Trace Elements, PPARs, and Metabolic Syndrome
Abstract
1. Introduction
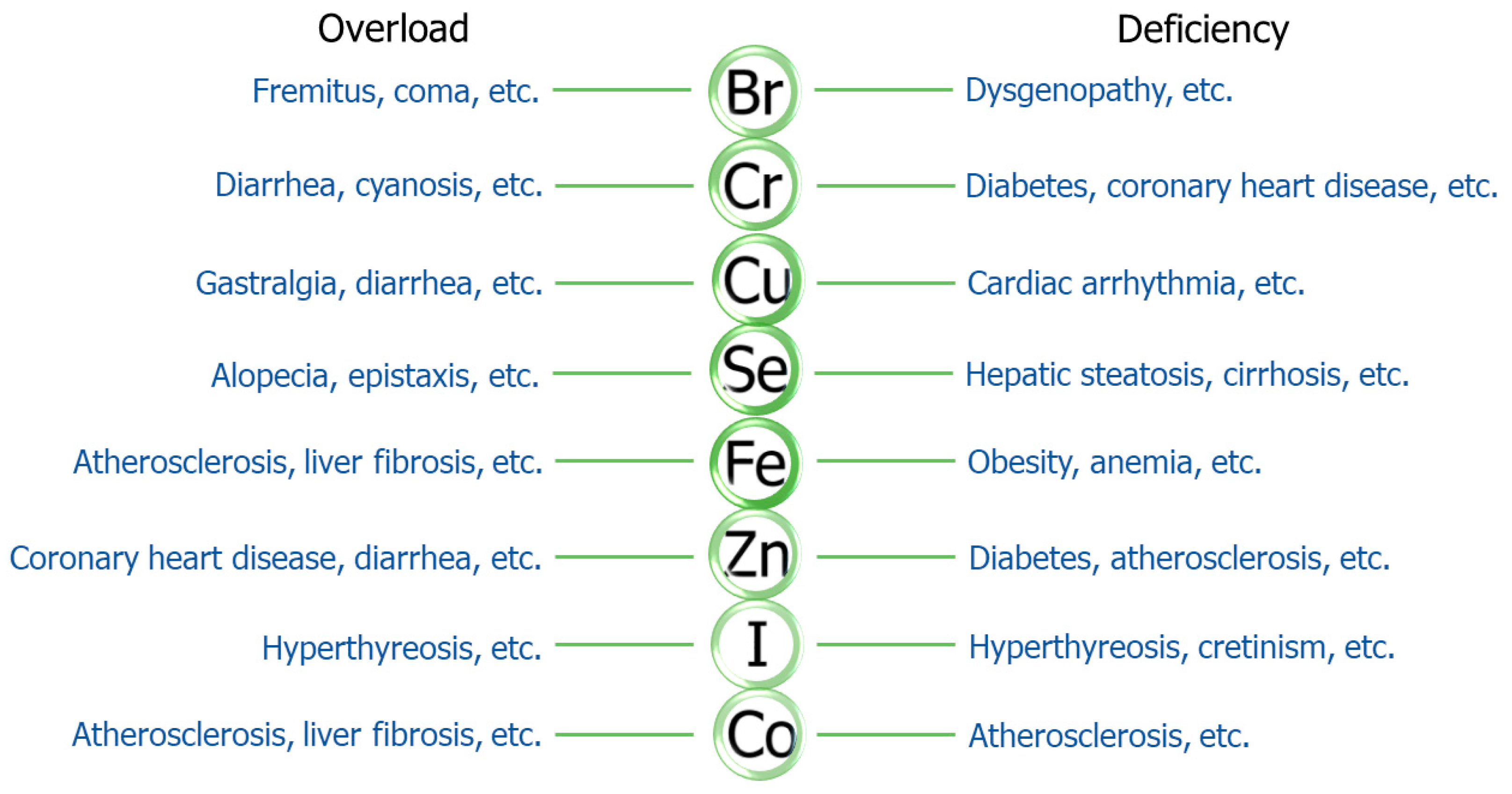
2. Trace Elements and Metabolic Syndrome
| Elements | Content in the Human Body | RDI | Reference |
|---|---|---|---|
| iron (Fe) | 3000–5000 mg | 15 mg for man 20 mg for woman | [41,42] |
| zinc (Zn) | 2500 mg | 15 mg for man 11.5 mg for woman | [43] |
| copper (Cu) | 100–150 mg | 2 mg | [44] |
| selenium (Se) | 14–21 mg | 50 μg | [45] |
| iodine (I) | 20–50 mg | 150 μg | [46] |
| molybdenum (Mo) | 9 mg | 0.1–0.5 mg | [47,48] |
| chromium (Cr) | 6 mg | 50 μg | [49] |
| cobalt (Co) | 1.1–1.5 mg | 5–45 μg | [50] |
| manganese (Mn) | 12–20 mg | 2.5–7 mg | [51] |
| silicon (Si) | 2000–3000 mg | 20–50 mg | [52] |
| boron (B) | 50 mg | 2–20 mg | [53,54] |
| vanadium(V) | 25 mg | 0.1–0.3 mg | [55] |
| nickel (Ni) | 6–10 mg | 0.3 mg | [56] |
| fluorine (F) | 2000–3000 mg | 0.5–1.0 mg | [52] |
| bromine (Br) | 200 mg | 1 mg | [40,57] |
| plumbum (Pb) | <10 μg/dL Blood | <0.1 mg | [58,59] |
| cadmium (Cd) | <1 mg/dL Blood | <70 μg | [60] |
| mercury (Hg) | <0.8 μg/dL Blood | <0.01 mg | [59,61] |
| arsenic (As) | <1 μg/dL Blood | 1 mg | [62] |
| aluminum (Al) | 50–100 mg | 1.8–8.4 mg | [63,64] |
| stannum (Sn) | 0.38 mg/dL Blood | 0.2–3.5 mg | [65] |
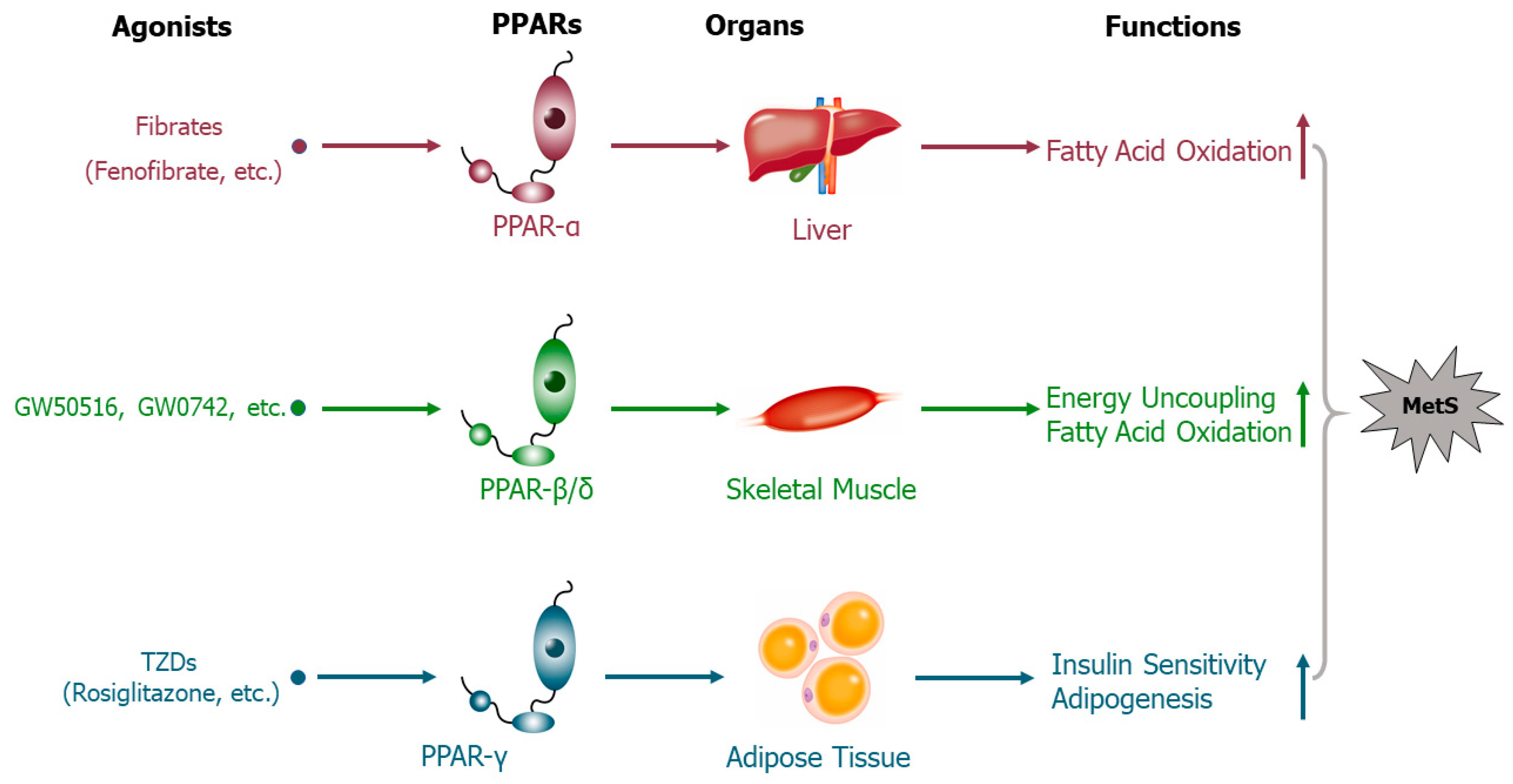
3. Peroxisome Proliferator-Activated Receptors Mediate the Effects of Trace Elements on Metabolic Syndrome
3.1. Iron
3.2. Zinc
3.3. Copper
3.4. Selenium
3.5. Other Essential Trace Elements
4. Trace Elements Supplementation and Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Puente, D.; López-Jiménez, T.; Cos-Claramunt, X.; Ortega, Y.; Duarte-Salles, T. Metabolic syndrome and risk of cancer: A study protocol of case–control study using data from the Information System for the Development of Research in Primary Care (SIDIAP) in Catalonia. BMJ Open 2019, 9, e025365. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, W.; Lun, Z.; Zhang, H.; Sun, Z.; Kanu, J.S.; Qiu, S.; Cheng, Y.; Liu, Y. Prevalence of metabolic syndrome in Mainland China: A meta-analysis of published studies. BMC Public Health 2016, 16, 296. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.T.J.; Gersch, T.M.; Segal, K.S.; Feig, D.I.; Lozada, J.L.; Nakagawa, T.; Kang, H.K.; Benner, J.S.; Sautin, Y.Y. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899. [Google Scholar] [PubMed]
- Wang, T.; Xu, M.; Bi, Y.; Ning, G. Interplay between diet and genetic susceptibility in obesity and related traits. Front. Med. 2018, 12, 601–607. [Google Scholar] [CrossRef]
- Aguilera, C.M.; Olza, J.; Gil, A. Genetic susceptibility to obesity and metabolic syndrome in childhood. Nutr. Hosp. 2013, 28, 44–55. [Google Scholar]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef]
- Silva, V.; Stanton, K.R.; Grande, A.J. Harmonizing the diagnosis of metabolic syndrome—Focusing on abdominal obesity. Metab. Syndr. Relat. Disord. 2013, 11, 102–108. [Google Scholar] [CrossRef]
- Tchernof, A.; Després, J.-P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Meshkani, R.; Adeli, K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin. Biochem. 2009, 42, 1331–1346. [Google Scholar] [CrossRef]
- Lebovitz, H.E. Type 2 Diabetes: An Overview. Clin. Chem. 2020, 8, 1339–1345. [Google Scholar] [CrossRef]
