Abstract
Mycobacteriophages possess different sets of lytic enzymes for disruption of the complex cell envelope of the mycobacteria host cells and release of the viral progeny. Lysin B (LysB) enzymes are mycolylarabinogalactan esterases that cleave the ester bond between the arabinogalactan and mycolic acids in the mycolylarabinogalactan-peptidoglycan (mAGP) complex in the cell envelope of mycobacteria. In the present study, four LysB enzymes were produced recombinantly and characterized with respect to their enzymatic and antibacterial activities. Examination of the kinetic parameters for the hydrolysis of para-nitrophenyl ester substrates, shows LysB-His6 enzymes to be active against a range of substrates (C4–C16), with a catalytic preference towards p-nitrophenyl laurate (C12). With p-nitrophenyl butyrate as substrate, LysB-His6 enzymes showed highest activity at 37 °C. LysB-His6 enzymes also hydrolyzed different Tween substrates with highest activity against Tween 20 and 80. Metal ions like Ca2+ and Mn2+ enhanced the enzymatic activity of LysB-His6 enzymes, while transition metal ions like Zn2+ and Cu2+ inhibited the enzymatic activity. The mycolylarabinogalactan esterase activity of LysB-His6 enzymes against mAGP complex was confirmed by LC-MS. LysB-His6 enzymes showed marginal antibacterial activity when tested alone against Mycobacterium smegmatis, however a synergetic activity was noticed when combined with outer membrane permealizers. These results confirm that LysB enzymes are lipolytic enzymes with potential application as antimycobacterials.
1. Introduction
Mycobacterial infections remain among the deadliest and disabling diseases globally, the most common being tuberculosis (TB), a respiratory contagious disease caused by a direct contact with the acid-fast bacterium, Mycobacterium tuberculosis (Mtb). The increasing emergence of antibiotic resistance makes the disease a public health crisis [1]. In 2018, an estimated 10 million people developed TB, with half million rifampicin-resistant new cases (of which 78% had multi-drug resistant TB). Moreover, 1.2 million deaths were reported due to infection with Mtb in HIV-negative patients and 251,000 deaths among HIV-positive individuals [2]. Despite the existence of curative chemotherapy, the alarming rise of multidrug-resistant (MDR) and extremely drug-resistant (XDR) TB has increased the demand for developing novel and effective antimycobacterials [3,4].
The major characteristic feature of mycobacteria is the unique structure of their cell envelope with up to 60% lipid content compared to 5–10% for Gram-positive and -negative bacteria [5]. The cell envelope of Mtb consists of an inner peptidoglycan layer that is covalently linked to arabinogalactan, which in turn is esterified with mycolic acids (MAs). MAs are long chain (C60–C90), α-branched, β-hydroxy fatty acids containing cyclopropane rings, double bonds, and oxygenated groups according to the species and genera [6]. MAs are found in unbound and bound forms. The unbound form comprises glycolipid esters of trehalose forming trehalose dimycolate (TDM, also called the cord factor) that has a key role in mycobacterial pathogenesis [7,8]. The bound MA is linked via ester bond to the terminal pentaarabinofuranosyl units of arabinogalactan (AG), the polysaccharide that together with peptidoglycan forms the insoluble mycolylarabinogalactan–peptidoglycan (mAGP) skeleton [9,10,11,12,13,14]. Both forms of MAs participate in the two leaflets of the mycobacterial outer membrane, the mycomembrane [15,16], which imparts hydrophobicity and decreased permeability to nutrients and antimycobacterials making TB difficult to treat [17]. It is also essential for cell viability, hence the target of antituberculosis drugs [18].
Mycobacteriophages infecting the mycobacteria are able to disrupt the bacterial cell envelope from inside with the help of their lytic enzymes and release the viral progeny at the end of the lytic cycle. When applied externally mycobacteriophage-derived lysins can induce cell lysis and are thus considered as promising alternatives to conventional TB therapies [19]. Phage lysins have some advantages over the conventional antibiotics, for example they can counter drug-resistant pathogens [20,21,22], while presenting low risk of developing bacterial resistance [23], and can exert cell lysis even when the bacteria are in dormant state [23]. Mycobacteriophages produce two kinds of lytic enzymes, Lysin A (LysA) peptidoglycan hydrolases [24,25] and Lysin B (LysB) lipolytic enzymes [26,27]. LysB is a mycolylarabinogalactan esterase that cleaves the ester bond between arabinogalactan and MA, hence compromising the link between the mycobacteria cell wall and the outer membrane and completing the cell lysis [26,28]. LysB has also the ability to hydrolyze unbound mycolic acids (TDM) in different mycobacteria species including Mtb [26]. Gigante and coworkers evaluated the role of the mycobacteriophage M6 LysB (LysB-Ms6) in the cell lysis and reported that lysis can still occur after infection with the lysB deletion mutant (ΔlysB) of the mycobacteriophage, however the new Ms6 ΔlysB phage progeny is not efficiently released into the medium. Further cryo-electron microscopy and tomographic analyses at 150 min post-adsorption of the mutant phage revealed that M. smegmatis cells are incompletely lysed with the phage particles being trapped intracellularly, in contrast to complete cell lysis observed upon infection with the wild type mycobacteriophage M6 [29]. Also, a ΔlysB mutant of the mycobacteriophage Giles was shown to be defective in the normal timing, progression, and completion of host cell lysis [28].
To date, crystal structure of only one LysB, from mycobacteriophage D29, has been determined at 2.0Å resolution [28]. The structure revealed its similarity to α/β hydrolase superfamily of proteins including cutinases, lipases, and esterases. The enzymatic activity of LysB-D29 relies on the presence of the catalytic triad Ser82–Asp166–His240 with serine being part of the pentapeptide [G-X-S-X-G]. Recently, we have screened the Actinobacteriophage database (https://phagesdb.org/) with ≈1885 fully-sequenced mycobacteriophage genomes for the presence of putative LysB proteins through multiple sequence alignment and phylogenetic relationship with the amino acid sequence of the LysB-D29 [30]. The multiple sequence alignment showed Ser and Asp residues to be absolutely conserved in contrast to His which was weakly conserved through slight shifts either by one position towards the N-terminal end or the C-terminal end by 3 positions [28,30,31,32].
LysB candidates from 8 different mycobacteriophages (D29, Omega, Saal, Obama12, Enkosi, Echild, DS6A and Pumpkin) with homology ranging between 76 and 30% were selected for the present study, out of which only four LysB enzymes (LysB-D29, -Omega, -Saal and -Obama12) could be recombinantly produced in Escherichia coli. This paper presents the production and characterization of these LysB enzymes with respect to their enzymatic and antibacterial activities. The esterase activity using p-nitrophenyl esters, and lipase activity with Tween substrates were determined. The antibacterial activity of the enzymes was measured alone as well as in combination with anti-TB drugs (Rifampicin, Isoniazid, Pyrazinamide, and Ciprofloxacin), and with antimicrobial cationic polypeptides (Colistin and Protamine sulfate) against Mycobacterium smegmatis, a surrogate strain to the pathogenic Mtb.
2. Results
2.1. Cloning and Expression of LysB-His6 Genes
Cloning and transformation of the DNA sequences encoding LysB-His6 proteins (D29, Omega, Saal, Obama12, Enkosi, Echild, DS6A and Pumpkin) into E. coli BL21(DE3) resulted in successful expression of only 4 LysB proteins (D29, Omega, Obama12, and Saal) in soluble, active form in the recombinant bacteria grown in LB or auto-induction media. SDS-PAGE analysis of the enzymes revealed molecular weights consistent with predicted (https://web.expasy.org/protparam/) molecular masses of 29.3 kDa for LysB-D29, 31.5 kDa for LysB-Omega, 37.4 kDa for LysB-Saal, and 36.7 kDa for LysB-Obama12 with His6 tags (Supplementary Materials Figure S1). Expression of the other 4 LysB proteins (Enkosi, Echild, DS6A and Pumpkin) was not successful in spite of several attempts including different culture media, growth temperature post-induction, IPTG concentration, and even E. coli strains.
2.2. Activity of the LysB-His6 Enzymes Against p-Nitrophenyl Esters
Assay of the esterase activities of the recombinant enzymes against p-nitrophenyl esters, with different carbon chain lengths (C4–C18) showed that the enzymes exhibited varying trends in kinetic parameters against the different substrates (Table 1).

Table 1.
Kinetic parameters of Lysin B (LysB)-His6 enzymes against para-nitrophenyl substrates with different carbon chain lengths.
The highest activities (Vmax) for all the enzymes were obtained with the shortest ester pNPB (C4); the highest Vmax recorded was 122.3 U/mg for LysB-D29 followed by the LysB-Omega (111.8 U/mg), while LysB-Saal and LysB-Obama12 exhibited significantly lower Vmax values. There was a significant drop in Vmax values on increasing the acyl chain length beyond C4, however increase in reaction rate to varying degrees was observed with pNPL (C12) for LysB-Omega, LysB-Saal, and LysB-Obama12, followed again by a decrease in Vmax. The trend in Km values with the different substrates varied among the four enzymes, LysB-D29 exhibiting the lowest value with pNPL, LysB-Omega, and LysB-Obama12 with pNPP and LysB-Saal with pNPM. Interestingly, the highest catalytic efficiency (kcat/Km) for LysB-D29, LysB-Omega and LysB-Saal was observed with the C12 substrate, pNPL; the highest value being for LysB-Saal (4.31 µM–1. min–1). On the other hand, the highest kcat/Km for LysB-Obama12 was 2.72 µM–1. min–1 with the C4 substrate pNPB. For all the LysB-His6 enzymes no activity was detected with pNPS (C18).
