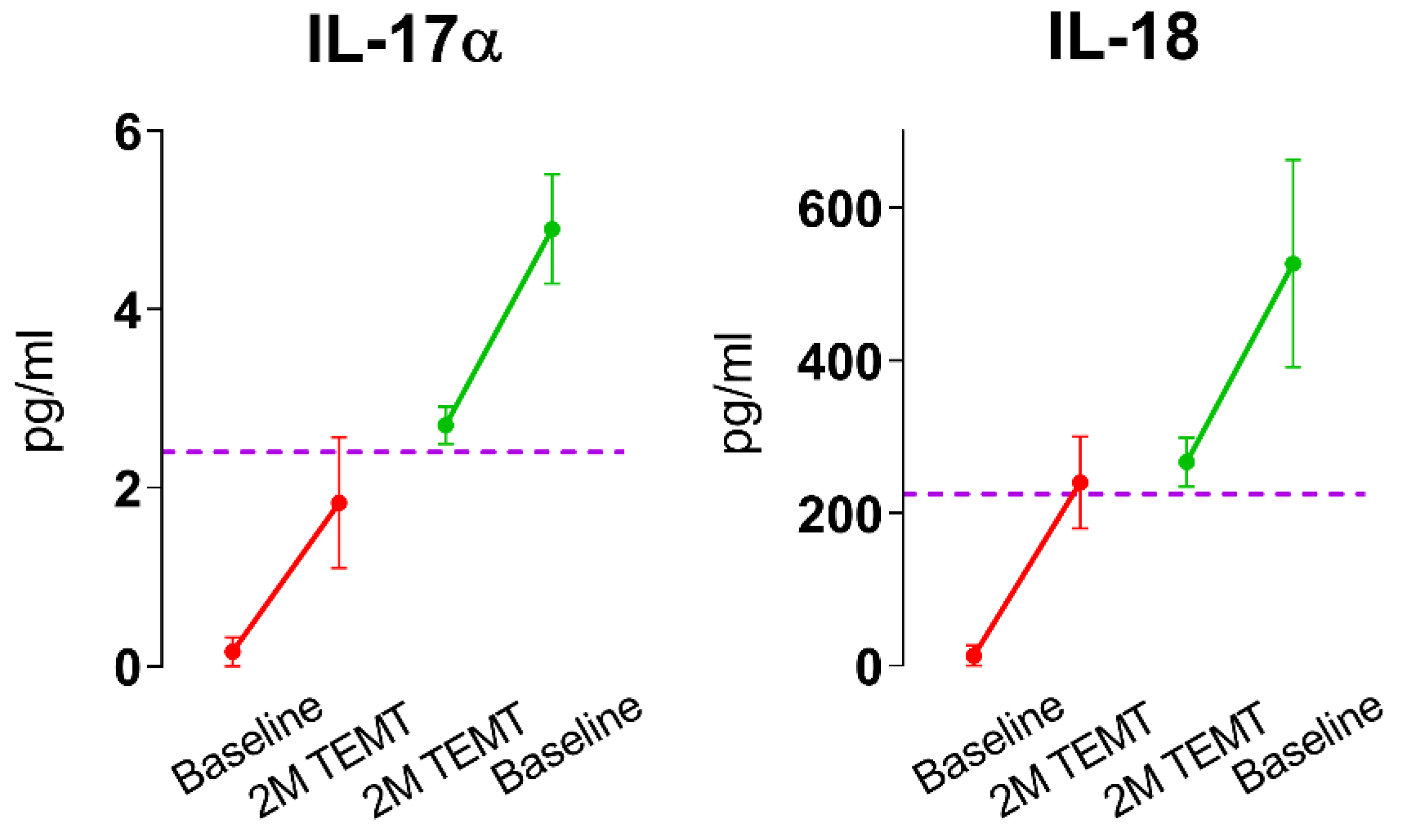
6. TEMT Rebalances the Human Brain’s Immune System in AD Subjects to Reduce Inflammation
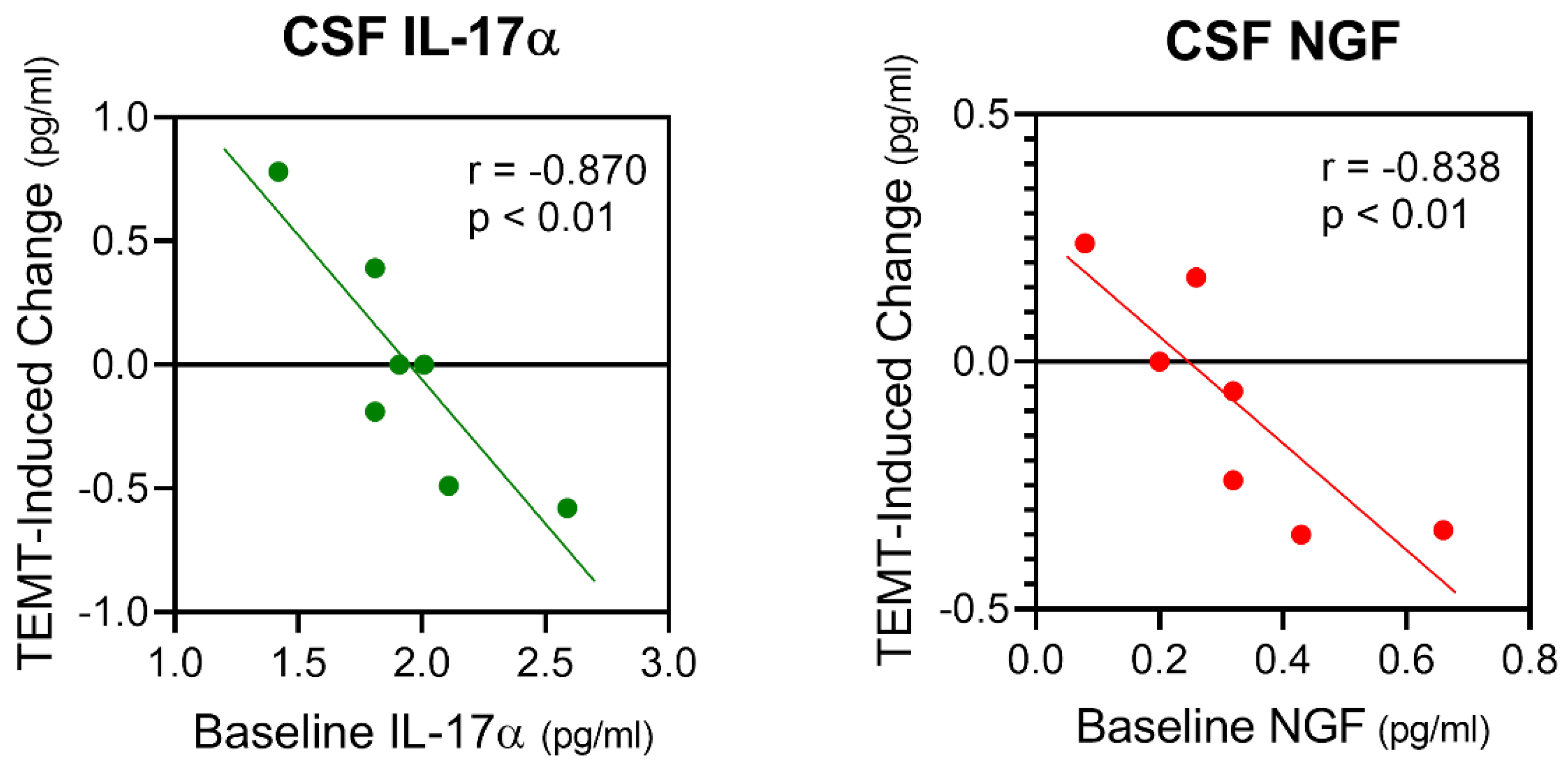
7. Possible Mechanisms of TEMT-Induced Rebalancing of Human Brain/CSF Cytokines
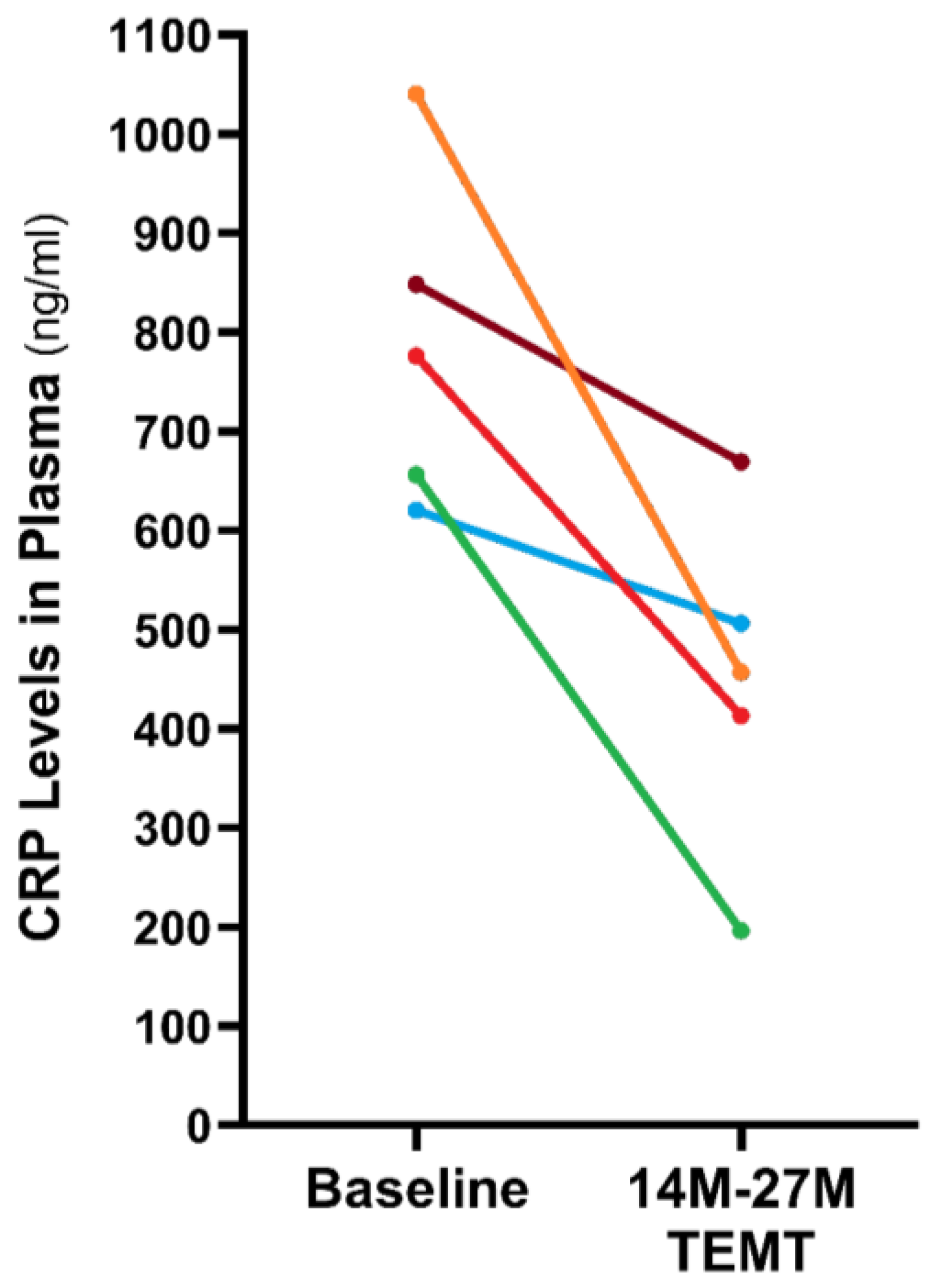
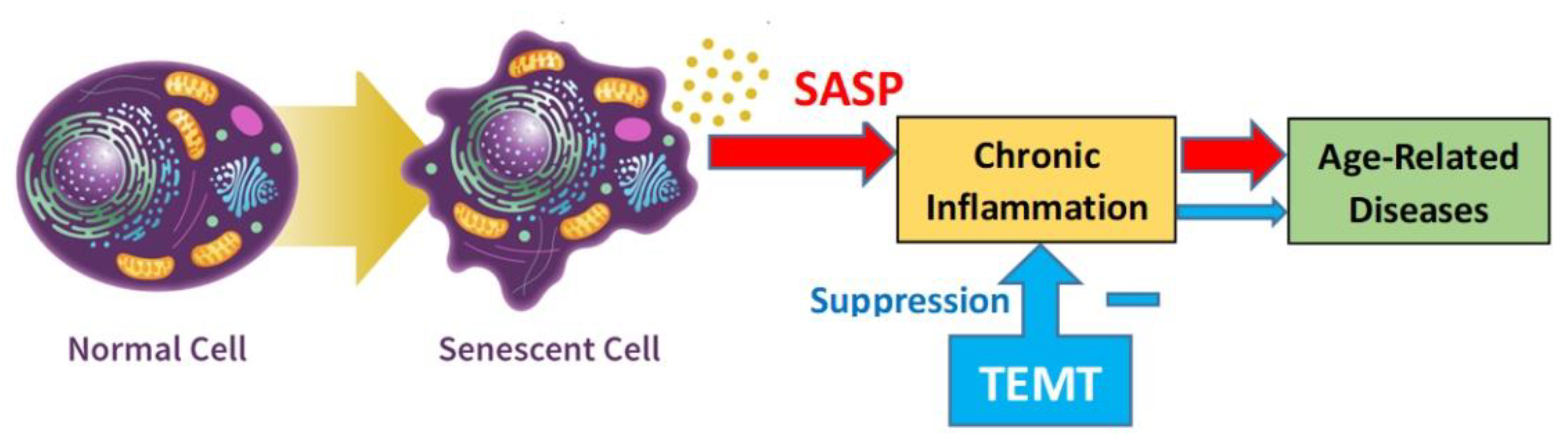
8. Potential Human Benefits of Rejuvenating the Balance in Cytokine Levels within Blood and Brain
9. General Discussion
10. Conclusions
11. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiley, C.; Campisi, J. The metabolic roots of senescence: Mechanisms and opportunities for intervention. Nat. Metab. 2021, 3, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Bernier, M.; Aon, M.; Cortassa, S.; Kim, E.; Fang, E.; Palacios, H.; Ali, A.; Navas-Enamorando, I.; Di Francesco, A.; et al. Nicotinamide improves aspects of health span, but not lifespan in mice. Cell Metab. 2018, 27, 667–676.e4. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Scheibye-Knudsen, M.; Longo, D.; de Cabo, R. Animal models of aging research: Implications for human aging and age-related diseases. Annu. Rev. Anim. Biosci. 2015, 3, 283–303. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A. Aging across the tree of life: The importance of a comparative perspective for the use of animal models in aging. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2680–2689. [Google Scholar] [CrossRef]
- Barzilai, N.; Crandall, J.; Kritchevsky, S.; Espeland, M. Metformin as a tool to target aging. Cell Metab. 2016, 23, 1060–1065. [Google Scholar] [CrossRef]
- Triggle, C.R.; Mohammed, I.; Bshesh, K.; Marei, I.; Ye, K.; Ding, H.; MacDonald, R.; Hollenberg, M.D.; Hill, M.A. Metformin: Is it a drug for all reasons and diseases? Metabolism 2022, 133, 155223. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Brutsaert, E.F.; Anghel, V.; Zhang, K.; Bloomgarden, N.; Pollak, M.; Mar, J.C.; Hawkins, M.; Crandall, J.P.; Barzilai, N. Metformin regulates metabolic and non-metabolic pathways in skeletal muscle and subcutaneous adipose tissues of older adults. Aging Cell 2018, 17, e12723. [Google Scholar] [CrossRef]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; D’Amico, D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R.; et al. NAD(+) repletion improves mitochondrial and stem cell function and enhances lifespan in mice. Science 2016, 352, 1436–1443. [Google Scholar] [CrossRef]
- Reiten, O.; Wilvang, M.; Mitchell, S.; Hu, Z.; Fang, E. Preclinical and clinical evidence of NAD(+) precursors in health, disease, and ageing. Mech. Ageing Dev. 2021, 199, 111567. [Google Scholar] [CrossRef]
- Kraig, E.; Linehan, L.A.; Liang, H.; Romo, T.Q.; Liu, Q.; Wu, Y.; Benavides, A.D.; Curiel, T.J.; Javors, M.A.; Musi, N.; et al. A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Exp. Gerontol. 2018, 105, 53–69. [Google Scholar] [CrossRef]
- Coppe, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Gasek, N.; Kuchel, G.; Kirkland, J.; Xu, M. Strategies for targeting senescent cells in human disease. Nat. Aging 2021, 1, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, E.; Misra, A.; Netto, J.; Tchkonia, T.; Kirkland, J. Strategies for late phase preclinical and early clinical trials of senolytics. Mech. Ageing Dev. 2021, 200, 111591. [Google Scholar] [CrossRef]
