eIF4E and Interactors from Unicellular Eukaryotes
Abstract
1. Introduction
2. eIF4E
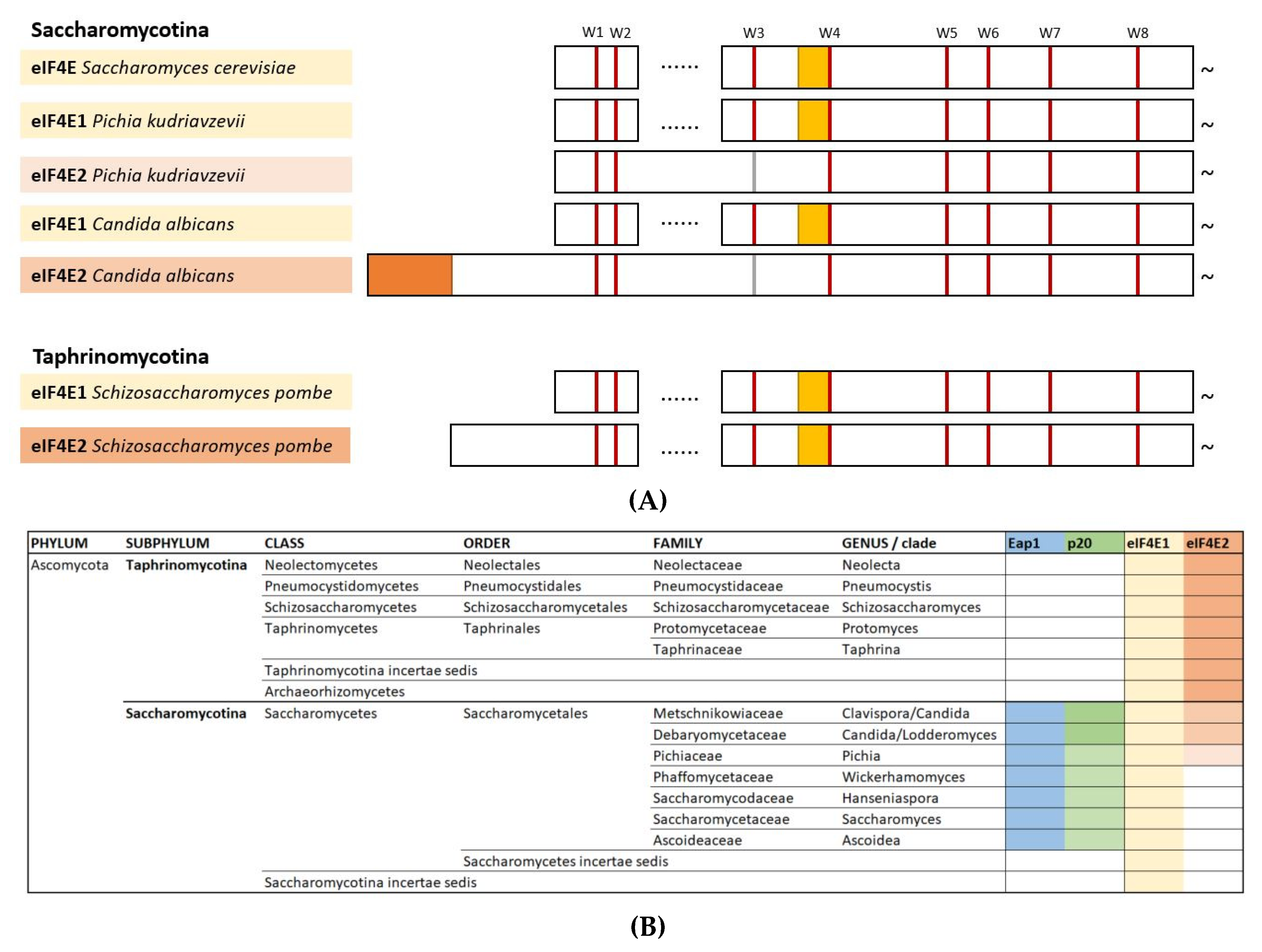
2.1. eIF4E from Yeast Species
- 1.)
- One (sometimes two) copies of the eIF4E1 gene, e.g., S. cerevisiae.
- 2.)
- Besides eIF4E1, one eIF4E2 gene is thought to act as an eIF4E-inhibitor or might be required for translating specific mRNAs under stress conditions (e.g., C. albicans or S. pombe). While eIF4E1 is essential for survival, eIF4E2 is not [38].
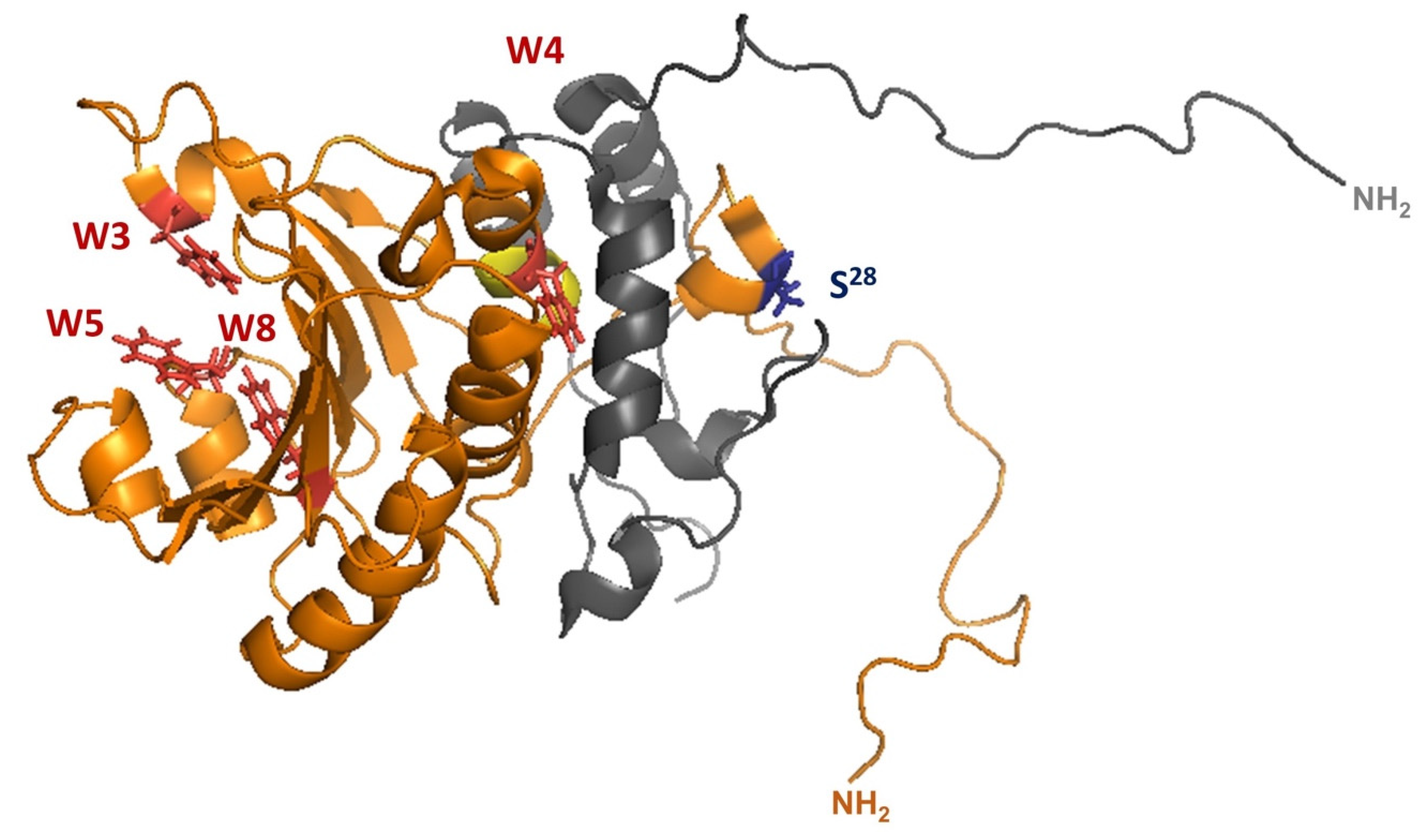
2.1.1. Yeast eIF4E1 Is Phosphorylated
2.1.2. eIF4E2 from Saccharomycotina
2.1.3. eIF4E2 from Taphrinomycotina
3. Yeast eIF4E Interactors
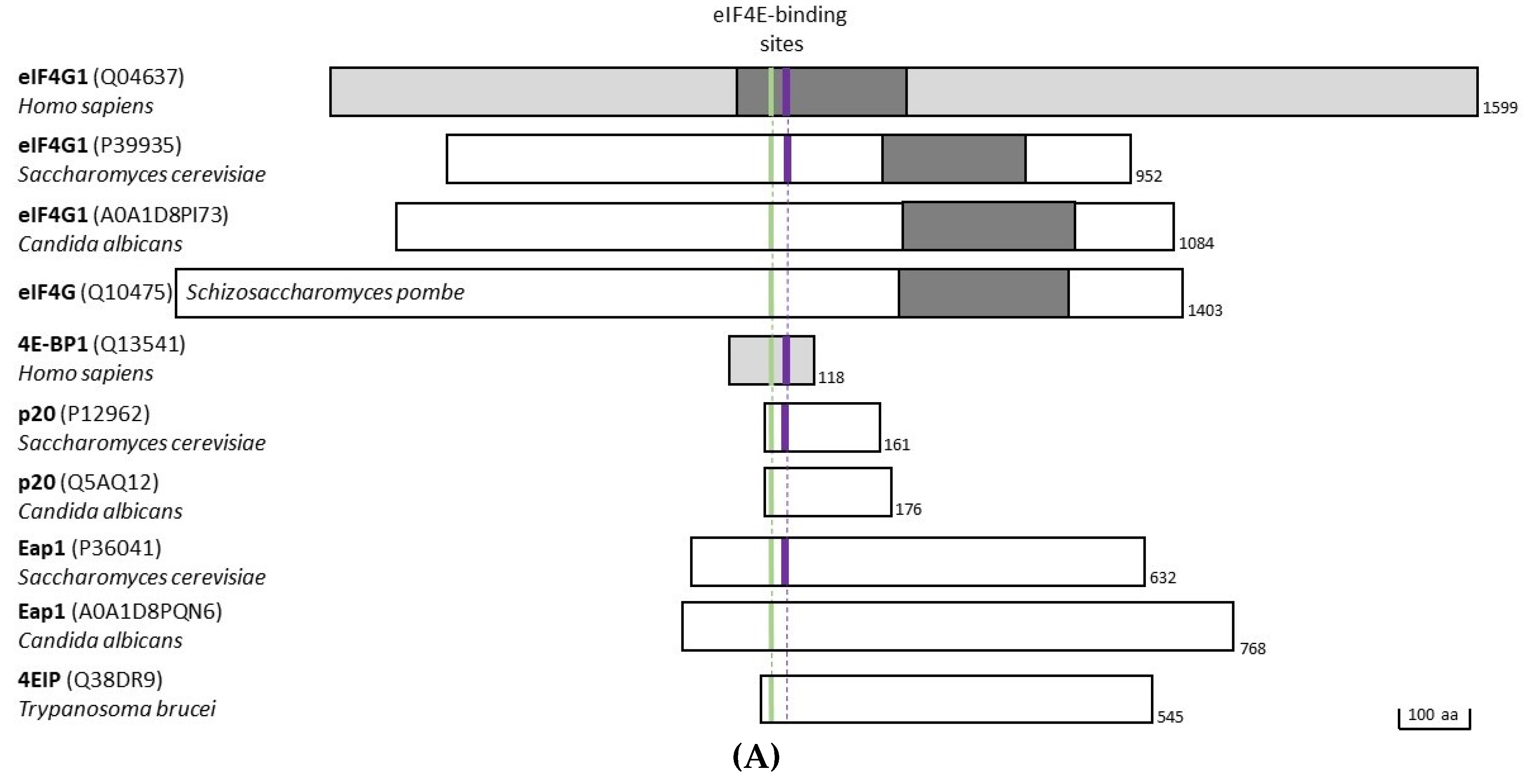
3.1. eIF4G
3.2. Eap1
3.3. p20
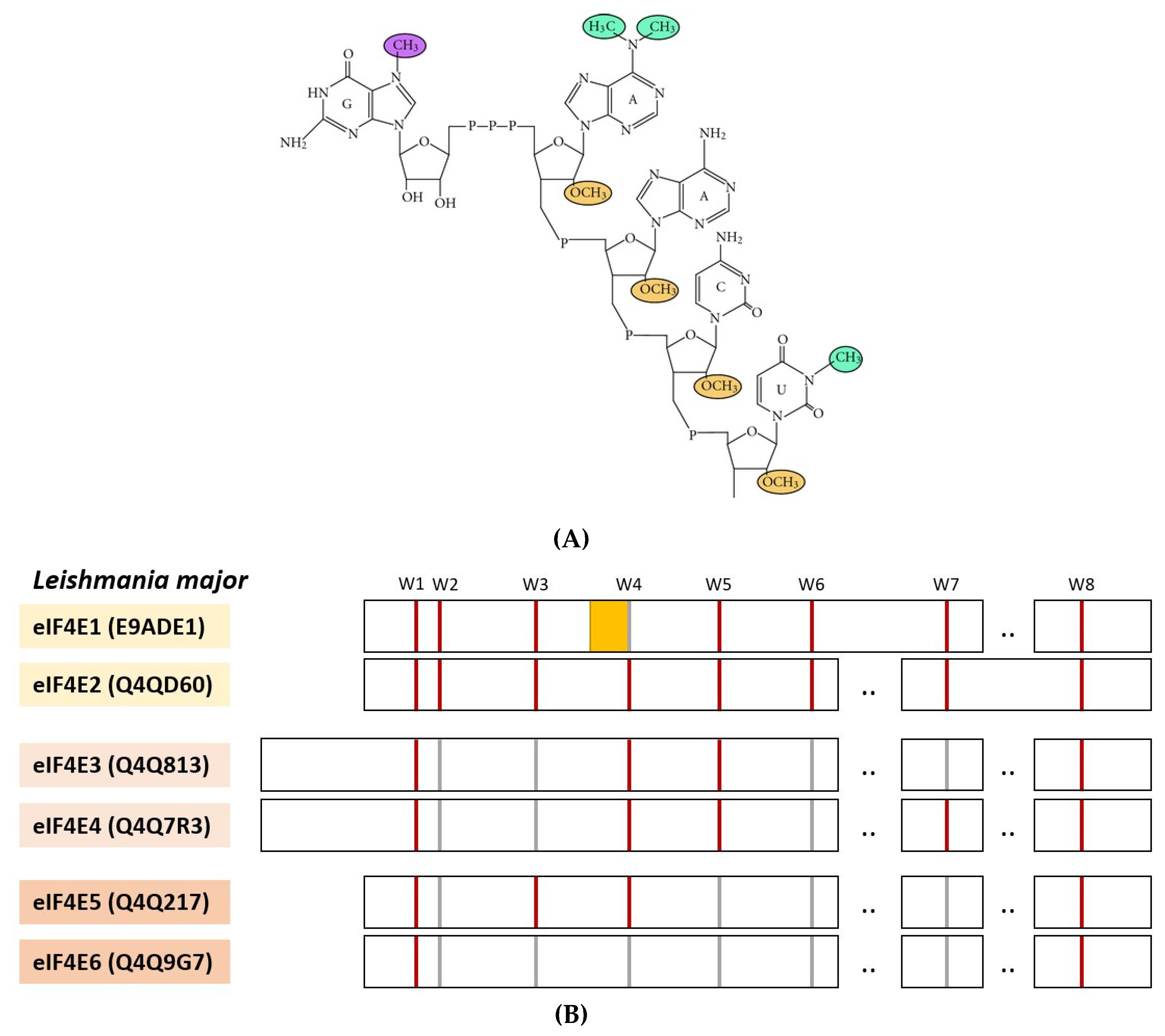
4. eIF4E and Interactors from Protozoan Parasites
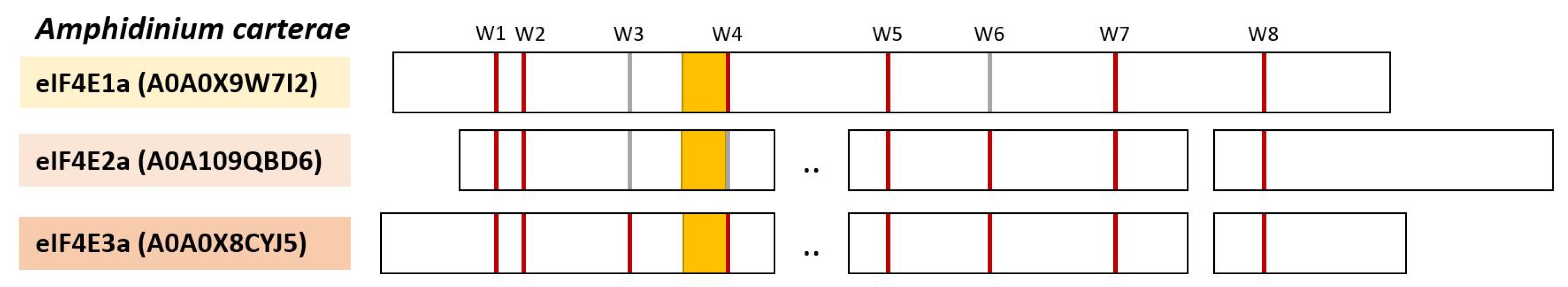
5. eIF4Es from Dinoflagellates
6. Summary and Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Valzania, L.; Ono, H.; Ignesti, M.; Cavaliere, V.; Bernardi, F.; Gamberi, C.; Gargiulo, G. Drosophila 4EHP is essential for the larval-pupal transition and required in the prothoracic gland for ecdysone biosynthesis. Dev. Biol. 2016, 410, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.-L.; Kim, C.S.; Uszkoreit, S.; Wickstrom, S.A.; Schumacher, B. Somatic Niche Cells Regulate the CEP-1/p53-Mediated DNA Damage Response in Primordial Germ Cells. Dev. Cell 2019, 50, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Minshall, N.; Schernthaner-Reiter, M.H.; Weil, D.; Standart, N. CPEB Interacts with an Ovary-specific eIF4E and 4E-T in Early Xenopus Oocytes. J. Biol. Chem. 2007, 282, 37389–37401. [Google Scholar] [CrossRef] [PubMed]
- Hernández, G.; Miron, M.; Han, H.; Liu, N.; Magescas, J.; Tettweiler, G.; Lasko, P. Mextli is a novel eukaryotic translation initiation factor 4E-binding protein that promotes translation in Drosophila melanogaster. Mol. Cell. Biol. 2013, 33, 2854–2864. [Google Scholar] [CrossRef]
- Rieckher, M.; Markaki, M.; Princz, A.; Schumacher, B.; Tavernarakis, N. Maintenance of Proteostasis by P Body-Mediated Regulation of eIF4E Availability during Aging in Caenorhabditis elegans. Cell Rep. 2018, 25, 199–211. [Google Scholar] [CrossRef]
- Dinkova, T.D.; Keiper, B.D.; Korneeva, N.L.; Aamodt, E.J.; Rhoads, R.E. Translation of a small subset of Caenorhabditis elegans mRNAs is dependent on a specific eukaryotic translation initiation factor 4E isoform. Mol. Cell. Biol. 2005, 25, 100–113. [Google Scholar] [CrossRef]
- Keiper, B.D.; Lamphear, B.J.; Deshpande, A.M.; Jankowska-Anyszka, M.; Aamodt, E.J.; Blumenthal, T.; Rhoads, R.E. Functional characterization of five eIF4E isoforms in Caenorhabditis elegans. J. Biol. Chem. 2000, 275, 10590–10596. [Google Scholar] [CrossRef]
- Huggins, H.P.; Subash, J.S.; Stoffel, H.; Henderson, M.A.; Hoffman, J.L.; Buckner, D.S.; Sengupta, M.S.; Boag, P.R.; Lee, M.-H.; Keiper, B.D. Distinct roles of two eIF4E isoforms in the germline of Caenorhabditis elegans. J. Cell Sci. 2020, 237990. [Google Scholar] [CrossRef]
- Friday, A.J.; Henderson, M.A.; Morrison, J.K.; Hoffman, J.L.; Keiper, B.D. Spatial and temporal translational control of germ cell mRNAs mediated by the eIF4E isoform IFE-1. J. Cell Sci. 2015, 128, 4487–4498. [Google Scholar] [CrossRef]