2.3. Activity of the LysB-His6 Enzymes Against Tween Substrates
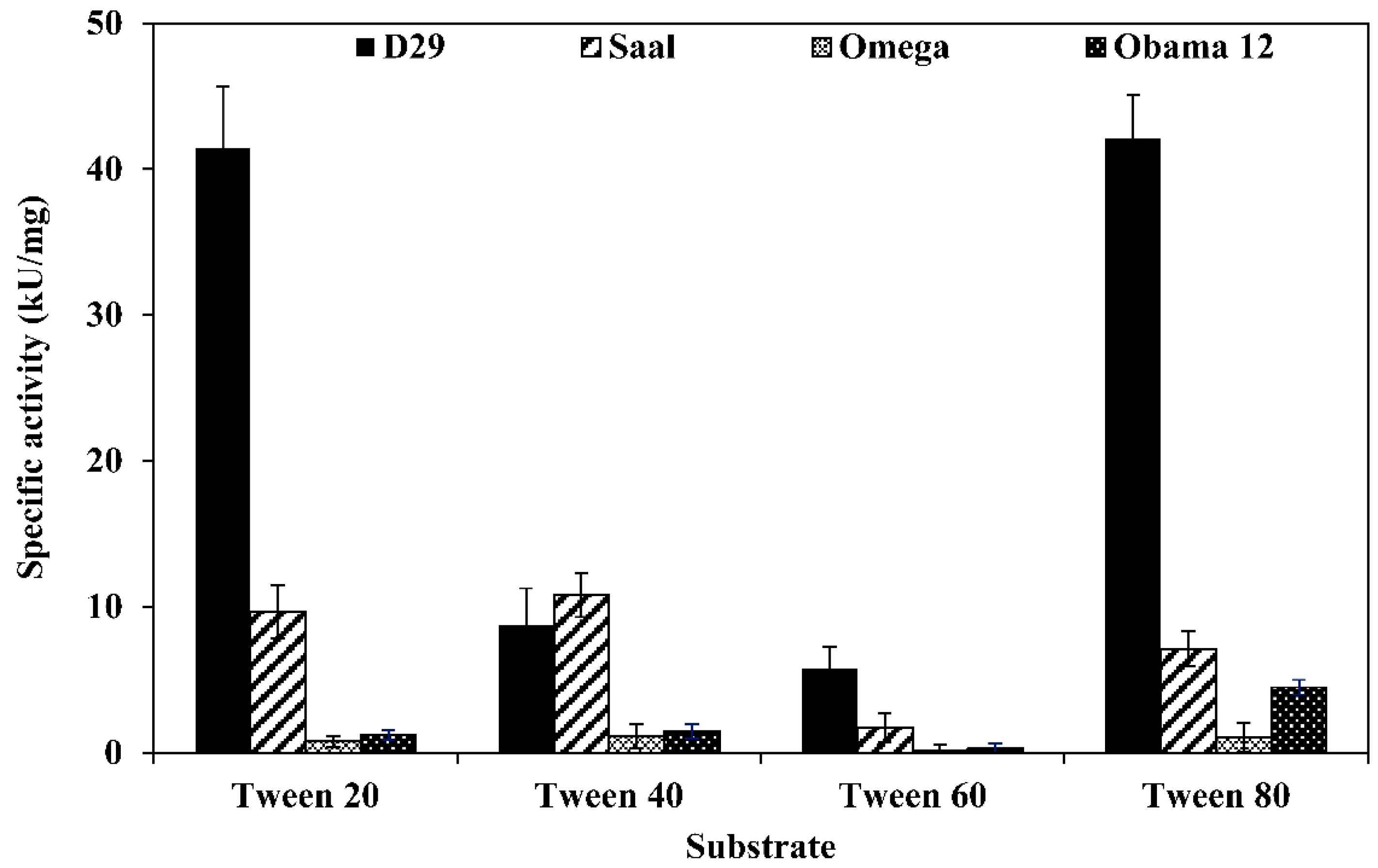
LysB-D29 was the most active enzyme against Tween substrates with prominent activities of 42.1 and 41.4 kU/mg against Tween 80 and 20, respectively. On the other hand, LysB-Saal exhibited highest activity against Tween 40 and 20 with activity values of 10.8 and 9.6 kU/mg, respectively, while LysB-Obama12 showed highest activity against Tween 80 (4.5 kU/mg) and lowest against Tween 60 (0.31 kU/mg). LysB-Omega exhibited relatively low activity against Tween substrates; the highest activity was 1.14 kU/mg against Tween 40 and 1.03 kU/mg against Tween 80 (Figure 1).
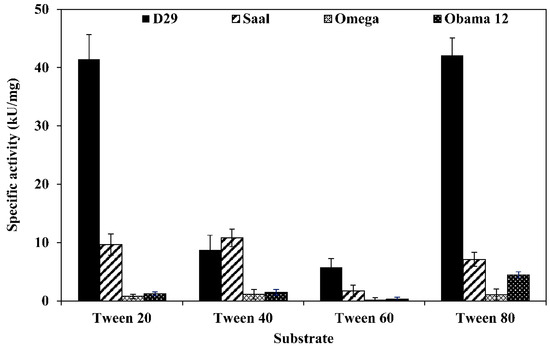
Figure 1.
Specific activities of LysB-His6 enzymes against different Tween substrates. Fifty microliters of LysB-His6 solution (containing 0.175 µg of the enzyme) were mixed with 250 µL of the assay buffer (0.33% Tween, 50 mM Tris-HCl, 33 mM CaCl2, pH 8) and incubated at 30 °C for 1 h. The absorbance at 400 nm was recorded at 1 min intervals. One unit of lipase activity is defined as the amount of LysB-His6 enzyme that increases the optical density OD400nm of 0.01/min under the assay conditions.
2.4. Data Correlation of Esterase and Lipase Activities
The correlation coefficients (−1 < r <1) for the esterase activity data sets against p-nitrophenyl esters and Tweens were calculated to determine if there is positive correlation (large values of one set are associated with large values of the other set) or negative correlation (large values of one set are associated with small values of the other set) or if there is no correlation at all between data sets. Table 2 shows the correlation coefficients displayed in the correlation matrix. Results for Tween 20, 60 and 80 hydrolysis exhibited strong correlation only with pNPB and pNPP, while Tween 40 showed strong correlation with all the tested pNP substrates.

Table 2.
Correlation matrix for the esterase and lipase activity data sets against p-nitrophenyl esters and Tweens, respectively.
2.5. LysB-His6 Activity Characteristics
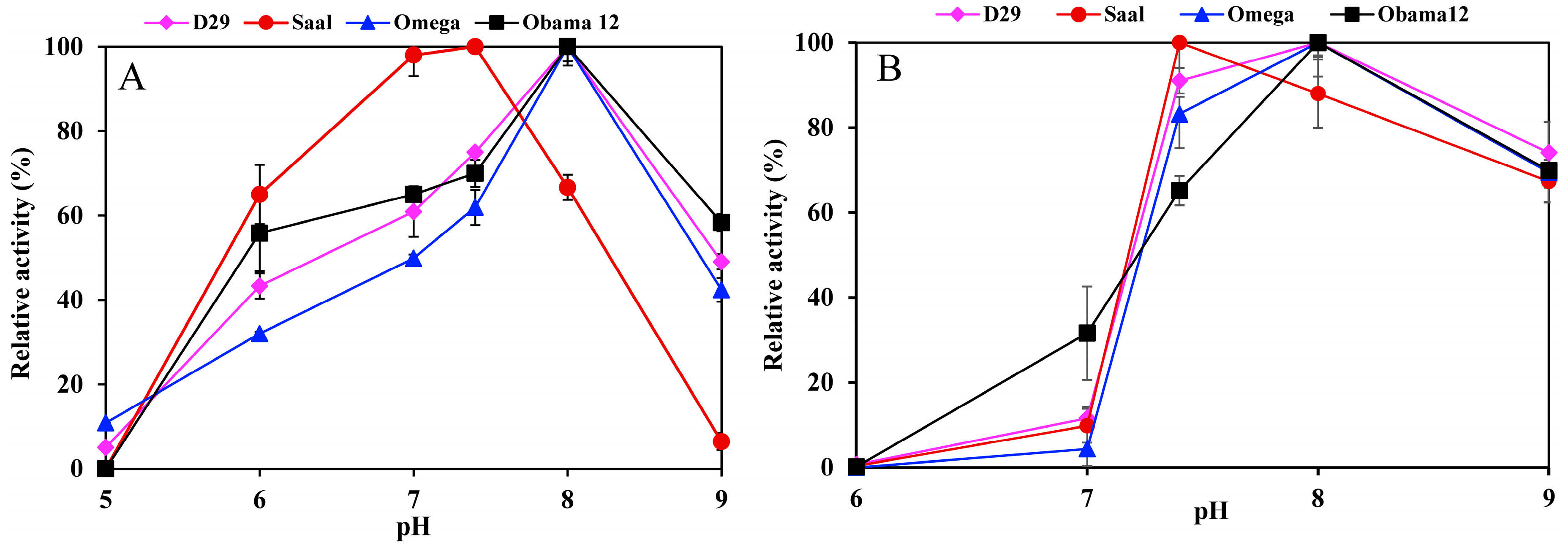
Determination of the esterase activity profiles of the LysB-His6 enzymes against p-nitrophenyl substrates in a pH range of 5–9 showed LysB-Omega, LysB-Saal and LysB-Obama12 to be optimally active at pH 8, and LysB-D29 at pH 7.4 (Figure 2A). Further increase in pH resulted in a sharp decrease in enzyme activities. The optimum pH for lipase activity against Tween substrates was 8 except for LysB-Saal that was optimally active at pH 7.4 (Figure 2B).
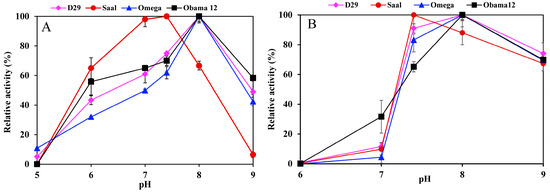
Figure 2.
pH activity profile of LysB-His6 enzymes in pH range 5–9 using 20 mM phosphate buffer (pH 5–7) and 20 mM Tris–HCl (pH 7.4–9) against: (A) 1 mM pNPB at 37 °C (the 100% activity corresponded to 10, 9.77, 0.0524 and 0.0145 U/mg in case of pNPB for LysB-D29, –Omega, –Saal and –Obama12, respectively), (B) 0.33% Tween 80 at 30 °C (the 100% activity corresponded to 42.1, 1.058, 7.11 and 4.49 kU/mg in case of Tween 80 for LysB-D29, –Omega, –Saal and –Obama12, respectively). Symbols: LysB-D29 (♦), LysB-Saal (●), LysB-Omega (▲) and LysB-Obama12 (■). The experimental details are described in Section 4.4.1.
