Structural Requirements of Benzofuran Derivatives Dehydro-δ- and Dehydro-ε-Viniferin for Antimicrobial Activity Against the Foodborne Pathogen Listeria monocytogenes
Abstract
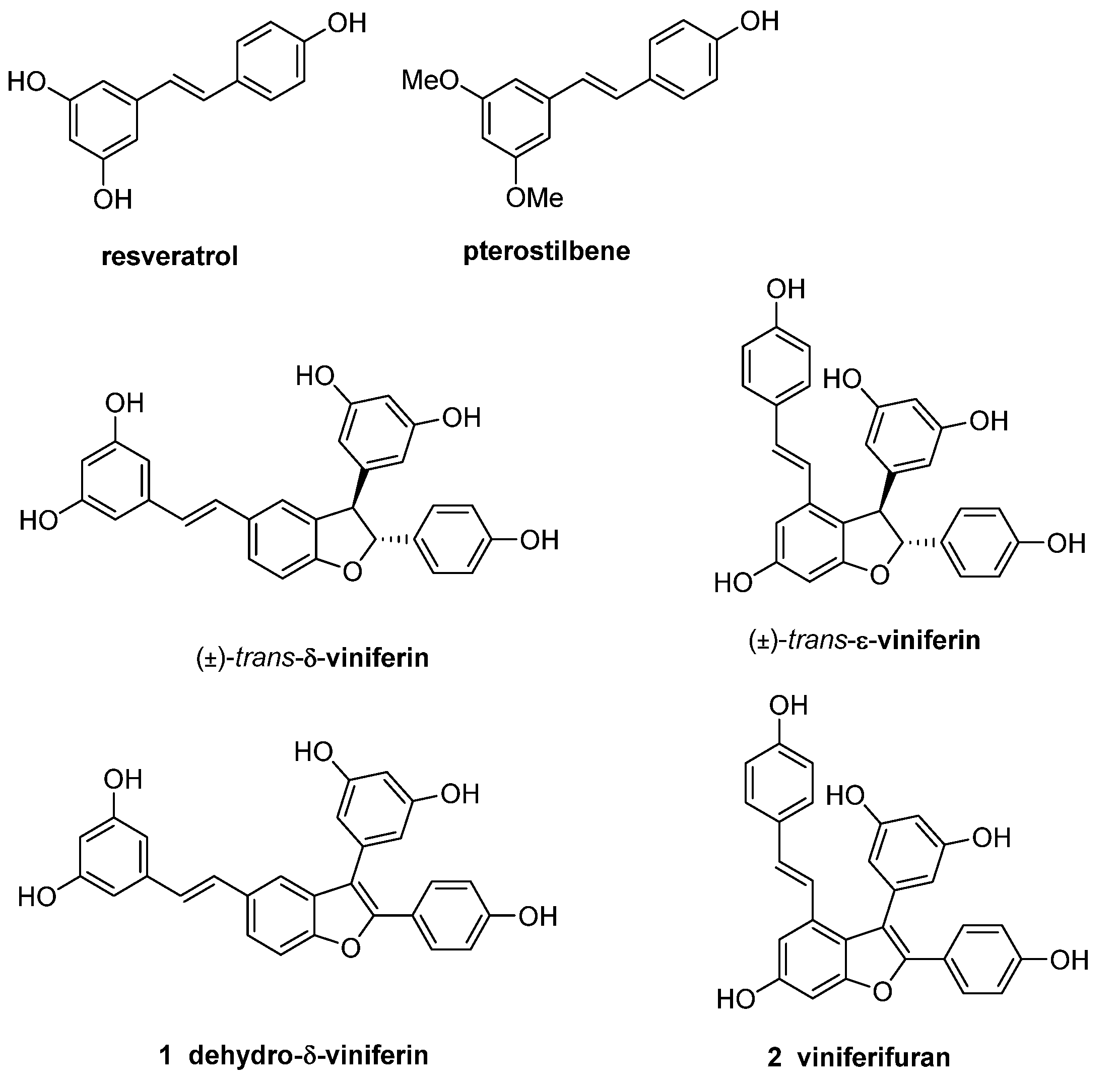
1. Introduction
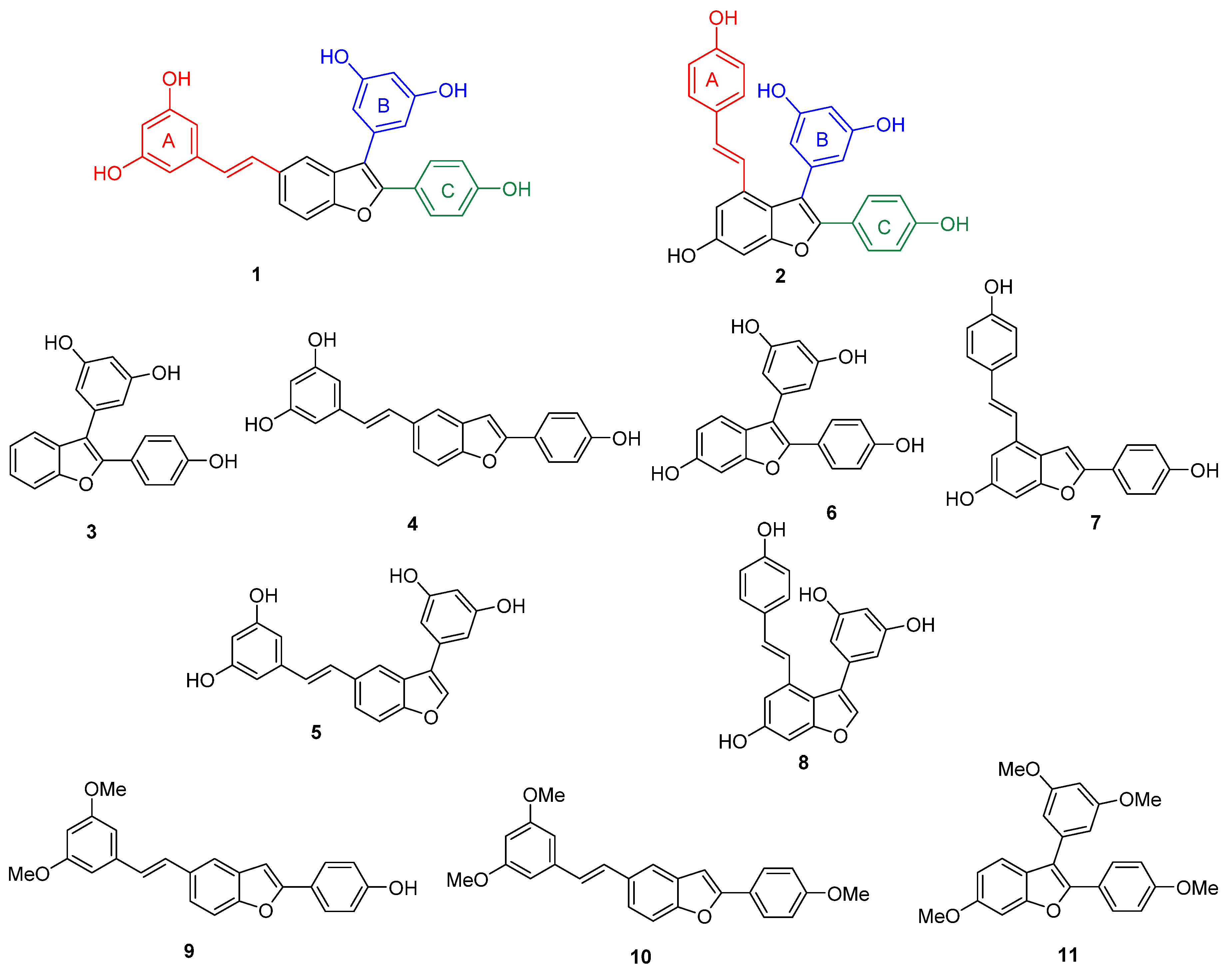
2. Results
3. Discussion
4. Materials and Methods
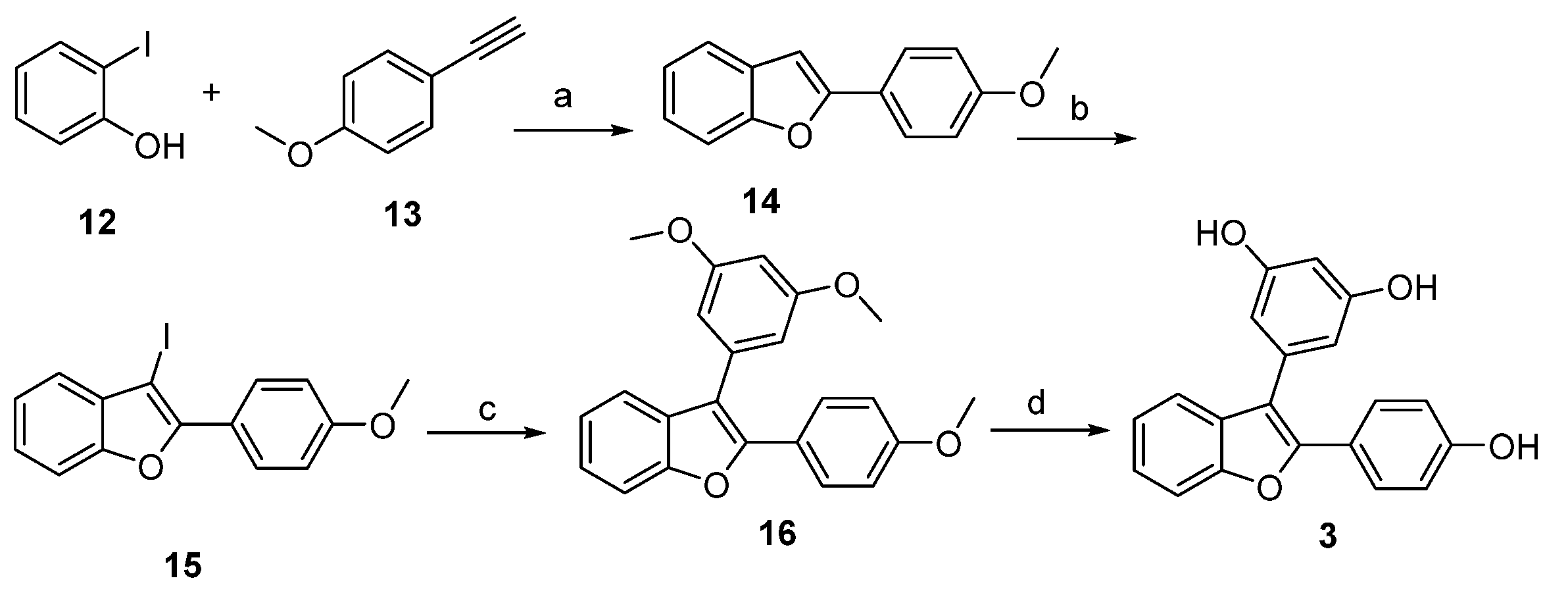
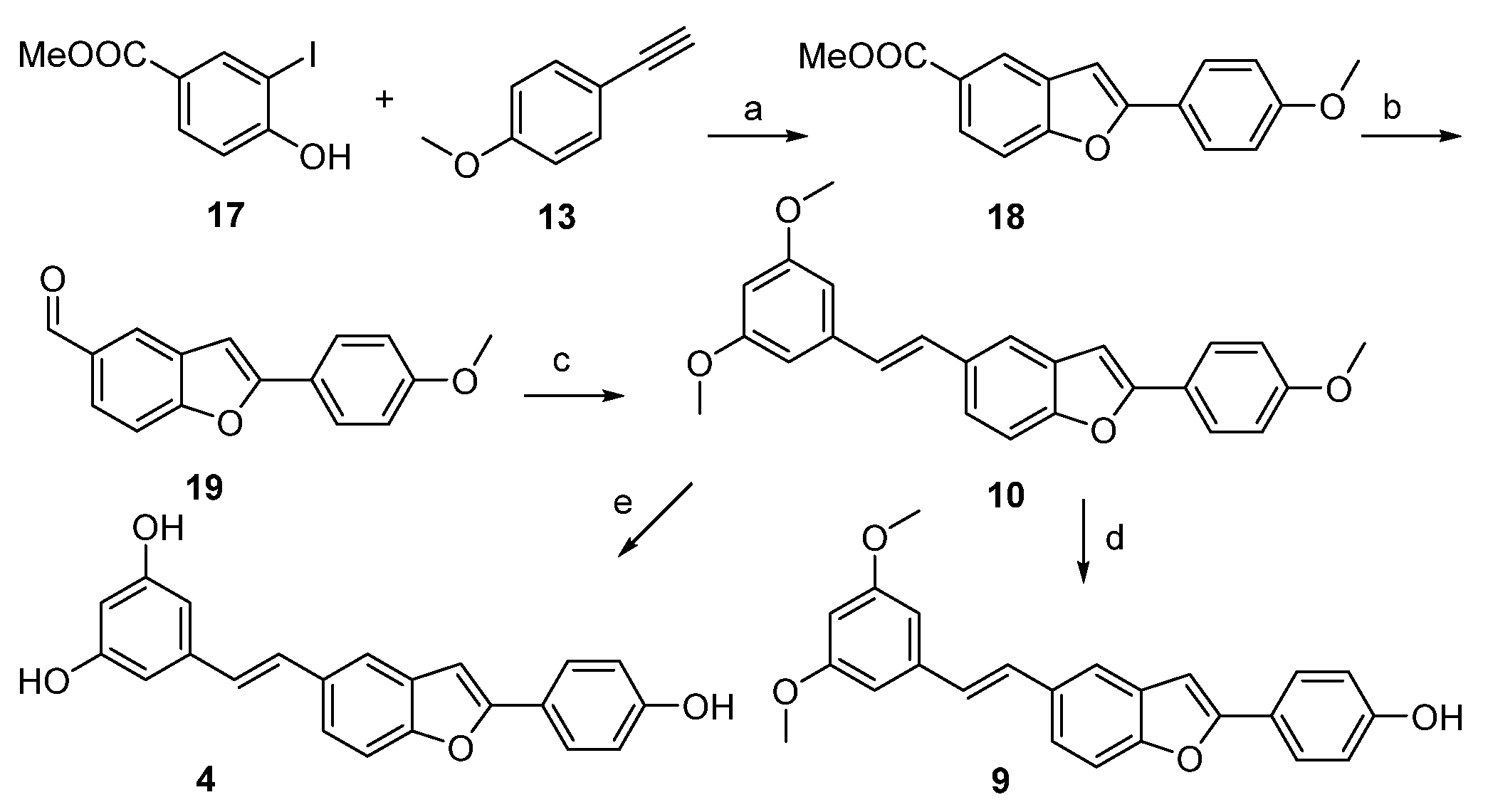
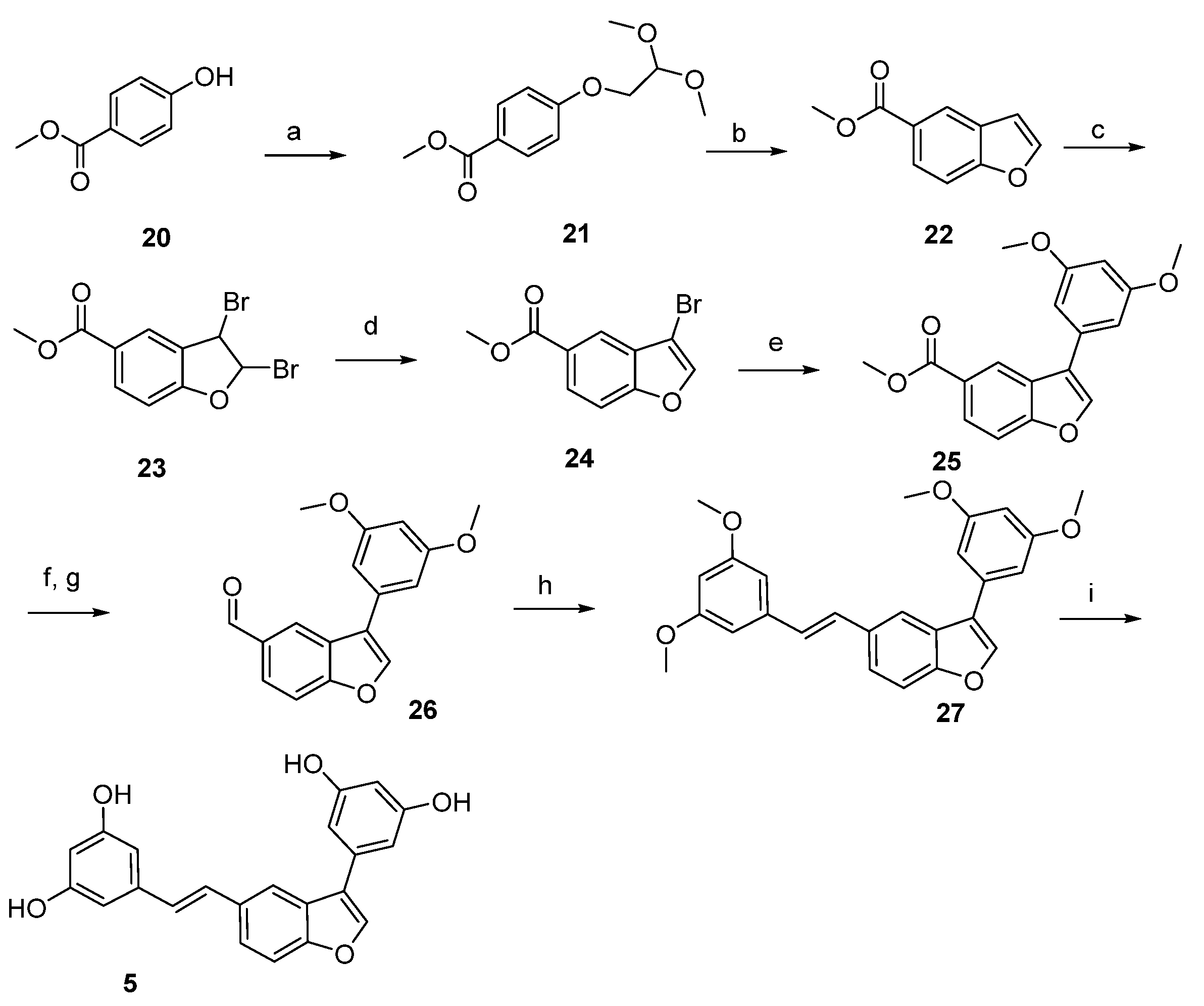
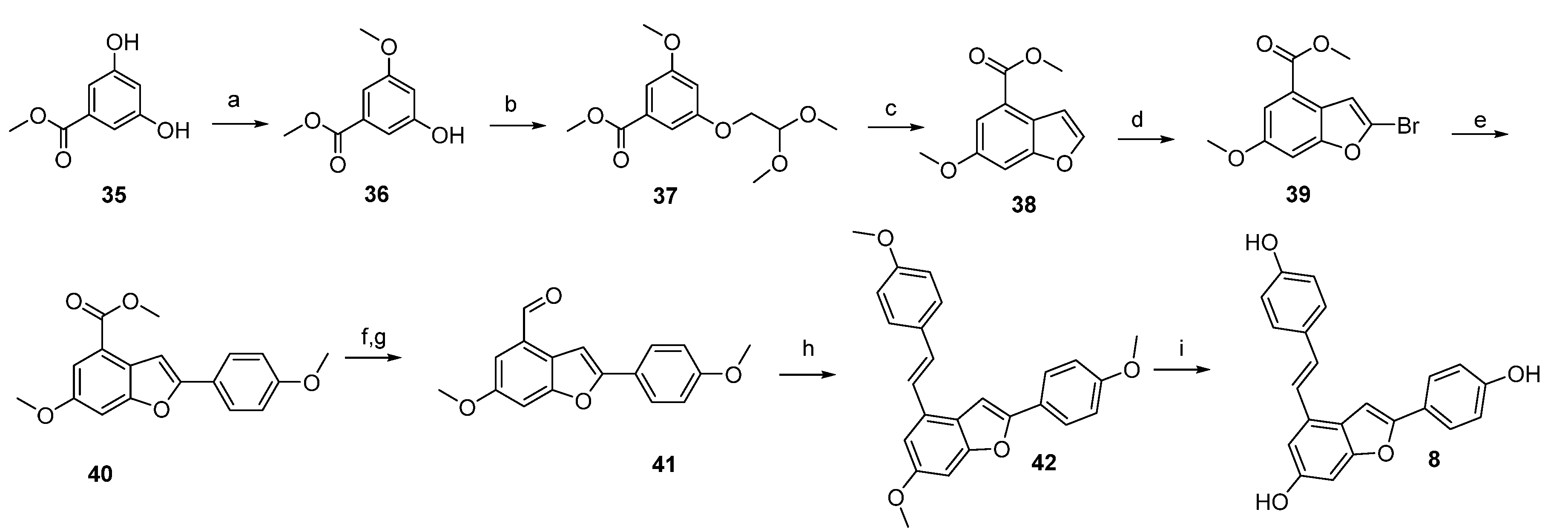
4.1. Chemical Synthesis
4.2. Evaluation of the Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)
4.3. Cytotoxicity on Human Skin Normal WS1 Fibroblast Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khanam, H. Bioactive benzofuran derivatives: A review. Eur. J. Med. Chem. 2015, 97, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.; Harmalkar, D.S.; Xu, X.; Jang, K.; Lee, K. Bioactive benzofuran derivatives: Moracins A-Z in medicinal chemistry. Eur. J. Med. Chem. 2015, 90, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Hiremathad, A.; Patil, M.R.; Chethana, K.R.; Chand, K.; Amelia Santos, M.; Keri, R.S. Benzofuran: An emerging scaffold for antimicrobial agents. RSC Adv. 2015, 5, 96809–96828. [Google Scholar] [CrossRef]
- Mattio, L.M.; Dallavalle, S.; Musso, L.; Filardi, R.; Franzetti, L.; Pellegrino, L.; D’Incecco, P.; Mora, D.; Pinto, A.; Arioli, S. Antimicrobial activity of resveratrol-derived monomers and dimers against foodborne pathogens. Sci. Rep. 2019, 9, 19525. [Google Scholar] [CrossRef]
- Markina, N.A.; Chen, Y.; Larock, R.C. Efficient microwave-assisted one-pot three-component synthesis of 2,3-disubstituted benzofurans under Sonogashira conditions. Tetrahedron 2013, 69, 2701–2713. [Google Scholar] [CrossRef]
- Jaseer, E.A.; Prasad, D.J.C.; Sekar, G. Domino synthesis of 2-arylbenzo[b]furans by copper (II)-catalyzed coupling of o-iodophenols and aryl acetylenes. Tetrahedron 2010, 66, 2077–2082. [Google Scholar] [CrossRef]