- Orr, M.; Gonzales, M.; Garbino, V.; Kautz, T.; Palavinci, J.; Lopez-Cruzan, M.; Dehkordi, S.; Matthews, J.; Habil, Z.; Xu, P.; et al. Senolytic therapy to modulate the progression of Alzheimer’s Disease (STomP-AD)—Outcomes from a first clinical trial of senolytic therapy for Alzheimer’s disease. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Lin, J.-R.; Sin-Chan, P.; Napolioni, V.; Torres, G.G.; Mitra, J.; Zhang, Q.; Jabalameli, M.R.; Wang, Z.; Nguyen, N.; Gao, T.; et al. Rare genetic coding variants associated with human longevity and protection against age-related diseases. Nat. Aging 2021, 1, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.G.; Barzilai, N. New horizons in life extension, health span extension, and exceptional longevity. Age Ageing 2022, 51, 1–6. [Google Scholar] [CrossRef]
- Buettner, D.; Skemp, S. Blue zones: Lessons from the world’s longest lived. Am. J. Lifestyle Med. 2016, 10, 318–321. [Google Scholar] [CrossRef]
- Deelen, J.; Beekman, M.; Uh, H.-W.; Broer, L.; Ayers, K.L.; Tan, Q.; Kamatani, Y.; Bennet, A.M.; Tamm, R.; Trompet, S.; et al. Genome-wide association meta-analysis of human longevity identified a novel locus conferring survival beyond 90 years of age. Hum. Mol. Genet. 2014, 23, 4420–4432. [Google Scholar] [CrossRef]
- Candore, G.; Caruso, C.; Jirillo, E.; Magrone, T.; Vasto, S. Low grade inflammation as a common pathogenetic denominator in age-related diseases: Novel drug targets for anti-ageing strategies and successful ageing achievement. Curr. Pharm. Des. 2010, 16, 584–596. [Google Scholar] [CrossRef]
- Taipa, R.; das Neves, S.P.; Sousa, A.L.; Fernandes, J.; Pinto, C.; Correia, A.P.; Santos, E.; Pinto, P.S.; Carneiro, P.; Costa, P.; et al. Proinflammatory and anti-inflammatory cytokines in the CSF of patients with Alzheimer’s disease and their correlation with cognitive decline. Neurobiol. Aging 2019, 76, 125–132. [Google Scholar] [CrossRef]
- Neves, J.; Sousa-Victor, P. Regulation of inflammation as an anti-aging intervention. FEBS J. 2020, 287, 43–52. [Google Scholar] [CrossRef]
- Ostan, R.; Bucci, L.; Capri, M.; Salvioli, S.; Scurti, M.; Pini, E.; Monti, D.; Franceschi, C. Immunosenescence and immunogenetics of human longevity. Neuroimmunomodulation 2008, 15, 224–240. [Google Scholar] [CrossRef]
- Sayed, N.; Huang, Y.; Nguyen, K.; Krejciova-Rajaniemi, Z.; Grawe, A.P.; Gao, T.; Tibshirani, R.; Hastie, T.; Alpert, A.; Cui, L.; et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbility, immunosenescence, frailty and cardiovascular aging. Nat. Aging 2021, 1, 598–615. [Google Scholar] [CrossRef]
- Zhou, L.; Ge, M.; Zhang, Y.; Wu, X.; Leng, M.; Gan, C. Centenarians alleviate inflammaging by changing the ratio and secretory phenotypes of T helper 17 and regulatory T cells. Front. Pharmacol. 2022, 13, 877709. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.; Gibson, D.; McGilligan, V.; McNerlan, S.; Alexander, H.; Ross, O. Age and Age-Related Diseases: Role of inflammation Triggers and Cytokines. Front. Immunol. 2019, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, J. Immune system aging and the aging-related diseases in the COVID-19 era. Immunol. Lett. 2022, 243, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Krabble, K.; Pedersen, M.; Bruunsgaard, H. Inflammatory mediators in the elderly. Exp. Gerontol. 2004, 39, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.-S.; Youn, J.-C.; Shin, E.-C.; Park, S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J. Transl. Med. 2011, 9, 113. [Google Scholar] [CrossRef]