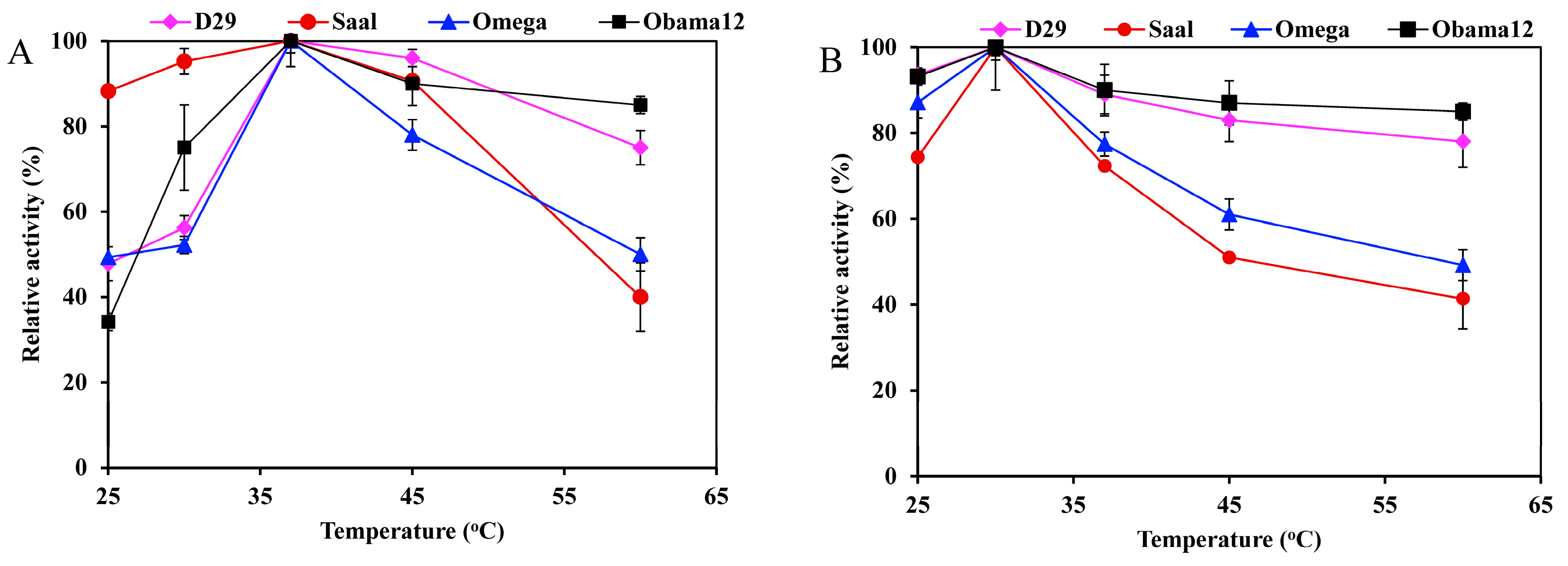
The optimum temperature for the esterase activity against pNPB was 37 °C for all the enzymes; LysB-Obama12 and LysB-D29 retained over 80% activity at temperatures up to 60 °C (Figure 3A). On the other hand, the optimum temperature for lipase activity against Tween 80 was 30 °C, and 50% of the activity was lost at 60 °C (Figure 3B).
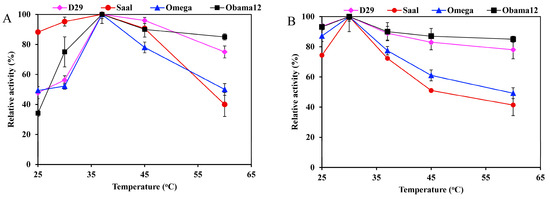
Figure 3.
Temperature activity profiles of LysB-His6 enzymes against: (A) 1 mM pNPB, and (B) 0.33% Tween 80. The lipase activity assay was performed in a temperature range of 25–60 °C. Symbols: LysB-D29 (♦), LysB-Saal (●), LysB-Omega (▲) and LysB-Obama12 (■). The 100% activity corresponded to 10, 9.77, 0.0524 and 0.015 U/mg in case of pNPB, and 42.1, 1.058, 7.11, and 4.49 kU/mg in case of Tween 80 for LysB-D29, -Omega, -Saal, and -Obama12, respectively. The experimental details are described in Section 4.4.2.
Effect of different additives including metal ions, EDTA and PMSF on the enzyme activities was also investigated. Mn2+ and Ca2+ ions stimulated the esterase activity of LysB-D29, -Omega and -Saal enzymes against pNPB but had an inhibitory effect on LysB-Obama12. Zn2+ and Cu2+ ions, on the other hand, decreased the activity of all the enzymes, and drastic inhibition of the activity was seen with phenylmethane sulfonyl fluoride (PMSF) (Table 3).

Table 3.
Effect of different additives including metal ions, EDTA and phenylmethane sulfonyl fluoride (PMSF) on the esterase and lipase activities of LysB-His6 enzymes against 1 mM pNPB and 0.33% Tween 80, respectively.
The metal ions (except for Mn2+) showed adverse effects on the lipase activity against Tween 80 of LysB-D29, Zn2+, and Cu2+ ions being the most inhibitory. In contrast, the lipase activity against Tween 80 of LysB-Omega, was boosted to 300%, 225%, and 212% of the original activity with Zn2+, Mn2+ and K+, respectively. Regarding LysB-Saal, Zn2+ totally abolished its activity, however EDTA enhanced the activity to 256% of original activity. The activity of LysB-Obama12 was stimulated by all the metal ions (except Zn2+ and Cu2+) as well as EDTA. The lipase activity of all LysB-His6 enzymes was inhibited by PMSF (Table 3).
2.6. Hydrolysis of mAGP
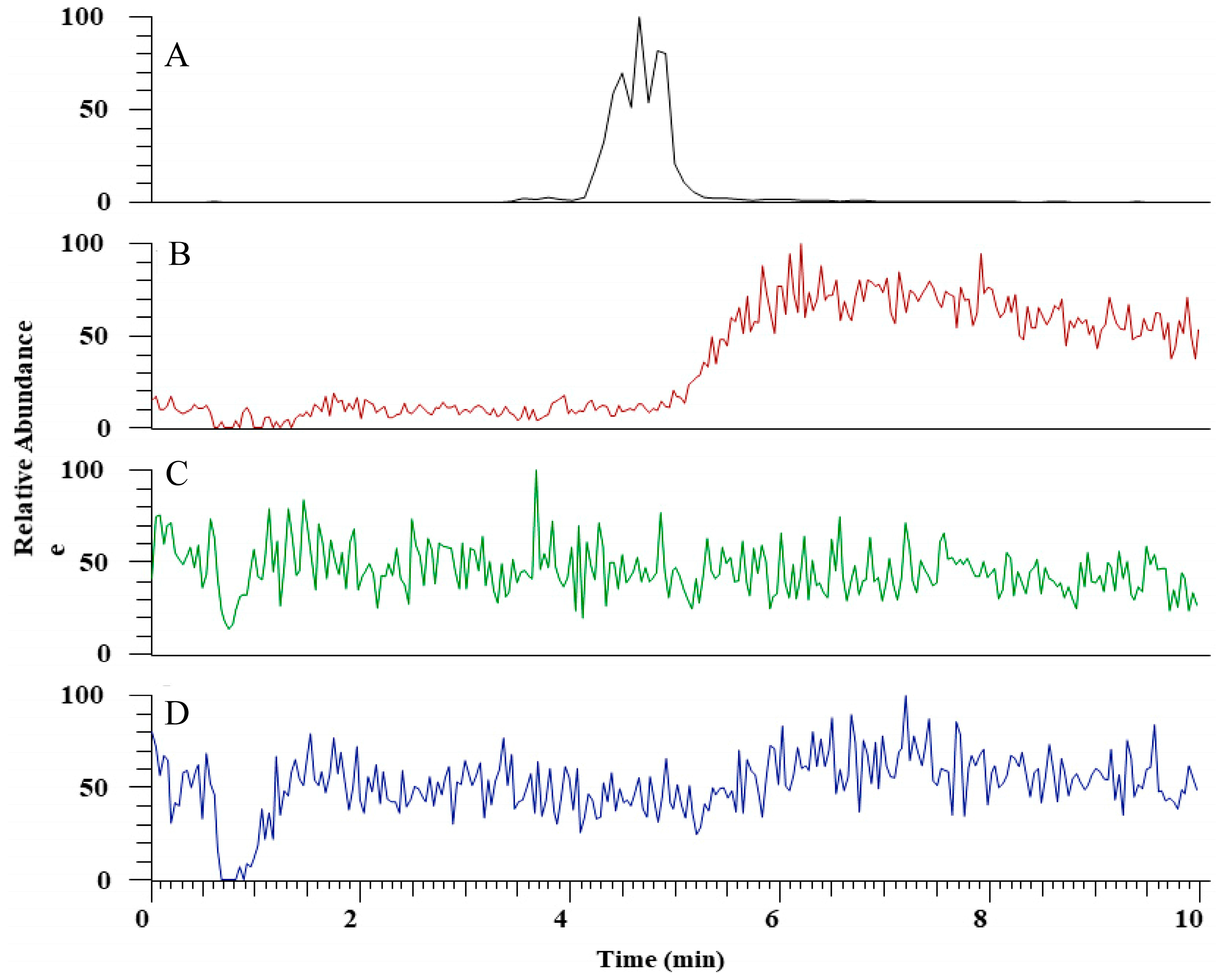
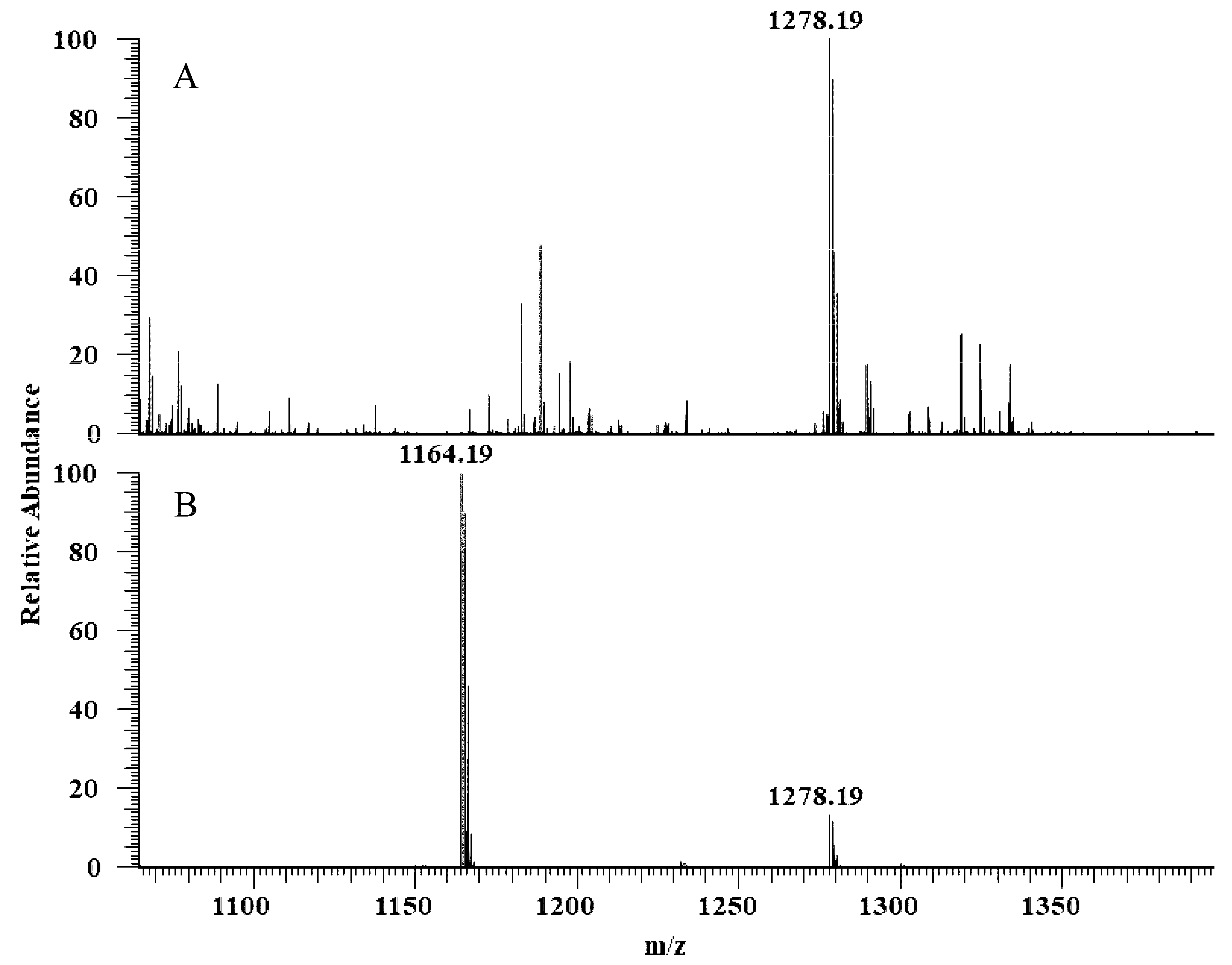
The hydrolytic activity of LysB-His6 enzymes against mAGP was evaluated using LC-MS in negative-ion mode. LysB attacks the ester bond between the arabinosyl units and mycolic acid, resulting in free mycolic acid. Figure 4 shows HPLC chromatogram for the different samples. mAGP treated with LysB-D29 reveals a peak corresponding to the liberated mycolic acid, while no peak was observed in the sample treated with Rhizopus oryzae lipase and the negative control. The observed m/z value of 1278.19 confirmed the presence of mycolic acid in LysB-D29 treated mAGP samples (Figure 5).
