Fractalkine Regulates HEC-1A/JEG-3 Interaction by Influencing the Expression of Implantation-Related Genes in an In Vitro Co-Culture Model
Abstract
1. Introduction
2. Results
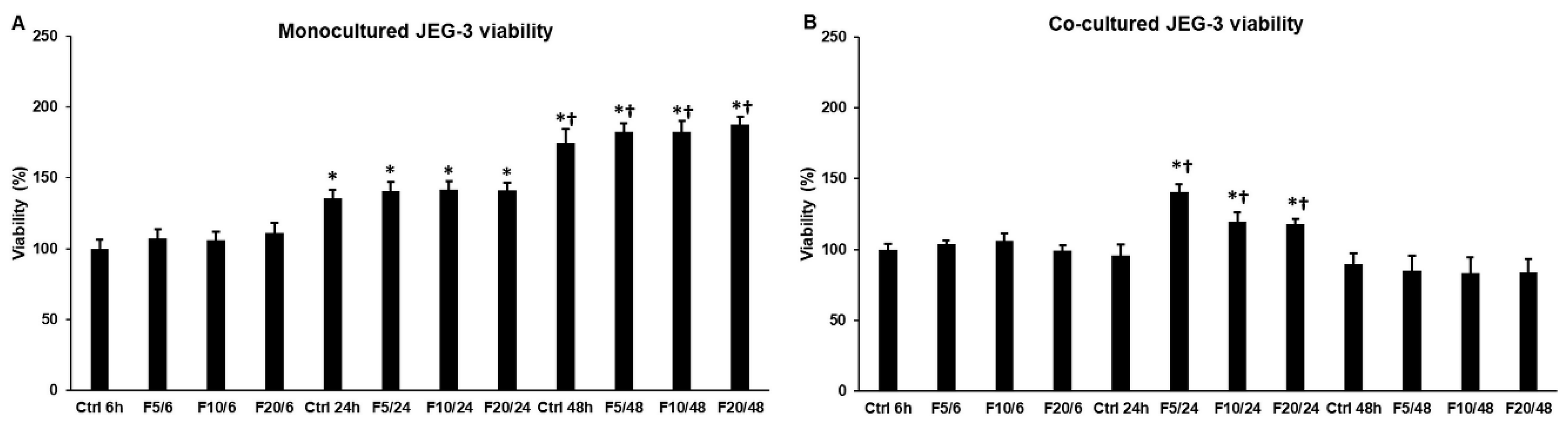
2.1. Effect of Fractalkine on the Viability of JEG-3 Cells in Mono- and Co-Cultures
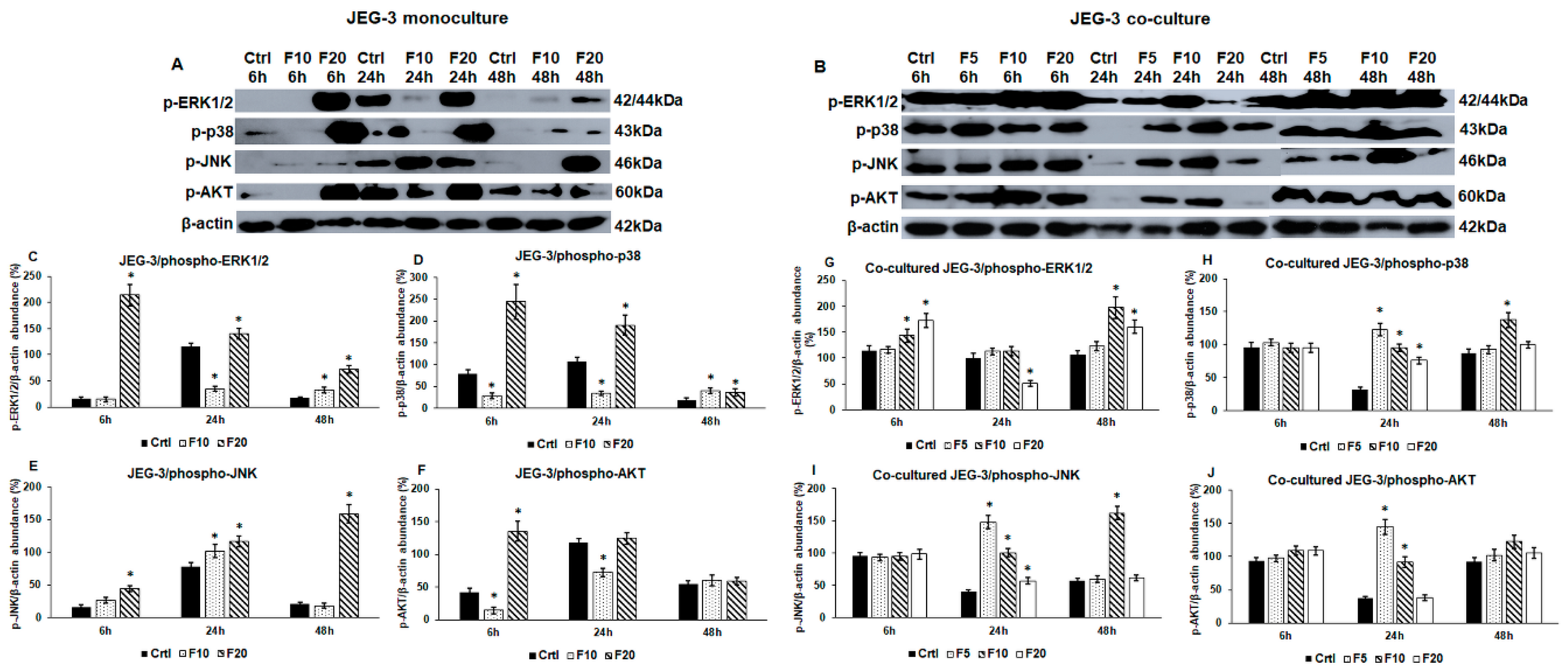
2.2. Fractalkine Changes the Activation of ERK1/2, p38, JNK and AKT Signalling Pathways in Mono- and Co-Cultured JEG-3 Cells
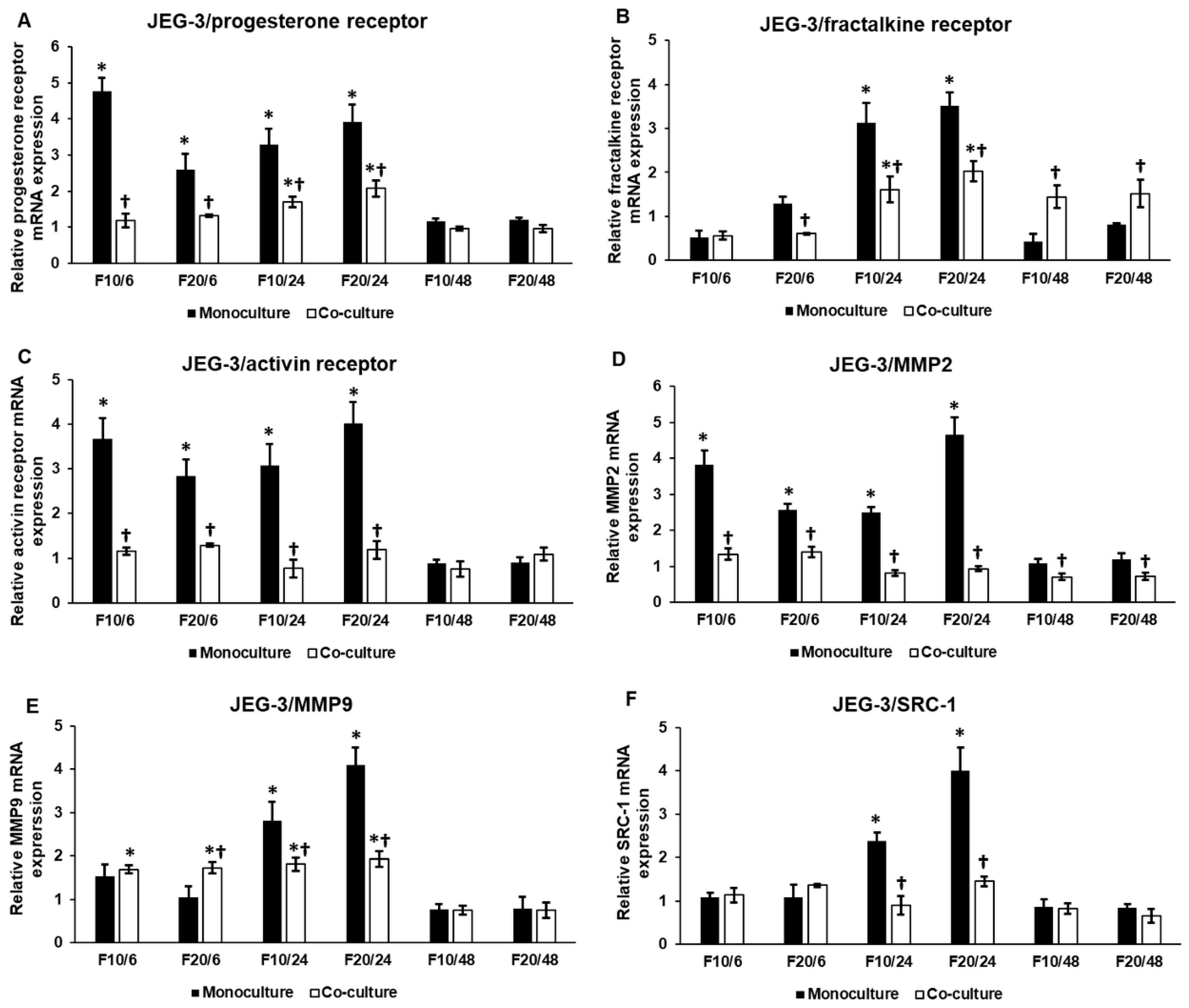
2.3. Fractalkine Exerts Different Effects on the mRNA Expression of Proliferation, Differentiation and Invasion Regulating Genes in Mono- and Co-Cultured JEG-3 Cells
2.4. Western Blot Analysis of the Implantation-Related Genes Reveals Alterations Between Fractalkine Treated Mono- and Co-Cultured JEG-3 Cells
2.5. The Presence of HEC-1A Cells Contributes to the Action of Fractalkine on JEG-3 Cells by Changing the Expressions of Activin, Follistatin and BMP2
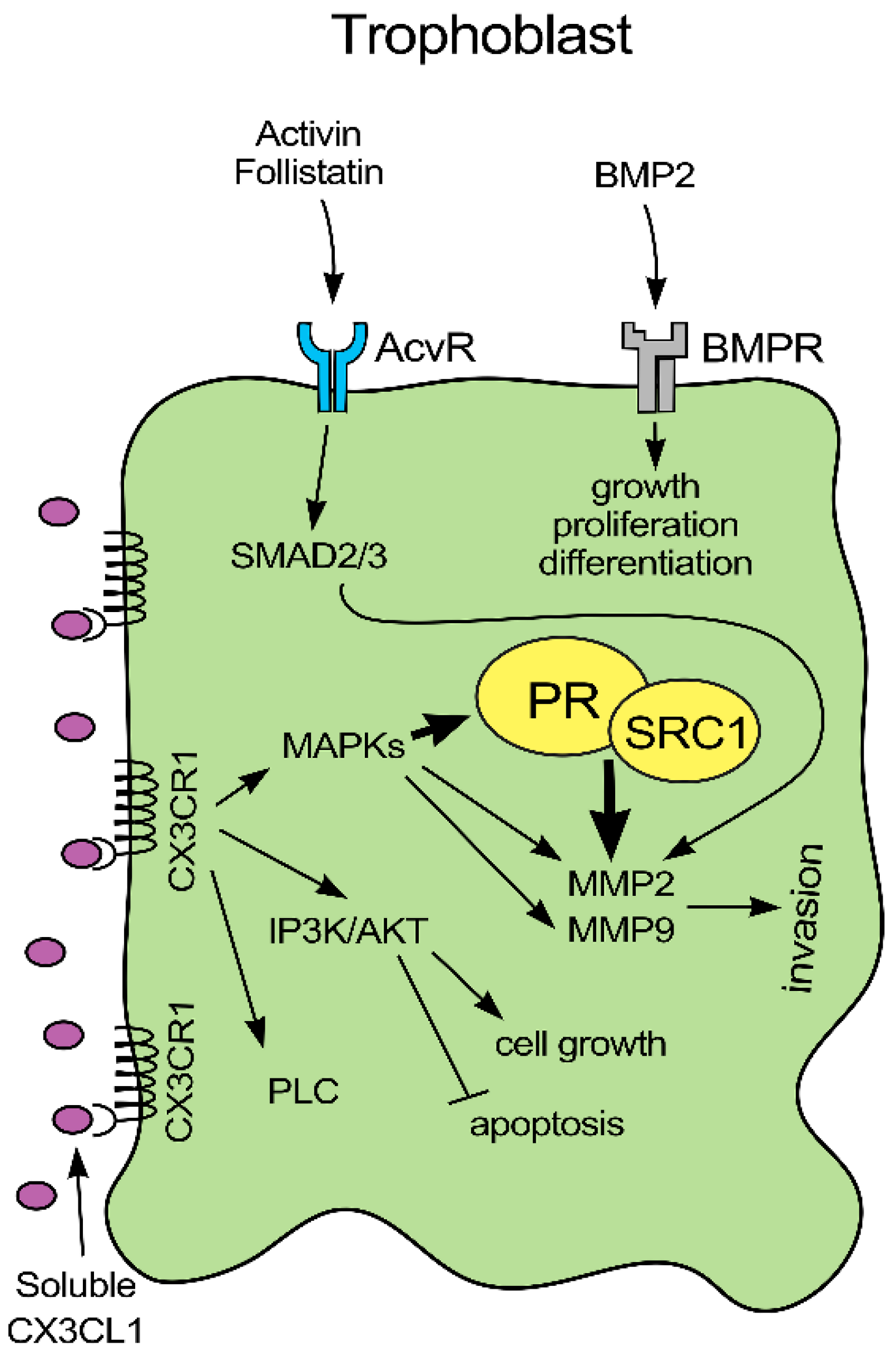
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Treatments
4.2. Cell Viability Assay
4.3. Real-Time PCR
4.4. Immunoblotting
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AcvR | activin receptor |
| AKT | protein kinase B |
| BMP2 | bone morphogenetic protein 2 |
| BMPR | bone morphogenetic protein receptor |
| CNS | central nervous system |
| CX3CL1 | fractalkine |
| CX3CR1 | fractalkine receptor |
| ERK | extracellular signal-regulated protein kinase |
| FKN | fractalkine |
| JNK | c-Jun N-terminal kinase |
| MAPK | mitogen-activated protein kinase |
| MMP | matrix metalloproteinase |
| NFκB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| PI3K | phosphatidylinositol 3 kinase |
| PKC | protein kinase C |
| PLC | phospholipase C |
| PR | progesterone receptor |
| SMAD2/3 | homologues of the Drosophila protein, mothers against decapentaplegic (Mad) and the caenorhabtidis elegans protein (Sma) |
| SRC-1 | steroid receptor coactivator-1 |
References
- Wang, H.; Zhang, S.; Lin, H.; Kong, S.; Wang, S.; Wang, H.; Armant, D.R. Physiological and molecular determinants of embryo implantation. Mol. Asp. Med. 2013, 34, 939–980. [Google Scholar]
- Aplin, J.D.; Ruane, P.T. Embryo-epithelium interactions during implantation at a glance. J. Cell Sci. 2017, 130, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Velasco, J.A.; Arici, A. Chemokines in human reproduction. Immunol. Allergy Clin. N. Am. 2002, 22, 567–583. [Google Scholar] [CrossRef]