- Song, A.; Labella, S.; Korneeva, N.L.; Keiper, B.D.; Aamodt, E.J.; Zetka, M.; Rhoads, R.E. A C. elegans eIF4E-family member upregulates translation at elevated temperatures of mRNAs encoding MSH-5 and other meiotic crossover proteins. J. Cell Sci. 2010, 123, 2228–2237. [Google Scholar] [CrossRef]
- Henderson, M.A.; Cronland, E.; Dunkelbarger, S.; Contreras, V.; Strome, S.; Keiper, B.D. A germline-specific isoform of eIF4E (IFE-1) is required for efficient translation of stored mRNAs and maturation of both oocytes and sperm. J. Cell Sci. 2009, 122, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Keiper, B.D.; Kawasaki, I.; Fan, Y.; Kohara, Y.; Rhoads, R.E.; Strome, S. Faculty of 1000 evaluation for An isoform of eIF4E is a component of germ granules and is required for spermatogenesis in C. elegans. Development 2001, 128, 3899–3912. [Google Scholar] [PubMed]
- Matsuo, H.; Li, H.; McGuire, A.M.; Fletcher, C.M.; Gingras, A.C.; Sonenberg, N.; Wagner, G. Structure of translation factor eIF4E bound to m7GDP and interaction with 4E-binding protein. Nat. Genet. 1997, 4, 686–689. [Google Scholar]
- Marcotrigiano, J.; Gingras, A.C.; Sonenberg, N.; Burley, S.K. Cocrystal Structure of the Messenger RNA 5′ Cap-Binding Protein (eIF4E) Bound to 7-methyl-GDP. Cell 1997, 89, 951–961. [Google Scholar] [CrossRef]
- Hernández, G.; Altmann, M.; Sierra, J.M.; Urlaub, H.; Del Corral, R.D.; Schwartz, P.; Rivera-Pomar, R. Functional analysis of seven genes encoding eight translation initiation factor 4E (eIF4E) isoforms in Drosophila. Mech. Dev. 2005, 122, 529–543. [Google Scholar] [CrossRef]
- Hernández, G.; Vazquez-Pianzola, P. Functional diversity of the eukaryotic translation initiation factors belonging to eIF4 families. Mech. Dev. 2005, 122, 865–876. [Google Scholar] [CrossRef]
- Joshi, B.; Lee, K.; Maeder, D.L.; Jagus, R. Phylogenetic analysis of eIF4E-family members. BMC Evol. Biol. 2005, 5, 48. [Google Scholar] [CrossRef]
- Tettweiler, G.; Kowanda, M.; Lasko, P.; Sonenberg, N.; Hernández, G. The Distribution of eIF4E-Family Members across Insecta. Comp. Funct. Genom. 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- Cho, P.F.; Poulin, F.; Cho-Park, Y.A.; Cho-Park, I.B.; Chicoine, J.D.; Lasko, P.; Sonenberg, N. A New Paradigm for Translational Control: Inhibition via 5′-3′ mRNA Tethering by Bicoid and the eIF4E Cognate 4EHP. Cell 2005, 121, 411–423. [Google Scholar] [CrossRef]
- Hernández, G.; Han, H.; Gandin, V.; Fabian, L.; Ferreira, T.; Zuberek, J.; Sonenberg, N.; Brill, J.A.; Lasko, P. Eukaryotic initiation factor 4E-3 is essential for meiotic chromosome segregation, cytokinesis and male fertility in Drosophila. Development 2012, 139, 3211–3220. [Google Scholar] [CrossRef]
- Jones, G.D.; Williams, E.; Place, A.; Jagus, R.; Bachvaroff, T.R. The alveolate translation initiation factor 4E family reveals a custom toolkit for translational control in core dinoflagellates. BMC Evol. Biol. 2015, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Osborne, M.J.; Borden, K.L.B. The eukaryotic translation initiation factor eIF4E in the nucleus: Taking the road less traveled. Immunol. Rev. 2015, 263, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Volpon, L.; Osborne, M.J.; Borden, K.L. Biochemical and Structural Insights into the Eukaryotic Translation Initiation Factor eIF4E. Curr. Protein Pept. Sci. 2019, 20, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Hussain, M.A.; Ahmad, Z. Candida albicans: A Model Organism for Studying Fungal Pathogens. ISRN Microbiol. 2012, 2012, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ross-Kaschitza, D.; Saxena, M.; Altmann, M. eIF4E Is an Important Determinant of Adhesion and Pseudohyphal Growth of the Yeast S. cerevisiae. PLoS ONE 2012, 7, e50773. [Google Scholar] [CrossRef]