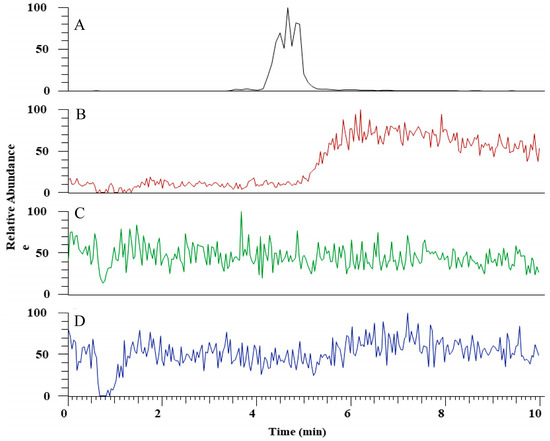
Figure 4.
HPLC chromatogram for (A) mycolic acid standard, (B) mycolylarabinogalactan–peptidoglycan (mAGP) treated with LysB-D29, (C) mAGP treated with Rhizopus oryzae lipase, and (D) mAGP treated with buffer as negative control. The experimental details are described in Section 4.6.2.
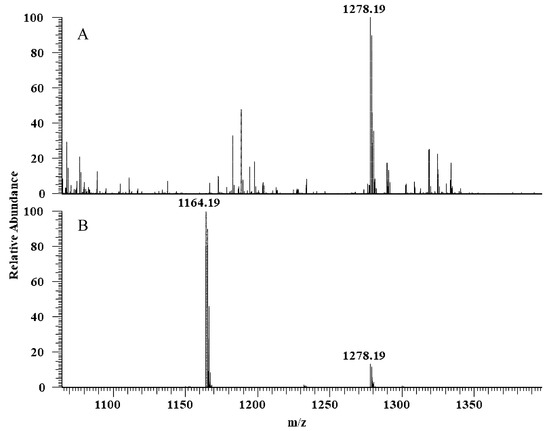
Figure 5.
Full scan negative ions mass spectra of (A) mAGP treated with LysB-D29 with m/z 1278 corresponds to keto-mycolic acid, and (B) mycolic acid standard with m/z 1164 and 1278 corresponding to mycolic acid and keto-mycolic acid, respectively.
2.7. Antibacterial Activity
LysB-His6 enzymes did not show any antibacterial activity against M. smegmatis as a function of MIC and MBC, when tested in different media. However, a marginal Log10 reduction of 1.1, 1.32, 1.44, and 1.36 for LysB-D29, -Omega, -Saal, and -Obama12, respectively could be detected with 100 µg/mL of LysB-His6 in Cation Adjusted Mueller-Hinton Broth (CAMHB) (Table 4). Moreover, combining LysB-His6 enzymes with different anti-TB drugs did not exert synergistic effect. On the other hand, the inhibitory effect of LysB-His6 enzymes was enhanced by addition of half MIC values of Colistin and Protamine sulfate, respectively; the highest reduction being obtained with Obama12 (Table 4 and Supplementary Materials Table S1). Incubation of M. smegmatis cells with Colistin and Protamine sulfate at half MIC values did not result in a decrease in the Log10 values.

Table 4.
Log10 reduction of M. smegmatis after treatment with 100 µg/mL of LysB-His6 enzymes alone and in combination with Colistin (1 µg/mL) and Protamine sulfate (10 µg/mL), respectively in Cation Adjusted Mueller–Hinton Broth (CAMHB), NA: not available.
2.8. Thermostability of LysB-His6 Enzymes
Thermostability of the enzymes measured as the melting temperature (Tm) using Nano DSF, showed LysB-Omega to be the most stable enzyme with a Tm value of 57.7 °C followed by LysB-D29 (Tm of 54.7 °C). On the other hand, LysB-Obama12 and -Saal showed lower Tm values of 47.9 °C and 45.7 °C, respectively (Table 5).

Table 5.
Nano-differential scanning fluorimetry (DSF) melting temperature for LysB-His6 enzymes.
3. Discussion
Despite the availability of ≈1885 mycobacteriophage complete genome sequences available on the Actinobacteriophage database (https://phagesdb.org/) (accessed April 2020), the knowledge of their lytic enzymes is fairly limited. Concerning LysB enzymes, crystal structure of only LysB-D29 is available so far and some studies on the antimycobacterial properties of a few other LysB candidates have been reported [26,27,28,32,33]. The present study was started by selection of 8 LysB candidates which exhibited 30–76% sequence analogy with LysB-D29. Only 4 (including LysB-D29) of the 8 LysB enzymes could be expressed and purified to homogeneity. Payne and coworkers have earlier observed high variations in expression and solubility levels of LysB proteins and were able to obtain good expression levels of only LysB-D29 among trials with expression of several LysB enzymes [28].
LysB enzymes hydrolyze the ester bonds between the mycolic acid and arabinogalactan as well as the disaccharide trehalose. The three LysB enzymes characterized so far have exhibited structural relatedness and activity patterns similar to the lipolytic enzymes belonging to the α/β hydrolase family including esterases, lipases, and cutinases [32]. While esterases catalyze the hydrolysis of glycerol esters with short acyl chains and lipases act on long chain triacylglycerol substrates [34], cutinases act on wide range of substrates from soluble p-nitrophenyl esters to insoluble long-chain triglyceride esters besides its natural substrate cutin [35,36]. LysB enzymes are considered to be a link between lipases and cutinases as they have the ability to hydrolyze soluble esters and emulsified triglycerides [27]. As reported earlier, amino acid sequence analysis revealed the cutinase motif to be conserved among the majority of LysB enzymes [30,31]. Our recent study has further reported several common structural features of Lys B enzymes with the α/β hydrolase members [30].
The lipolytic activities i.e., esterase and lipase activities of LysB-His6 enzymes were evaluated against p-nitrophenyl esters and Tween substrates, respectively, both with varying carbon chain lengths. In general, the activity levels of the different enzymes varied significantly with respect to the different substrates. Measurement of the esterase activity against p-nitrophenyl esters (C4–C16) showed 3 of the enzymes to exhibit highest catalytic efficiency towards C12 (pNPL) (Table 1), which is in accordance with the earlier report on LysB-Ms6 having the highest catalytic efficiency of 1.14 µM–1. min–1 against pNPL [27]. The decrease in the esterase activity of LysB-D29 and other LysB enzymes with increasing chain lengths of the p-nitrophenyl ester substrates is also in agreement with the previous report on LysB-D29 showing the highest activity against the C4 substrate pNPB (0.72 U/mg) [28]. It was further reported that the Km values of LysB-Ms6 and -Bxz2 decreased with the increase in carbon chain length of the pNP esters [33]. LysB-Bxz2 showed 10-fold higher esterase activity than Ms6 but with lower catalytic efficiency [33]. This might be attributed to the different conformations of the active sites of LysB proteins (tunnel, shallow bowl, deep, superficial funnels and deep buried cave) influencing the binding of different substrates as suggested in our previous study [30]. We noted that LysB enzymes with longer hydrophobic acyl binding site, e.g., LysB-D29 (tunnel) and LysB-Omega (shallow bowl) have higher Vmax values against long fatty substrates than the LysB enzymes with shorter ones, e.g., LysB-Saal (deep funnel) and LysB-Obama12 (inverted tunnel).
Tunnel-shaped active sites of LysB-D29 and -Obama12 confer long, open hydrophobic cervix that accept long chain fatty substrates (Supplementary Materials Figure S3a). However, the inverted positioning of pNP ligands upon docking to LysB-Obama12 active site (Supplementary Materials Figure S3b) directs the long hydrophobic ligand tail towards the narrow hydrophilic mouth of the tunnel where no sufficient hydrophobic acyl binding site is provided. This might explain the highest activity of LysB-D29 on pNPP (C16) among the tested LysB-His6 enzymes in contrast to LysB-Obama12 with the lowest activity against the same substrate (Table 1). Furthermore, the shallow bowl conformation, was shown earlier to accept long substrates up to pNPS with C18 acyl chain length [37]; it provides a long hydrophobic superficial groove for fitting the long hydrophobic tail of pNP ligands, which might account for LysB-Omega achieving the second highest specific activity on pNPP (Supplementary Materials Figure S3c). On the other hand, LysB-Saal has a deep funnel conformation, which was reported earlier to suit bulky ligands with acyl chain length up to C14, with a narrow opposing hydrophobic wall wrapping the fatty pNP tail (Supplementary Materials Figure S3d). The narrow and short acyl binding site of LysB-Saal might explain its diminished activity against fatty substrates with long acyl side chain and its highest affinity towards pNPM (Table 1) [30]. In contrast, shorter substrates (up to C8) are better accepted by shorter and shallower funnel conformation e.g., LysB-Bxz2 [30,31,37].
Tweens 20, 40, 60, and 80 are esters of lauric (C12), palmitic (C16), stearic (C18), and oleic (C18) acids. Tween 20 and 80 turned out to be the best substrates for most of the enzymes, probably due to the relatively lower melting point of the corresponding fatty acids, especially for oleic acid. However, LysB-Saal showed higher activity against Tween 40 (10.8 kU/mg) (Figure 1). The higher enzymatic activity recorded against Tweens compared with p-nitrophenyl substrates may be attributed to the high emulsifying properties of the detergents forming stable oil-in-water emulsions, allowing for greater interactions between the substrate and the enzyme [38,39]. It is worthy to note that the mycobacterial cutinases as well as phospholipase A isolated from M. smegmatis show the same patterns of activity correlation between Tweens and pNP substrates [40,41,42,43].