- Bazan, J.F.; Bacon, K.B.; Hardiman, G.; Wang, W.; Soo, K.; Rossi, D.; Greaves, D.R.; Zlotnik, A.; Schall, T.J. A new class of membrane-bound chemokine with a CX3C motif. Nature 1997, 385, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Lloyd, C.; Zhou, H.; Dolich, S.; Deeds, J.; Gonzalo, J.A.; Vath, J.; Gosselin, M.; Ma, J.; Dussault, B.; et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature 1997, 387, 611–617. [Google Scholar] [CrossRef]
- Sheridan, G.K.; Murphy, K.J. Neuron-glia crosstalk in health and disease: Fractalkine and CX3CR1 take centre stage. Open Biol. 2013, 3, 130181. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Kotela, A.; Deszczyński, J.; Kotela, I.; Szukiewicz, D. The Chemokine CX3CL1 (Fractalkine) and its Receptor CX3CR1: Occurrence and Potential Role in Osteoarthritis. Arch. Immunol. Ther. Exp. 2014, 62, 395–403. [Google Scholar] [CrossRef]
- Nagira, M.; Imai, T.; Yoshida, R.; Takagi, S.; Iwasaki, M.; Baba, M.; Tabira, Y.; Akagi, J.; Nomiyama, H.; Yoshie, O. A lymphocyte-specific CC chemokine, secondary lymphoid tissue chemokine (SLC), is a highly efficient chemoattractant for B cells and activated T cells. Eur. J. Immunol. 1998, 28, 1516–1523. [Google Scholar] [CrossRef]
- Cardona, A.E.; Pioro, E.P.; Sasse, M.E.; Kostenko, V.; Cardona, S.M.; Dijkstra, I.M.; Huang, D.R.; Kidd, G.; Dombrowski, S.; Dutta, R.; et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006, 9, 917–924. [Google Scholar] [CrossRef]
- Pandur, E.; Tamási, K.; Pap, R.; Varga, E.; Miseta, A.; Sipos, K. Fractalkine Induces Hepcidin Expression of BV-2 Microglia and Causes Iron Accumulation in SH-SY5Y Cells. Cell. Mol. Neurobiol. 2019, 39, 985–1001. [Google Scholar] [CrossRef]
- Hannan, N.J.; Jones, R.L.; Critchley, H.O.D.; Kovacs, G.J.; Rogers, P.A.W.; Affandi, B.; Salamonsen, L.A. Coexpression of fractalkine and its receptor in normal human endometrium and in endometrium from users of progestin-only contraception supports a role for fractalkine in leukocyte recruitment and endometrial remodeling. J. Clin. Endocrinol. Metab. 2004, 89, 6119–6129. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhao, P.; Yang, L.; Chen, Y.; Yan, J.; Duan, E.; Qiao, J. Fractalkine is expressed in the human ovary and increases progesterone biosynthesis in human luteinised granulosa cells. Reprod. Biol. Endocrinol. 2011, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kervancioglu Demirci, E.; Salamonsen, L.A.; Gauster, M. The role of CX3CL1 in fetal-maternal interaction during human gestation. Cell Adhes. Migr. 2016, 10, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Jones, R.L.; White, C.A.; Salamonsen, L.A. The Chemokines, CX3CL1, CCL14, and CCL4, Promote Human Trophoblast Migration at the Feto-Maternal Interface. Biol. Reprod. 