- Cullen, P.J.; Sprague, G.F. The Regulation of Filamentous Growth in Yeast. Genetics 2012, 190, 23–49. [Google Scholar] [CrossRef]
- Youk, H.; Lim, W.A. Secreting and Sensing the Same Molecule Allows Cells to Achieve Versatile Social Behaviors. Science 2014, 343, 1242782. [Google Scholar] [CrossRef]
- Decker, C.J.; Parker, R. P-Bodies and Stress Granules: Possible Roles in the Control of Translation and mRNA Degradation. Cold Spring Harb. Perspect Biol. 2012, 4, a012286. [Google Scholar] [CrossRef]
- Brengues, M.; Parker, R. Accumulation of polyadenylated mRNA, Pab1p, eIF4E, and eIF4G with P-bodies in Saccharomyces cerevisiae. Mol. Biol. Cell 2007, 18, 2592–2602. [Google Scholar] [CrossRef]
- Hoyle, N.P.; Castelli, L.M.; Campbell, S.G.; Holmes, L.E.; Ashe, M.P. Stress-dependent relocalization of translationally primed mRNPs to cytoplasmic granules that are kinetically and spatially distinct from P-bodies. J. Cell Biol. 2007, 179, 65–74. [Google Scholar] [CrossRef]
- Buchan, J.R.; Nissan, T.; Parker, R. Analyzing P-Bodies and Stress Granules in Saccharomyces cerevisiae. Meth. Enzymol. 2010, 470, 619–640. [Google Scholar]
- Altmann, M.; Handschin, C.; Trachsel, H. mRNA cap-binding protein: Cloning of the gene encoding protein synthesis initiation factor eIF-4E from Saccharomyces cerevisiae. Mol. Cell. Biol. 1987, 7, 998–1003. [Google Scholar] [CrossRef]
- Altmann, M.; Müller, P.P.; Pelletier, J.; Sonenberg, N.; Trachsel, H. A mammalian translation initiation factor can substitute for its yeast homologue in vivo. J. Biol. Chem. 1989, 264, 12145–12147. [Google Scholar]
- Altmann, M.; Sonenberg, N.; Trachsel, H. Translation in Saccharomyces cerevisiae: Initiation factor 4E-dependent cell-free system. Mol. Cell. Biol. 1989, 9, 4467–4472. [Google Scholar] [CrossRef]
- Altmann, M.; Trachsel, H. Altered mRNA cap recognition activity of initiation factor 4E in the yeast cell cyde division mutant cdc33. Nucleic Acids Res. 1989, 17, 5923–5931. [Google Scholar] [CrossRef]
- Altmann, M.; Linder, P. Power of Yeast for Analysis of Eukaryotic Translation Initiation. J. Biol. Chem. 2010, 285, 31907–31912. [Google Scholar] [CrossRef]
- Yoffe, Y.; Zuberek, J.; Lerer, A.; Lewdorowicz, M.; Stepinski, J.; Altmann, M.; Darzynkiewicz, E.; Shapira, M. Binding Specificities and Potential Roles of Isoforms of Eukaryotic Initiation Factor 4E in Leishmania. Eukaryot. Cell 2006, 5, 1969–1979. [Google Scholar] [CrossRef]
- Ptushkina, M.; Malys, N.; McCarthy, J.E.G. eIF4E isoform 2 in Schizosaccharomyces pombe is a novel stress-response factor. EMBO Rep. 2004, 5, 311–316. [Google Scholar] [CrossRef]
- Gross, J.D.; Moerke, N.J.; Von Der Haar, T.; Lugovskoy, A.A.; Sachs, A.B.; McCarthy, J.E.; Wagner, G. Ribosome loading onto the mRNA cap is driven by conformational coupling between eIF4G and eIF4E. Cell 2003, 115, 739–750. [Google Scholar] [CrossRef]
- Zanchin, N.I.T.; McCarthy, J.E.G. Characterization of thein VivoPhosphorylation Sites of the mRNA·Cap-binding Complex Proteins Eukaryotic Initiation Factor-4E and p20 inSaccharomyces cerevisiae. J. Biol. Chem. 1995, 270, 26505–26510. [Google Scholar] [CrossRef]