- Hu, W.T.; Howell, J.C.; Ozturk, T.; Gangishetti, U.; Kollhoff, A.L.; Hatcher-Martin, J.M.; Anderson, A.M.; Tyor, W.R. CSF cytokines in aging, multiple sclerosis, and dementia. Front. Immunol. 2019, 10, 480. [Google Scholar] [CrossRef]
- Arranz, L.; Lord, J.; De la Fuente, M. Preserved ex vivo inflammatory status and cytokine responses in naturally long-lived mice. AGE 2010, 32, 451–466. [Google Scholar] [CrossRef]
- Fila, M. Re-balancing of inflammation and Abeta immunity as a therapeutic for Alzheimer’s disease—View from the bedside. CNS Neurol. Disord. Drug. Targets 2010, 9, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, E.; Camats-Perna, J.; Silva, M.; Valmas, M.; Huat, T.; Medeiros, R. Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflamm. 2018, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Zhou, C.; Shanshan, X.; Bai, J.; Shoufen, J.; Xiaosa, L.; Wang, H.; Tan, Q. Mechanism of repetitive transgenic magentic stimulation for depression. Shanghai Arch. Psychiatry 2018, 30, 84–92. [Google Scholar]
- Ma, J.; Zhang, Z.; Kang, L.; Geng, D.; Wang, Y.; Wang, M.; Cui, H. Repetitive transcranial magnetic stimulation (rTMS) influences spatial cognition and modulates hippocampal structural synaptic plasticity in aging mice. Exp. Gerontol. 2014, 58, 256–268. [Google Scholar] [CrossRef]
- Cheng, C.-M.; Li, C.-T.; Tsai, S.-J. Current updates on newer forms of transcranial magnetic stimulation in major depression. Adv. Exp. Med. Biol. 2021, 1305, 333–349. [Google Scholar]
- Gonteman, F. A systematic review assessing patient-related predictors of response to transcranial magnetic stimulation in major depressive disorder. Neuropsychiatr. Dis. Treat. 2023, 19, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, S.; van der Werf, Y.; van Campen, A.; Arns, M.; Sack, A.; Hoogendoorn, A.; van den Heuve, O. Repetitive transcranial magnetic stimulation for obsessive-compulsive disorder: A systematic review and pairwise/network meta-analysis. J. Affect. Disord. 2022, 302, 302–312. [Google Scholar] [CrossRef]
- Harmelech, T.; Roth, Y.; Tendler, A. Transcranial magnetic stimulation in obsessive-compulsive disorder. Psychiatr. Clin. N. Am. 2022, 46, 133–166. [Google Scholar] [CrossRef]
- Sabbagh, M.; Sadowsky, C.; Tousi, B.; Agronin, M.; Alva, G.; Armon, C. Effects of a combined transcranial magnetic stimulation (TMS) and cognitive training intervention in patients with Alzheimer’s disease. Alzheimer’s Dement. 2020, 10, 641–650. [Google Scholar] [CrossRef]
- Weiler, M.; Stieger, K.; Long, J.; Rapp, P. Transcranial magnetic stimulation in Alzheimer’s Disease: Are we ready? Eneuro 2020, 7. [Google Scholar] [CrossRef]
- Somaa, F.; de Graaf, T.; Sack, A. Transcranial magnetic stimulation in the treatment of neurological diseases. Front. Neurol. 2022, 13, 793253. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G.W.; Sanchez-Ramos, J.; Mori, T.; Mamcarz, M.; Lin, X.; Runfeldt, M.; Wang, L.; Zhang, G.; Sava, V.; Tan, J.; et al. Electromagnetic field treatment protects against and reverses cognitive impairment in Alzheimer’s disease mice. J. Alzheimer’s Dis. 2010, 19, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G.; Mori, T.; Dorsey, M.; Gonzalez, R.; Tajiri, N.; Borlongan, C. Electromagnetic treatment to old Alzheimer’s mice reverses β-amyloid deposition, modifies cerebral blood flow, and provides selected cognitive benefit. PLoS ONE 2012, 7, e35751. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G. Transcranial electromagnetic treatment against Alzheimer’s disease: Why it has the potential to trump Alzheimer’s disease drug development. J. Alzheimer’s Dis. 2012, 32, 243–266. [Google Scholar] [CrossRef]