- Lu, C.-W.; Yang, K.-C.; Chang, H.-H.; Lee, L.-T.; Chen, C.-Y.; Huang, K.-C. Sarcopenic obesity is closely associated with metabolic syndrome. Obes. Res. Clin. Pract. 2013, 7, e301–e307. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, Y.; Liu, X.; Li, M.; Wu, B.; Li, Y.; Liang, Y.; Shao, X.; Holthöfer, H.; Zou, H. Insulin resistance and metabolic syndrome in normal-weight individuals. Endocrine 2014, 46, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- Anke, M.K. Essential and Toxic Effects of Macro, Trace, and Ultratrace Elements in the Nutrition of Animals; Wiley: Weinheim, Germany, 2008. [Google Scholar]
- Dongiovanni, P.; Fracanzani, A.L.; Fargion, S.; Valenti, L. Iron in fatty liver and in the metabolic syndrome: A promising therapeutic target. J. Hepatol. 2011, 55, 920–932. [Google Scholar] [CrossRef]
- Standeven, K.F.; Hess, K.; Carter, A.M.; Rice, G.I.; Grant, P.J. Neprilysin, obesity and the metabolic syndrome. Int. J. Obes. 2010, 35, 1031–1040. [Google Scholar] [CrossRef]
- Obeid, O.; Elfakhani, M.; Hlais, S.; Iskandar, M.; Batal, M.; Mouneimne, Y.; Adra, N.; Hwalla, N. Plasma Copper, Zinc, and Selenium Levels and Correlates with Metabolic Syndrome Components of Lebanese Adults. Biol. Trace Elem. Res. 2008, 123, 58–65. [Google Scholar] [CrossRef]
- Raimundo, J.; Costa, P.M.; Vale, C.; Costa, M.H.; Moura, I. Metallothioneins and trace elements in digestive gland, gills, kidney and gonads of Octopus vulgaris. Comp. Biochem. Physiol. Part C 2010, 152, 139–146. [Google Scholar] [CrossRef]
- Anderson, R.A. Trace elements and cardiovascular diseases. Basic Clin. Pharmacol. Toxicol. 1986, 59, 317–324. [Google Scholar] [CrossRef]
- Muskiet, F.D.; Muskiet, F.A.J. Lipids, fatty acids and trace elements in plasma and erythrocytes of pediatric patients with homozygous sickle cell disease. Clin. Chim. Acta 1984, 142, 1–10. [Google Scholar] [CrossRef]
- Zadik, Z. Vitamins and Trace Elements are Important for the Integrity of the Endocrine System. J. Pediatr. Endocrinol. Metab. 2009, 22, 579–580. [Google Scholar] [CrossRef] [PubMed]
- Abu-El-Zahab, H.; Aal, W.E.A.; Awadallah, R.; Mikhail, T.M.; Zakaria, K. The correlation between serum total cholesterol and some trace elements in serum, liver and heart of rats fed high cholesterol diet. Food Nahrung 1991, 35, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Houtman, J.P. Trace elements and cardiovascular diseases. J. Cardiovasc. Risk 1996, 3, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef]
- Yu, H.; Feng, S.; Chao, P.; Lin, A. Anti-inflammatory effects of pioglitazone on iron-induced oxidative injury in the nigrostriatal dopaminergic system. Neuropathol. Appl. Neurobiol. 2010, 36, 612–622. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Zrieq, R.; Al-Quraishy, S.; Abdel Moneim, A.E. Selenium nanoparticles attenuate oxidative stress and testicular damage in streptozotocin-induced diabetic rats. Molecules 2016, 21, 1517. [Google Scholar] [CrossRef]
- Lei, L.; Xiaoyi, S.; Fuchang, L. Effect of dietary copper addition on lipid metabolism in rabbits. Food Nutr. Res. 2017, 61, 1348866. [Google Scholar] [CrossRef]
- Sogou Encyclopedia. Available online: https://baike.sogou.com/v213556.htm?fromTitle=%E5%BE%AE%E9%87%8F%E5%85%83%E7%B4%A0 (accessed on 29 December 2019).