2006, 74, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Bérangère Ré, D.; Przedborski, S. Fractalkine: Moving from chemotaxis to neuroprotection. Nat. Neurosci. 2006, 9, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Widmann, C.; Gibson, S.; Jarpe, M.B.; Johnson, G.L. Mitogen-activated protein kinase: Conservation of a three-kinase module from yeast to human. Physiol. Rev. 1999, 79, 143–180. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Chan, T.O.; Rittenhouse, S.E.; Tsichlis, P.N. AKT/PKB and Other D3 Phosphoinositide-Regulated Kinases: Kinase Activation by Phosphoinositide-Dependent Phosphorylation. Annu. Rev. Biochem. 1999, 68, 965–1014. [Google Scholar] [CrossRef]
- Hers, I.; Vincent, E.E.; Tavaré, J.M. Akt signalling in health and disease. Cell. Signal. 2011, 23, 1515–1527. [Google Scholar] [CrossRef]
- Alessandro, R.; Kohn, E.C. Signal transduction targets in invasion. Clin. Exp. Metastasis 2002, 19, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Salamonsen, L.A. Tissue injury and repair in the female human reproductive tract. Reproduction 2003, 125, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Nasu, K.; Nishida, M.; Ito, H.; Bing, S.; Miyakawa, I. Regulation of proliferation, motility, and contractility of human endometrial stromal cells by platelet-derived growth factor. J. Clin. Endocrinol. Metab. 2005, 90, 3560–3567. [Google Scholar] [CrossRef] [PubMed]
- Chiu, D.; Ma, K.; Scott, A.; Duronio, V. Acute activation of Erk1/Erk2 and protein kinase B/akt proceed by independent pathways in multiple cell types. FEBS J. 2005, 272, 4372–4384. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, M.; Yamamoto, T.; Kohno, M.; Takeichi, M.; Nishida, E. Requirement for ERK MAP kinase in mouse preimplantation development. Development 2007, 134, 2751–2759. [Google Scholar] [CrossRef] [PubMed]
- Halasz, M.; Szekeres-Bartho, J. The role of progesterone in implantation and trophoblast invasion. J. Reprod. Immunol. 2013, 97, 43–50. [Google Scholar] [CrossRef]
- Hagan, C.R.; Daniel, A.R.; Dressing, G.E.; Lange, C.A. Role of phosphorylation in progesterone receptor signaling and specificity. Mol. Cell. Endocrinol. 2012, 357, 43–49. [Google Scholar] [CrossRef]
