Serenoa repens and Urtica dioica Fixed Combination: In-Vitro Validation of a Therapy for Benign Prostatic Hyperplasia (BPH)
Abstract
1. Introduction
2. Results
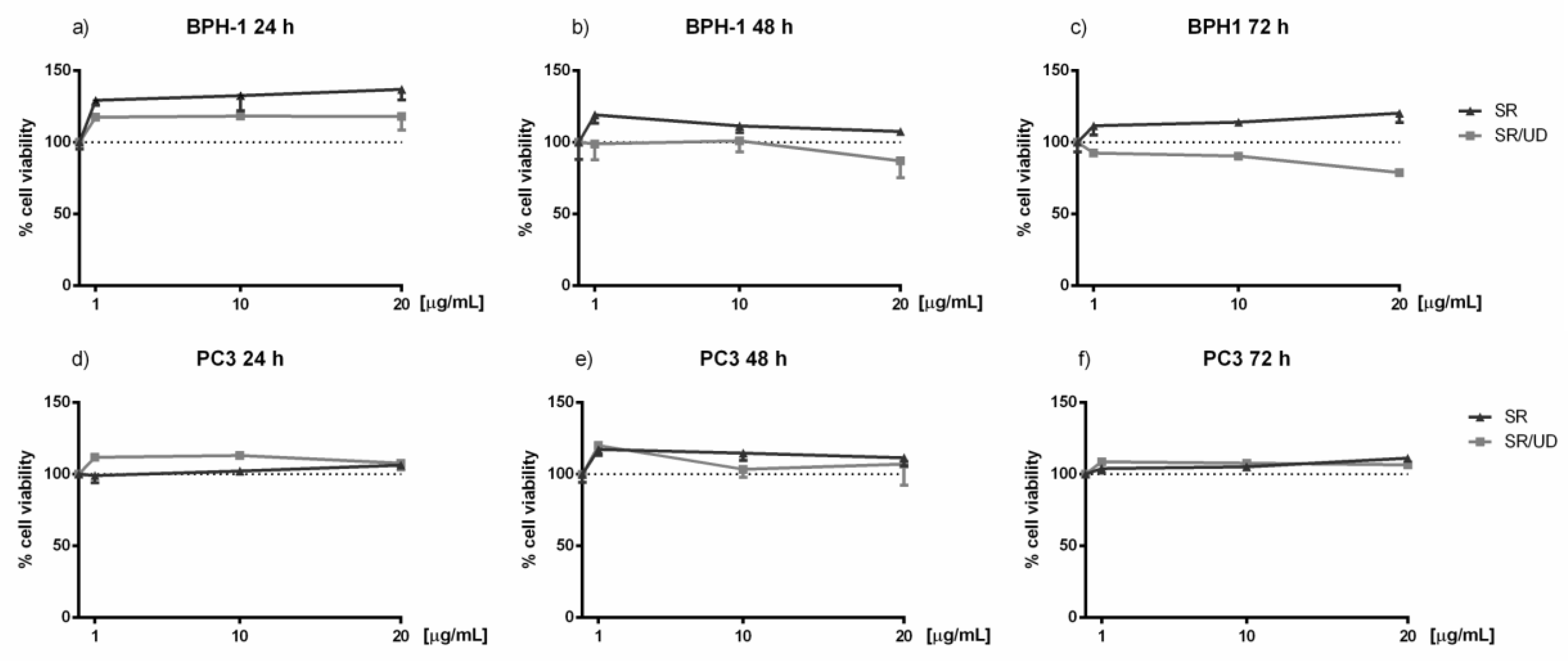