LysB-His6 enzymes were shown to be active over a wide range of temperature (with optimum of 37 °C against pNPB and 30 °C against Tween 80) and in the pH range of 7.4–8, which seems to be similar to the activity pattern of different lipolytic enzymes reported earlier [44]. The melting temperatures of LysB-His6 enzymes ranged between 45–60 °C which correlates well with both lipase and esterase activities of the enzymes; increasing the reaction temperature above 60 °C, resulted in drop of the enzymatic activity of more than 60% for some of the enzymes. As reported earlier for LysB-Ms6 [27], the metal ions Mn2+, Ca2+, and Mg2+ generally showed a stimulating effect on the esterase and lipase activities of most LysB-His6 enzymes, while the transition metal ions, Zn2+ and Cu2+ had an inhibitory effect (Table 3). Activation by Ca2+ and inhibition by Zn2+ and Cu2+ have also been reported for esterase/lipase from Geobacillus thermoleovorans [45]. The effect of Ca2+ is related to the structural stabilization and activation of lipases [46,47], while Zn2+ and Cu2+ ions lead to activity loss due to the metal ion catalyzed oxidation of the sensitive amino acids such as histidine that is an important part of the catalytic triad in the α/β hydrolases [48,49]. EDTA had a slight inhibitory effect on the esterase activity that may suggest chelation of some stimulatory metal ions present in the samples [50]. On the other hand, the reason underlying the significant increase in the lipase activity of most of the enzymes with EDTA is not entirely clear but may be related either to increased flexibility of the enzyme or complexation of potential inhibitory compounds in the reaction. The activity inhibition by PMSF is attributed to its interaction with the conserved nucleophilic Ser residue in the catalytic triad, which is typical of esterases, lipases as well as proteases, hence confirming that LysB enzymes belong to the group of serine hydrolases [27,51].
The hydrolytic activity of LysB-His6 enzymes against the isolated mAGP substrate is also in accordance with the previously reported mycolylarabinogalactan esterase activity of other LysB homologous enzymes Ms6 [26], Bxz2 [33], D29 [28], and Bxb1 [52]. This confirms that the LysB enzymes are capable of hydrolyzing the ester bonds not only from inside the cells when viral progeny is released but also when applied from outside.
Earlier investigations on LysB-Ms6, -Bxz2 [33], LysA-D29 [53], BTCU-1 LysA and B [54], LysA-Ms6 [55], and LysA-TM4 [56] have revealed no significant antibacterial activity against M. smegmatis except for a combination of BTCU-1 LysA and BTCU-1 LysB that showed higher antibacterial activity (minimum bactericidal concentration (MBC) of 20–40 µg/mL) than LysA alone (MBC > 80 µg/mL) against M. smegmatis. Although the mechanism is not entirely clear, the synergistic effect is most likely due to the ability of the enzymes to penetrate through the peptidoglycan layer and disrupt the integrity of the mycolic acid linkage to the arabinogalactan-peptidoglycan layer.
LysB-His6 enzymes in this study did not exhibit significant antibacterial activity either alone or in combination with anti-TB drugs. The activity was determined without Tween 80, which has been suggested to be required for removal of M. smegmatis cell clumps and aggregates due to surface hydrophobicity and also for promoting the antibacterial activity of LysB enzymes [33,57,58,59,60]. There is also a possibility that the antibacterial activity observed in the presence of Tween 80 could be due to the action of the oleic acid released by hydrolysis of the surfactant by LysB [26,33]. The differences in the antibacterial activity among the LysB-His6 enzymes tested in this study are very subtle when compared with the high diversity of their catalytic profiles (in terms of esterase and lipase activities), which is clearly related to the diversity of active site conformations among LysB enzymes.
While LysB-His6 enzymes did not exert notable inhibitory effect against M. smegmatis cells, half MIC values of the polycationic peptides colistin and protamine sulfate enhanced the Log10 reduction of M. smegmatis treated with 100 µg/mL LysB-His6 enzymes (Table 5). Colistin and Protamine sulfate are cyclic cationic polypeptides with a hydrophobic tail, which destabilize the bacterial outer membrane by quenching the divalent cations (Ca2+ and Mg2+) from the lipopolysaccharides and phospholipids creating pores and subsequently cell death [61]. The antibacterial activity of cationic polypeptides extends beyond Gram-negative bacteria to different species of Mycobacteria including M. avium [62], M. aurum, M. vaccae [63], M. xenopi [64], M. smegmatis [65], and Mtb [60,65]. Due to the permeabilizing effect of Colistin on Mycobacteria cell membrane, it has been used in combination with anti-TB drugs to facilitate their uptake [66,67].
Even with the combination of LysB-His6 and polycationic peptides, we could not detect MIC/MBC values. For achieving significant antimycobacterial activity, a combination of antimicrobial peptides (outer membrane permeabilizer), LysB (mAGP hydrolase) and LysA (peptidoglycan hydrolase) should be considered. Earlier reports have demonstrated that fusions of antimicrobial peptide with either LysA or LysB did not exert antimycobacterial activity, however mixing the two fusion proteins together resulted in enhanced antimycobacterial activity even for intracellular mycobacteria in lung macrophages cell line [68].
4. Materials and Methods
4.1. Bacterial Strains, Vectors, Media, and Reagents
The genes encoding LysB enzymes from different mycobacteriophages (D29, Omega, Obama12, Enkosi, Echild, DS6A, Pumpkin and Saal) were codon optimized, fused with a hexa-histidine tag (His6) at the C-terminus (Supplementary Materials Figure S2) and ordered as gBlock gene fragments from Integrated DNA Technologies (IDT, Leuven, Belgium). Escherichia coli BL21(DE3) expression host and pET22b(+) expression vector were purchased from Novagen (Madison, WI, USA). Luria–Bertani (LB) medium was obtained from Saveen & Werner AB (Limhamn, Sweden). Isopropyl β-D-1-thiogalactopyranoside (IPTG), EcoRI, NdeI and T4 DNA ligase were products of Thermo Fisher Scientific (Waltham, MA). Ampicillin, p-nitrophenyl butyrate (pNPB; C4), p-nitrophenyl octanoate (pNPO; C8), p-nitrophenyl laurate (pNPL; C12), p-nitrophenyl myristate (pNPM; C14), p-nitrophenyl palmitate (pNPP; C16) and p-nitrophenyl stearate (pNPS; C18) were procured from Sigma-Aldrich (St Louis, MO, USA).
M. smegmatis mc2 155 (ATCC 700084) was procured from American Type Culture Collection (ATCC) (Manassas, VA, USA) and grown in Middlebrook 7H9 broth medium (Difco, Detroit, MI, USA) supplemented with 0.2% glycerol, 0.05% Tween 80 and ADC (Albumin-Dextrose Complex) at 37 °C, 200 rpm. Glycerol stocks of M. smegmatis were prepared and stored at −80 °C. The bacteria were also grown on Middlebrook 7H10 agar medium supplemented with OADC (Oleic acid-Albumin-Dextrose Complex), 0.5% glycerol and 0.05% Tween 80.
4.2. Cloning and Expression of LysB-His6 Genes
Cloning and expression of LysB-His6 gBlocks were performed as reported earlier [30]. This involved cloning in EcoRI and NdeI restriction sites of pET22b(+) expression vector, ligation with T4 DNA ligase, and transformation of the ligation mixtures into E. coli BL21(DE3) expression host. The bacteria were grown overnight at 37 °C on LB agar plates supplemented with 100 µg/mL ampicillin. The plasmids (pET22b(+)-LysB-His6) extracted from the transformant colonies were sequenced (GATC Biotech AB, Solna, Sweden), and those with correct sequences were used for protein expression in E. coli BL21(DE3), which was grown overnight at 37 °C, 200 rpm in LB medium supplemented with the antibiotic as above. The recombinant cells were suspended in 50% glycerol, distributed in xx μL aliquots and stored at −80 °C.
Small scale production of the recombinant LysB-His6 enzymes was done in LB medium supplemented with 100 μg/mL ampicillin. The respective glycerol stocks were inoculated into 10 mL of the medium in 50 mL sterile falcon tubes and grown overnight at 37 °C and 200 rpm, and 5 mL of the culture were used to inoculate 50 mL medium in 250 mL Erlenmeyer flask and grown under the same conditions. When the optical density (OD600nm) reached 0.5−0.6, the cells were induced for protein expression using IPTG at a final concentration of 1mM and reducing the cultivation temperature to 30 °C. After 4 h, the cells were harvested by centrifugation at 3900× g and 4 °C for 15 min (Sigma 3−16PK, Sigma Laborzentrifugen GmbH, Osterode am Harz, Germany).
Protein production at a larger scale was performed in auto-induction medium (1% tryptone, 0.5%, yeast extract, 25 mM Na2HPO4, 25 mM KH2PO4, 25 mM (NH4)2SO4, 2 mM MgSO4, 0.05% glucose and 0.2% α-lactose) supplemented with 100 µg/mL ampicillin. One liter of the auto-induction medium in 5 L Erlenmeyer flask was inoculated with 15 mL of recombinant cell culture grown in LB medium as above, incubated at 37 °C and 180 rpm for 4 h, and then at 30 °C for 24 h prior to harvesting the cells by centrifugation at 6000× g and 4 °C for 20 min (Sorvall Lynx 4000 centrifuge, Thermo Scientific, Waltham, MA, USA).
4.3. Purification of LysB-His6 Enzymes
The cell pellet obtained above from 1 L auto-induction medium was suspended in 50 mM Tris-HCl buffer, pH 8 supplemented with 50 mM NaCl and a cocktail of protease inhibitors (Calbiochem) and then sonicated on ice (5 × 60 s, cycle 0.5) using UP400S sonicator (Dr. Hielscher GmbH, Stahnsdorf, Teltow, Germany). After removal of the cell debris by centrifugation (18,500× g, 30 min, 4 °C, Sorvall RC5C, Sorvall Instruments, Dupont, Wilmington, DE, USA), the clarified lysate was subjected to immobilized metal ion affinity chromatography (IMAC) for purification of the LysB-His6 enzymes using 5 mL HisTrap FFTM nickel column (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). Briefly, 15 mL of the clarified lysate was applied on the column pre-equilibrated with the binding buffer (50 mM Tris-HCl, 0.3 M NaCl, 20 mM imidazole, pH 8), the unbound proteins were washed out using a wash buffer (50 mM Tris-HCl, 0.3 M NaCl, 40 mM imidazole, pH 8), and finally the bound proteins were eluted with the elution buffer (50 mM Tris-HCl, 0.3 M NaCl, 0.5 M imidazole, pH 8). The purified proteins were dialyzed against dialysis buffer (50 mM Tris-HCl, 50 mM NaCl, 50% glycerol, pH 8), analyzed by SDS-PAGE and quantified with bicinchoninic acid (BCA) reagent using bovine serum albumin (BSA) as a standard (Sigma-Aldrich, St Louis, MO, USA), prior to storage at −20 °C.