- Gaide Chevronnay, H.P.; Selvais, C.; Emonard, H.; Galant, C.; Marbaix, E.; Henriet, P. Regulation of matrix metalloproteinases activity studied in human endometrium as a paradigm of cyclic tissue breakdown and regeneration. Biochim. Biophys. Acta-Proteins Proteom. 2012, 1824, 146–156. [Google Scholar] [CrossRef]
- Goldman, S. Differential activity of the gelatinases (matrix metalloproteinases 2 and 9) in the fetal membranes and decidua, associated with labour. Mol. Hum. Reprod. 2003, 9, 367–373. [Google Scholar] [CrossRef]
- Davidson, B.; Givant-Horwitz, V.; Lazarovici, P.; Risberg, B.; Nesland, J.M.; Trope, C.G.; Schaefer, E.; Reich, R. Matrix metalloproteinases (MMP), EMMPRIN (extracellular matrix metalloproteinase inducer) and mitogen-activated protein kinases (MAPK): Co-expression in metastatic serous ovarian carcinoma. Clin. Exp. Metastasis 2003, 20, 621–631. [Google Scholar] [CrossRef]
- Loesch, M.; Zhi, H.Y.; Hou, S.W.; Qi, X.M.; Li, R.S.; Basir, Z.; Iftner, T.; Cuenda, A.; Chen, G. p38γ MAPK cooperates with c-Jun in trans-activating matrix metalloproteinase 9. J. Biol. Chem. 2010, 285, 15149–15158. [Google Scholar] [CrossRef] [PubMed]
- Onogi, A.; Naruse, K.; Sado, T.; Tsunemi, T.; Shigetomi, H.; Noguchi, T.; Yamada, Y.; Akasaki, M.; Oi, H.; Kobayashi, H. Hypoxia inhibits invasion of extravillous trophoblast cells through reduction of matrix metalloproteinase (MMP)-2 activation in the early first trimester of human pregnancy. Placenta 2011, 32, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Staun-Ram, E.; Goldman, S.; Gabarin, D.; Shalev, E. Expression and importance of matrix metalloproteinase 2 and 9 (MMP-2 and -9) in human trophoblast invasion. Reprod. Biol. Endocrinol. 2004, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Refaat, B. Role of activins in embryo implantation and diagnosis of ectopic pregnancy: A review. Reprod. Biol. Endocrinol. 2014, 12, 1–8. [Google Scholar] [CrossRef]
- Zhao, H.J.; Klausen, C.; Li, Y.; Zhu, H.; Wang, Y.L.; Leung, P.C.K. Bone morphogenetic protein 2 promotes human trophoblast cell invasion by upregulating N-cadherin via non-canonical SMAD2/3 signaling. Cell Death Dis. 2018, 9, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.B.; Krueger, J.S.; Kondapaka, S.B.; Diglio, C.A. Mitogen-activated protein kinase (MAPK) regulates the expression of progelatinase B (MMP-9) in breast epithelial cells. Int. J. Cancer 1999, 82, 268–273. [Google Scholar] [CrossRef]
- Wijayarathna, R.; de Kretser, D.M. Activins in reproductive biology and beyond. Hum. Reprod. Update 2016, 22, 342–357. [Google Scholar] [CrossRef]