2.1. Activity of SR and SR/UD in Cell Proliferation
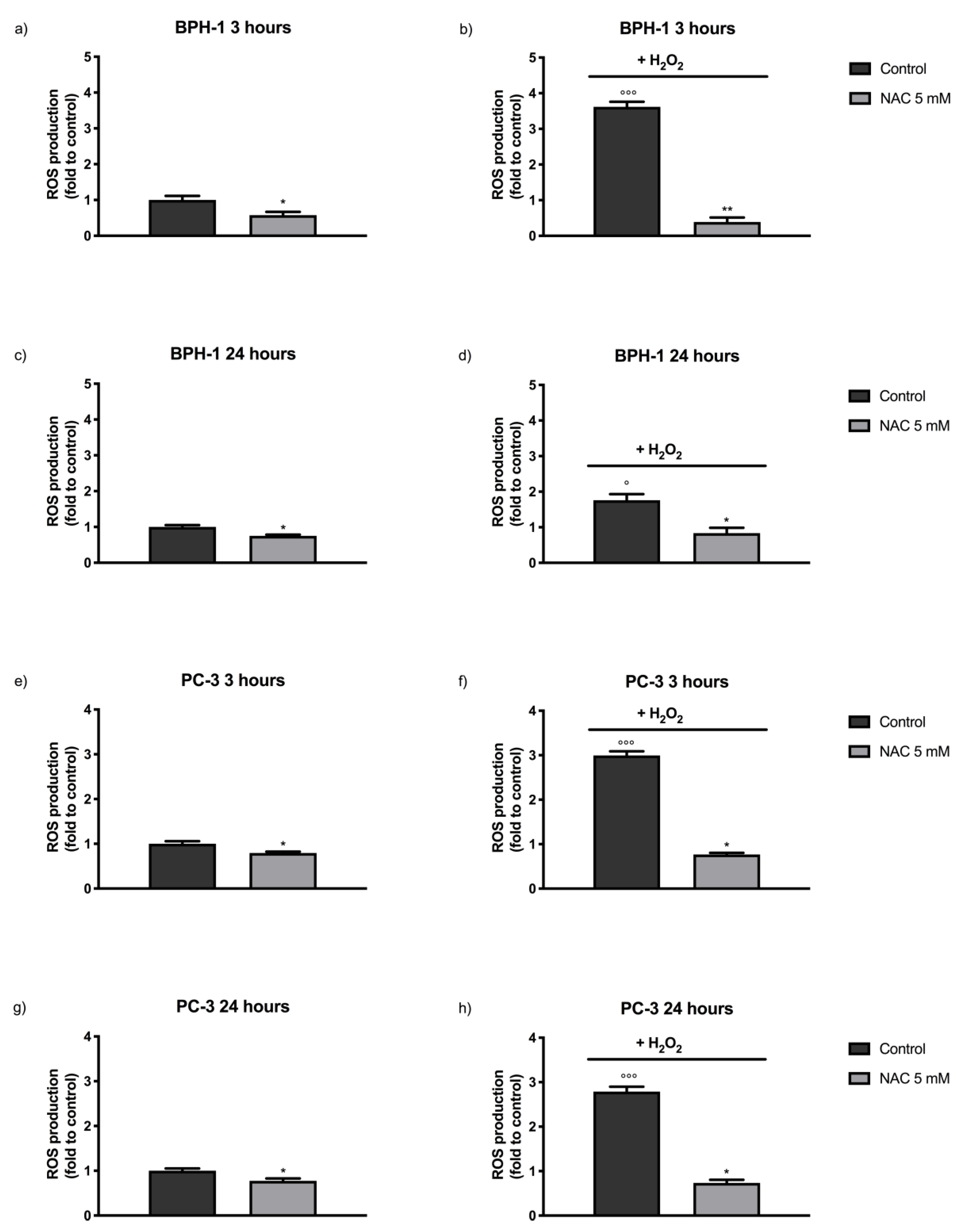
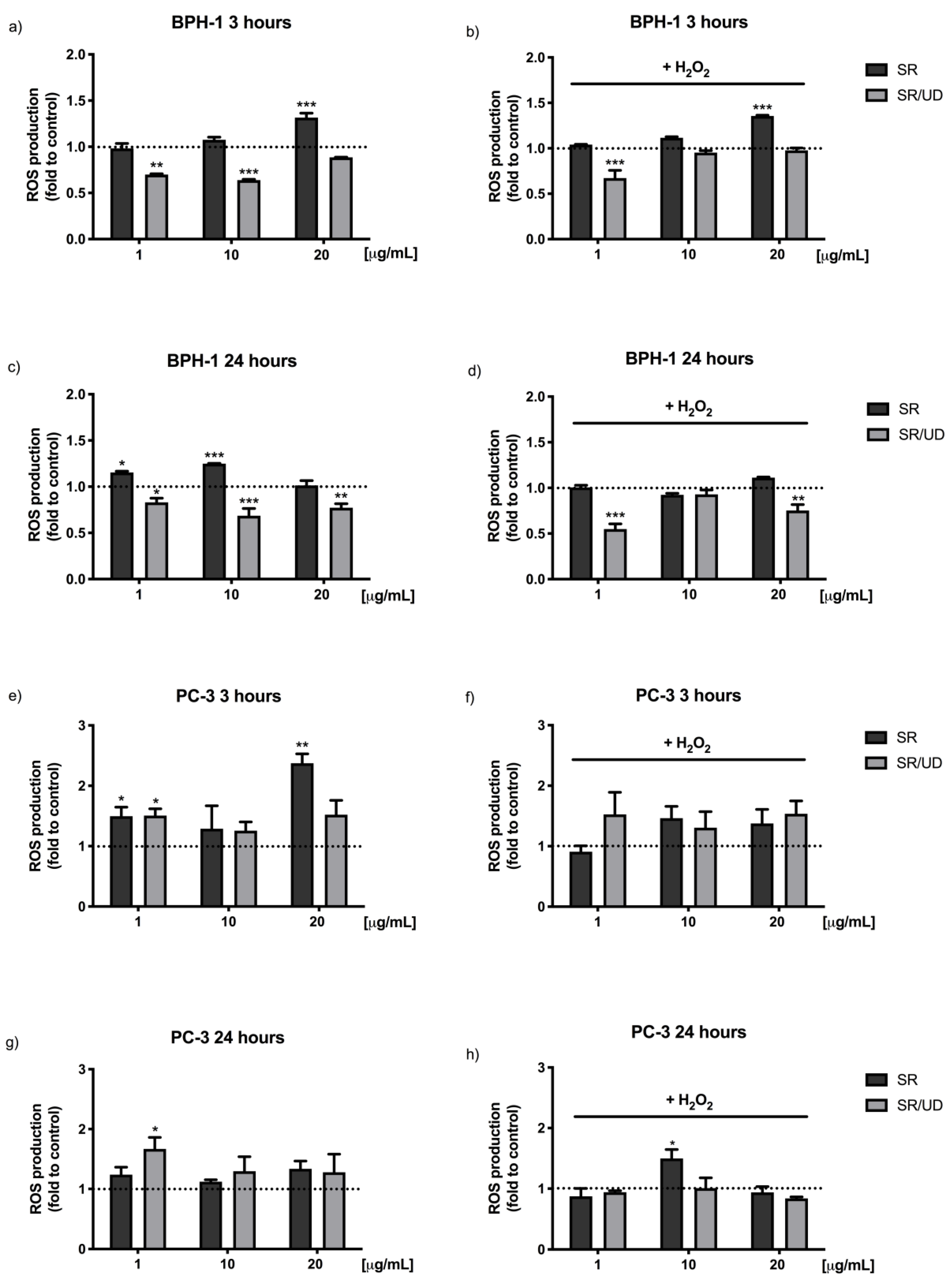
2.2. Antioxidant Effect of SR and SR/UD