- Fraga, C.G. Relevance, essentiality and toxicity of trace elements in human health. Mol. Asp. Med. 2005, 26, 235–244. [Google Scholar] [CrossRef]
- Czarnek, K.; Terpiłowska, S.; Siwicki, A.K. Review paper Selected aspects of the action of cobalt ions in the human body. Cent. Eur. J. Immunol. 2015, 2, 236–242. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans. A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Squadrone, S.; Brizio, P.; Chiaravalle, E.; Abete, M. Sperm whales (Physeter macrocephalus), found stranded along the Adriatic coast (Southern Italy, Mediterranean Sea), as bioindicators of essential and non-essential trace elements in the environment. Ecol. Ind. 2015, 58, 418–425. [Google Scholar] [CrossRef]
- Bomar, M.G.; Pai, M.-T.; Tzeng, S.-R.; Li, S.S.-C.; Zhou, P. Structure of the ubiquitin-binding zinc finger domain of human DNA Y-polymerase η. Embo Rep. 2007, 8, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, J.; Chen, X.; Zeng, X.; Wang, Y.; Dong, J.; Chen, J. Maternal iodine supplementation improves motor coordination in offspring by modulating the mGluR1 signaling pathway in mild iodine deficiency-induced hypothyroxinemia rats. J. Nutr. Biochem. 2018, 58, 80. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, C.; Karim, Z. Iron metabolism: State of the art. Transfus. Clin. Boil. 2013, 34, 17–25. [Google Scholar]
- Huwait, E.; Kumosani, T.; Moselhy, S.; Mosaoa, R.; Yaghmoor, S. Relationship between soil cobalt and vitamin B12 levels in the liver of livestock in Saudi Arabia: Role of competing elements in soils. Afr. Health Sci. 2015, 15, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Silvera, S.A.N.; Rohan, T.E. Trace elements and cancer risk: A review of the epidemiologic evidence. Cancer Causes Control 2007, 18, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Leonhard-Marek, S. Why do trace elements have an influence on fertility? Tierarztl. Praxis. Ausg. G Grosstiere Nutztiere 2000, 28, 60–65. [Google Scholar]
- Qin, J. The biological necessity of bromine. Guangdong Trace Elem. Sci. 2011, 18, 1–10. [Google Scholar]
- Eijk, H.G.V.; Wiltink, W.F.; Bos, G.; Goossens, J.P. Measurement of the iron content in human liver specimens. Clin. Chim. Acta 1974, 50, 275–280. [Google Scholar] [CrossRef]
- Xu, S. Relation of Trace Element Iron and Human Health. Food Nutr. China 2007, 12, 53–56. [Google Scholar]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Wu, D.; Lu, G. Copper and Health of Human. Food Nutr. China 2003, 2, 56–57. [Google Scholar]
- Gao, N.; Chen, W.; Zou, L. The newest study advance of microelement selenium and human health. J. Shenyang Med. Coll. 2003, 5, 259–261. [Google Scholar]
- Li, Y.; Liu, X. Iodine and Health of Human Body. Stud. Trace Elem. Health 2004, 1, 58–62. [Google Scholar]
- Hu, X. Molybdenum and Health of Human. Chem. World 2002, 43, 166–168. [Google Scholar]
- Barceloux, D.G. Molybdenum. Clin. Toxicol. 1999, 37, 231. [Google Scholar] [CrossRef]
- Gao, H. Physiological Function and Metabolism of Chromium. Shanxi Med. J. 2007, 36, 895–896. [Google Scholar]
- Qingren, L.I.; Bin, S.U.; Shengchuan, L.I. Trace Element Cobalt, Nickel and Human Health. Guangdong Trace Elem. Sci. 2008, 15, 66–70. [Google Scholar]
- Yang, X.; Wang, G.; Zhang, Z. Manganese and Health of Human. Med. Recapitul. 2006, 12, 1134–1136. [Google Scholar]
- Bin, S.U.; Qingren, L.I.; Fan, D. The Relation between Iodine, Fluorine, Silicon and Human Health. Guangdong Trace Elem. Sci. 2008, 15, 10–13. [Google Scholar]
- Zhong, B. The Role of Boron in Life Science and Its Effect on Human Health. World Elem. Med. 2005, 12, 49–50. [Google Scholar]
- Nielsen, F.H. Boron in human and animal nutrition. Plant. Soil 1997, 193, 199–208. [Google Scholar] [CrossRef]
- Byrne, A.R.; Kosta, L. Vanadium in foods and in human body fluids and tissues. Sci. Total Environ. 1978, 10, 17–30. [Google Scholar] [CrossRef]
- Wei, Y.; Huan, Q.; Su, X. Review on the Toxicological Effect and the Mechanism of Nikel to Human Health. Environ. Sci. Manag. 2008, 33, 45–48. [Google Scholar]
- Zeng, Z.H.; Zeng, X.P. Relation between halogen group elements, F, Cl, Br, I and human health. Hum. Geol. 2002, 21, 221–227. [Google Scholar]
- Peng, N. Plumbum and Health of Human. Mod. Prev. Med. 2004, 31, 91–94. [Google Scholar]
- Emsley, J. Nature’s Building Blocks: An A-Z Guide to the Elements; OUP Oxford: Oxford, UK, 2011; pp. 87–88. [Google Scholar]
- Guo, R.; Wang, E.; Wang, L. Determine of Cadmium in Human Whole Blood. Chin. J. Coal Ind. Med. 2001, 4, 941–942. [Google Scholar]
- Hunag, X. Biotoxicity and Environmental Pollution of Mercury. Mod. Enterp. Educ. 2012, 15, 255. [Google Scholar]
- Wang, X.H.; Bian, J.C. The trace element arsenic and human health. Foreign Med. Sci. Sect. Medgeogr. 2005, 26, 101–105. [Google Scholar]
- Krízek, M.; Senft, V.; Motán, J. Aluminum and the human body. Casopís Lékar Ceskych 1997, 136, 544–547. [Google Scholar]
- Matsuda, R.; Sasaki, K.; Sakai, H.; Aoyagi, Y.; Saeki, M.; Hasegawa, Y.; Hidaka, T.; Ishii, K.; Mochizuki, E.; Yamamoto, T. Estimation of daily dietary intake of aluminum. Shokuhinseigaku Zasshi J. Food Hyg. Soc. Jpn. 2001, 42, 18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steel Grade. Available online: https://www.steel-grades.com/Element/Tin.html (accessed on 29 December 2019).
- Datz, C.; Müller, E.; Aigner, E. Iron overload and nonalcoholic fatty liver disease. Minerva Endocrinol. 2016, 42, 173. [Google Scholar]
- Arthur, M. Iron overload and liver fibrosis. J. Gastroenterol. Hepatol. 1996, 11, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Kew, M.C. Hepatic iron overload and hepatocellular carcinoma. Liver Cancer 2014, 3, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kumfu, S.; Chattipakorn, S.; Fucharoen, S.; Chattipakorn, N. Mitochondrial calcium uniporter blocker prevents cardiac mitochondrial dysfunction induced by iron overload in thalassemic mice. Biometals 2012, 25, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Aigner, E.; Feldman, A.; Datz, C. Obesity as an emerging risk factor for iron deficiency. Nutrients 2014, 6, 3587–3600. [Google Scholar] [CrossRef] [PubMed]
- Cruz, K.J.C.; Morais, J.B.S.; de Oliveira, A.R.S.; Severo, J.S.; do Nascimento Marreiro, D. The effect of zinc supplementation on insulin resistance in obese subjects: A systematic review. Biol. Trace Elem. Res. 2017, 176, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Little, P.J.; Bhattacharya, R.; Moreyra, A.E.; Korichneva, I.L. Zinc and cardiovascular disease. Nutrition 2010, 26, 1050–1057. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Pivina, L.; Doşa, M.D.; Semenova, Y.; Aaseth, J. The Role of Zinc and Copper in Insulin Resistance and Diabetes Mellitus. Curr. Med. Chem. 2019, 26, 1. [Google Scholar] [CrossRef]
- Vincent, J.B. Effects of chromium supplementation on body composition, human and animal health, and insulin and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 483–489. [Google Scholar] [CrossRef]
- Moskalyk, R.; Alfantazi, A. Review of present cobalt recovery practice. Min. Metall. Explor. 2000, 17, 205–216. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Esmaieli, M.; Khodadadi, A.A.; Ganjkhanlou, Y.; Asheghali, D. Direct electron transfer and biocatalytic activity of iron storage protein molecules immobilized on electrodeposited cobalt oxide nanoparticles. Microchim. Acta 2011, 173, 317–322. [Google Scholar] [CrossRef]
