A Tribute to Lewis Wolpert and His Ideas on the 50th Anniversary of the Publication of His Paper ‘Positional Information and the Spatial Pattern of Differentiation’. Evidence for a Timing Mechanism for Setting Up the Vertebrate Anterior-Posterior (A-P) Axis
Abstract
1. Introduction: Lewis Wolpert and His Ideas
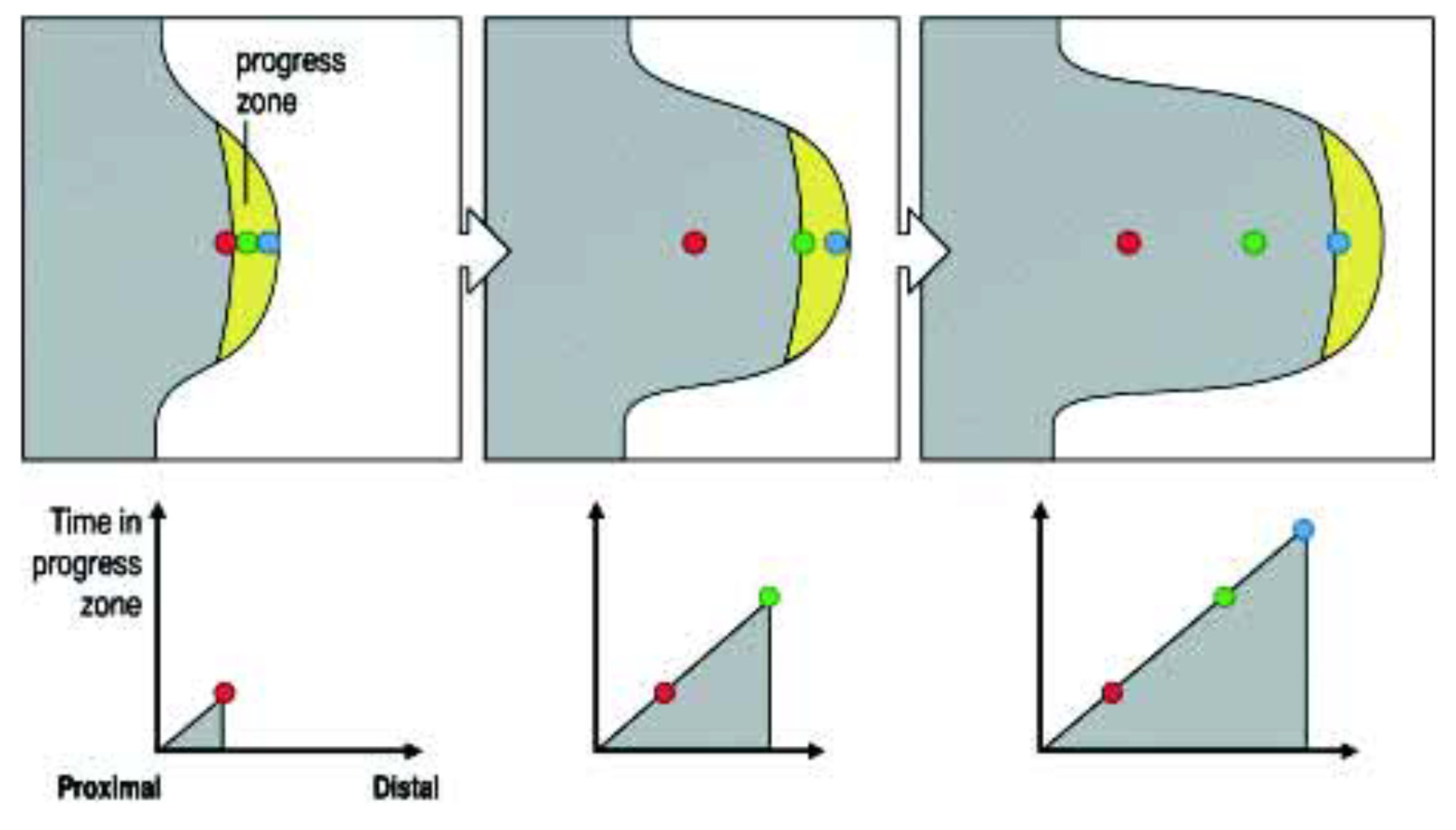
2. Wolpert’s ‘Progress Zone’ Model
3. The Vertebrate A-P Axis Is Also Generated in A Timed Manner
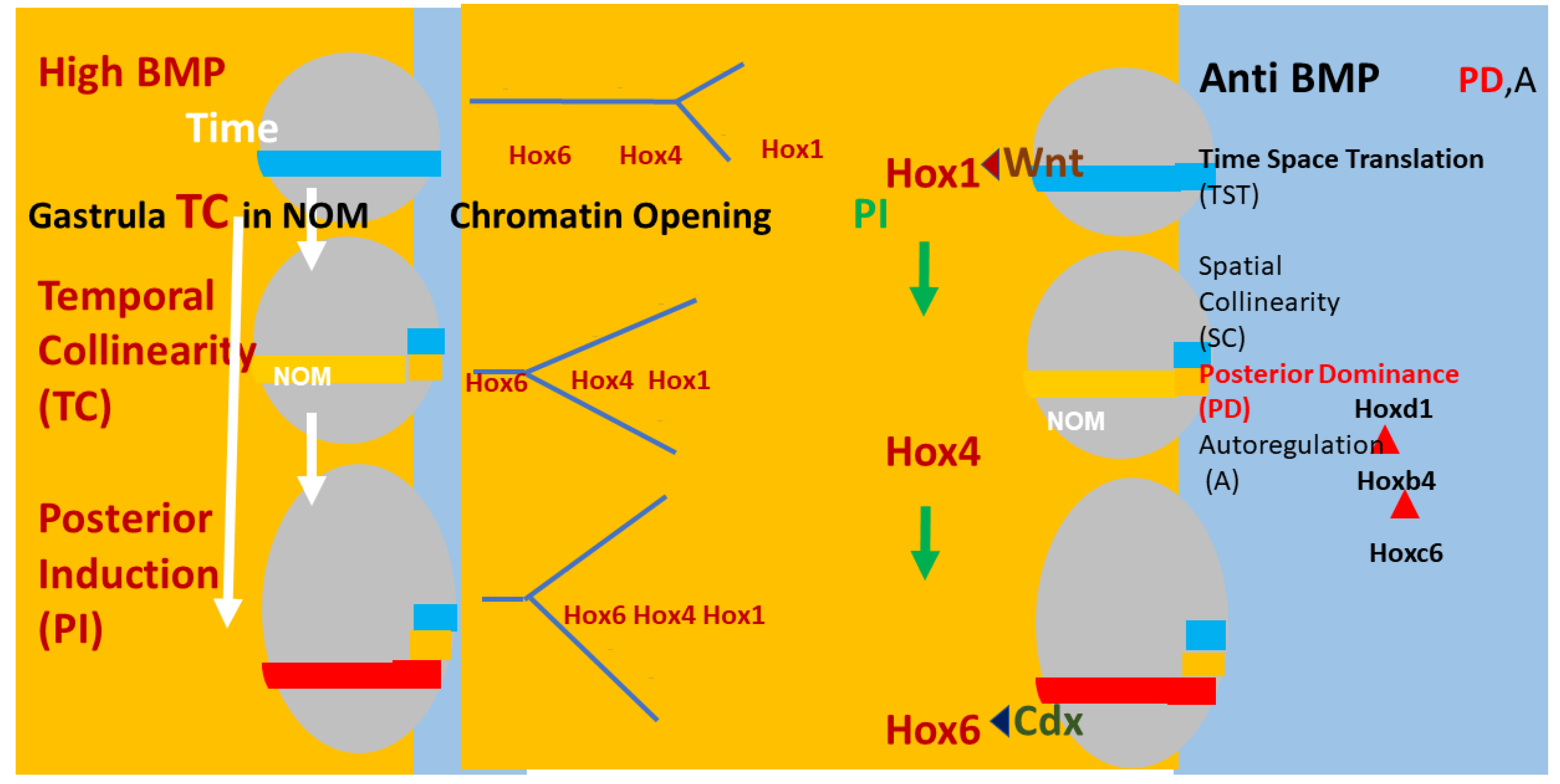
4. What Is the Nature of the Vertebrate Axial Timing Mechanism?
5. Do P-D Limb Patterning and A-P Body Axis Patterning Have A Common Basis?
6. Questions for the Future
7. Hearty Congratulations to Lewis on These Important Anniversaries
Funding
Acknowledgments
Conflicts of Interest
References
- Wolpert, L. Positional Information and the Spatial Pattern of Cellular Differentiation. J Theor. Biol. 1969, 25, 1–47. [Google Scholar] [CrossRef]
- Summerbell, D.; Lewis, J.; Wolpert, L. Positional Information in Chick Limb Morphogenesis. Nature 1973, 244, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, L.; Tickle, C.; Sampford, M.; Lewis, J.H. The effect of cell killing by X-Irradiationn on pattern formation in the chick limb. Development 1979, 50, 175–198. [Google Scholar]
- Wolpert, L. Limb patterning: Reports of Model’s Death Exaggerated. Curr. Biol. 2002, 12, R628–R630. [Google Scholar] [CrossRef]
- Wolpert, L. The Progress Zone Model for specifying Positional Information. Int. J. Dev. Biol. 2002, 46, 869–870. [Google Scholar] [PubMed]
- Tabin, C.; Wolpert, L. Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes Dev. 2007, 21, 1433–1442. [Google Scholar] [CrossRef]
- Brown, A.; Wolpert, L. The development of handedness in left/right asymmetry. Development 1990, 109, 1–9. [Google Scholar]
- Dudley, A.T.; Ros, M.A.; Tabin, C.J. A re-examination of proximodistal patterning during vertebrate limb development. Nature 2002, 418, 539–544. [Google Scholar] [CrossRef]
- Saunders, J.W. Is the Progress Zone Model a Victim of Progress? Cell 2002, 110, 541–543. [Google Scholar] [CrossRef]
- Towers, M.; Tickle, C. Growing models of vertebrate limb Development. Development 2009, 136, 179–190. [Google Scholar] [CrossRef]
- Maden, M. Intercalary regeneration in the amphibian limb and the rule of distal transformation. Development 1980, 56, 201–209. [Google Scholar]