- Arendash, G. Review of the evidence that Transcranial Electromagnetic Treatment will be a safe and effective therapeutic against Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 53, 753–771. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Bradshaw, P.C.; Mamcartz, M.; Lin, X.; Wang, L.; Cao, C.; Arendash, G. Long-term electromagnetic field treatment enhances brain mitochondrial function of both Alzheimer’s transgenic mice and normal mice: A mechanism for electromagnetic field-induced cognitive benefit? Neuroscience 2011, 185, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Arendash, G. Electromagnetic field treatment enhances neuronal activity: Linkage to cognitive benefit and therapeutic implications for Alzheimer’s Disease. J. Alzheimer’s Dis. Park. 2011, 1, 2. [Google Scholar] [CrossRef]
- Wyde, M.; Cesta, M.; Blystone, C.; Elmore, S.; Foster, P.; Hooth, M.; Kissling, G.; Malarkey, D.; Sills, R.; Stout, M.; et al. Report of partial findings from the national toxicology program carcinogenesis studies of cell phone radiofrequency radiation in Hsd: Sprague Dawley SD rats. bioRxiv 2018, 055699. [Google Scholar] [CrossRef]
- Olsson, A.; Bouaoun, L.; Auvinen, A.; Johansen, C.; Mathiesen, T.; Melin, B.; Lahkola, A.; Larjavaara, S.; Villegier, A.-S.; Byrnes, G.; et al. Survival of glioma patients in relation to mobile phone use in Denmark, Finland and Sweden. J. Neuro. Oncol. 2019, 141, 139–149. [Google Scholar] [CrossRef]
- Schuz, J.; Waldemar, G.; Olsen, J.; Johansen, C. Risks for central nervous system diseases among mobile phone subscribers: A Danish retrospective cohort study. PLoS ONE 2009, 4, e4389. [Google Scholar] [CrossRef]
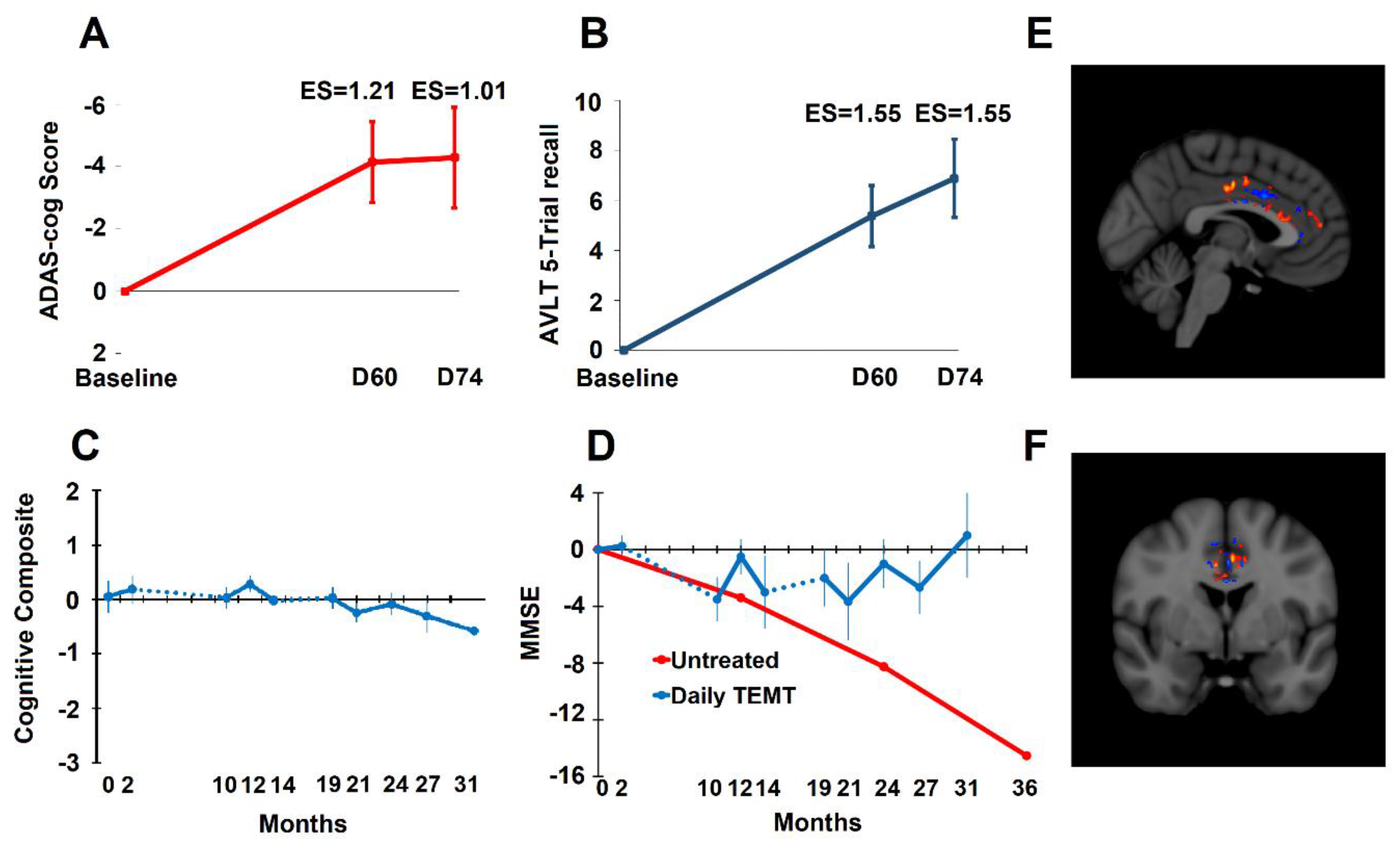
- Arendash, G.; Abulaban, H.; Steen, S.; Andel, R.; Wang, Y.; Bai, Y.; Baranowski, R.; McGarity, J.; Scritsmier, L.; Lin, X.; et al. Transcranial electromagnetic treatment stops Alzheimer’s cognitive decline over a 2½ year period: A pilot study. Medicines 2022, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Arendash, G.; Cao, C.; Abulaban, H.; Baranowski, R.; Wisniewski, G.; Becerra, L.; Andel, R.; Lin, X.; Zhang, X.; Wittwer, D.; et al. A Clinical Trial of Transcranial Electromagnetic Treatment in Alzheimer’s Disease: Cognitive Enhancement and Associated Changes in Cerebrospinal Fluid, Blood, and Brain Imaging. J. Alzheimer’s Dis. 2019, 71, 57–82. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Abulaban, H.; Baranowski, R.; Wang, Y.; Bai, Y.; Lin, X.; Shen, N.; Zhang, X.; Arendash, G.W. Transcranial Electromagnetic Treatment “rebalances” blood and brain cytokines levels in Alzheimer’s patients: A new mechanism for reversal of their cognitive impairment. Front. Aging Neurosci. 2022, 14, 829049. [Google Scholar] [CrossRef]
- Tan, K.-S.; Libon, D.; Rascovsky, K.; Grossman, M.; Xie, S.W. Differential longitudinal decline on the mini-mental state examination in Frontotemporal Lobar Degeneration and Alzheimer’s Disease. Alzheimer Dis. Assoc. Disord. 2013, 27, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Banaceur, S.; Banasr, S.; Sakly, M.; Abdelmelek, H. Whole body exposure to 2.4GHz WIFI signals: Effects on cognitive impairment in adult triple transgenic mouse models of Alzheimer’s disease (3xTg-AD). Behav. Brain Res. 2013, 240, 197–201. [Google Scholar] [CrossRef]