- Schade, S.G.; Felsher, B.F.; Bernier, G.M.; Conrad, M.E. Interrelationship of cobalt and iron absorption. J. Lab. Clin. Med. 1970, 75, 435–441. [Google Scholar] [PubMed]
- Valberg, L.; Flanagan, P.R.; Chamberlain, M.J. Effects of iron, tin, and copper on zinc absorption in humans. Am. J. Clin. Nutr. 1984, 40, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.K.; Amy, N.K. Effect of molybdenum-deficient and low iron diets on xanthine oxidase activity and iron status in rats. J. Nutr. 1984, 114, 1652–1659. [Google Scholar] [CrossRef] [PubMed]
- Wegner, M.; Singer, L.; Ophaug, R.; Magil, S. The interrelation of fluoride and iron in anemia. Proc. Soc. Exp. Biol. Med. 1976, 153, 414–418. [Google Scholar] [CrossRef]
- Ruliffson, W.; Burns, L.; Hughes, J. The Effect of Fluoride Ion on Fe 59 Iron Levels in Blood of Rats. Trans. Kansas Acad. Sci. 1903 1963, 66, 52–58. [Google Scholar] [CrossRef]
- Mendes, B.I.S.; Oliveira-Santos, M.; Ferreira, M.J.V. Sodium fluoride in cardiovascular disorders: A systematic review. J. Nucl. Cardiol. 2019, 1–13. [Google Scholar] [CrossRef]
- Leone, N.; Courbon, D.; Ducimetiere, P.; Zureik, M. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology 2006, 17, 308–314. [Google Scholar] [CrossRef]
- Pérez-Granados, A.M.; Vaquero, M.P. Silicon, aluminium, arsenic and lithium: Essentiality and human health implications. J. Nutr. Health Aging 2002, 6, 154–162. [Google Scholar]
- Kim, J.-W.; Park, S.-Y.; You, Y.-H.; Ham, D.-S.; Lee, S.-H.; Yang, H.K.; Jeong, I.-K.; Ko, S.-H.; Yoon, K.-H. Suppression of ROS production by exendin-4 in PSC attenuates the high glucose-induced islet fibrosis. PLoS ONE 2016, 11, e0163187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.j.; Li, P.X.; Guo, X.H.; Huang, Q.B. Role of moesin, Src, and ROS in advanced glycation end product-induced vascular endothelial dysfunction. Microcirculation 2017, 24, e12358. [Google Scholar] [CrossRef]
- Pilarczyk, B.; Pilarczyk, R.; Tomza-Marciniak, A.; Hendzel, D.; Bąkowska, M.; Stankiewicz, T. Evaluation of selenium status and its distribution in organs of free living foxes (Vulpes vulpes) from an Se deficient area. Pol. J. Vet. Sci. 2011, 14, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Galal, G.M.; Ahmad, N.S.; Mohammad, A.; Bakrey, R. Serum Selenium Level in Patients with Chronic Liver Disease. Sohag Med. J. 2017, 21, 125–133. [Google Scholar] [CrossRef]
- Fu, X.; Zhong, Z.; Hu, F.; Zhang, Y.; Huang, B. The protective effects of selenium-enriched Spirulina platensis on chronic alcohol-induced liver injury in mice. Food Funct. 2018, 9, 3155–3165. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, F.; Skinner, E.; MacIntyre, F.; Ijomah, G.; Holliday, J. Plasma bromine concentrations and lipid profiles. Clin. Chim. Acta 1991, 204, 301–304. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, W.; Cheng, Y.; Liu, C.; Chen, S. Bromide alleviates fatty acid-induced lipid accumulation in mouse primary hepatocytes through the activation of PPARα signals. J. Cell. Mol. Med. 2019, 23, 4464–4474. [Google Scholar] [CrossRef] [PubMed]
- Devirian, T.A.; Volpe, S.L. The physiological effects of dietary boron. Crit. Rev. Food Sci. Nutr. 2003, 43, 219–231. [Google Scholar] [CrossRef]
- Dubois, V.; Eeckhoute, J.; Lefebvre, P.; Staels, B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J. Clin. Investig. 2017, 127, 1202–1214. [Google Scholar] [CrossRef]
- Chen, L.; Yang, G. PPARs integrate the mammalian clock and energy metabolism. PPAR Res. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.P. The roles of PPARs in human diseases. Nucleosides Nucleotides Nucleic Acids 2018, 37, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Botta, M.; Audano, M.; Sahebkar, A.; Sirtori, C.R.; Mitro, N.; Ruscica, M. PPAR agonists and metabolic syndrome: An established role? Int. J. Mol. Sci. 2018, 19, 1197. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Corsini, A.; Sirtori, C.; Ruscica, M. PPAR-α agonists are still on the rise: An update on clinical and experimental findings. Expert Opin. Investig. Drugs 2017, 26, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shen, W.; Zhang, Q.; Hu, Y.; Chen, Y. Up-regulation of Hepatic VLDLR via PPARα Is Required for the Triglyceride-Lowering Effect of Fenofibrate. J. Lipid Res. 2014, 55, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Foufelle, F.; Scotto, C.; Dauça, M.; Wahli, W. Differential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-alpha,-beta, and-gamma in the adult rat. Endocrinology 1996, 137, 354–366. [Google Scholar] [CrossRef]
- Holst, D.; Luquet, S.; Nogueira, V.; Kristiansen, K.; Leverve, X.; Grimaldi, P.A. Nutritional regulation and role of peroxisome proliferator-activated receptor δ in fatty acid catabolism in skeletal muscle. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2003, 1633, 43–50. [Google Scholar] [CrossRef]
- Dimopoulos, N.; Watson, M.; Green, C.J.; Hundal, H.S. The PPARdelta agonist, GW501516, promotes fatty acid oxidation but has no direct effect on glucose utilisation or insulin sensitivity in rat L6 skeletal muscle cells. Febs Lett. 2007, 581, 4743–4748. [Google Scholar] [CrossRef]
- Dressel, U.; Allen, T.L.; Pippal, J.B.; Rohde, P.R.; Lau, P.; Muscat, G. The Peroxisome Proliferator-Activated Receptor β/δ Agonist, GW501516, Regulates the Expression of Genes Involved in Lipid Catabolism and Energy Uncoupling in Skeletal Muscle Cells. Mol. Endocrinol. 2003, 17, 2477–2493. [Google Scholar] [CrossRef]
- Jones, J.R.; Barrick, C.; Kim, K.-A.; Lindner, J.; Blondeau, B.; Fujimoto, Y.; Shiota, M.; Kesterson, R.A.; Kahn, B.B.; Magnuson, M.A. Deletion of PPARγ in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 6207–6212. [Google Scholar] [CrossRef]
- Colca, J.R. The TZD insulin sensitizer clue provides a new route into diabetes drug discovery. Expert Opin. Drug Discov. 2015, 10, 1259–1270. [Google Scholar] [CrossRef]
- Ross, S.A.; Dzida, G.; Vora, J.; Khunti, K.; Kaiser, M.; Ligthelm, R.J. Impact of weight gain on outcomes in type 2 diabetes. Curr. Med. Res. Opin. 2011, 27, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Lipscombe, L.L.; Gomes, T.; Lévesque, L.E.; Hux, J.E.; Juurlink, D.N.; Alter, D.A. Thiazolidinediones and Cardiovascular Outcomes in Older Patients With Diabetes. Jama 2007, 298, 2634. [Google Scholar] [CrossRef] [PubMed]
- Festuccia, W.; Laplante, M.; Berthiaume, M.; Gelinas, Y.; Deshaies, Y. PPARγ agonism increases rat adipose tissue lipolysis, expression of glyceride lipases, and the response of lipolysis to hormonal control. Diabetologia 2006, 49, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Teruel, T.; Hernandez, R.; Rial, E.; Martín-Hidalgo, A.; Lorenzo, M. Rosiglitazone up-regulates lipoprotein lipase, hormone-sensitive lipase and uncoupling protein-1, and down-regulates insulin-induced fatty acid synthase gene expression in brown adipocytes of Wistar rats. Diabetologia 2005, 48, 1180–1188. [Google Scholar] [CrossRef]
- Lazar, M.A. Reversing the curse on PPARγ. J. Clin. Investig. 2018, 128, 2202–2204. [Google Scholar] [CrossRef]
- Xu, L.; Ma, X.; Verma, N.K.; Wang, D.; Mueller, E. Ablation of PPARγ in subcutaneous fat exacerbates age-associated obesity and metabolic decline. Aging Cell 2018, 17, e12721. [Google Scholar] [CrossRef]