- Jones, R.L.; Kaitu’u-Lino, T.J.; Nie, G.; Sanchez-Partida, L.G.; Findlay, J.K.; Salamonsen, L.A. Complex expression patterns support potential roles for maternally derived activins in the establisment of pregnancy in mouse. Reproduction 2006, 132, 799–810. [Google Scholar] [CrossRef]
- Thompson, T.B.; Lerch, T.F.; Cook, R.W.; Woodruff, T.K.; Jardetzky, T.S. The structure of the follistatin: Activin complex reveals antagonism of both type I and type II receptor binding. Dev. Cell 2005, 9, 535–543. [Google Scholar] [CrossRef]
- Hemmati-Brivanlou, A.; Thomsen, G.H. Ventral mesodermal patterning in Xenopus embryos: Expression patterns and activities of BMP-2 and BMP-4. Dev. Genet. 1995, 17, 78–89. [Google Scholar] [CrossRef]
- Sidis, Y.; Mukherjee, A.; Keutmann, H.; Delbaere, A.; Sadatsuki, M.; Schneyer, A. Biological activity of follistatin isoforms and follistatin-like-3 is dependent on differential cell surface binding and specificity for activin, myostatin, and bone morphogenetic proteins. Endocrinology 2006, 147, 3586–3597. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Abt, A.; Meucci, O. Bilaminar co-culture of primary rat cortical neurons and glia. J. Vis. Exp. 2011, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Wu, Y.M.; Ji, Z.; Gao, X.Y.; Pan, S.Y. A modified technique for culturing primary fetal rat cortical neurons. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.; Hippensteel, R.; Shimizu, S.; Nicolai, J.; Fatatis, A.; Meucci, O. Interactions between Chemokines: Regulation of fractalkine/CX 3CL1 homeostasis by SDF/CXCL12 in cortical neurons. J. Biol. Chem. 2010, 285, 10563–10571. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.M.; Walker, R.V.; Parker, S.; Rhodes, N.P.; Hunt, J.A.; Freemont, A.J.; Hoyland, J.A. Intervertebral Disc Cell-Mediated Mesenchymal Stem Cell Differentiation. Stem Cells 2006, 24, 707–716. [Google Scholar] [CrossRef]
- Hatori, K.; Nagai, A.; Heisel, R.; Ryu, J.K.; Kim, S.U. Fractalkine and fractalkine receptors in human neurons and glial cells. J. Neurosci. Res. 2002, 69, 418–426. [Google Scholar] [CrossRef]
- White, G.E.; Greaves, D.R. Fractalkine: A survivor’s guide chemokines as antiapoptotic mediators. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 589–594. [Google Scholar] [CrossRef]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional Microglia–Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018, 12, 1–26. [Google Scholar] [CrossRef]
- Zhuang, Q.; Ou, J.; Zhang, S.; Ming, Y. Crosstalk between the CX3CL1/CX3CR1 Axis and Inflammatory Signaling Pathways in Tissue Injury. Curr. Protein Pept. Sci. 2019, 20, 844–854. [Google Scholar] [CrossRef]