- Jeong, Y.; Kang, G.; Kwon, J.; Choi, H.; Pack, J.; Kim, N.; Lee, Y.; Le, H. 1950 MHz electromagnetic fields ameliorate A-beta pathology in Alzheimer’s disease mice. Curr. Alzheimer Res. 2015, 12, 481–492. [Google Scholar] [CrossRef]
- Son, Y.; Kim, J.; Jeong, Y.; Jeong, Y.; Kwon, J.; Choi, H.-D. Long-term RF exposure on behavior and cerebral glucose metabolism in 5xFAD mice. Neurosci. Lett. 2018, 666, 64–69. [Google Scholar] [CrossRef]
- Walsh, D.; Tseng, B.; Rydel, R.; Podlisny, M.; Selkoe, D. The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry 2000, 39, 10831–10839. [Google Scholar] [CrossRef]
- Zhao, P.-W.; Jiang, W.-G.; Wang, L.; Jiang, Z.-Y.; Shan, Y.-X.; Jiang, Y.-F. Plasma levels of IL-37 and correlation with TNF-α, IL-17A, and disease activity during DMARD treatment of Rheumatoid Arthritis. PLoS ONE 2014, 9, e95346. [Google Scholar] [CrossRef]
- Cao, W.; Zheng, H. Peripheral immune system in aging and Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 51. [Google Scholar] [CrossRef]
- Kustrimovic, N.; Marino, F.; Cosentino, M. Peripheral immunity, immunoaging and neuroinflammation in Parkinson’s Disease. Curr. Med. Chem. 2019, 26, 3719–3753. [Google Scholar] [CrossRef] [PubMed]
- Webers, A.; Heneka, M.; Gleeson, P. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell Biol. 2020, 98, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Badimon, L.; Montecucco, F.; Lüscher, T.; Libby, P.; Camici, G.G. Inflammation, aging, and cardiovascular disease: JACC review topic of the week. J. Am. Coll. Cardiol. 2022, 79, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Perez, J.; Morillas-Ruiz, J. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J. 2012, 2012, 756357. [Google Scholar] [CrossRef]
- Sopova, K.; Gatsiou, K.; Stellos, K.; Laske, C. Dysregulation of neurotrophic and haematopoietic growth factors in Alzheimer’s disease: From pathophysiology to novel treatment strategies. Curr. Alzheimer Res. 2014, 11, 27–39. [Google Scholar] [CrossRef]
- ADAPT-FS Research Group. Follow-up evaluation of cognitive function in the randomized Alzheimer’s disease anti-inflammatory prevention trial and its follow-up study. Alzheimer’s Dement. 2015, 11, 216–225.e1. [Google Scholar] [CrossRef]
- Laske, C.; Stellos, K.; Stransky, E.; Leyhe, T.; Gawaz, M. Decreased plasma levels of granulocyte-colony stimulating factor (G-CSF) in patients with early Alzheimer’s disease. J. Alzheimer’s Dis. 2009, 17, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Loewenstein, D.A.; Lin, X.; Zhang, C.; Wang, L.; Duara, R.; Wu, Y.; Giannini, A.; Bai, G.; Cai, J.; et al. High blood caffeine levels in MCI linked to lack of progression to dementia. J. Alzheimer’s Dis. 2012, 30, 559–572. [Google Scholar] [CrossRef]
- Zheng, F.; Xie, W. High-sensitivity C-reactive protein and cognitive decline: The English Longitudinal Study of Ageing. Psychol. Med. 2018, 48, 1381–1389. [Google Scholar] [CrossRef]
- Luo, J.; Yin, Z.; Lyu, T.; Wang, J.; Shi, X. Association between the hypersensitive C-reactive protein and activities of daily living among elderly adults in longevity areas of China. Zhonghua Yu Fang Yi Xue Za Zhi 2016, 50, 605–610. [Google Scholar] [CrossRef]