- TuTunchi, H.; Ostadrahimi, A.; Saghafi-Asl, M.; Maleki, V. The effects of oleoylethanolamide, an endogenous PPAR-α agonist, on risk factors for NAFLD: A systematic review. Obes. Rev. 2019, 20, 1057–1069. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Melnichenko, A.A.; Myasoedova, V.A.; Ivanova, E.A. Peroxisome Proliferator-Activated Receptor (PPAR) Gamma Agonists as Therapeutic Agents for Cardiovascular Disorders: Focus on Atherosclerosis. Curr. Pharm. Design 2016, 23, 1119–1124. [Google Scholar]
- Bonomo, L.D.F.; Silva, M.; Oliveira, R.D.P.; Silva, M.E.; Pedrosa, M.L. Iron overload potentiates diet-induced hypercholesterolemia and reduces liver ppar-α expression in hamsters. J. Biochem. Mol. Toxicol. 2012, 26, 224–229. [Google Scholar] [CrossRef]
- Minamiyama, Y.; Takemura, S.; Kodai, S.; Shinkawa, H.; Tsukioka, T.; Ichikawa, H.; Naito, Y.; Yoshikawa, T.; Okada, S. Iron restriction improves type 2 diabetes mellitus in Otsuka Long-Evans Tokushima fatty rats. Am. J. Physiol. Metab. 2010, 298, E1140–E1149. [Google Scholar] [CrossRef]
- Bao, B.; Prasad, A.S.; Beck, F.W.; Fitzgerald, J.T.; Snell, D.; Bao, G.W.; Singh, T.; Cardozo, L.J. Zinc decreases C-reactive protein, lipid peroxidation, and inflammatory cytokines in elderly subjects: A potential implication of zinc as an atheroprotective agent. Am. J. Clin. Nutr. 2010, 91, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Meerarani, P.; Reiterer, G.; Toborek, M.; Hennig, B. Zinc modulates PPARγ signaling and activation of porcine endothelial cells. J. Nutr. 2003, 133, 3058–3064. [Google Scholar] [CrossRef]
- Kang, X.; Zhong, W.; Liu, J.; Song, Z.; McClain, C.J.; Kang, Y.J.; Zhou, Z. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4α and peroxisome proliferator-activated receptor-α. Hepatology 2009, 50, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Slinko, S.; Piraino, G.; Hake, P.W.; Ledford, J.R.; O’Connor, M.; Lahni, P.; Solan, P.D.; Wong, H.R.; Zingarelli, B. Combined zinc supplementation with proinsulin C-peptide treatment decreases the inflammatory response and mortality in murine polymicrobial sepsis. Shock 2014, 41, 292. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-L.; Luo, Z.; Pan, Y.-X.; Zheng, J.-L.; Zhu, Q.-L.; Sun, L.-D.; Zhuo, M.-Q.; Hu, W. Differential induction of enzymes and genes involved in lipid metabolism in liver and visceral adipose tissue of juvenile yellow catfish Pelteobagrus fulvidraco exposed to copper. Aquat. Toxicol. 2013, 136, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wei, X.; Chen, T.; Wang, W.; Xia, X.; Miao, J.; Yin, S. Studies of the mechanism of fatty liver formation in Takifugu fasciatus following copper exposure. Ecotoxicol. Environ. Saf. 2019, 181, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jia, T.; Liu, R.; Xu, S. The antagonistic effect of selenium on cadmium-induced apoptosis via PPAR-γ/PI3K/Akt pathway in chicken pancreas. J. Hazard. Mater. 2018, 357, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, Z.; Li, Y.; Hu, X.; Shen, P.; Fu, Y.; Cao, Y.; Zhang, N. Selenium deficiency deteriorate the inflammation of S. aureus infection via regulating NF-κB and PPAR-γ in mammary gland of mice. Biol. Trace Elem. Res. 2016, 172, 140–147. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sk, U.H.; He, P.; Peters, J.M.; Amin, S. Synthesis of isosteric selenium analog of the PPARβ/δ agonist GW501516 and comparison of biological activity. Bioorg. Med. Chem. Lett. 2010, 20, 4050–4052. [Google Scholar] [CrossRef]
- Modarres, S.Z.; Heidar, Z.; Foroozanfard, F.; Rahmati, Z.; Aghadavod, E.; Asemi, Z. The effects of selenium supplementation on gene expression related to insulin and lipid in infertile polycystic ovary syndrome women candidate for in vitro fertilization: A randomized, double-blind, placebo-controlled trial. Biol. Trace Elem. Res. 2018, 183, 218–225. [Google Scholar] [CrossRef]
- Rayman, M.P.; Blundell-Pound, G.; Pastor-Barriuso, R.; Guallar, E.; Steinbrenner, H.; Stranges, S. A randomized trial of selenium supplementation and risk of type-2 diabetes, as assessed by plasma adiponectin. PLoS ONE 2012, 7, e45269. [Google Scholar] [CrossRef] [PubMed]
- Nido, S.A.; Shituleni, S.A.; Mengistu, B.M.; Liu, Y.; Khan, A.Z.; Gan, F.; Kumbhar, S.; Huang, K. Effects of selenium-enriched probiotics on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in mice fed a high-fat diet. Biol. Trace Elem. Res. 2016, 171, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Li, D.; Chen, M.; Jiang, L.; Liu, X.; Li, Q.; Geng, C.; Sun, X.; Yang, G.; Zhang, L. Citreoviridin induces myocardial apoptosis through PPAR-γ-mTORC2-mediated autophagic pathway and the protective effect of thiamine and selenium. Chem.-Biol. Interact. 2019, 311, 108795. [Google Scholar] [CrossRef] [PubMed]
- Aceves, C.; García-Solís, P.; Arroyo-Helguera, O.; Vega-Riveroll, L.; Delgado, G.; Anguiano, B. Antineoplastic effect of iodine in mammary cancer: Participation of 6-iodolactone (6-IL) and peroxisome proliferator-activated receptors (PPAR). Mol. Cancer 2009, 8, 33. [Google Scholar] [CrossRef]
- Alfaro, Y.; Delgado, G.; Cárabez, A.; Anguiano, B.; Aceves, C. Iodine and doxorubicin, a good combination for mammary cancer treatment: Antineoplastic adjuvancy, chemoresistance inhibition, and cardioprotection. Mol. Cancer 2013, 12, 45. [Google Scholar] [CrossRef]
- Turgut, M.; Cinar, V.; Pala, R.; Tuzcu, M.; Orhan, C.; Telceken, H.; Sahin, N.; Deeh, P.B.D.; Komorowski, J.R.; Sahin, K. Biotin and chromium histidinate improve glucose metabolism and proteins expression levels of IRS-1, PPAR-γ, and NF-κB in exercise-trained rats. J. Int. Soc. Sports Nutr. 2018, 15, 45. [Google Scholar] [CrossRef]
- Xue, F.; Zheng, Y. The Effect of Chromiun and Sport on Serum Lipid Level. J. Nanjing Sport Inst. 2005, 3, 13–16. [Google Scholar]
- Sahin, K.; Tuzcu, M.; Orhan, C.; Sahin, N.; Kucuk, O.; Ozercan, I.H.; Juturu, V.; Komorowski, J.R. Anti-diabetic activity of chromium picolinate and biotin in rats with type 2 diabetes induced by high-fat diet and streptozotocin. Br. J. Nutr. 2013, 110, 197–205. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Chen, C.-J.; Liu, C.-H.; Mao, F.C. Chromium attenuates high-fat diet-induced nonalcoholic fatty liver disease in KK/HlJ mice. Biochem. Biophys. Res. Commun. 2010, 397, 459–464. [Google Scholar] [CrossRef]
- Daoud, G.; Simoneau, L.; Masse, A.; Rassart, E.; Lafond, J. Expression of cFABP and PPAR in trophoblast cells: Effect of PPAR ligands on linoleic acid uptake and differentiation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2005, 1687, 181–194. [Google Scholar] [CrossRef]
- Razeghi, P.; Young, M.E.; Abbasi, S.; Taegtmeyer, H. Hypoxia in vivo decreases peroxisome proliferator-activated receptor α-regulated gene expression in rat heart. Biochem. Biophys. Res. Commun. 2001, 287, 5–10. [Google Scholar] [CrossRef]
- Isaac, A.O.; Kawikova, I.; Bothwell, A.L.; Daniels, C.K.; Lai, J.C. Manganese treatment modulates the expression of peroxisome proliferator-activated receptors in astrocytoma and neuroblastoma cells. Neurochem. Res. 2006, 31, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Miriyala, S.; Fang, F.; Bakthavatchalu, V.; Noel, T.; Schell, D.; Wang, C.; St Clair, W.H.; St Clair, D.K. Manganese superoxide dismutase deficiency triggers mitochondrial uncoupling and the Warburg effect. Oncogene 2015, 34, 4229–4237. [Google Scholar] [CrossRef] [PubMed]