- Studer, R.A.; A Rodriguez-Mias, R.; Haas, K.M.; I Hsu, J.; Viéitez, C.; Solé, C.; Swaney, D.L.; Stanford, L.B.; Liachko, I.; Böttcher, R.; et al. Evolution of protein phosphorylation across 18 fungal species. Science 2016, 354, 229–232. [Google Scholar] [CrossRef]
- Altmann, M.; Edery, I.; Trachsel, H.; Sonenberg, N. Site-directed mutagenesis of the tryptophan residues in yeast eukaryotic initiation factor 4E. Effects on cap binding activity. J. Biol. Chem. 1988, 263, 17229–17232. [Google Scholar]
- Ptushkina, M. A second eIF4E protein in Schizosaccharomyces pombe has distinct eIF4G-binding properties. Nucleic Acids Res. 2001, 29, 4561–4569. [Google Scholar] [CrossRef]
- Ptushkina, M.; Fierro, I.; Heuvel, J.V.D.; Vasilescu, S.; Birkenhäger, R.; Mita, K.; McCarthy, J.E.G. Schizosaccharomyces pombeHas a Novel Eukaryotic Initiation Factor 4F Complex Containing a Cap-binding Protein with the Human eIF4E C-terminal Motif KSGST. J. Biol. Chem. 1996, 271, 32818–32824. [Google Scholar] [CrossRef]
- Patrick, R.M.; Mayberry, L.K.; Choy, G.; Woodard, L.E.; Liu, J.S.; White, A.; Mullen, R.A.; Tanavin, T.M.; Latz, C.A.; Browning, K.S. Two Arabidopsis loci encode novel eukaryotic initiation factor 4E isoforms that are functionally distinct from the conserved plant eukaryotic initiation factor 4E. Plant Physiol. 2014, 164, 1820–1830. [Google Scholar] [CrossRef]
- Barnes, C.A.; MacKenzie, M.M.; Johnston, G.C.; Singer, R.A. Efficient translation of an SSA1-derived heat-shock mRNA in yeast cells limited for cap-binding protein and eIF-4F. Mol. Genet. Genom. 1995, 246, 619–627. [Google Scholar] [CrossRef]
- Cawley, A.; Warwicker, J. EIF4E-binding protein regulation of mRNAs with differential 5′-UTR secondary structure: A polyelectrostatic model for a component of protein-mRNA interactions. Nucleic Acids Res. 2012, 40, 7666–7675. [Google Scholar] [CrossRef][Green Version]
- Grüner, S.; Peter, D.; Weber, R.; Wohlbold, L.; Chung, M.Y.; Weichenrieder, O.; Izaurralde, E. The Structures of eIF4E-eIF4G Complexes Reveal an Extended Interface to Regulate Translation Initiation. Mol. Cell 2016, 64, 467–479. [Google Scholar] [CrossRef]
- Grüner, S.; Weber, R.; Peter, D.; Chung, M.-Y.; Igreja, C.; Valkov, E.; Izaurralde, E. Structural motifs in eIF4G and 4E-BPs modulate their binding to eIF4E to regulate translation initiation in yeast. Nucleic Acids Res. 2018, 46, 6893–6908. [Google Scholar] [CrossRef]
- Peter, D.; Igreja, C.; Weber, R.; Wohlbold, L.; Weiler, C.; Ebertsch, L.; Izaurralde, E. Molecular Architecture of 4E-BP Translational Inhibitors Bound to eIF4E. Mol. Cell 2015, 57, 1074–1087. [Google Scholar] [CrossRef]
- Wells, S.E.; Hillner, P.E.; Vale, R.D.; Sachs, A.B. Circularization of mRNA by Eukaryotic Translation Initiation Factors. Mol. Cell 1998, 2, 135–140. [Google Scholar] [CrossRef]
- Marcotrigiano, J.; Lomakin, I.B.; Sonenberg, N.; Pestova, T.V.; Hellen, C.U.T.; Burley, S.K. A Conserved HEAT Domain within eIF4G Directs Assembly of the Translation Initiation Machinery. Mol. Cell 2001, 7, 193–203. [Google Scholar] [CrossRef]
- Jivotovskaya, A.V.; Valášek, L.; Hinnebusch, A.G.; Nielsen, K.H. Eukaryotic translation initiation factor 3 (eIF3) and eIF2 can promote mRNA binding to 40S subunits independently of eIF4G in yeast. Mol. Cell. Biol. 2006, 26, 1355–1372. [Google Scholar] [CrossRef]