- Roselló-Díez, A.; Arques, C.G.; Delgado, I.; Giovinazzo, G.; Torres, M. Diffusible signals and epigenetic timing cooperate in late proximo-distal limb patterning. Development 2014, 141, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Lopez, P.; Chinnaiya, K.; Campa, V.M.; Delgado, I.; Ros, M.A.; Towers, M. An intrinsic timer specifies distal structures of the vertebrate limb. Nat. Commun. 2015, 6, 8108. [Google Scholar] [CrossRef] [PubMed]
- Lewandoski, M.; Sun, X.; Martin, G.R. Fgf8 signalling from the AER is essential for normal limb development. Nat. Genet. 2000, 26, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Zakany, J.; Duboule, D. The role of Hox genes during vertebrate limb development. Curr. Opin. Genet. Dev. 2007, 17, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Niswander, L.; Jeffrey, S.; Martin, G.R.; Tickle, C. A positive feedback loop coordinates growth and patterning in the vertebrate limb. Nature 1994, 371, 609–612. [Google Scholar] [CrossRef]
- Cooper, K.L.; Kuang-Hsien Hu, J.; ten Berge, D.; Fernandez Teran, M.; Ros, M.A.; Tabin, C.J. Initiation of Proximal-Distal Patterning in the Vertebrate Limb by Signals and Growth. Science 2011, 332, 1083–1086. [Google Scholar] [CrossRef]
- Sheth, R.; Félix Bastida, M.; Kmita, M.; Ros, M. Self-Regulation, A New Facet of Hox Genes’ Function. Dev. Dyn. 2014, 243, 182–191. [Google Scholar] [CrossRef]
- Deschamps, J.; Duboule, D. Embryonic timing, axial stem cells, chromatin dynamics, and the Hox clock. Genes Dev. 2017, 31, 1406–1416. [Google Scholar] [CrossRef]
- Nieuwkoop, P.D. Activation and organisation of the central nervous system in Amphibians. III. Synthesis of a working hypothesis. J. Exp. Zool. 1952, 120, 83–108. [Google Scholar] [CrossRef]
- Eyal Giladi, H. Dynamic aspects of neural induction in Amphibia. Arch. Biol. 1954, 65, 179–259. [Google Scholar]
- Collier, J.R.; McInerney, D.; Schnell, S.; Maini, P.K.; Gavaghan, A.; Houston, P.; Stern, C.D. A cell cycle model for somitogenesis: Mathematical formulation and numerical simulation. J. Theor. Biol. 2000, 207, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Selleck, M.A.J.; Stern, C.D. Fate mapping and cell lineage analysis of Hensen’s node in the chick embryo. Development 1991, 112, 615–626. [Google Scholar] [PubMed]
- Gaunt, S.J.; Strachan, L. Temporal colinearity in expression of anterior Hox genes in developing chick embryos. Dev. Dyn. 1996, 207, 270–280. [Google Scholar] [CrossRef]