4.4. Activity Assays of LysB-His6 Enzymes
4.4.1. Activity Against p-Nitrophenyl Esters
The esterase activity of the purified LysB-His6 enzymes was measured against p-nitrophenyl esters with variable carbon chain length as substrates. Stock solutions (250 mM) of pNP-esters were prepared in dichloromethane and stored at −20 °C. For the assay, 180 µL of pNP-esters (final concentration of 1 mM dissolved in 20 mM Tris-HCl, 100 mM NaCl, 0.1% Triton X-100, pH 8) was mixed with 20 µL LysB-His6 enzyme solution (containing 0.175 µg enzyme). The reaction mixture was incubated at 37 °C and the release of p-nitrophenol was followed at 410 nm with 1 min intervals for 30 min (MultiskanTM GO Microplate Spectrophotometer, Thermo Scientific, Vantaa, Finland). Each assay was performed in three independent replicates, using the corresponding buffer as negative control unless otherwise stated.
The activity was calculated and quantified with the following equation using calibration curve for p-nitrophenol.
where ε is the extinction coefficient of p-nitrophenol which was determined under the assay conditions and at different pH values. One unit (U) of enzyme activity corresponds to the amount of enzyme liberating 1 µmol of p-nitrophenol per min under the assay conditions.
Optimum conditions for the enzyme activity were determined using 1 mM pNPB as substrate. To determine the optimum temperature, the assay was performed in a temperature range of 25–60 °C, while optimum pH was determined in a pH range of 5–9 using 20 mM sodium phosphate buffer (pH 5–7) and 20 mM Tris-HCl (pH 7.4–9), respectively.
The substrate specificity of the LysB-His6 enzymes was measured using pNP esters with variable carbon chain length ranging from C4 to C18. The kinetic parameters were determined by running the enzymatic activity assay using the substrates in a concentration range of 10 µM–4 mM and calculated according to the Michaelis-Menten model.
The effect of metal ions (5 mM Ca2+, Mn2+, Cu2+, Mg2+, Zn2+, K+, Na+), 5 mM EDTA and 10 mM phenylmethane sulfonyl fluoride (PMSF), respectively, was determined by incubation with LysB-His6 enzymes at room temperature for 2 h, and then determining the residual esterase activity using pNPB as substrate under the standard assay conditions at pH8 and 37 °C. The relative activity (%) was calculated in relation to the activity of the enzymes incubated under the same conditions but without any metal ions.
4.4.2. Activity Against Tween Substrates
The lipase activity of LysB-His6 enzymes was determined against Tween 20, 40, 60, and 80 as substrates [32], which are esters of lauric, palmitic, stearic, and oleic acids, respectively. The assay is based on the cleavage of Tween into alcohol and the corresponding fatty acids, which in the presence of calcium ions form insoluble calcium salts of fatty acids that can be measured turbidimetrically at 400 nm. In a microtiter plate, 250 µL of the assay buffer (composed of 0.33% Tween dissolved in 50 mM Tris-HCl, 33 mM CaCl2, pH 8) was mixed with 50 µL of LysB-His6 enzymes (final amount of 0.175 µg in the reaction mixture) and incubated at 30 °C for 1 h. The absorbance at 400 nm was recorded at 1 min intervals (MultiskanTM GO Microplate Spectrophotometer). One unit of lipase activity is defined as the amount of LysB-His6 enzyme that increases the optical density OD400nm of 0.01/min under the assay conditions.
Optimum temperature, pH, as well as the effect of metal ions and enzyme inhibitors on the lipase activity were also determined as described above using Tween 80 as substrate.
A correlation matrix between the esterase activities against different pNP substrates and the lipase activity against different Tweens was performed using IBM SPSS Statistics 25 (IBM, Armonk, NY, USA).
4.5. Determination of Protein Stability
Stability of LysB-His6 enzymes was determined using Nano-Differential Scanning Fluorimetry (DSF) on a Prometheus NT.48 instrument (NanoTemper Technologies, GmbH, Germany). Ten microliter samples of 2 μM LysB-His6 enzymes in 50 mM Tris-HCl buffer (pH 8) were loaded into UV capillaries (NanoTemper Technologies), and subjected to increasing temperature from 20 to 95 °C with a temperature gradient of 1 °C/min. Protein unfolding was detected by tracking changes in the intrinsic tryptophan/tyrosine fluorescence (350 nm/330 nm fluorescence ratio) as a function of temperature increase. Data was analyzed using the ThermControl software V 2.0.4 (NanoTemper Technologies) and melting temperature (Tm) was calculated.
4.6. Hydrolysis of M. smegmatis mAGP
4.6.1. Preparation of Mycolylarabinogalactan-Peptidoglycan (mAGP) Substrate
mAGP was extracted from M. smegmatis according to the method described earlier [33] with modification. Briefly, M. smegmatis cells were grown overnight in 1L of LB medium supplemented with 0.05% Tween 80 at 37 °C and 200 rpm. The cells were harvested by centrifugation at 3900× g and 4 °C for 20 min and washed three times with phosphate buffered saline (PBS) pH 7.4. The extractable lipids of the cell pellet were extracted with a 2:1 mixture of chloroform/methanol (15 mL per g cell pellet) at 55 °C overnight, followed by 3 successive washing steps with MilliQ quality water. Subsequently, the pellet was resuspended in 30 mL of 2% SDS and stirred gently overnight at room temperature and then collected by centrifugation (3900× g, 4 °C, 20 min), resuspended again in 30 mL of 2% SDS and refluxed for 1 h. SDS was removed through five successive washings (with MilliQ quality water)/centrifugation steps. The pellet was then washed with 30 mL of 80% acetone followed by 30 mL of diethyl ether, 30 mL of 80% acetone, and twice with water at the end. The resulting mAGP was lyophilized and stored at 4 °C for further experiments.
4.6.2. Treatment of mAGP with LysB-His6 Enzymes
The purified mAGP (1 mg) was suspended in 1 mL PBS pH 7.4 containing 0.2% Triton X-100 through sonication (2 × 60 s, cycle 0.5), followed by addition of LysB-His6 (100 µg), and incubation for 24 h at room temperature with shaking at 300 rpm. The liberated mycolic acid was extracted with 3 mL of diethyl ether and washed with water. The organic phase (upper layer) was separated from the aqueous phase by centrifugation (3900× g, 4 °C, 5 min), collected and air dried. After drying, the samples were dissolved in 1 mL of organic solvent (80% n-propanol, 20% hexane) and analyzed by liquid chromatography–mass spectrometry (LC-MS) using an Accela 600 high-pressure liquid chromatograph (HPLC) coupled to an LTQ-Orbitrap hybrid mass spectrometer (Thermo Scientific). The LC was equipped with a C18 reversed-phase column (Phenomenex Kinetex XB-C18 50 × 2.1 mm, 1.7 µm particle size) and elution was performed with a mobile phase comprising 40% solvent A (100% methanol) and 60% solvent B (80% n-propanol and 20% hexane) at a flow rate of 0.2 mL/min over 10 min. The MS was operated in electrospray ionization mode with detection of negatively charged ions in the m/z range 500–1500 Da at a resolution of ∼100,000. Negative control (buffer replacing the LysB-His6 enzymes) and a control using Rhizopus oryzae lipase were included in the assay.
4.7. Antibacterial Activity of LysB-His6 Enzymes Against M. smegmatis
4.7.1. In Vitro Antibacterial Activity Testing Using Different Culture Media
M. smegmatis was cultivated in 7H9 medium supplied with ADC, 0.2% glycerol and 0.05% Tween 80 at 37 °C, 200 rpm until mid-log phase. The cells were incubated with different concentrations (20–200 µg/mL) of LysB-His6 enzymes and Rhizopus oryzae lipase, respectively, in a microtiter plate in a final reaction volume of 200 µL; 7H9 medium without any enzyme served as negative control. The microtiter plates were incubated at 37 °C for 24 h and examined for growth. For viable counting plating assay, 100 µL aliquot from each well was diluted in PBS containing 0.05% Tween 80 and plated on 7H10 agar supplemented with OADC (Oleic acid-Albumin-Dextrose Complex), 0.5% glycerol and 0.05% Tween 80. The plates were incubated at 37 °C for 48 h followed by colony counting. Since 7H9 medium contains mono- and divalent cations (Na+, K+, Mg2+, Zn2+, and Ca2+), we noticed formation of a precipitate upon addition of LysB-His6 enzymes which might be due to formation of oleic acid salts upon hydrolysis of Tween 80 by LysB-His6 enzymes. Also, adding LysB-His6 enzymes to Tween 80 free 7H9 medium resulted in a faint turbidity, so we decided to exclude 7H9 medium when testing the antibacterial activity of LysB-His6 enzymes. Instead, Mueller-Hinton broth (MHB), Cation Adjusted Mueller-Hinton Broth (CAMHB), and LB culture media, respectively, were tested.
4.7.2. In Vitro Testing of the Combined Effect of LysB-His6 Enzymes with Anti-TB Drugs
The following antibiotics were tested: Rifampicin (RIF), Isoniazid (INH), Ciprofloxacin (CIP), and Pyrazinamide (PYZ). Firstly, the minimum inhibitory concentrations (MIC) and the minimum bactericidal concentrations (MBC) of those antibiotics were determined. For MIC, antibiotics stock solutions were serially diluted (2-fold) in MHB and mixed with M. smegmatis (1 × 106 CFU/mL) in a total volume of 200 µL, the reaction mixture was incubated at 37 °C for 24 h and examined for bacterial growth. MIC was defined as the lowest antibiotic concentration of the antibiotic that inhibited the growth of M. smegmatis (no visible growth at the end of the experiment).
MBC was evaluated by plating 100 μL suspension from the wells with no visible growth on 7H10 agar medium and incubating at 37 °C for 48 h and checking for growth. MBC was defined as the lowest antibiotic concentration that showed no growth. Positive controls (100 μL of bacterial culture + 100 μL MHB) and negative controls (MHB mixed with antibiotics) were included. The experiment was performed in triplicates independently. To test the combined effect of LysB-His6 enzymes with anti-TB drugs, modified checkerboard technique was applied [69]. Briefly, in a microtiter plate, antibiotics (half the MIC values) were diluted two-fold vertically (columns) in MHB, while LysB-His6 enzymes were diluted two-fold horizontally (rows). M. smegmatis cells were added to the wells to a final volume of 200 µL to give (1 × 106 CFU/mL). The plates were incubated at 37 °C for 24 h and examined for growth. The combination effect is synergistic when the effect of the combined drugs is significantly greater than the effects of each drug individually, antagonistic when the combination effect is lower than the effect of each drug individually or additive when the effect is equal to the sum of the separate effect of each drug [70].