- Yao, T.; Yue, D.; Larock, R.C. Synthesis of 2,3-disubstituted benzofurans by the palladium-catalyzed coupling of 2-iodoanisoles and terminal alkynes. Org. Synth. 2014, 91, 283–292. [Google Scholar] [CrossRef]
- Kraus, G.A.; Gupta, V. A new synthetic strategy for the synthesis of bioactive stilbene dimers. A direct synthesis of amurensin H. Tetrahedron Lett. 2009, 50, 7180–7183. [Google Scholar] [CrossRef]
- Vo, D.D.; Elofsson, M. Total Synthesis of Viniferifuran, Resveratrol-Piceatannol Hybrid, Anigopreissin A and Analogues—Investigation of Demethylation Strategies. Adv. Synth. Catal. 2016, 358, 4085–4092. [Google Scholar] [CrossRef]
- Liu, J.; Do, T.J.; Simmons, C.J.; Lynch, J.C.; Gu, W.; Ma, Z.; Xuc, W.; Tang, W. Total synthesis of diptoindonesin G and its analogues as selective modulators of estrogen receptors. Org. Biomol. Chem. 2016, 14, 8927–8930. [Google Scholar] [CrossRef]
- Saitoh, M.; Kunitomo, J.; Kimura, E.; Iwashita, H.; Uno, Y.; Onishi, T.; Uchiyama, N.; Kawamoto, T.; Tanaka, T.; Mol, C.D.; et al. (2-{3-[4-(Alkylsulfinyl)phenyl]-1-benzofuran-5-yl}-5-methyl-1,3,4-oxadiazole