- Gaunt, S.J. Evolutionary shifts of vertebrate structures and Hox expression up and down the axial series of segments: A consideration of possible mechanisms. Int. J. Dev. Biol. 2000, 44, 109–117. [Google Scholar] [PubMed]
- Gamse, J.; Sive, H. Vertebrate anteroposterior patterning: The Xenopus Neurectoderm as a paradigm. BioEssays 2000, 22, 976–986. [Google Scholar] [CrossRef]
- Gamse, J.T.; Sive, H. Early anteroposterior division of the presumptive neurectoderm in Xenopus. Mech. Dev. 2001, 104, 21–36. [Google Scholar] [CrossRef]
- Vasiliauskas, D.; Stern, C.D. Patterning the Embryonic Axis: FGF Signaling and How Vertebrate Embryos Measure Time. Cell 2001, 106, 133–136. [Google Scholar] [CrossRef]
- Wacker, S.A.; Jansen, H.J.; McNulty, C.L.; Houtzager, E.; Durston, A.J. Timed interactions between the Hox expressing non-organiser mesoderm and the Spemann organiser generate positional information during vertebrate gastrulation. Dev. Biol. 2004, 268, 207–219. [Google Scholar] [CrossRef][Green Version]
- Tucker, J.A.; Mintzer, K.A.; Mullins, M.C. The BMP signaling gradient patterns dorsoventral tissue in a temporally progressive manner along the anteroposterior axis. Dev. Cell 2008, 14, 108–119. [Google Scholar] [CrossRef]
- Stern, C.D.; Charité, J.; Deschamps, J.; Duboule, D.; Durston, A.J.; Kmita, M.; Nicolas, J.F.; Palmeirim, I.; Smith, J.C.; Wolpert, L. Head-tail patterning of the vertebrate embryo: One, two or many unresolved problems? Int. J. Dev. Biol. 2006, 50, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Durston, A.J.; Zhu, K. A time space translation hypothesis for vertebrate axial patterning. Semin. Cell Dev. Biol. 2015, 42, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.S.; de Almeida, I.; Belmonte, J.M.; Glazier, J.A.; Stern, C.D. Somites without a clock. Science 2014, 343, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, M.; Mullins, M.C. Anteroposterior and dorsoventral patterning are coordinated by an identical patterning clock. Development 2013, 140, 19708. [Google Scholar] [CrossRef]
- Wymeersch, F.J.; Skylaki, S.; Huang, Y.; Watson, J.A.; Economou, C.; Carylyn Marek-Johnston, C.; Tomlinson, S.R.; Wilson, V. Transcriptionally dynamic progenitor populations organised around a stable niche drive axial patterning. Development 2019, 146, dev168161. [Google Scholar] [CrossRef]