- Shen, X.-N.; Niu, L.-D.; Wang, Y.-J.; Cao, X.-P.; Liu, Q.; Tan, L.; Zhang, C.; Yu, J.-T. Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review of 170 studies. J. Neurol. Neurosurg. Psychiatry 2019, 90, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Clouston, S.; Smith, D. Elevated C-reactive protein in Alzheimer’s Disease without depression in older adults: Finds from the health and retirement study. Gerontol. Biol. Sci. Med. Sci. 2022, 77, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Al-Baradie, R.; Pu, S.; Liu, D.; Zeinolabediny, Y.; Ferris, G.; Sanfel, C.; Corpas, R.; Garcia-Lara, E.; Alsagaby, S.; Alshehri, B.; et al. Monomeric C-reactive protein localized in the cerebral tissue of damaged vascular brain regions is associated with neuro-inflammation and neurodegeneration—An immunohistochemical study. Front. Immunol. 2021, 12, 644213. [Google Scholar] [CrossRef]
- Harris, T.B.; Ferrucci, L.; Tracy, R.P.; Corti, M.C.; Wacholder, S.; Ettinger, W.H., Jr.; Heimovitz, H.; Cohen, H.J.; Wallace, R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am. J. Med. 1999, 106, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Wassel, C.; Barrett-Connor, E.; Laughlin, G. Association of circulating C-reactive protein and interleukin-6 with longevity into the 80s and 90s: The Rancho Bernardo Study. J. Clin. Endocrinol. Metab. 2010, 95, 4748–4755. [Google Scholar] [CrossRef] [PubMed]
- Karsten, E.; Breen, E.; Herbert, B. Red blood cells are dynamic reservoirs of cytokines. Sci. Rep. 2018, 8, 3101. [Google Scholar] [CrossRef]
- Segwa, R.; Kaur, K. Microwave absorption in oligomers of ethylene glycol. Indian J. Biochem. Biophys. 1999, 36, 325–329. [Google Scholar]
- Nasir, N.; Al Ahmad, M. Cells electrical characterization: Dielectrical properties, mixture, and modeling theories. J. Eng. 2020, 2020, 9475490. [Google Scholar] [CrossRef]
- Nguyen, T.; Plam, V.; Baulin, V.; Croft, R.; Crawford, U.; Ivanova, E. The effect of a high frequency electromagnetic field in the microwave range on red blood cells. Sci. Rep. 2017, 7, 10798. [Google Scholar] [CrossRef]
- Barbour, R.; Kling, K.; Anderson, J.; Banducci, K.; Cole, T.; Diep, L. Red blood cells are the major source of alpha-synuclein in blood. Neurodegen. Dis. 2008, 5, 55–59. [Google Scholar] [CrossRef]
- Caprari, P.; Scuteri, A.; Salvati, A.; Bauco, C.; Cantafora, A.; Masella, R. Aging and red blood cell membrane: A study of centenarians. Exp. Gerontol. 1999, 34, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Rabini, R.; Moretti, N.; Staffolani, R.; Salvolini, E.; Nanetti, L.; Franceschi, C.; Mazzanti, L. Reduced susceptibility to peroxidation of erythrocyte plasma membranes from centenarians. Exp. Gerontol. 2002, 37, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Puca, A.A.; Andrew, P.; Novelli, V.; Anselmi, C.V.; Somalvico, F.; Cirillo, N.A.; Chatgilialoglu, C.; Ferreri, C. Fatty acid profile of erythrocyte membranes as possible biomarker of longevity. Rejuvenation Res. 2008, 11, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; O’Brien, M.; Mau, T.; Qi, N.; Yung, R. Adipose tissue senescence and inflammation in aging is reversed by the young milieu. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, S.; Ye, Y.; Ren, J.; Chen, R.; Li, W.; Li, J.; Zhao, L.; Zhao, Q.; Sun, G.; et al. Heterochronic parabiosis induces stem cell revitalization and systemic rejuvenation across aged tissues. Cell Stem Cell 2022, 29, 990–1005. [Google Scholar] [CrossRef] [PubMed]
- Conboy, M.; Conboy, I.; Rando, T. Heterochronic parabiosis: Historical perspective and methodological considerations for studies of aging and longevity. Aging Cell 2013, 12, 525–530. [Google Scholar] [CrossRef]