- Jones, R.L.; Salamonsen, L.A.; Findlay, J.K. Potential roles for endometrial inhibins, activins and follistatin during human embryo implantation and early pregnancy. Trends Endocrinol. Metab. 2002, 13, 144–150. [Google Scholar] [CrossRef]
- Minas, V.; Loutradis, D.; Makrigiannakis, A. Factors controlling blastocyst implantation. Reprod. Biomed. Online 2005, 10, 205–216. [Google Scholar] [CrossRef]
- de Ruijter-Villani, M.; Stout, T. The Role of Conceptus-maternal Signalling in the Acquisition of Uterine Receptivity to Implantation in Mammals. Reprod. Domest. Anim. 2015, 50, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Wetendorf, M.; DeMayo, F.J. Progesterone receptor signaling in the initiation of pregnancy and preservation of a healthy uterus. Int. J. Dev. Biol. 2014, 58, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Hannan, N.J.; Paiva, P.; Dimitriadis, E.; Salamonsen, L.A. Models for Study of Human Embryo Implantation: Choice of Cell Lines? Biol. Reprod. 2010, 82, 235–245. [Google Scholar] [CrossRef]
- Hannan, N.J.; Salamonsen, L.A. CX3CL1 and CCL14 Regulate Extracellular Matrix and Adhesion Molecules in the Trophoblast: Potential Roles in Human Embryo Implantation. Biol. Reprod. 2008, 79, 58–65. [Google Scholar] [CrossRef]
- Pandur, E.; Varga, E.; Tamási, K.; Pap, R.; Nagy, J.; Sipos, K. Effect of inflammatory mediators lipopolysaccharide and lipoteichoic acid on iron metabolism of differentiated SH-SY5Y cells alters in the presence of BV-2 microglia. Int. J. Mol. Sci. 2019, 20, 17. [Google Scholar] [CrossRef]
- Rothbauer, M.; Patel, N.; Gondola, H.; Siwetz, M.; Huppertz, B.; Ertl, P. A comparative study of five physiological key parameters between four different human trophoblast-derived cell lines. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Franco, H.L.; Jeong, J.W.; Tsai, S.Y.; Lydon, J.P.; DeMayo, F.J. In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin. Cell Dev. Biol. 2008, 19, 178–186. [Google Scholar] [CrossRef]
- Szwarc, M.M.; Kommagani, R.; Lessey, B.A.; Lydon, J.P. The p160/Steroid Receptor Coactivator Family: Potent Arbiters of Uterine Physiology and Dysfunction1. Biol. Reprod. 2014, 91, 1–11. [Google Scholar] [CrossRef]
- Jones, R.L. Expression of activin receptors, follistatin and betaglycan by human endometrial stromal cells; consistent with a role for activins during decidualization. Mol. Hum. Reprod. 2002, 8, 363–374. [Google Scholar] [CrossRef]