- Berset, C.; Zurbriggen, A.; Djafarzadeh, S.; Altmann, M.; Trachsel, H. RNA-binding activity of translation initiation factor eIF4G1 from Saccharomyces cerevisiae. RNA 2003, 9, 871–880. [Google Scholar] [CrossRef]
- Goyer, C.; Altmann, M.; Lee, H.S.; Blanc, A.; Deshmukh, M.; Woolford, J.L.; Trachsel, H.; Sonenberg, N. TIF4631 and TIF4632: Two yeast genes encoding the high-molecular-weight subunits of the cap-binding protein complex (eukaryotic initiation factor 4F) contain an RNA recognition motif-like sequence and carry out an essential function. Mol. Cell. Biol. 1993, 13, 4860–4874. [Google Scholar] [CrossRef][Green Version]
- Clarkson, B.K.; Gilbert, W.V.; Doudna, J.A. Functional Overlap between eIF4G Isoforms in Saccharomyces cerevisiae. PLoS ONE 2010, 5, e9114. [Google Scholar] [CrossRef]
- Park, E.-H.; Zhang, F.; Warringer, J.; Sunnerhagen, P.; Hinnebusch, A.G. Depletion of eIF4G from yeast cells narrows the range of translational efficiencies genome-wide. BMC Genom. 2011, 12, 68. [Google Scholar] [CrossRef]
- Costello, J.; Kershaw, C.J.; Castelli, L.M.; Talavera, D.; Rowe, W.; Sims, P.F.G.; Ashe, M.P.; Grant, C.M.; Hubbard, S.J.; Pavitt, G. Dynamic changes in eIF4F-mRNA interactions revealed by global analyses of environmental stress responses. Genome Biol. 2017, 18, 201. [Google Scholar] [CrossRef]
- Rhoads, R.E. Regulation of eukaryotic protein synthesis by initiation factors. J. Biol. Chem. 1993, 268, 3017–3020. [Google Scholar]
- Keiper, B.D.; Gan, W.; E Rhoads, R. Protein synthesis initiation factor 4G. Int. J. Biochem. Cell Biol. 1999, 31, 37–41. [Google Scholar] [CrossRef]
- Hershey, P.E.C.; McWhirter, S.M.; Gross, J.D.; Wagner, G.; Alber, T.; Sachs, A.B. The Cap-binding Protein eIF4E Promotes Folding of a Functional Domain of Yeast Translation Initiation Factor eIF4G1. J. Biol. Chem. 1999, 274, 21297–21304. [Google Scholar] [CrossRef]
- Cosentino, G.P.; Schmelzle, T.; Haghighat, A.; Helliwell, S.B.; Hall, M.N.; Sonenberg, N. Eap1p, a novel eukaryotic translation initiation factor 4E-associated protein in Saccharomyces cerevisiae. Mol. Cell. Biol. 2000, 20, 4604–4613. [Google Scholar] [CrossRef]
- Sezen, B.; Seedorf, M.; Schiebel, E. The SESA network links duplication of the yeast centrosome with the protein translation machinery. Genome Res. 2009, 23, 1559–1570. [Google Scholar] [CrossRef]
- Chial, H.J.; Stemm-Wolf, A.J.; McBratney, S.; Winey, M. Yeast Eap1p, an eIF4E-associated protein, has a separate function involving genetic stability. Curr. Biol. 2000, 10, 1519–1522. [Google Scholar] [CrossRef][Green Version]
- Deloche, O.; De La Cruz, J.; Kressler, D.; Doère, M.; Linder, P. A membrane transport defect leads to a rapid attenuation of translation initiation in Saccharomyces cerevisiae. Mol. Cell 2004, 13, 357–366. [Google Scholar] [CrossRef]
- Meier, K.D.; Deloche, O.; Kajiwara, K.; Funato, K.; Riezman, H. Sphingoid base is required for translation initiation during heat stress in Saccharomyces cerevisiae. Mol. Biol. Cell 2006, 17, 1164–1175. [Google Scholar] [CrossRef]
- Blewett, N.H.; Goldstrohm, A.C. A Eukaryotic Translation Initiation Factor 4E-Binding Protein Promotes mRNA Decapping and Is Required for PUF Repression. Mol. Cell. Biol. 2012, 32, 4181–4194. [Google Scholar] [CrossRef]