Derivatives as Novel Inhibitors of Glycogen Synthase Kinase-3ß with Good Brain Permeability. J. Med. Chem. 2009, 52, 6270–6286. [Google Scholar] [CrossRef]
- Kim, I.; Choi, J. A versatile approach to oligostilbenoid natural products—Synthesis of permethylated analogues of viniferifuran, malibatol A., and shoreaphenol. Org. Biomol. Chem. 2009, 7, 2788–2795. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, A.E.G.; Öberg, C.T.; Hillgren, J.M.; Elofsson, M. Total Synthesis of the Resveratrol Oligomers (±)-Ampelopsin B and (±)-ϵ-Viniferin. Eur. J. Org. Chem. 2016, 3, 426–429. [Google Scholar] [CrossRef]
- Iino, T.; Tsukahara, D.; Kamata, K.; Sasaki, K.; Ohyama, S.; Hosaka, H.; Hasegawa, T.; Chiba, M.; Nagata, Y.; Eiki, J.; et al. Discovery of potent and orally active 3-alkoxy-5-phenoxy-N-thiazolyl benzamides as novel allosteric glucokinase activators. Bioorg. Med. Chem. 2009, 17, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Domingues, F. The antimicrobial action of resveratrol against Listeria monocytogenes in food-based models and its antibiofilm properties. J. Sci. Food Agric. 