- Jones, R.L.; Salamonsen, L.A.; Findlay, J.K. Activin A promotes human endometrial stromal cell decidualization in vitro. J. Clin. Endocrinol. Metab. 2002, 87, 4001–4004. [Google Scholar] [CrossRef] [PubMed]
- Massimiani, M.; Lacconi, V.; La Civita, F.; Ticconi, C.; Rago, R.; Campagnolo, L. Molecular signaling regulating endometrium–blastocyst crosstalk. Int. J. Mol. Sci. 2020, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Maia-Filho, V.O.A.; Rocha, A.M.; Ferreira, F.P.; Bonetti, T.C.S.; Serafini, P.; Motta, E.L.A. Matrix metalloproteinases 2 and 9 and E-cadherin expression in the endometrium during the implantation window of infertile women before in vitro fertilization treatment. Reprod. Sci. 2015, 22, 416–422. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, J.; Qiao, F.; Yin, X. Impact of silencing MMP9 gene on the biological behaviors of trophoblasts. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Hess, A.P.; Hamilton, A.E.; Talbi, S.; Dosiou, C.; Nyegaard, M.; Nayak, N.; Genbecev-Krtolica, O.; Mavrogianis, P.; Ferrer, K.; Kruessel, J.; et al. Decidual Stromal Cell Response to Paracrine Signals from the Trophoblast: Amplification of Immune and Angiogenic Modulators1. Biol. Reprod. 2007, 76, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Garin, A.; Pellet, P.; Deterre, P.; Debre, P.; Combadière, C. Cloning and functional characterization of the human fractalkine receptor promoter regions. Biochem. J. 2002, 368, 753–760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhurke, A.S.; Bagchi, I.C.; Bagchi, M.K. Progesterone-Regulated Endometrial Factors Controlling Implantation. Am. J. Reprod. Immunol. 2016, 75, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef]
- Zhao, H.J.; Chang, H.M.; Zhu, H.; Klausen, C.; Li, Y.; Leung, P.C.K. Bone morphogenetic protein 2 promotes human trophoblast cell invasion by inducing activin A production. Endocrinology 2018, 159, 2815–2825. [Google Scholar] [CrossRef]
- Treviño, L.S.; Bingman, W.E.; Edwards, D.P.; Nl, W. The requirement for p42/p44 MAPK activity in progesterone receptor-mediated gene regulation is target gene-specific. Steroids 2013, 78, 542–547. [Google Scholar] [CrossRef][Green Version]
- Tamm, K.; Rõõm, M.; Salumets, A.; Metsis, M. Genes targeted by the estrogen and progesterone receptors in the human endometrial cell lines HEC1A and RL95-2. Reprod. Biol. Endocrinol. 2009, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bars-Cortina, D.; Riera-Escamilla, A.; Gou, G.; Piñol-Felis, C.; Motilva, M.J. Design, optimization and validation of genes commonly used in expression studies on DMH/AOM rat colon carcinogenesis model. PeerJ 2019, 2019, 1–18. [Google Scholar] [CrossRef] [PubMed]