- Lu, Y.; He, M.; Zhang, Y.; Xu, S.; Zhang, L.; He, Y.; Chen, C.; Liu, C.; Pi, H.; Yu, Z.; et al. Differential Pro-Inflammatory Responses of Astrocytes and Microglia Involve STAT3 Activation in Response to 1800 MHz Radiofrequency Fields. PLoS ONE 2014, 9, e108318. [Google Scholar] [CrossRef]
- Kudo, M.; Fujita, K.; Niyaz, M.; Matsuyama, N. Immunohistochemical findings that exposure to 915 MHz Global System for Mobile Communications (GSM) mobile phone microwaves activates microglia in rat brain. J. Tokyo Med. Univ. 2007, 65, 29–36. [Google Scholar]
- Brillaud, E.; Piotrowski, A.; de Seze, R. Effect of an acute 900 MHz GSM exposure on glia in the rat brain: A time-dependent study. Toxicology 2007, 16, 23–33. [Google Scholar] [CrossRef]
- Ammari, M.; Brillaud, E.; Gamez, C.; Lecomte, A.; Sakly, M.; Abdelmelek, H.; de Seze, R. Effect of a chronic GSM 900 MHz exposure on glia in the rat brain. Biomed. Pharmacother. 2008, 62, 273–281. [Google Scholar] [CrossRef]
- Thorlin, T.; Rouquette, J.-M.; Hamnerius, Y.; Hansson, E.; Persson, M.; Björklund, U.; Rosengren, L.; Rönnbäck, L. Exposure of cultured astroglial and microglial brain cells to 900 MHz microwave radiation. Radiat. Res. 2006, 166, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Stopa, E.G.; Berzin, T.; Kim, S.; Song, P.; Kuo-LeBlanc, V.; Rodriguez-Wolf, M.; Baird, A.; Johanson, C. Human choroid plexus growth factors: What are the implications for CSF dynamics in Alzheimer’s disease? Exp. Neurol. 2001, 167, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Xu, H.; Lehtinen, M. Macrophages on the margin: Choroid plexus immune responses. Trends Neurosci. 2021, 44, 864–875. [Google Scholar] [CrossRef]
- Boassa, D.; Stamer, W.; Yool, A. Ion channel function of aquaportin-1 natively expressed in choroid plexus. J. Neurosci. 2006, 26, 7811–7819. [Google Scholar] [CrossRef] [PubMed]
- Hub, J.; Aponte-Santamaria, C.; Grubmuller, H.; de Groot, B. Voltage-regulated water flux through aquaporin channels in silico. Biophys. J. 2010, 99, L97–L99. [Google Scholar] [CrossRef] [PubMed]
- Lio, D.; Scola, L.; Crivello, A.; Colonna-Romano, G.; Candore, G.; Bonafè, M.; Cavallone, L.; Franceschi, C.; Caruso, C. Gender-specific association between -1082 IL-10 promoter polymorphism and longevity. Genes Immun. 2002, 3, 30–33. [Google Scholar] [CrossRef]
- Lio, D.; Scola, L.; Crivello, A.; Colonna-Romano, G.; Candore, G.; Bonafe, M.; Cavallone, L.; Marchegiani, F.; Oliviera, F.; Franceschi, C.; et al. Inflammation, genetics, and longevity: Further studies on the protective effects in men of IL-10-1082 promoter SNP and its interaction with TNF-α-308 promoter SNP. J. Med. Genet. 2003, 40, 296–299. [Google Scholar] [CrossRef]
- Justice, J.; Nambiar, A.; Tchkonia, T.; LeBrasseur, N.; Pascual, R.; Hashmi, S.; Prata, L.; Masternak, M.; Kritchevsky, S.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis; Results from a first-in-human, open label, pilot study. eBiomedicine 2018, 40, 554–563. [Google Scholar] [CrossRef]
- Hickson, L.J.; Prata, L.G.L.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. eBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef]
- Hattori, Y.; Suzuki, K.; Hattori, S.; Kasai, K. Metformin inhibits cytokine-induced nuclear factor κB activation via AMP-activated protein kinase activation in vascular endothelial cells. Hypertension 2006, 47, 1183–11888. [Google Scholar] [CrossRef]