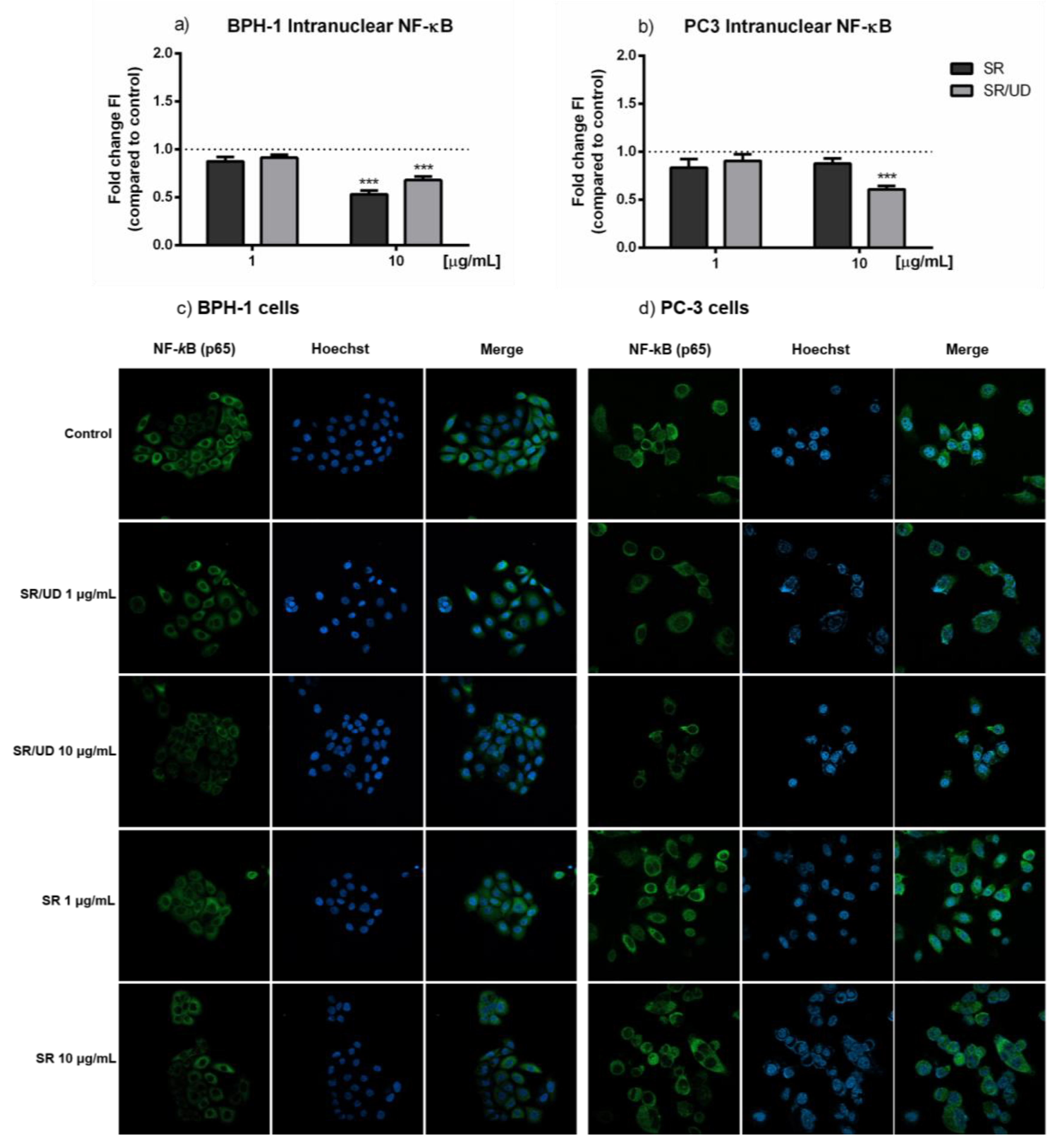
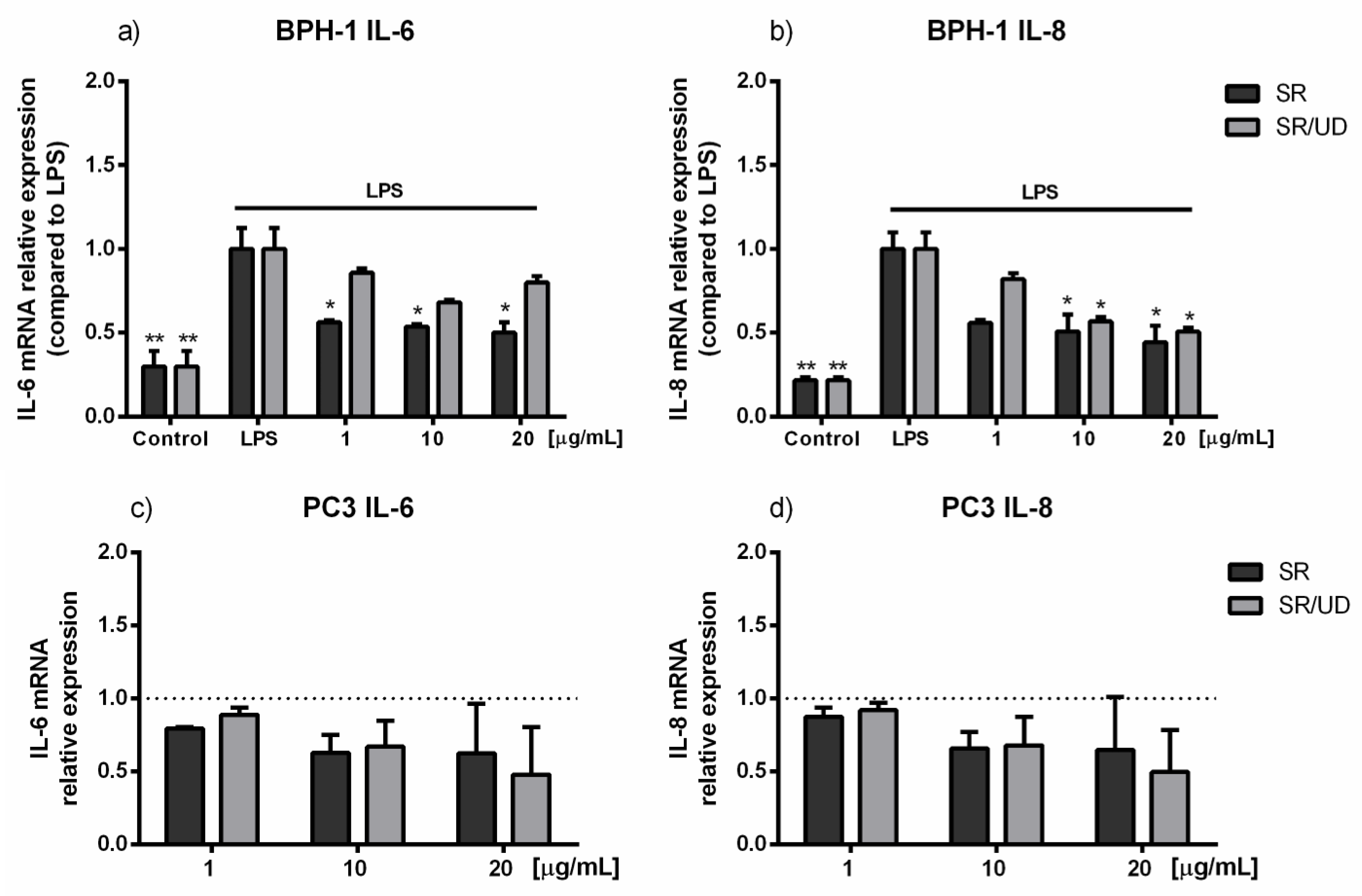
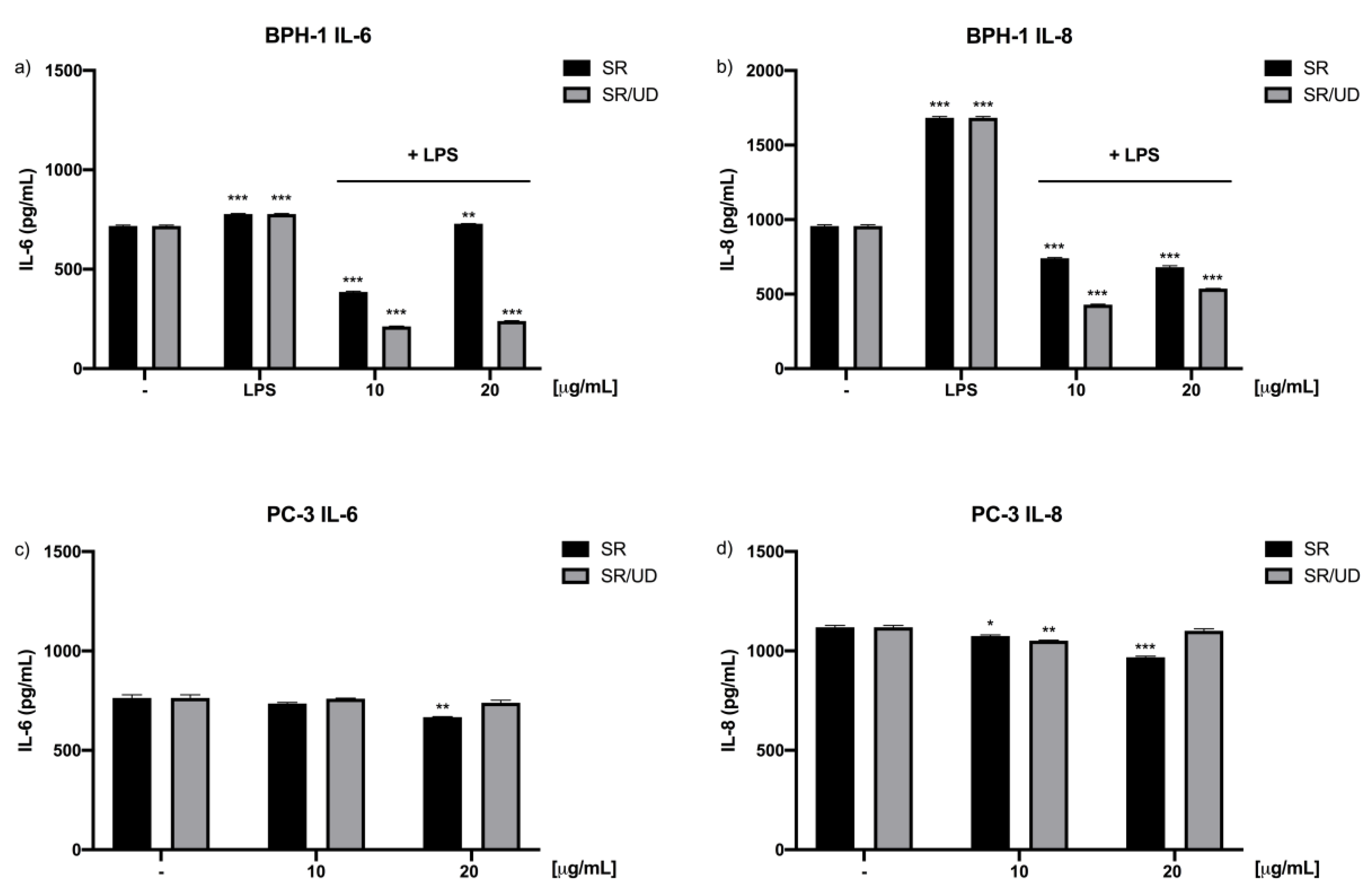
2.3. Anti-Inflammatory Effect of SR and SR/UD on BPH-1 Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Cultures
5.2. SR Composition
5.3. SR/UD Composition
- lipophilic extract from fruits of S. repens [160 mg WS® 1473; drug–extract ratio 10.0–14.3:1; extraction solvent: 90% ethanol (m/m)]
- aqueous ethanolic extract from roots of U. dioica [120mg WS® 1031, drug–extract ratio 7.6–12.5:1; extraction solvent: 60% ethanol (m/m)].