- ImageJ. Available online: https://imagej.nih.gov/ij/ (accessed on 23 September 1997).


| Primer | Sequence 5′ → 3′ |
|---|---|
| Progesterone receptor A/B forward | CCAAAGGCCGCAAATTCT |
| Progesterone receptor A/B reverse | TGAGGTCAGAAAGGTCATCG |
| Fractalkine receptor forward | CCATTAGTCTGGGCGTCTGG |
| Fractalkine receptor reverse | GTCACCCAGACACTCGTTGT |
| Activin receptor 1B forward | CGTTTGCCGTCTTTCTTATC |
| Activin receptor 1B reverse | ACCAGTTTGATTGGTTCTGT |
| MMP2 forward | GTCGCCCATCATCAAGTT |
| MMP2 reverse | GCATCTTCTTTAGTGTGTCCT |
| MMP9 forward | CGGACCAAGGATACAGTTTG |
| MMP9 reverse | AAGCGGTACATAGGGTACAT |
| SRC-1 forward | AGACCCAACCTTTATTCCCA |
| SRC-1 reverse | GGTGTTACTTGAACAGGCAT |
| Activin A forward | GAACTTATGGAGCAGACCTC |
| Activin A reverse | GGACTTTTAGGAAGAGCCAG |
| Follistatin forward | CAAAGCAAAGTCCTGTGAAG |
| Follistatin reverse | CCTCTCCCAACCTTGAAATC |
| BMP2 forward | TAAGTTCTATCCCCACGGAG |
| BMP2 reverse | AGCATCTTGCATCTGTTCTC |
| β-actin forward | AGAAAATCTGGCACCACACC |
| β-actin reverse | GGGGTGTTGAAGGTCTCAAA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pap, R.; Montskó, G.; Jánosa, G.; Sipos, K.; Kovács, G.L.; Pandur, E. Fractalkine Regulates HEC-1A/JEG-3 Interaction by Influencing the Expression of Implantation-Related Genes in an In Vitro Co-Culture Model. Int. J. Mol. Sci. 2020, 21, 3175. https://doi.org/10.3390/ijms21093175
Pap R, Montskó G, Jánosa G, Sipos K, Kovács GL, Pandur E. Fractalkine Regulates HEC-1A/JEG-3 Interaction by Influencing the Expression of Implantation-Related Genes in an In Vitro Co-Culture Model. International Journal of Molecular Sciences. 2020; 21(9):3175. https://doi.org/10.3390/ijms21093175
Chicago/Turabian StylePap, Ramóna, Gergely Montskó, Gergely Jánosa, Katalin Sipos, Gábor L. Kovács, and Edina Pandur. 2020. "Fractalkine Regulates HEC-1A/JEG-3 Interaction by Influencing the Expression of Implantation-Related Genes in an In Vitro Co-Culture Model" International Journal of Molecular Sciences 21, no. 9: 3175. https://doi.org/10.3390/ijms21093175
APA StylePap, R., Montskó, G., Jánosa, G., Sipos, K., Kovács, G. L., & Pandur, E. (2020). Fractalkine Regulates HEC-1A/JEG-3 Interaction by Influencing the Expression of Implantation-Related Genes in an In Vitro Co-Culture Model. International Journal of Molecular Sciences, 21(9), 3175. https://doi.org/10.3390/ijms21093175