2016, 96, 4531–4535. [Google Scholar] [CrossRef] [PubMed]
- Hammerum, A.M.; Heuer, O.E. Human health hazards from antimicrobial-resistant Escherichia coli of animal origin. Clin. Infect. Dis. 2009, 48, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.K.; Donato, J.; Wang, H.H.; Cloud-Hansen, K.A.; Davies, J.; Handelsman, J. Call of wild: Antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 2010, 8, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Molla, M.N.; Cantor, C.R.; Collins, J.J. Bacterial charity work leads to population wide-resistance. Nature 2010, 467, 82–85. [Google Scholar] [CrossRef]
- Akinwumi, B.C.; Bordun, K.A.M.; Anderson, H.D. Biological activities of stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef]
- Ma, D.S.L.; Tan, L.T.; Chan, K.; Yap, W.; Pusparajah, P.; Chuah, L.; Ming, L.C.; Khan, T.M.; Lee, L.; Goh, B. Resveratrol-Potential antibacterial agent against foodborne pathogens. Front. Pharmacol. 2018, 9, 102. [Google Scholar] [CrossRef]
- Alvarez-Martinez, F.J.; Barrajon-Catalan, E.; Encinar, J.A.; Rodriguez-Diaz, J.C.; Micol, V. Antimicrobial Capacity of Plant Polyphenols against Gram-positive Bacteria: A Comprehensive Review. Curr. Med. Chem. 2019, 26, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Chibane, L.B.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Qi, H.; Jiang, Y.; Gao, H. Antibacterial Activity and Membrane-Disruptive Mechanism of 3-p-trans-Coumaroyl-2-hydroxyquinic Acid, a Novel Phenolic Compound from Pine Needles of Cedrus deodara, against Staphylococcus aureus. Molecules 2016, 21, 1084. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]

| Compound | L. monocytogenes Scott A | L. monocytogenes Scott A | WS1 |
|---|---|---|---|
| MIC (MBC) µg/mL a | MIC (µM) a | IC50 (µM) b | |
| resveratrol | 200 (-) c | 876 | >200 |
| pterostilbene | 64 (128) | 250 | 57 ± 10 |
| 1 dehydro-δ-viniferin | 2 (16) | 4.42 | 37.0 ± 1.4 |