5.4. Cells Viability Assay
5.5. ROS Assay
5.6. Nuclear Factor-kappa B Translocation Assay
5.7. RNA Expression/Quantitative Real-Time PCR
5.8. IL-6 and IL-8 ELISA
5.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Madersbacher, S.; Sampson, N.; Culig, Z. Pathophysiology of Benign Prostatic Hyperplasia and Benign Prostatic Enlargement: A Mini-Review. Gerontology 2019, 65, 458–464. [Google Scholar] [CrossRef]
- Vuichoud, C.; Loughlin, K.R. Benign prostatic hyperplasia: Epidemiology, economics and evaluation. Can. J. Urol. 2015, 22, 1–6. [Google Scholar]
- Roehrborn, C.G.; Boyle, P.; Gould, A.L.; Waldstreicher, J. Serum prostate-specific antigen as a predictor of prostate volume in men with benign prostatic hyperplasia. Urology 1999, 53, 581–589. [Google Scholar] [CrossRef]
- Untergasser, G.; Madersbacher, S.; Berger, P. Benign prostatic hyperplasia: Age-related tissue-remodeling. Exp. Gerontol. 2005, 40, 121–128. [Google Scholar] [CrossRef]
- Lepor, H. Pathophysiology of lower urinary tract symptoms in the aging male population. Rev. Urol. 2005, 7, S3–S11. [Google Scholar]
- Eliaz, I.; Weil, E.; Wilk, B. Improvements in Self-reported Lower Urinary Tract Symptoms with Prostate Health Supplement. Altern. Ther. Health Med. 2018, 24, 26–32. [Google Scholar] [PubMed]
- Mobley, D.; Feibus, A.; Baum, N. Benign prostatic hyperplasia and urinary symptoms: Evaluation and treatment. Postgrad Med. 2015, 127, 301–307. [Google Scholar] [CrossRef]
- Penna, G.; Mondaini, N.; Amuchastegui, S.; Degli Innocenti, S.; Carini, M.; Giubilei, G.; Fibbi, B.; Colli, E.; Maggi, M.; Adorini, L. Seminal Plasma Cytokines and Chemokines in Prostate Inflammation: Interleukin 8 as a Predictive Biomarker in Chronic Prostatitis/Chronic Pelvic Pain Syndrome and Benign Prostatic Hyperplasia. Eur. Urol. 2007, 51, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, B.; Lee, R.; Te, A.; Kaplan, S. Role of Inflammation in Benign Prostatic Hyperplasia. Rev. Urol. 2011, 13, 147–150. [Google Scholar] [PubMed]
- Nunzio, C.D.; Albisinni, S.; Gacci, M.; Tubaro, A. The Role of Inflammation in the Progression of Benign Prostatic Hyperplasia. Curr. Bladder. Dysfunct. Rep. 2013, 8, 142–149. [Google Scholar] [CrossRef]
- Fibbi, B.; Penna, G.; Morelli, A.; Adorini, L.; Maggi, M. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia: BPH and inflammation. Int. J. Androl. 2009, 33, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Li, X.; Cheng, G.; Li, N.; Hou, Z.; Suo, J.; Wang, J.; Za, X. The effects of ROS in prostatic stromal cells under hypoxic environment. Aging Male 2015, 18, 84–88. [Google Scholar] [CrossRef]
- Berger, A.P.; Kofler, K.; Bektic, J.; Rogatsch, H.; Steiner, H.; Bartsch, G.; Klocker, H. Increased growth factor production in a human prostatic stromal cell culture model caused by hypoxia. Prostate 2003, 57, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Paterniti, I.; Campolo, M.; Cordaro, M.; Siracusa, R.; Filippone, A.; Esposito, E.; Cuzzocrea, S. Effects of different natural extracts in an experimental model of benign prostatic hyperplasia (BPH). Inflamm. Res. 2018, 67, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.M.; Das, A.K. A scientific basis for the therapeutic effects of Pygeum africanum and Serenoa repens. Urol. Res. 2000, 28, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Latil, A.; Libon, C.; Templier, M.; Junquero, D.; Lantoine-Adam, F.; Nguyen, T. Hexanic lipidosterolic extract of Serenoa repens inhibits the expression of two key inflammatory mediators, MCP-1/CCL2 and VCAM-1, in vitro. BJU Int. 2012, 110, E301–E307. [Google Scholar] [CrossRef] [PubMed]
- Novara, G.; Giannarini, G.; Alcaraz, A.; Cózar-Olmo, J.-M.; Descazeaud, A.; Montorsi, F.; Ficarra, V. Efficacy and Safety of Hexanic Lipidosterolic Extract of Serenoa repens (Permixon) in the Treatment of Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia: Systematic Review and Meta-analysis of Randomized Controlled Trials. Eur. Urol. Focus 2016, 2, 553–561. [Google Scholar] [CrossRef]