- Faiella, A.; Zappavigna, V.; Mavilio, F.; Boncinelli, E. Inhibition of retinoic acid induced activation of 3’ human HOXB genes by antisense oligonucleotides affects sequential activation of genes located upstream in the four HOX clusters. Proc. Natl. Acad. Sci. USA 1994, 91, 5335–5339. [Google Scholar] [CrossRef]
- Hooiveld, M.H.; Morgan, R.; Der Rieden, P.I.; Houtzager, E.; Pannese, M.; Damen, K.; Boncinelli, E.; Durston, A. Novel colinear interactions between vertebrate Hox genes. Int. J. Dev. Biol. 1999, 43, 665–674. [Google Scholar]
- McNulty, C.L.; Peres, J.N.; Bardine, N.; van den Akker, W.M.R.; Durston, A.J. Knockdown of the complete Hox paralogous group 1 leads to dramatic hindbrain and neural crest defects. Development 2005, 132, 2861–2871. [Google Scholar] [CrossRef]
- Zhu, K.; Spaink, H.P.; Durston, A.J. Collinear Hox-Hox interactions are involved in patterning the vertebrate anteroposterior (A-P) axis. PLoS ONE 2017, 12, e0175287. [Google Scholar] [CrossRef]
- Zhu, K.; Spaink, H.P.; Durston, A.J. Hoxc6 loss of function truncates the main body axis in Xenopus. Cell Cycle 2017, 16, 1136–1138. [Google Scholar] [CrossRef]
- Durston, A.J. What are the roles of retinoids, other morphogens, and Hox genes in setting up the vertebrate body axis? Genesis 2019, 19, e23296. [Google Scholar] [CrossRef] [PubMed]
- Bardine, N.; Lamers, G.; Wacker, S.; Donow, C.; Knoechel, W.; Durston, A.J. Vertical signalling involves transmission of Hox information from gastrula mesoderm to neurectoderm. PLoS ONE 2014, 9, e115208. [Google Scholar] [CrossRef] [PubMed]
- Durston, A.J. Some Questions and Answers about the Role of Hox Temporal Collinearity in Vertebrate Axial Patterning. Front. Cell Dev. Biol. 2019, 7, 257. [Google Scholar] [CrossRef] [PubMed]
- Tarchini, B.; Duboule, D. Control of Hoxd genes collinearity during early limb development. Dev. Cell 2006, 10, 93–103. [Google Scholar] [CrossRef]
- Pizette, S.; Niswander, L. BMPs negatively regulate structure and function of the limb apical ectodermal ridge. Development 1999, 126, 883–894. [Google Scholar]
- Fromental-Ramain, C.; Warot, X.; Lakkaraju, S.; Favier, B.; Haack, H.; Birling, C.; Dierich, A.; Dolle, P.; Chambon, P. Specific and redundant functions of the paralogous Hoxa-9 and Hoxd-9 genes in forelimb and axial skeleton patterning. Development 1996, 122, 461–472. [Google Scholar]
- Wellik, D.M.; Capecchi, M.R. Hox10 and Hox11 Genes Are Required to Globally Pattern the Mammalian Skeleton. Science 2003, 301, 363–367. [Google Scholar] [CrossRef]