- Rendl, L.M.; Bieman, M.A.; Vari, H.K.; A Smibert, C. The eIF4E-Binding Protein Eap1p Functions in Vts1p-Mediated Transcript Decay. PLoS ONE 2012, 7, e47121. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Yao, Z.; Jin, M.; Namkoong, S.; Yin, Z.; Lee, J.H.; Klionsky, D.J. Dhh1 promotes autophagy-related protein translation during nitrogen starvation. PLoS Biol. 2019, 17, e3000219. [Google Scholar] [CrossRef]
- Cridge, A.; Castelli, L.M.; Smirnova, J.B.; Selley, J.; Rowe, W.; Hubbard, S.J.; McCarthy, J.E.; Ashe, M.P.; Grant, C.M.; Pavitt, G. Identifying eIF4E-binding protein translationally-controlled transcripts reveals links to mRNAs bound by specific PUF proteins. Nucleic Acids Res. 2010, 38, 8039–8050. [Google Scholar] [CrossRef]
- Castelli, L.M.; Talavera, D.; Kershaw, C.J.; Mohammad-Qureshi, S.S.; Costello, J.; Rowe, W.; Sims, P.F.G.; Grant, C.M.; Hubbard, S.J.; Ashe, M.P.; et al. The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation. PLoS Genet. 2015, 11, e1005233. [Google Scholar] [CrossRef]
- Ross, D.; Altmann, M. eIF4Es and Their Interactors from Yeast Species. In Evolution of the Protein Synthesis Machinery and Its Regulation; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Albuquerque, C.P.; Smolka, M.B.; Payne, S.H.; Bafna, V.; Eng, J.; Zhou, H. A multidimensional chromatography technology for in-depth phosphoproteome analysis. Mol. Cell. Proteomics 2008, 7, 1389–1396. [Google Scholar] [CrossRef]
- Hughes, J.M.; Ptushkina, M.; Karim, M.; Koloteva, N.; Von Der Haar, T.; McCarthy, J.E.G. Translational Repression by Human 4E-BP1 in Yeast Specifically Requires Human eIF4E as Target. J. Biol. Chem. 1999, 274, 3261–3264. [Google Scholar] [CrossRef]
- Hernández, G.; Altmann, M.; Lasko, P. Origins and evolution of the mechanisms regulating translation initiation in eukaryotes. Trends Biochem. Sci. 2010, 35, 63–73. [Google Scholar] [CrossRef]
- Altmann, M.; Schmitz, N.; Berset, C.; Trachsel, H. A novel inhibitor of cap-dependent translation initiation in yeast: p20 competes with eIF4G for binding to eIF4E. EMBO J. 1997, 16, 1114–1121. [Google Scholar] [CrossRef]
- De la Cruz, J.; Iost, I.; Kressler, D.; Linder, P. The p20 and Ded1 proteins have antagonistic roles in eIF4E-dependent translation in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1997, 94, 5201–5206. [Google Scholar] [CrossRef]
- Arndt, N.; Ross-Kaschitza, D.; Kojukhov, A.; Komar, A.A.; Altmann, M. Properties of the ternary complex formed by yeast eIF4E, p20 and mRNA. Sci. Rep. 2018, 8, 6707. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.C. Identification in the Ancient Protist Giardia lamblia of Two Eukaryotic Translation Initiation Factor 4E Homologues with Distinctive Functions†. Eukaryot. Cell 2005, 4, 948–959. [Google Scholar] [CrossRef]
- Tuteja, R. Identification and bioinformatics characterization of translation initiation complex eIF4F components and poly(A)-binding protein from Plasmodium falciparum. Commun. Integr. Biol. 2009, 2, 45–260. [Google Scholar] [CrossRef]
- Zinoviev, A.; Shapira, M. Evolutionary conservation and diversification of the translation initiation apparatus in trypanosomatids. Comp. Funct. Genomics 2012. [Google Scholar] [CrossRef]
- Reolon, L.W.; Vichier-Guerre, S.; De Matos, B.M.; Dugué, L.; Assunção, T.R.D.S.; Zanchin, N.I.T.; Pochet, S.; Guimaraes, B.G. Crystal structure of the Trypanosoma cruzi EIF4E5 translation factor homologue in complex with mRNA cap-4. Nucleic Acids Res. 2019, 47, 5973–5987. [Google Scholar] [CrossRef] [PubMed]