- Vela-Navarrete, R.; Alcaraz, A.; Rodríguez-Antolín, A.; Miñana López, B.; Fernández-Gómez, J.M.; Angulo, J.C.; Castro Díaz, D.; Romero-Otero, J.; Brenes, F.J.; Carballido, J.; et al. Efficacy and safety of a hexanic extract of Serenoa repens (Permixon®) for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH): Systematic review and meta-analysis of randomised controlled trials and observational studies. BJU Int. 2018, 122, 1049–1065. [Google Scholar] [CrossRef]
- Ranjan, M.; Diffley, P.; Stephen, G.; Price, D.; Walton, T.J.; Newton, R.P. Comparative study of human steroid 5alpha-reductase isoforms in prostate and female breast skin tissues: Sensitivity to inhibition by finasteride and epristeride. Life Sci. 2002, 71, 115–126. [Google Scholar] [CrossRef]
- Vacherot, F.; Azzouz, M.; Gil-Diez-de-Medina, S.; Colombel, M.; Taille, A.D.L.; Belda, M.-A.L.; Abbou, C.C.; Raynaud, J.-P.; Chopin, D.K. Induction of apoptosis and inhibition of cell proliferation by the lipido-sterolic extract of Serenoa repens (LSESr, Permixon®) in benign prostatic hyperplasia. Prostate 2000, 45, 259–266. [Google Scholar] [CrossRef]
- Cai, T.; Cui, Y.; Yu, S.; Li, Q.; Zhou, Z.; Gao, Z. Comparison of Serenoa repens With Tamsulosin in the Treatment of Benign Prostatic Hyperplasia: A Systematic Review and Meta-Analysis. Am. J. Mens Health 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Pavone, C.; Abbadessa, D.; Tarantino, M.L.; Oxenius, I.; Laganà, A.; Lupo, A.; Rinella, M. Associating Serenoa repens, Urtica dioica and Pinus pinaster. Safety and efficacy in the treatment of lower urinary tract symptoms. Prospective study on 320 patients. Urologia 2010, 77, 43–51. [Google Scholar] [CrossRef]
- Esposito, S.; Bianco, A.; Russo, R.; Di Maro, A.; Isernia, C.; Pedone, P.V. Therapeutic Perspectives of Molecules from Urtica dioica Extracts for Cancer Treatment. Molecules 2019, 24, 2753. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Available online: https://www.hindawi.com/journals/omcl/2017/8416763/ (accessed on 2 November 2020).
- Soh, N. Recent advances in fluorescent probes for the detection of reactive oxygen species. Anal. Bioanal. Chem. 2006, 386, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The Nuclear Factor NF-κB Pathway in Inflammation. Cold Spring Harb. Perspect Biol. 2009, 1. [Google Scholar] [CrossRef]
- Kim, J.-H.; Han, I.-H.; Kim, Y.-S.; Noh, C.-S.; Ryu, J.-S. Proliferation of prostate epithelia induced by IL-6 from stroma reacted with Trichomonas vaginalis. Parasite Immunol. 2018, 40, e12531. [Google Scholar] [CrossRef]
- Penna, G.; Fibbi, B.; Amuchastegui, S.; Cossetti, C.; Aquilano, F.; Laverny, G.; Gacci, M.; Crescioli, C.; Maggi, M.; Adorini, L. Human Benign Prostatic Hyperplasia Stromal Cells As Inducers and Targets of Chronic Immuno-Mediated Inflammation. J. Immunol. 2009, 182, 4056–4064. [Google Scholar] [CrossRef]
- Penna, G.; Fibbi, B.; Amuchastegui, S.; Corsiero, E.; Laverny, G.; Silvestrini, E.; Chavalmane, A.; Morelli, A.; Sarchielli, E.; Vannelli, G.B.; et al. The vitamin D receptor agonist elocalcitol inhibits IL-8-dependent benign prostatic hyperplasia stromal cell proliferation and inflammatory response by targeting the RhoA/Rho kinase and NF-kB pathways. Prostate 2009, 69, 480–493. [Google Scholar] [CrossRef]
- Silvestri, I.; Cattarino, S.; Aglianò, A.; Nicolazzo, C.; Scarpa, S.; Salciccia, S.; Frati, L.; Gentile, V.; Sciarra, A. Effect of Serenoa repens (Permixon®) on the expression of inflammation-related genes: Analysis in primary cell cultures of human prostate carcinoma. J. Inflamm. 2013, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Begley, L.A.; Kasina, S.; Mehra, R.; Adsule, S.; Admon, A.J.; Lonigro, R.J.; Chinnaiyan, A.M.; Macoska, J.A. CXCL5 Promotes Prostate Cancer Progression. Neoplasia 2008, 10, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J. Benign prostatic hyperplasia: Multiple factors for prostate tissue change with aging. Korean J. Urol. 2005, 46, 547–554. [Google Scholar]