- Tickle, C. Making digit patterns in the vertebrate limb. Nat. Rev. Mol. Cell Biol. 2006, 7, 45–53. [Google Scholar] [CrossRef]
- Davis, A.P.; Witte, D.P.; Hsieh-Li, H.M.; Potter, S.S.; Capecchi, M.R. Absence of Radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature 1995, 375, 791–795. [Google Scholar] [CrossRef]
- Dollé, P.; Dierich, A.; LeMeur, M.; Schimmang, T.; Schuhbaur, B.; Chambon, P.; Duboule, D. Disruption of the Hoxd-13 gene induces localized heterochrony leading to mice with neotenic limbs. Cell 1993, 75, 431–441. [Google Scholar] [CrossRef]
- Fromental-Ramain, C.; Warot, X.; Messadecq, N.; LeMeur, M.; Chambon, P. Hoxa-13 and Hoxd-13 play a crucial role in the patterning of the limb autopod. Development 1996, 122, 2997–3011. [Google Scholar] [PubMed]
- Roselló-Díez, A.; Ros, M.A.; Torres, M. Diffusible signals, not autonomous mechanisms, determine the main proximodistal limb subdivision. Science 2011, 332, 1086–1088. [Google Scholar] [CrossRef] [PubMed]
- Kessel, M.; Gruss, P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell 1991, 67, 89–104. [Google Scholar] [CrossRef]
- Mahapatra, P.K.; Mohanty-Hejmadi, P. Vitamin A-mediated homeotic transformation of tail to limbs, limb suppression and abnormal tail regeneration in the Indian jumping frog Polypedates maculatus develop. Growth Differ. 1994, 36, 307–313. [Google Scholar] [CrossRef]
- Kondo, M.; Yamamoto, T.; Takahashi, S.; Taira, M. Comprehensive analyses of hox gene expression in Xenopus laevis embryos and adult tissues. Dev. Growth Differ. 2017, 59, 526–539. [Google Scholar] [CrossRef]
- Kondo, M.; Matsuo, M.; Igarashi, K.; Haramoto, Y.; Yamamoto, T.; Yasuoka, Y.; Taira, M. De novo transcription of multiple Hox cluster genes takes place simultaneously in early Xenopus tropicalis embryos. Biol. Open 2019, 8, bio038422. [Google Scholar] [CrossRef]
- Durston, A.J. Vertebrate hox temporal collinearity: Does it exist and what is its function? Cell Cycle 2019, 18, 523–530. [Google Scholar] [CrossRef]
- Durston, A.J. Hox Temporal Collinearity: Misleading Fallacy or Essential Developmental Mechanism? Preprints 2019, 2019060082. (accessed on 6 April 2020). [Google Scholar] [CrossRef]
- Pascoal, S.; Palmeirim, I. Watching out for chick limb development. Integr. Comp. Biol. 2007, 47, 382–389. [Google Scholar] [CrossRef]
- Jouve, C.; Iimura, T.; Pourquie, O. Onset of the segmentation clock in the chick embryo: Evidence for oscillations in the somite precursors in the primitive streak. Development 2002, 129, 1107–1117. [Google Scholar]