- Liao, W.; Xu, J.; Li, B.; Ruan, Y.; Li, T.; Liu, J. Deciphering the roles of metformin in Alzheimer’s Disease: A snapshot. Front. Pharmacol. 2022, 12, 728315. [Google Scholar] [CrossRef] [PubMed]
- Johansson, O. Disturbance of the immune system by electromagnetic fields—A potentially underlying cause for cellular damage and tissue repair reduction which could lead to disease and impairment. Pathophysiology 2009, 16, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Gatta, L.; Pinto, R.; Ubaldi, V.; Pace, L.; Galloni, P.; Lovisolo, G.A.; Marino, C.; Pioli, C. Effects of in vivo exposure to GSM-modulated 900 MHz radiation on mouse peripheral lymphocytes. Radiat. Res. 2003, 160, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Nasta, F.; Prisco, M.; Pinto, R.; Lovisolo, G.; Marino, C.; Pioli, C. Effects of GSM-modulated radiofrequency electromagnetic fields on B-cell peripheral differentiation and antibody production. Radiat. Res. 2006, 165, 664–670. [Google Scholar] [CrossRef]
- Rosada, M.; Simki, M.; Mattsson, M.M.-O.; Pioli, C. Immune-modulating perspectives for low frequency electromagnetic fields in innate immunity. Front. Public Health 2018, 6, 85. [Google Scholar] [CrossRef]
- Tuchi, H.; Novak, W.; Molla-Dyafari, H. In vitro effects of GSM modulated radiofrequency fields on human immune cells. Bioelectromagnetics 2006, 27, 188–196. [Google Scholar] [CrossRef]
- Ho, J.; Hendi, A. Recent trends in life expectancy across high income countries: Retrospective observational study. Br. Med. J. 2018, 362, k2562. [Google Scholar] [CrossRef]
- Mazzuco, G.; Campostrini, S. Life expectancy drop in 2020. Estimates based on human mortality database. PLoS ONE 2022, 17, e0262846. [Google Scholar] [CrossRef]

| Methformin | TEMT |
|---|---|
| Reduces only pro-inflammatory cytokines in | Rebalances both pro- and anti-inflammatory |
| blood (no effect on anti-inflammatoy cytokines) | cytokines in both blood and brain |
| Decreases CRP only in blood | Decreases CRP in both blood and brain |
| Has side effects and some reports show an | No long-term side effects found so far, but is new |
| increased risk of AD and PD | and has not been investigated much clinically |
| Approved only for type 2 diabetes, but may | Not approved for any indication so far, but has |
| have other disease indications | FDA “breakthrough” designation for AD |
| Old drug; much investigated | New bioengineered technology |
| Two mechanisms of action against aging | Three mechanisms of action against aging |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arendash, G.; Cao, C. Transcranial Electromagnetic Wave Treatment: A Fountain of Healthy Longevity? Int. J. Mol. Sci. 2023, 24, 9652. https://doi.org/10.3390/ijms24119652
Arendash G, Cao C. Transcranial Electromagnetic Wave Treatment: A Fountain of Healthy Longevity? International Journal of Molecular Sciences. 2023; 24(11):9652. https://doi.org/10.3390/ijms24119652
Chicago/Turabian StyleArendash, Gary, and Chuanhai Cao. 2023. "Transcranial Electromagnetic Wave Treatment: A Fountain of Healthy Longevity?" International Journal of Molecular Sciences 24, no. 11: 9652. https://doi.org/10.3390/ijms24119652
APA StyleArendash, G., & Cao, C. (2023). Transcranial Electromagnetic Wave Treatment: A Fountain of Healthy Longevity? International Journal of Molecular Sciences, 24(11), 9652. https://doi.org/10.3390/ijms24119652