- Freire, E.R.; Sturm, N.R.; Campbell, D.A.; Neto, O.P.D.M. The Role of Cytoplasmic mRNA Cap-Binding Protein Complexes in Trypanosoma brucei and Other Trypanosomatids. Pathogens 2017, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Neto, O.P.D.M.; Lima, T.D.C.D.C.; Xavier, C.C.; Nascimento, L.M.; Romão, T.P.; Assis, A.L.; Pereira, M.M.C.; Reis, C.R.S.; Papadopoulou, B. The unique Leishmania EIF4E4 N-terminus is a target for multiple phosphorylation events and participates in critical interactions required for translation initiation. RNA Biol. 2015, 12, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, R.; Tupperwar, N.; Drory-Retwitzer, M.; Shapira, M. Deletion of a Single LeishIF4E-3 Allele by the CRISPR-Cas9 System Alters Cell Morphology and Infectivity of Leishmania. mSphere 2019, 4. [Google Scholar] [CrossRef]
- Moura, D.M.; Reis, C.R.; Xavier, C.C.; da Costa Lima, T.D.; Lima, R.P.; Carrington, M.; de Melo Neto, O.P. Two related trypanosomatid eiF4G homologues have functional differences compatible with distinct roles during translation initiation. RNA Biol. 2015, 12, 305–319. [Google Scholar] [CrossRef]
- Yoffe, Y.; Léger, M.; Zinoviev, A.; Zuberek, J.; Darzynkiewicz, E.; Wagner, G.; Shapira, M. Evolutionary changes in the Leishmania eIF4F complex involve variations in the eIF4E-eIF4G interactions. Nucleic Acids Res. 2009, 37, 3243–3253. [Google Scholar] [CrossRef]
- Freire, E.R.; Vashisht, A.A.; Malvezzi, A.M.; Zuberek, J.; Langousis, G.; Saada, E.A.; Nascimento, J.D.F.; Stepinski, J.; Darzynkiewicz, E.; Hill, K.; et al. eIF4F-like complexes formed by cap-binding homolog TbEIF4E5 with TbEIF4G1 or TbEIF4G2 are implicated in post-transcriptional regulation in Trypanosoma brucei. RNA 2014, 20, 1272–1286. [Google Scholar] [CrossRef]
- Freire, E.R.; Malvezzi, A.M.; Vashisht, A.A.; Zuberek, J.; Saada, E.A.; Langousis, G.; Nascimento, J.D.F.; Moura, D.; Darzynkiewicz, E.; Hill, K.; et al. Trypanosoma brucei Translation Initiation Factor Homolog EIF4E6 Forms a Tripartite Cytosolic Complex with EIF4G5 and a Capping Enzyme Homolog. Eukaryot. Cell 2014, 13, 896–908. [Google Scholar] [CrossRef]
- Zinoviev, A.; Léger, M.; Wagner, G.; Shapira, M. A novel 4E-interacting protein in Leishmania is involved in stage-specific translation pathways. Nucleic Acids Res. 2011, 39, 8404–8415. [Google Scholar] [CrossRef]
- Meleppattu, S.; Arthanari, H.; Zinoviev, A.; Boeszoermenyi, A.; Wagner, G.; Shapira, M.; Léger-Abraham, M. Structural basis for LeishIF4E-1 modulation by an interacting protein in the human parasite Leishmania major. Nucleic Acids Res. 2018, 46, 3791–3801. [Google Scholar] [CrossRef]
- Meleppattu, S.; Kamus-Elimeleh, D.; Zinoviev, A.; Cohen-Mor, S.; Orr, I.; Shapira, M. The eIF3 complex of Leishmania-subunit composition and mode of recruitment to different cap-binding complexes. Nucleic Acids Res. 2015, 43, 6222–6235. [Google Scholar] [CrossRef]
- Freire, E.R.; Moura, D.M.; Bezerra, M.J.R.; Xavier, C.C.; Morais-Sobral, M.C.; Vashisht, A.; Rezende, A.M.; Wohlschlegel, J.A.; Sturm, N.R.; Neto, O.P.D.M.; et al. Trypanosoma brucei EIF4E2 cap-binding protein binds a homolog of the histone-mRNA stem-loop-binding protein. Curr. Genet. 2017, 64, 821–839. [Google Scholar] [CrossRef]
- Zinoviev, A.; Manor, S.; Shapira, M. Nutritional stress affects an atypical cap-binding protein in Leishmania. RNA Biol. 2012, 9, 1450–1460. [Google Scholar] [CrossRef]