- Kajita, D.; Nakamura, M.; Matsumoto, Y.; Ishikawa, M.; Hashimoto, Y.; Fujii, S. Design and synthesis of silicon-containing fatty acid amide derivatives as novel peroxisome proliferator-activated receptor (PPAR) agonists. Bioorg. Med. Chem. Lett. 2015, 25, 3350–3354. [Google Scholar] [CrossRef] [PubMed]
- Gunasinghe, M.A.; Kim, A.T.; Kim, S.M. Inhibitory Effects of Vanadium-Binding Proteins Purified from the Sea Squirt Halocynthia roretzi on Adipogenesis in 3T3-L1 Adipocytes. Appl. Biochem. Biotechnol. 2019, 189, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, Y.; Liu, F.; Zhang, F.; Ding, W. Vanadium (IV)-chlorodipicolinate inhibits 3T3-L1 preadipocyte adipogenesis by activating LKB1/AMPK signaling pathway. J. Inorg. Biochem. 2016, 162, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Wu, Y.; Wang, N.; Wang, Z.; Zhao, P.; Yang, X. Is the hypoglycemic action of vanadium compounds related to the suppression of feeding? Biol. Trace Elem. Res. 2014, 157, 242–248. [Google Scholar] [CrossRef]
- Kawakami, T.; Hanao, N.; Nishiyama, K.; Kadota, Y.; Inoue, M.; Sato, M.; Suzuki, S. Differential effects of cobalt and mercury on lipid metabolism in the white adipose tissue of high-fat diet-induced obesity mice. Toxicol. Appl. Pharmacol. 2012, 258, 32–42. [Google Scholar] [CrossRef]
- Yadav, S.; Anbalagan, M.; Shi, Y.; Wang, F.; Wang, H. Arsenic inhibits the adipogenic differentiation of mesenchymal stem cells by down-regulating peroxisome proliferator-activated receptor gamma and CCAAT enhancer-binding proteins. Toxicology 2013, 27, 211–219. [Google Scholar] [CrossRef]
- Wauson, E.M.; Langan, A.S.; Vorce, R.L. Sodium arsenite inhibits and reverses expression of adipogenic and fat cell-specific genes during in vitro adipogenesis. Toxicol. Sci. 2002, 65, 211–219. [Google Scholar] [CrossRef]
- Hou, H.; Yu, Y.; Shen, Z.; Liu, S.; Wu, B. Hepatic transcriptomic responses in mice exposed to arsenic and different fat diet. Environ. Sci. Pollut. Res. 2017, 24, 10621–10629. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Vann, D.R.; Doulias, P.-T.; Wang, T.; Landesberg, G.; Li, X.; Ricciotti, E.; Scalia, R.; He, M.; Hand, N.J. Hepatic metal ion transporter ZIP8 regulates manganese homeostasis and manganese-dependent enzyme activity. J. Clin. Investig. 2017, 127, 2407–2417. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Mahowald, N.; Bond, T.; Chuang, P.; Artaxo, P.; Siefert, R.; Chen, Y.; Schauer, J. Combustion iron distribution and deposition. Glob. Biogeochem. Cycl. 2008, 22. [Google Scholar] [CrossRef]
- Weinborn, V.; Valenzuela, C.; Olivares, M.; Arredondo, M.; Weill, R.; Pizarro, F. Prebiotics increase heme iron bioavailability and do not affect non-heme iron bioavailability in humans. Food Funct. 2017, 8, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Furuyama, K.; Kaneko, K. Heme as a Magnificent Molecule with Multiple Missions: Heme Determines Its Own Fate and Governs Cellular Homeostasis. Tohoku J. Exp. Med. 2007, 213, 1–16. [Google Scholar] [CrossRef]
- Jensen, J.; Swaminathan, S.; Tosti, C.; Szulcl, K.; Hultman, K.; Wu, E.; Nunezl, A.; Sheth, S.; Brittenham, G.; Brown, T. Quantification of ferritin iron in presence of hemosiderin. In Proceedings of the International Society of Magnetic Resonance in Medicine, Berlin, Germany, 19–25 May 2007. [Google Scholar]
- Kiela, P.R.; Ghishan, F.K. Physiology of intestinal absorption and secretion. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 145–159. [Google Scholar] [CrossRef]
- Milic, S.; Mikolasevic, I.; Orlic, L.; Devcic, E.; Starcevic-Cizmarevic, N.; Stimac, D.; Kapovic, M.; Ristic, S. The role of iron and iron overload in chronic liver disease. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 2144. [Google Scholar] [CrossRef]
- Kato, I.; Dnistrian, A.; Schwartz, M.; Toniolo, P.; Koenig, K.; Shore, R.; Zeleniuch-Jacquotte, A.; Akhmedkhanov, A.; Riboli, E. Risk of Iron Overload among Middle-aged Women. Int. J. Vitam. Nutr. Res. 2000, 70, 119–125. [Google Scholar] [CrossRef]
- Choi, J.S.; Koh, I.-U.; Lee, H.J.; Kim, W.H.; Song, J. Effects of excess dietary iron and fat on glucose and lipid metabolism. J. Nutr. Biochem. 2013, 24, 1634–1644. [Google Scholar] [CrossRef]
- Hatunic, M.; Finucane, F.M.; Brennan, A.M.; Norris, S.; Pacini, G.; Nolan, J.J. Effect of iron overload on glucose metabolism in patients with hereditary hemochromatosis. Metabolism 2010, 59, 380–384. [Google Scholar] [CrossRef]
- Valenzuela, R.; Rincóncervera, M.Á.; Echeverría, F.; Barrera, C.; Espinosa, A.; Hernándezrodas, M.C.; Ortiz, M.; Valenzuela, A.; Videla, L.A. Iron-induced pro-oxidant and pro-lipogenic responses in relation to impaired synthesis and accretion of long-chain polyunsaturated fatty acids in rat hepatic and extrahepatic tissues. Nutrition 2018, 45, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Fausther, M.; Pritchard, M.T.; Popov, Y.V.; Bridle, K. Contribution of liver nonparenchymal cells to hepatic fibrosis: Interactions with the local microenvironment. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef]
- Huang, Y.; Deng, X.; Liang, J. Modulation of hepatic stellate cells and reversibility of hepatic fibrosis. Exp. Cell Res. 2017, 352, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Gardi, C.; Arezzini, B.; Fortino, V.; Comporti, M. Effect of free iron on collagen synthesis, cell proliferation and MMP-2 expression in rat hepatic stellate cells. Biochem. Pharmacol. 2002, 64, 1139–1145. [Google Scholar] [CrossRef]
- Dias, H.B.; Krause, G.C.; Squizani, E.D.; Lima, K.G.; Schuster, A.D.; Pedrazza, L.; de Souza Basso, B.; Martha, B.A.; de Mesquita, F.C.; Nunes, F.B. Fructose-1, 6-bisphosphate reverts iron-induced phenotype of hepatic stellate cells by chelating ferrous ions. Biometals 2017, 30, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.A.; Dawod, A.E.A.; Nasr, M.A. Role of iron in diabetes mellitus and its complications. Menoufia Med. J. 2016, 29, 11. [Google Scholar]
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; Banasiak, W.; Polonski, L.; Filippatos, G. Iron deficiency: An ominous sign in patients with systolic chronic heart failure. Eur. Heart J. 2010, 31, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Frassinetti, S.; Bronzetti, G.L.; Caltavuturo, L.; Cini, M.; Croce, C.D. The role of zinc in life: A review. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 597. [Google Scholar] [CrossRef]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef]
- Gammoh, N.Z.; Rink, L. Zinc in infection and inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef]
- Abregú, F.M.G.; Gobetto, M.N.; Juriol, L.V.; Caniffi, C.; Elesgaray, R.; Tomat, A.L.; Arranz, C. Developmental programming of vascular dysfunction by prenatal and postnatal zinc deficiency in male and female rats. J. Nutr. Biochem. 2018, 56, 89–98. [Google Scholar] [CrossRef]
- Samadi, A.; Isikhan, S.Y.; Tinkov, A.A.; Lay, I.; Doşa, M.D.; Skalny, A.V.; Skalnaya, M.G.; Chirumbolo, S.; Bjørklund, G. Zinc, copper, and oxysterol levels in patients with type 1 and type 2 diabetes mellitus. Clin. Nutr. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Elcik, D.; Dogan, A.; Cetinkaya, Z.; Cetin, A.; Inanc, M.T.; Topsakal, R.; Kalay, N.; Eryol, N.K.; Oguzhan, A. The relation of serum trace elements and coronary atherosclerotic progression. Trace Elem. Electrolytes 2019, 36, 210–214. [Google Scholar] [CrossRef]