| 2 viniferifuran | 16 (>512) | 35.4 | 33.0 ± 1.4 |
| 3 | 16 (64) | 50.3 | 98.7 ± 1.8 |
| 4 | 256 (>512) | 743 | 97.8 ± 3.0 |
| 5 | 16 (64) | 44.4 | 96.8 ± 4.5 |
| 6 | 64 (>512) | 191 | 98.5 ± 2.0 |
| 7 | 8 (64) | 23.2 | 45.0 ± 1.2 |
| 8 | 64 (>512) | 178 | 85.0 ± 4.6 |
| 9 | >512 (>512) | 1370 | 95.0 ± 2.3 |
| 10 | >256 (>256) | 662 | >100 |
| 11 | >256 (>256) | 656 | >100 |
| Chlorhexidine | 8 (32) | 15.8 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catinella, G.; Mattio, L.M.; Musso, L.; Arioli, S.; Mora, D.; Beretta, G.L.; Zaffaroni, N.; Pinto, A.; Dallavalle, S. Structural Requirements of Benzofuran Derivatives Dehydro-δ- and Dehydro-ε-Viniferin for Antimicrobial Activity Against the Foodborne Pathogen Listeria monocytogenes. Int. J. Mol. Sci. 2020, 21, 2168. https://doi.org/10.3390/ijms21062168
Catinella G, Mattio LM, Musso L, Arioli S, Mora D, Beretta GL, Zaffaroni N, Pinto A, Dallavalle S. Structural Requirements of Benzofuran Derivatives Dehydro-δ- and Dehydro-ε-Viniferin for Antimicrobial Activity Against the Foodborne Pathogen Listeria monocytogenes. International Journal of Molecular Sciences. 2020; 21(6):2168. https://doi.org/10.3390/ijms21062168
Chicago/Turabian StyleCatinella, Giorgia, Luce M. Mattio, Loana Musso, Stefania Arioli, Diego Mora, Giovanni Luca Beretta, Nadia Zaffaroni, Andrea Pinto, and Sabrina Dallavalle. 2020. "Structural Requirements of Benzofuran Derivatives Dehydro-δ- and Dehydro-ε-Viniferin for Antimicrobial Activity Against the Foodborne Pathogen Listeria monocytogenes" International Journal of Molecular Sciences 21, no. 6: 2168. https://doi.org/10.3390/ijms21062168
APA StyleCatinella, G., Mattio, L. M., Musso, L., Arioli, S., Mora, D., Beretta, G. L., Zaffaroni, N., Pinto, A., & Dallavalle, S. (2020). Structural Requirements of Benzofuran Derivatives Dehydro-δ- and Dehydro-ε-Viniferin for Antimicrobial Activity Against the Foodborne Pathogen Listeria monocytogenes. International Journal of Molecular Sciences, 21(6), 2168. https://doi.org/10.3390/ijms21062168