- Dos Santos Rodrigues, F.H.; Firczuk, H.; Breeze, A.L.; Cameron, A.D.; Walko, M.; Wilson, A.J.; McCarthy, J.E. The Leishmania PABP1-eIF4E4 interface: A novel 5′-3′ interaction architecture for trans-spliced mRNAs. Nucleic Acids Res. 2019, 47, 1493–1504. [Google Scholar] [CrossRef]
- Dhalia, R.; Reis, C.R.; Freire, E.R.; Rocha, P.O.; Katz, R.; Muniz, J.; Standart, N.; Neto, O.P.D.M. Translation initiation in Leishmania major: Characterisation of multiple eIF4F subunit homologues. Mol. Biochem. Parasitol. 2005, 140, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Tupperwar, N.; Shrivastava, R.; Shapira, M. LeishIF4E1 Deletion Affects the Promastigote Proteome, Morphology, and Infectivity. mSphere 2019, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Terrao, M.; Marucha, K.K.; Mugo, E.; Droll, R.; Minia, I.; Egler, F.; Braun, J.; Clayton, C. The suppressive cap-binding complex factor 4EIP is required for normal differentiation. Nucleic Acids Res. 2018, 46, 8993–9010. [Google Scholar] [CrossRef] [PubMed]
- Gorgoni, B.; Andrews, S.; Schaller, A.; Schümperli, D.; Gray, N.; Müller, B. The stem–loop binding protein stimulates histone translation at an early step in the initiation pathway. RNA 2005, 11, 1030–1042. [Google Scholar] [CrossRef]
- Allard, P.; Yang, Q.; Marzluff, W.F.; Clarke, H.J. The stem-loop binding protein regulates translation of histone mRNA during mammalian oogenesis. Dev. Biol. 2005, 286, 195–206. [Google Scholar] [CrossRef]
- Sarjeant, W.A.S.; Taylor, F.J.R. The Biology of Dinoflagellates; Stony Brook University: Stony Brook, NY, USA, 1989. [Google Scholar]
- Jagus, R.; Bachvaroff, T.R.; Joshi, B.; Place, A.R. Diversity of Eukaryotic Translational Initiation Factor eIF4E in Protists. Comp. Funct. Genom. 2012, 2012, 1–21. [Google Scholar] [CrossRef]

| eIF4E Source | eIF4E-Binding Protein | eIF4E-Binding Motif | Function/Significance | Reference |
|---|---|---|---|---|
| LmeIF4E1 | Lm4E-IP | 7-YTREELL-13 | Inhibitory complex | [90,91] |
| LmeIF4E1 | LmeIF3a | eIF4G3 not involved | [83,92] | |
| TbeIF4E2 | TbSLBP-2 (binds to stem-loop motif in 3′UTR of histone mRNA) | Central part of TbSLBP-2 which does not carry the canonical 4E-binding motif | Translation of histone mRNA | [93] |
| LmeIF4E3 | LmeIF4G4 | 22-LADILAFRDT-31 | eIF4F complex, dissociates upon heat stress | [94] |
| LmeIF4E4/(LmeIF4E1) | LmeIF4G3 | 20-YPGFSLDE- 27 | eIF4F complex (LmeIF4A1 also detected) | [87] |
| LmeIF4E4 | LmPAB1 | 5′-3′-mRNA circularization | [84,95] | |
| TbeIF4E5 | TbeIF4G1 TbeIF4G2 | [88] | ||
| TbeIF4E6 | TbeIF4G5 (TbG5-IP) | [89] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ross-Kaschitza, D.; Altmann, M. eIF4E and Interactors from Unicellular Eukaryotes. Int. J. Mol. Sci. 2020, 21, 2170. https://doi.org/10.3390/ijms21062170
Ross-Kaschitza D, Altmann M. eIF4E and Interactors from Unicellular Eukaryotes. International Journal of Molecular Sciences. 2020; 21(6):2170. https://doi.org/10.3390/ijms21062170
Chicago/Turabian StyleRoss-Kaschitza, Daniela, and Michael Altmann. 2020. "eIF4E and Interactors from Unicellular Eukaryotes" International Journal of Molecular Sciences 21, no. 6: 2170. https://doi.org/10.3390/ijms21062170
APA StyleRoss-Kaschitza, D., & Altmann, M. (2020). eIF4E and Interactors from Unicellular Eukaryotes. International Journal of Molecular Sciences, 21(6), 2170. https://doi.org/10.3390/ijms21062170