4.7.3. In Vitro Testing the Combined Effect of LysB-His6 Enzymes with Cationic Antimicrobial Polypeptides (Colistin and Protamine Sulfate)
The MIC and MBC values for Colistin (Col) and Protamine sulfate (Prot) were determined as described earlier in CAMHB medium, and modified checkerboard technique was performed to determine the combinatorial effect of LysB-His6 enzymes with Col and Prot as described above. M. smegmatis cells incubated with half MIC values of Colistin and Protamine sulfate were included as controls.
5. Conclusions
Our study clearly demonstrates that LysB enzymes are lipolytic enzymes (combining features of esterases and lipases) that hydrolyse a range of fatty substrates besides the mAGP complex, their natural substrate in the mycobacterial cell envelope. When applied externally to M. smegmatis cells, LysB-His6 enzymes showed marginal antimycobacterial activity. However, combining outer membrane permealizers with LysB-His6 enzymes enhanced the antimycobacterial activity. The potential application of LysB enzymes to disrupt and kill the mycobacterial cells is currently being investigated in combination with both LysA and antimicrobial peptides.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/9/3176/s1. Figure S1: SDS-PAGE of purified LysB-His6 enzymes. L1 Precision Plus ProteinTM All Blue Prestained Standards (Bio-Rad), L2: LysB-D29 (MW: 29.3 kDa), L3: LysB-Omega (MW: 31.5 kDa), L4: LysB-Saal (MW: 37.4 kDa), L5: LysB-Obama12 (MW: 36.7 kDa). Figure S2. Nucleotide sequences of the codon optimized genes used in the current study. Figure S3: 3D conformations of poses of pNP ligands upon docking to LysB proteins. Each diagram illustrates (in order) the overall surface of protein with its ligand, the shape of active site with the docked ligand, the interactions of ligand atoms with different residues of the corresponding protein. Hydrophilic residues (pink color), hydrophobic residues (green color), pNP ligands (black color). Orientation pose: ligand-protein conformation where the catalytic Ser faces ligand’s ester bond (C=O) with no H-bond formation. NDP: (No Detected Pose) neither binding nor orientation pose were detected. Stars indicate catalytic triad residues of LysB-D29. Table S1: Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) values of the antibiotics used in the antibacterial activity assay against M. smegmatis.
Author Contributions
Conceptualization, T.D. and R.H.-K.; methodology, A.A., A.H.K. and C.G.; validation, R.H.-K. and T.D.; formal analysis, A.A., A.H.K. and C.G.; investigation, A.A. and A.H.K.; resources, R.H.-K. and T.D.; data curation, A.A., A.H.K. and C.G.; writing—original draft preparation, A.A.; writing—review and editing, A.A., A.H.K., T.D. and R.H.-K.; visualization, A.A. and A.H.K.; supervision, R.H.-K. and T.D.; project administration, R.H.-K.; funding acquisition, R.H.-K. and T.D. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support from the Swedish Research Council (Vetenskapsrådet, Grant No. 2016−05898) is gratefully acknowledged.
Acknowledgments
We are grateful to Maria Gourdon for running Nano-DSF analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Muller, B.; Borrell, S.; Rose, G.; Gagneux, S. The heterogeneous evolution of multidrug–resistant Mycobacterium tuberculosis. Trends Genet. 2013, 29, 160–169. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2019; WHO/CDS/TB/2019.15 Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1 (accessed on 22 April 2020).
- Marrakchi, H.; Laneelle, M.A.; Daffe, M. Mycolic acids: Structures, biosynthesis, and beyond. Chem. Biol. 2014, 21, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Mitnick, C.D.; McGee, B.; Peloquin, C.A. Tuberculosis pharmacotherapy: Strategies to optimize patient care. Expert Opin. Pharmacother. 2009, 10, 381–401. [Google Scholar] [CrossRef] [PubMed]
- Neyrolles, O.; Guilhot, C. Recent advances in deciphering the contribution of Mycobacterium tuberculosis lipids to pathogenesis. Tuberculosis 2011, 91, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Aoyagi, Y.; Ridell, M.; Minnikin, D.E. Separation and characterization of individual mycolic acids in representative mycobacteria. Microbiology 2001, 147, 1825–1837. [Google Scholar] [CrossRef]
- Brennan, P.J. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis 2003, 83, 91–97. [Google Scholar] [CrossRef]
- Bhamidi, S.; Scherman, M.S.; Rithner, C.D.; Prenni, J.E.; Chatterjee, D.; Khoo, K.H.; McNeil, M.R. The identification and location of succinyl residues and the characterization of the interior arabinan region allow for a model of the complete primary structure of Mycobacterium tuberculosis mycolyl arabinogalactan. J. Biol. Chem. 2008, 283, 12992–13000. [Google Scholar] [CrossRef]
- Hoffmann, C.; Leis, A.; Niederweis, M.; Plitzko, J.M.; Engelhardt, H. Disclosure of the mycobacterial outer membrane: Cryo–electron tomography and vitreous sections reveal the lipid bilayer structure. Proc. Natl. Acad. Sci. USA 2008, 105, 3963–3967. [Google Scholar] [CrossRef]
- Niederweis, M. Nutrient acquisition by mycobacteria. Microbiology 2008, 154, 679–692. [Google Scholar] [CrossRef]
- Daffe, M. The Global Architecture of the Mycobacterial Cell Envelope. In Mycobacterial Cell Envelope; ASM Press: Washingtin, DC, USA, 2008; pp. 3–11. [Google Scholar]
- Mcneil, M.; Daffe, M.; Brennan, P.J. Evidence for the nature of the link between the arabinogalactan and peptidoglycan of mycobacterial cell–walls. J. Biol. Chem. 1990, 265, 18200–18206. [Google Scholar]
- Brennan, P.J.; Nikaido, H. The envelope of Mycobacteria. Annu. Rev. Biochem. 1995, 64, 29–63. [Google Scholar] [CrossRef] [PubMed]
- Daffe, M. Structure of the cell envelope of Mycobacterium tuberculosis. Med. Mal. Infect. 1996, 26, 891–897. [Google Scholar] [CrossRef]
- Zuber, B.; Chami, M.; Houssin, C.; Dubochet, J.; Griffiths, G.; Daffe, M. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J. Bacteriol. 2008, 190, 5672–5680. [Google Scholar] [CrossRef] [PubMed]
- Sani, M.; Houben, E.N.G.; Geurtsen, J.; Pierson, J.; De Punder, K.; Van Zon, M.; Wever, B.; Piersma, S.R.; Jimenez, C.R.; Daffe, M.; et al. Direct visualization by Cryo–EM of the mycobacterial capsular layer: A labile structure containing ESX–1–secreted proteins. PLoS Path. 2010, 6, e1000794. [Google Scholar] [CrossRef] [PubMed]
- Jarlier, V.; Nikaido, H. Permeability barrier to hydrophilic solutes in Mycobacterium chelonei. J. Bacteriol. 1990, 172, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Vilcheze, C.; Jacobs, W.R. The mechanism of isoniazid killing: Clarity through the scope of genetics. Annu. Rev. Microbiol. 2007, 61, 35–50. [Google Scholar] [CrossRef]
- Danelishvili, L.; Young, L.S.; Bermudez, L.E. In vivo efficacy of phage therapy for Mycobacterium avium infection as delivered by a nonvirulent mycobacterium. Microb. Drug Resist. 2006, 12, 1–6. [Google Scholar] [CrossRef]
- Peng, L.; Chen, B.W.; Luo, Y.A.; Wang, G.Z. Effect of mycobacteriophage to intracellular mycobacteria in vitro. Chin. Med. J. 2006, 119, 692–695. [Google Scholar] [CrossRef]
- Yacoby, I.; Shamis, M.; Bar, H.; Shabat, D.; Benhar, I. Targeting antibacterial agents by using drug–carrying filamentous bacteriophages. Antimicrob. Agents Chemother. 2006, 50, 2087–2097. [Google Scholar] [CrossRef]
- Gan, Y.L.; Wu, T.T.; Liu, P.; Guo, S.L. Characterization and classification of Bo4 as a cluster G mycobacteriophage that can infect and lyse M. tuberculosis. Arch. Microbiol. 2014, 196, 209–218. [Google Scholar] [CrossRef]
- Coates, A.R.; Hu, Y. Novel approaches to developing new antibiotics for bacterial infections. Br. J. Pharmacol. 2007, 152, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Hatfull, G.F.; Pedulla, M.L.; Jacobs–Sera, D.; Cichon, P.M.; Foley, A.; Ford, M.E.; Gonda, R.M.; Houtz, J.M.; Hryckowian, A.J.; Kelchner, V.A.; et al. Exploring the mycobacteriophage metaproteome: Phage genomics as an educational platform. PLoS Genet. 2006, 2, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Pimentel, M.; Moniz–Pereira, J. Expression of mycobacteriophage Ms6 lysis genes is driven by two sigma(70)–like promoters and is dependent on a transcription termination signal present in the leader RNA. J. Bacteriol. 2002, 184, 3034–3043. [Google Scholar] [CrossRef] [PubMed]
- Gil, F.; Grzegorzewicz, A.E.; Catalao, M.J.; Vital, J.; McNeil, M.R.; Pimentel, M. Mycobacteriophage Ms6 LysB specifically targets the outer membrane of Mycobacterium smegmatis. Microbiology 2010, 156, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Gil, F.; Catalao, M.J.; Moniz–Pereira, J.; Leandro, P.; McNeil, M.; Pimentel, M. The lytic cassette of mycobacteriophage Ms6 encodes an enzyme with lipolytic activity. Microbiology 2008, 154, 1364–1371. [Google Scholar] [CrossRef]
- Payne, K.; Sun, Q.A.; Sacchettini, J.; Hatfull, G.F. Mycobacteriophage Lysin B is a novel mycolylarabinogalactan esterase. Mol. Microbiol. 2009, 73, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Gigante, A.M.; Hampton, C.M.; Dillard, R.S.; Gil, F.; Catalao, M.J.; Moniz-Pereira, J.; Wright, E.R.; Pimentel, M. The Ms6 Mycolyl-Arabinogalactan Esterase LysB is Essential for an Efficient Mycobacteriophage-Induced Lysis. Viruses 2017, 9, 343. [Google Scholar] [CrossRef]