- Peres, J.; McNulty, C.; Durston, A.J. Interaction between X-Delta-2 and Hox genes regulates segmentation and patterning of the anteroposterior axis. Mech. Dev. 2006, 123, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Riedel-Kruse, H.; Müller, C.; Oates, A.C. Synchrony dynamics during initiation, failure and rescue of the segmentation clock. Science 2007, 317, 1911–1915. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | A-P Main Axis | PD-AP Limb |
|---|---|---|
| Morphogens | ||
| FGF | + (Hox6) | + (AER) |
| Retinoid | + (Hox1) | + (proximal) |
| Wnt | + | + |
| BMP | + Axis connection D-V-A-P | + Axis connection D-V-P-D |
| Chromatin | ||
| Histone acetylation | + | + |
| Hox genes | + (1–13) TC,SC. | + (9–13) TC, SC. |
| Timer | + | + |
| Stage dependence | Early anterior | Early proximal |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durston, A.J. A Tribute to Lewis Wolpert and His Ideas on the 50th Anniversary of the Publication of His Paper ‘Positional Information and the Spatial Pattern of Differentiation’. Evidence for a Timing Mechanism for Setting Up the Vertebrate Anterior-Posterior (A-P) Axis. Int. J. Mol. Sci. 2020, 21, 2552. https://doi.org/10.3390/ijms21072552
Durston AJ. A Tribute to Lewis Wolpert and His Ideas on the 50th Anniversary of the Publication of His Paper ‘Positional Information and the Spatial Pattern of Differentiation’. Evidence for a Timing Mechanism for Setting Up the Vertebrate Anterior-Posterior (A-P) Axis. International Journal of Molecular Sciences. 2020; 21(7):2552. https://doi.org/10.3390/ijms21072552
Chicago/Turabian StyleDurston, Antony J. 2020. "A Tribute to Lewis Wolpert and His Ideas on the 50th Anniversary of the Publication of His Paper ‘Positional Information and the Spatial Pattern of Differentiation’. Evidence for a Timing Mechanism for Setting Up the Vertebrate Anterior-Posterior (A-P) Axis" International Journal of Molecular Sciences 21, no. 7: 2552. https://doi.org/10.3390/ijms21072552
APA StyleDurston, A. J. (2020). A Tribute to Lewis Wolpert and His Ideas on the 50th Anniversary of the Publication of His Paper ‘Positional Information and the Spatial Pattern of Differentiation’. Evidence for a Timing Mechanism for Setting Up the Vertebrate Anterior-Posterior (A-P) Axis. International Journal of Molecular Sciences, 21(7), 2552. https://doi.org/10.3390/ijms21072552