- Du, K.; Liu, M.-Y.; Zhong, X.; Wei, M.-J. Decreased circulating Zinc levels in Parkinson’s disease: A meta-analysis study. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Discovery of human zinc deficiency: 50 years later. J. Trace Elem. Med. Biol. 2012, 26, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Laity, J.H.; Lee, B.M.; Wright, P.E. Zinc finger proteins: New insights into structural and functional diversity. Curr. Opin. Struct. Biol. 2001, 11, 39–46. [Google Scholar] [CrossRef]
- Okine, B.N.; Gaspar, J.C.; Finn, D.P. PPARs and pain. Br. J. Pharmacol. 2019, 176, 1421–1442. [Google Scholar] [CrossRef]
- Takashi, H.; Tsutomu, M. Associations between Zinc Deficiency and Metabolic Abnormalities in Patients with Chronic Liver Disease. Nutrients 2018, 10, 88. [Google Scholar]
- Mousavi, S.N.; Faghihi, A.; Motaghinejad, M.; Shiasi, M.; Imanparast, F.; Amiri, H.L.; Shidfar, F. Zinc and Selenium Co-supplementation Reduces Some Lipid Peroxidation and Angiogenesis Markers in a Rat Model of NAFLD-Fed High Fat Diet. Boil. Trace Element Res. 2017, 181, 288–295. [Google Scholar] [CrossRef]
- Kar, K.; De, J.; Bhattyacharya, G. Study of Zinc in Cirrhosis of Liver. Ind. Med. Gaz. 2013, 75, 74–78. [Google Scholar]
- Katayama, K.; Kawaguchi, T.; Shiraishi, K.; Ito, T.; Suzuki, K. The Prevalence and Implication of Zinc Deficiency in Patients With Chronic Liver Disease. J. Clin. Med. Res. 2018, 10, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.C.; Luo, Z.; Hogstrand, C.; Xu, Y.H.; Song, Y.F. Zinc reduces hepatic lipid deposition and activates lipophagy via Zn 2+/MTF-1/PPARα and Ca 2+/CaMKKβ/AMPK pathways. Faseb J. 2018, 32, 6666–6680. [Google Scholar] [CrossRef] [PubMed]
- Sugino, H.; Kumagai, N.; Watanabe, S.; Toda, K.; Takeuchi, O.; Tsunematsu, S.; Morinaga, S.; Tsuchimoto, K. Polaprezinc attenuates liver fibrosis in a mouse model of non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2008, 23, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Timbol, A.B.; Roberto Razo, I.I.; Villaluna, R.A.; Ong, J. 740: Zinc Supplementation for Hepatic Encephalopathy in Chronic Liver Disease: A Meta-Analysis. Crit. Care Med. 2013, 41, A183. [Google Scholar] [CrossRef]
- Saner, G.; Süoğlu, Ö.D.; Yiğitbaşı, M.; Sökücü, S.; Elkabes, B. Zinc nutrition in children with chronic liver disease. J. Trace Elem. Exp. Med. 2000, 13, 271–276. [Google Scholar] [CrossRef]
- Umusig-Quitain, P.; Gregorio, G.V. High incidence of zinc deficiency among Filipino children with compensated and decompensated liver disease. J. Gastroenterol. Hepatol. 2010, 25, 387–390. [Google Scholar] [CrossRef]
- Haybar, H.; Shahrabi, S.; Rezaeeyan, H.; Shirzad, R.; Saki, N. Endothelial cells: From dysfunction mechanism to pharmacological effect in cardiovascular disease. Cardiovasc. Toxicol. 2019, 19, 13–22. [Google Scholar] [CrossRef]
- Jarosz, M.; Olbert, M.; Wyszogrodzka, G.; Młyniec, K.; Librowski, T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology 2017, 25, 11–24. [Google Scholar] [CrossRef]
- Shen, H.; Oesterling, E.; Stromberg, A.; Toborek, M.; MacDonald, R.; Hennig, B. Zinc deficiency induces vascular pro-inflammatory parameters associated with NF-κB and PPAR signaling. J. Am. Coll. Nutr. 2008, 27, 577–587. [Google Scholar] [CrossRef]
- Ogórek, M.; Gąsior, Ł.; Pierzchała, O.; Daszkiewicz, R.; Lenartowicz, M. Role of copper in the process of spermatogenesis. Postepy Higieny I Medycyny Doswiadczalnej 2017, 71, 663. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.-F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, I.; Dringen, R.; Mercer, J.F. Copper: Effects of deficiency and overload. In Interrelations between Essential Metal Ions and Human Diseases; Springer: Berlin, Germany, 2013; pp. 359–387. [Google Scholar]
- Husak, V.V.; Mosiichuk, N.M.; Kubrak, O.I.; Matviishyn, T.M.; Lushchak, V.I. Acute exposure to copper induces variable intensity of oxidative stress in goldfish tissues. Fish. Physiol. Biochem. 2018, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Stuart, M.; Bowman, T. Bioavailability of copper to rats from various foodstuffs and in the presence of different carbohydrates. Proc. Soc. Exp. Biol. Med. 1988, 23, 353. [Google Scholar]
- Shen, J. Micronutrients: Essential Minerals to Human Body. Food Sci. Technol. 1995, 3, 31–32. [Google Scholar]
- Herrera, C.; Pettiglio, M.A.; Bartnikas, T.B. Investigating the role of transferrin in the distribution of iron, manganese, copper, and zinc. JBIC J. Biol. Inorg. Chem. 2014, 19, 869–877. [Google Scholar] [CrossRef]
- Inesi, G. Molecular features of copper binding proteins involved in copper homeostasis. IUBMB Life 2017, 69, 211–217. [Google Scholar] [CrossRef]
- Frayn, K.N.; Arner, P.; Yki-Järvinen, H. Fatty acid metabolism in adipose tissue, muscle and liver in health and disease. Essays Biochem. 2006, 42, 89–103. [Google Scholar]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R.; Akil, M.; Bicer, M. Selenium: Its metabolism and relation to exercise. Pak. J. Pharm. Sci. 2016, 29, 1719–1725. [Google Scholar]
- Dos Reis, A.R.; El-Ramady, H.; Santos, E.F.; Gratão, P.L.; Schomburg, L. Overview of selenium deficiency and toxicity worldwide: Affected areas, selenium-related health issues, and case studies. In Selenium in Plants; Springer: Berlin, Germany, 2017; pp. 209–230. [Google Scholar]
- Jones, L.; Diehl, A.M. Diabetes and the Liver; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar]
- Ren, D.; Hu, Y.; Luo, Y.; Yang, X. Selenium-containing polysaccharides from Ziyang green tea ameliorate high-fructose diet induced insulin resistance and hepatic oxidative stress in mice. Food Funct. 2015, 6, 3342–3350. [Google Scholar] [CrossRef]
- Mueller, A.S.; Pallauf, J. Compendium of the antidiabetic effects of supranutritional selenate doses. In vivo and in vitro investigations with type II diabetic db/db mice. J. Nutr. Biochem. 2006, 17, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Donma, M.; Donma, O. Promising link between selenium and peroxisome proliferator activated receptor gamma in the treatment protocols of obesity as well as depression. Med. Hypotheses 2016, 89, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Rannem, T.; Ladefoged, K.; Hylander, E.; Christiansen, J.; Laursen, H.; Kristensen, J.; Linstow, M.; Beyer, N.; Liguori, R.; Dige-Petersen, H. The effect of selenium supplementation on skeletal and cardiac muscle in selenium-depleted patients. J. Parenter. Enter. Nutr. 1995, 19, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Dallak, M. A synergistic protective effect of selenium and taurine against experimentally induced myocardial infarction in rats. Arch. Physiol. Biochem. 2017, 123, 344–355. [Google Scholar] [CrossRef]
- Mirdamadi, A.; Rafiei, R.; Kahazaipour, G.; Fouladi, L. Selenium level in patients with heart failure versus normal individuals. Int. J. Prev. Med. 2019, 10. [Google Scholar] [CrossRef]
- Do Brasil, P.E.A.A.; de Souza, A.P.; Hasslocher-Moreno, A.M.; Xavier, S.S.; Passos, S.R.L.; Moreira, M.D.F.R.; de Oliveira, M.S.; da Silva, G.M.S.; Saraiva, R.M.; de Aguiar Cardoso, C.S.; et al. Selenium Treatment and Chagasic Cardiopathy (STCC): Study protocol for a double-blind randomized controlled trial. Trials 2014, 15, 388. [Google Scholar] [CrossRef]
- Zimmermann, M.B. The role of iodine in human growth and development. Semin. Cell Dev. Boil. 2011, 22, 645–652. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Micronutrient Deficiency. Our World in Data. 2017. Available online: https://ourworldindata.org/micronutrient-deficiency (accessed on 1 March 2020).