- Korany, A.H.; Abouhmad, A.; Bakeer, W.; Essam, T.; Amin, M.A.; Hatti-Kaul, R.; Dishisha, T. Comparative structural analysis of different mycobacteriophage-derived mycolylarabinogalactan esterases (Lysin B). Biomolecules 2020, 10, 45. [Google Scholar] [CrossRef]
- Henry, M.; Coffey, A.; O’Mahony, J.; Sleator, R.D. Comparative modelling of LysB from the mycobacterial bacteriophage Ardmore. Bioeng. Bugs 2011, 2, 88–95. [Google Scholar] [CrossRef]
- Catalao, M.J.; Pimentel, M. Mycobacteriophage Lysis Enzymes: Targeting the Mycobacterial Cell Envelope. Viruses 2018, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Grover, N.; Paskaleva, E.E.; Mehta, K.K.; Dordick, J.S.; Kane, R.S. Growth inhibition of Mycobacterium smegmatis by mycobacteriophage–derived enzymes. Enzym. Microb. Technol. 2014, 63, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T. Microbial carboxyl esterases: Classification, properties and application in biocatalysis. FEMS Microbiol. Rev. 2002, 26, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Longhi, S.; Cambillau, C. Structure-activity of cutinase, a small lipolytic enzyme. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 1999, 1441, 185–196. [Google Scholar] [CrossRef]
- Kodama, Y.; Masaki, K.; Kondo, H.; Suzuki, M.; Tsuda, S.; Nagura, T.; Shimba, N.; Suzuki, E.; Iefuji, H. Crystal structure and enhanced activity of a cutinase-like enzyme from Cryptococcus sp. strain S-2. Proteins 2009, 77, 710–717. [Google Scholar] [CrossRef]
- Pleiss, J.; Fischer, M.; Schmid, R.D. Anatomy of lipase binding sites: The scissile fatty acid binding site. Chem. Phys. Lipids 1998, 93, 67–80. [Google Scholar] [CrossRef]
- Plou, F.J.; Ferrer, M.; Nuero, O.M.; Calvo, M.V.; Alcalde, M.; Reyes, F.; Ballesteros, A. Analysis of Tween 80 as an esterase/lipase substrate for lipolytic activity assay. Biotechnol. Tech. 1998, 12, 183–186. [Google Scholar] [CrossRef]
- Gilham, D.; Lehner, R. Techniques to measure lipase and esterase activity in vitro. Methods 2005, 36, 139–147. [Google Scholar] [CrossRef]
- Saito, H.; Tomioka, H.; Watanabe, T.; Yoneyama, T. Mycobacteriocins produced by rapidly growing mycobacteria are Tween–hydrolyzing esterases. J. Bacteriol. 1983, 153, 1294–1300. [Google Scholar] [CrossRef]
- West, N.P.; Chow, F.M.E.; Randall, E.J.; Wu, J.; Chen, J.; Ribeiro, J.M.C.; Britton, W.J. Cutinase–like proteins of Mycobacterium tuberculosis: Characterization of their variable enzymatic functions and active site identification. FASEB J. 2009, 23, 1694–1704. [Google Scholar] [CrossRef]
- Tomioka, H. Purification and characterization of the Tween–hydrolyzing esterase of Mycobacterium smegmatis. J. Bacteriol. 1983, 155, 1249–1259. [Google Scholar] [CrossRef]
- Parker, S.K.; Curtin, K.M.; Vasil, M.L. Purification and characterization of mycobacterial phospholipase A: An activity associated with mycobacterial cutinase. J. Bacteriol. 2007, 189, 4153–4160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sukul, P.; Lupilov, N.; Leichert, L.I. Characterization of ML–005, a novel metaproteomics–derived esterase. Front. Microbiol. 2018, 9, 1925. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.A.; Knoll, M.; Abdel–Fattah, Y.R.; Schmid, R.D.; Lange, S. Molecular cloning and characterization of thermostable esterase and lipase from Geobacillus thermoleovorans YN isolated from desert soil in Egypt. Process Biochem. 2007, 42, 1090–1100. [Google Scholar] [CrossRef]
- Yip, Y.K.; Ramachandran, S.; Wagle, S.R. Studies on catalytic properties of purified high molecular–weight pancreatic lipase. Proc. Soc. Exp. Biol. Med. 1975, 149, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Hertadi, R.; Widhyastuti, H. Effect of Ca2+ Ion to The Activity and Stability of Lipase Isolated from Chromohalobacter japonicus BK–AB18. Procedia Chem. 2015, 16, 306–313. [Google Scholar] [CrossRef]
- Bozzo, G.G.; Raghothama, K.G.; Plaxton, W.C. Purification and characterization of two secreted purple acid phosphatase isozymes from phosphate–starved tomato (Lycopersicon esculentum) cell cultures. Eur. J. Biochem. 2002, 269, 6278–6286. [Google Scholar] [CrossRef]
- Rao, L.; Xue, Y.F.; Zheng, Y.Y.; Lu, J.R.; Ma, Y.H. A novel alkaliphilic Bacillus esterase belongs to the 13th bacterial lipolytic enzyme family. PLoS ONE 2013, 8, e60645. [Google Scholar] [CrossRef]
- Metin, K.; Ateslier, Z.B.B.; Basbulbul, G.; Biyik, H.H. Characterization of esterase activity in Geobacillus sp HBB–4. J. Basic Microbiol. 2006, 46, 400–409. [Google Scholar] [CrossRef]
- Wu, G.J.; Zhang, S.; Zhang, H.J.; Zhang, S.S.; Liu, Z.D. A novel esterase from a psychrotrophic bacterium Psychrobacter celer 3Pb1 showed cold–adaptation and salt–tolerance. J. Mol. Catal. B Enzym. 2013, 98, 119–126. [Google Scholar] [CrossRef]
- Barbeau, D. Investigating the Antimicrobial Activity of Two Mycobacteriophage-Encoded Lysterases; University of Pittsburgh: Pittsburgh, PA, USA, 2017. [Google Scholar]
- Joshi, H.; Seniya, S.P.; Suryanarayanan, V.; Patidar, N.D.; Singh, S.K.; Jain, V. Dissecting the structure–function relationship in lysozyme domain of mycobacteriophage D29-encoded peptidoglycan hydrolase. FEBS Lett. 2017, 591, 3276–3287. [Google Scholar] [CrossRef]
- Lai, M.J.; Liu, C.C.; Jiang, S.J.; Soo, P.C.; Tu, M.H.; Lee, J.J.; Chen, Y.H.; Chang, K.C. Antimycobacterial activities of endolysins derived From a Mycobacteriophage, BTCU–1. Molecules 2015, 20, 19277–19290. [Google Scholar] [CrossRef]
- Mahapatra, S.; Piechota, C.; Gil, F.; Ma, Y.F.; Huang, H.R.; Scherman, M.S.; Jones, V.; Pavelka, M.S.; Moniz–Pereira, J.; Pimentel, M.; et al. Mycobacteriophage Ms6 LysA: A peptidoglycan amidase and a useful analytical tool. Appl. Environ. Microbiol. 2013, 79, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.; Begley, M.; Neve, H.; Maher, F.; Ross, R.P.; McAuliffe, O.; Coffey, A.; O’Mahony, J.M. Cloning and expression of a mureinolytic enzyme from the mycobacteriophage TM4. FEMS Microbiol. Lett. 2010, 311, 126–132. [Google Scholar] [CrossRef]
- Connolly, L.E.; Edelstein, P.H.; Ramakrishnan, L. Why is long–term therapy required to cure tuberculosis? PLoS Med. 2007, 4, 435–442. [Google Scholar] [CrossRef]
- Ojha, A.K.; Trivelli, X.; Guerardel, Y.; Kremer, L.; Hatfull, G.F. Enzymatic hydrolysis of Trehalose Dimycolate Releases Free Mycolic Acids during Mycobacterial Growth in Biofilms. J. Biol. Chem. 2010, 285, 7380–17389. [Google Scholar] [CrossRef]
- Islam, M.S.; Richards, J.P.; Ojha, A.K. Targeting drug tolerance in mycobacteria: A perspective from mycobacterial biofilms. Expert Rev. Antiinfect. Ther. 2012, 10, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Kwa, A.L.; Tam, V.H.; Falagas, M.E. Polymyxins: A Review of the Current Status Including Recent Developments. Ann. Acad. Med. Singap. 2008, 37, 870–883. [Google Scholar] [PubMed]
- Rastogi, N.; Potar, M.C.; Henrotte, J.G.; Franck, G.; David, H.L. Further–studies on colistin (polymyxin E)–induced cell leakage in mycobacteria–Mg++ efflux in Mycobacterium avium and its effects on drug–susceptibility. Zent. Bakteriol. Mikrobiol. Hyg. Ser. A 1988, 268, 251–258. [Google Scholar] [CrossRef]
- Korycka–Machala, M.; Ziolkowski, A.; Rumijowska–Galewicz, A.; Lisowska, K.; Sedlaczek, L. Polycations increase the permeability of Mycobacterium vaccae cell envelopes to hydrophobic compounds. Microbiology 2001, 147, 2769–2781. [Google Scholar] [CrossRef][Green Version]
- Rastogi, N.; Henrotte, J.G.; David, H.L. Colistin (Polymyxin–E) induced cell leakage in Mycobacterium aurum. Zent. Bakteriol. Mikrobiol. Hyg. Ser. A 1987, 263, 548–551. [Google Scholar] [CrossRef]
- Mogi, T.; Murase, Y.; Mori, M.; Shiomi, K.; Omura, S.; Paranagama, M.P.; Kita, K. Polymyxin B identified as an inhibitor of alternative NADH dehydrogenase and malate: Quinone oxidoreductase from the Gram–positive bacterium Mycobacterium smegmatis. J. Biochem. 2009, 146, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Koen, N.; Van Breda, S.V.; Loots, D. Elucidating the antimicrobial mechanisms of colistin sulfate on Mycobacterium tuberculosis using metabolomics. Tuberculosis 2018, 111, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bax, H.I.; De Steenwinkel, J.E.M.; Kate, M.T.; Van der Meijden, A.; Verbon, A.; Bakker–Woudenberg, I.A.J.M. Colistin as a potentiator of anti–TB drug activity against Mycobacterium tuberculosis. J. Antimicrob. Chemother. 2015, 70, 2828–2837. [Google Scholar] [CrossRef] [PubMed]
- De Knegt, G.J.; Van der Meijden, A.; De Vogel, C.P.; Aarnoutse, R.E.; De Steenwinkel, J.E.M. Activity of moxifloxacin and linezolid against Mycobacterium tuberculosis in combination with potentiator drugs verapamil, timcodar, colistin and SQ109. Int. J. Antimicrob. Agents 2017, 49, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Miller, S. Composition for Use in Mycobacteria Therapy. U.S. Patent US20150344859A1, 3 December 2015. [Google Scholar]
- Hsieh, M.H.; Yu, C.M.; Yu, V.L.; Chow, J.W. Synergy Assessed by Checkerboard—A Critical Analysis. Diagn. Microbiol. Infect. 1993, 16, 343–349. [Google Scholar] [CrossRef]
- King, T.C.; Schlessinger, D.; Krogstad, D.J. The assessment of antimicrobial combinations. Rev. Infect. Dis. 1981, 3, 627–633. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).