- Liang, Z.; Xu, C.; Luo, Y.J. Association of iodized salt with goiter prevalence in Chinese populations: A continuity analysis over time. Mil. Med. Res. 2017, 4, 8. [Google Scholar] [CrossRef]
- Martin, J.; Wang, Z.Q.; Zhang, X.H.; Wachtel, D.; Volaufova, J.; Matthews, D.E.; Cefalu, W.T. Chromium Picolinate Supplementation Attenuates Body Weight Gain and Increases Insulin Sensitivity in Subjects With Type 2 Diabetes. Diabetes Care 2006, 29, 1826–1832. [Google Scholar] [CrossRef][Green Version]
- Thomas, V.L.K.; Gropper, S.S. Effect of chromium nicotinic acid supplementation on selected cardiovascular disease risk factors. Biol. Trace Elem. Res. 1997, 55, 297–305. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; McClain, C.; Feng, W. Cobalt chloride induces the reduction of fibroblast growth factor 21 in Caco-2 cells through HIF and PPAR-α and–γ pathway. FASEB J. 2011, 25. [Google Scholar] [CrossRef]
- Wang, Z.X.; Jiang, C.S.; Lei, L.; Wang, X.H.; Jin, H.J.; Qiao, W.; Quan, C. The role of Akt on arsenic trioxide suppression of 3T3-L1 preadipocyte differentiation. Cell Res. 2005, 15, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Murphy, F.T. Fortified Micronutrient Food and Beverage Additive. U.S. Patent Application No. 16/362,302, 15 August 2019. [Google Scholar]
- Mirza, M.A.; Shakeel, F.; Iqbal, Z. An overview of the regulatory and developmental strategies of chronotherapeutics. Ther. Innov. Regul. Sci. 2016, 50, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H.; Peppas, N.A. Chronobiology, drug delivery, and chronotherapeutics. Adv. Drug Deliv. Rev. 2007, 59, 828–851. [Google Scholar] [CrossRef]
- Hiriart, M.; Velasco, M.; Larqué, C.; Diaz-Garcia, C.M. Metabolic syndrome and ionic channels in pancreatic beta cells. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2014; Volume 95, pp. 87–114. [Google Scholar]
- Rauws, A. Pharmacokinetics of bromide ion—An overview. Food Chem. Toxicol. 1983, 21, 379–382. [Google Scholar] [CrossRef]
- Czerwinski, A.L. Bromide excretion as affected by chloride administration. J. Am. Pharm. Assoc. 1958, 47, 467–471. [Google Scholar] [CrossRef]
- AL-Fifi, Z.I.; Mujallid, M.I. Effect of circadian on the activities of ion transport ATPases and histological structure of kidneys in mice. Saudi J. Biol. Sci. 2019, 26, 963–969. [Google Scholar] [CrossRef]
- Shimura, M.; Akaike, N.; Harata, N. Circadian rhythm in intracellular Cl− activity of acutely dissociated neurons of suprachiasmatic nucleus. Am. J. Physiol. Cell Physiol. 2002, 282, C366–C373. [Google Scholar] [CrossRef][Green Version]
- Charoensuksai, P.; Xu, W. PPARs in rhythmic metabolic regulation and implications in health and disease. PPAR Res. 2010, 2010. [Google Scholar] [CrossRef]
- Maekawa, M.; Watanabe, A.; Iwayama, Y.; Kimura, T.; Hamazaki, K.; Balan, S.; Ohba, H.; Hisano, Y.; Nozaki, Y.; Ohnishi, T. Polyunsaturated fatty acid deficiency during neurodevelopment in mice models the prodromal state of schizophrenia through epigenetic changes in nuclear receptor genes. Transl. Psychiatry 2017, 7, e1229. [Google Scholar] [CrossRef]
- Burns, K.A.; Heuvel, J.P.V. Modulation of PPAR activity via phosphorylation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2007, 1771, 952–960. [Google Scholar] [CrossRef] [PubMed]
| Elements | Diseases or Models | Organ or Cells | Doses of Elements | Change of PPARs |
|---|---|---|---|---|
| Fe | Hyperlipidemia, Hamsters | Liver | 10 mg/d i.p. | PPAR-α↓ [113] |
| Diabetes, Rats | Pancreas | De | PPAR-β/δ↑ [114] | |
| Oxidative Stress, Rats | Central Nervous System | 3 mM | PPAR-γ↑ [26] | |
| Zn | Atherosclerosis | HAECs | 15 μM | PPAR-α↑ [115] |
| Inflammation | PPAECs | 12 μM | PPAR-γ↑ [116] | |
| Steatosis, Mice | Liver | 75 mg/L Liquid Diet | PPAR-α↑ [117] | |
| Sepsis, Mice | Lung | 1.3 mg/kg BW i.p. | PPAR-γ↑ [118] | |
| Cu | Rabbits | Liver, Muscle, Adipose Tissue | 5–45 mg/kg Diet | PPAR-α↑ [28] |
| Pufferfish | Liver | 24–98 μg/L Water | PPAR-γ↑ [119,120] | |
| Se | Chicken | Pancreas | 2 mg/kg Diet | PPAR-γ↑ [121] |
| Infection | Mammary Gland | De | PPAR-γ↓ [122] | |
| Proliferation | HaCaT Keratinocytes | 10 μM | PPAR-β/δ↑ [123] | |
| PCOS, Human | Lymphocytes | 200 μg/d p.o. | PPAR-γ↑ [124] | |
| Diabetes, Human | Macrophages | 100–300 μg/d p.o. | PPAR-γ↑ [125] | |
| HFD-fed Mice | Liver | 0.3 μg/d Diet | PPAR-α↑, PPAR-γ↓ [126] | |
| Heart Damage, Mice | Heart, H9c2 | 9 mg/L Water, 5μM | PPAR-γ↓ [127] | |
| I | Mammary Cancer, Rats | Tumor | 0.05% in Water | PPAR-α↓, PPAR-γ↑ [128,129] |
| Cr | Exercise-trained Rats | Liver, Muscle | 4 mg/kg BW i.g. | PPAR-γ↑, PPARβ/δ↑ [130,131] |
| Diabetes, Rats | Adipose Tissue | 80 μg/kg BW i.g. | PPAR-γ↑ [132] | |
| NAFLD, Mice | Liver | 80 μg/kg BW i.g. | PPAR-α↑ [133] | |
| Co | Hypoxia | Trophoblast Cells | 100μM | PPAR-α/β/γ↓ [134] |
| Hypoxia, Rats | Heart | 60 mg/kg BW i.p. | PPAR-α↓ [135] | |
| Mn | Neurotoxicity | U87, SK-N-SH | 4 mM | PPAR-α/β/γ↓ [136] |
| Oxidative Stress, Mice | Mitochondria | De | PPAR-α↑ [137] | |
| Si | - | - | - | PPAR-α/β/γ↑ [138] |
| V | Adipogenesis | 3T3-L1 | 2.5–10 μM | PPAR-γ↓ [139,140] |
| db/db Mice | Adipose Tissue | 0.05 mmol/kg BW i.g. | PPAR-γ↑ [141] | |
| Br | Hyperlipidemia | Hepatocytes | 1–10 μM | PPAR-α↑ [91] |
| Cd | Chicken | Pancreas | 150 mg/kg Diet | PPAR-γ↓ [121] |
| Hg | HFD-fed Mice | Adipocytes | 1 mg/kg BW s.c. | PPAR-α↓, PPARγ↓ [142] |
| As | - | hMETSCs | 0.2–4μM | PPAR-γ↓ [143] |
| Adipogenesis | C3H/10T1/2 | 6 μM | PPAR-γ↓ [144] | |
| HFD-fed Mice | Liver | 3 mg/L Water | PPAR-γ↓ [145] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Zou, Y.; Shen, Z.; Xiong, Y.; Zhang, W.; Liu, C.; Chen, S. Trace Elements, PPARs, and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2612. https://doi.org/10.3390/ijms21072612
Shi Y, Zou Y, Shen Z, Xiong Y, Zhang W, Liu C, Chen S. Trace Elements, PPARs, and Metabolic Syndrome. International Journal of Molecular Sciences. 2020; 21(7):2612. https://doi.org/10.3390/ijms21072612
Chicago/Turabian StyleShi, Yujie, Yixin Zou, Ziyue Shen, Yonghong Xiong, Wenxiang Zhang, Chang Liu, and Siyu Chen. 2020. "Trace Elements, PPARs, and Metabolic Syndrome" International Journal of Molecular Sciences 21, no. 7: 2612. https://doi.org/10.3390/ijms21072612
APA StyleShi, Y., Zou, Y., Shen, Z., Xiong, Y., Zhang, W., Liu, C., & Chen, S. (2020). Trace Elements, PPARs, and Metabolic Syndrome. International Journal of Molecular Sciences, 21(7), 2612. https://doi.org/10.3390/ijms21072612