- Norström, M.M.; Rådestad, E.; Sundberg, B.; Mattsson, J.; Henningsohn, L.; Levitsky, V.; Uhlin, M. Progression of benign prostatic hyperplasia is associated with pro-inflammatory mediators and chronic activation of prostate-infiltrating lymphocytes. Oncotarget 2016, 7, 23581–23593. [Google Scholar] [CrossRef]
- Vásquez-Velásquez, C.; Gasco, M.; Fano-Sizgorich, D.; Gonzales, G.F. Inflammatory pathway employed by Red Maca to treat induced benign prostatic hyperplasia in rats. Andrologia 2020, 52, e13516. [Google Scholar] [CrossRef]
- Kaplan, S.A. AUA Guidelines and Their Impact on the Management of BPH: An Update. Rev. Urol. 2004, 6, S46–S52. [Google Scholar]
- Pirozzi, L.; Sountoulides, P.; Castellan, P.; Presicce, F.; Lombardo, R.; Romero, M.; De Nunzio, C.; Tubaro, A.; Schips, L.; Cindolo, L. Current Pharmacological Treatment for Male LUTS due to BPH: Dutasteride or Finasteride? Curr. Drug Targets 2015, 16, 1165–1171. [Google Scholar] [CrossRef]
- Björndahl, L.; Giwercman, A.; Tournaye, H.; Weidner, W. Clinical Andrology: EAU/ESAU Course Guidelines; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-1-84184-747-4. [Google Scholar]
- Krzeski, T.; Kazón, M.; Borkowski, A.; Witeska, A.; Kuczera, J. Combined extracts of Urtica dioica and Pygeum africanum in the treatment of benign prostatic hyperplasia: Double-blind comparison of two doses. Clin. Ther. 1993, 15, 1011–1020. [Google Scholar]
- Engelhardt, P.F.; Seklehner, S.; Brustmann, H.; Lusuardi, L.; Riedl, C.R. Immunohistochemical expression of interleukin-2 receptor and interleukin-6 in patients with prostate cancer and benign prostatic hyperplasia: Association with asymptomatic inflammatory prostatitis NIH category IV. Scand. J. Urol. 2015, 49, 120–126. [Google Scholar] [CrossRef]
- Narazaki, M.; Kishimoto, T. The Two-Faced Cytokine IL-6 in Host Defense and Diseases. Int. J. Mol. Sci. 2018, 19, 3528. [Google Scholar] [CrossRef]
- Brat, D.J.; Bellail, A.C.; Van Meir, E.G. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. 2005, 7, 122–133. [Google Scholar] [CrossRef]
- Kramer, G.; Marberger, M. Could inflammation be a key component in the progression of benign prostatic hyperplasia? Curr. Opin Urol. 2006, 16, 25–29. [Google Scholar]
- Bouraoui, Y.; Ricote, M.; García-Tuñón, I.; Rodriguez-Berriguete, G.; Touffehi, M.; Rais, N.B.; Fraile, B.; Paniagua, R.; Oueslati, R.; Royuela, M. Pro-inflammatory cytokines and prostate-specific antigen in hyperplasia and human prostate cancer. Cancer Detect. Prev. 2008, 32, 23–32. [Google Scholar] [CrossRef]
- Adler, H.L.; McCurdy, M.A.; Kattan, M.W.; Timme, T.L.; Scardino, P.T.; Thompson, T.C. Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostatic carcinoma. J. Urol. 1999, 161, 182–187. [Google Scholar] [CrossRef]
- George, D.J.; Halabi, S.; Shepard, T.F.; Sanford, B.; Vogelzang, N.J.; Small, E.J.; Kantoff, P.W. The prognostic significance of plasma interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: Results from cancer and leukemia group B 9480. Clin. Cancer Res. 2005, 11, 1815–1820. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.P.; Ertunc, O.; Kulac, I.; Baena-Del Valle, J.A.; De Marzo, A.M.; Sfanos, K.S. IL8 Expression Is Associated with Prostate Cancer Aggressiveness and Androgen Receptor Loss in Primary and Metastatic Prostate Cancer. Mol. Cancer Res. 2020, 18, 153–165. [Google Scholar] [CrossRef]
- Ghafar, M.A.; Puchner, P.J.; Anastasiadis, A.G.; Cabelin, M.A.; Buttyan, R. Does the prostatic vascular system contribute to the development of benign prostatic hyperplasia? Curr. Urol. Rep. 2002, 3, 292–296. [Google Scholar] [CrossRef]
- Chughtai, B.; Lee, R.; Te, A.; Kaplan, S. Inflammation and Benign Prostatic Hyperplasia: Clinical Implications. Curr. Urol. Rep. 2011, 12, 274–277. [Google Scholar] [CrossRef]
- Cervellati, F.; Cervellati, C.; Romani, A.; Cremonini, E.; Sticozzi, C.; Belmonte, G.; Pessina, F.; Valacchi, G. Hypoxia induces cell damage via oxidative stress in retinal epithelial cells. Free Radic. Res. 2014, 48, 303–312. [Google Scholar] [CrossRef]
- Ravenna, L.; Principessa, L.; Verdina, A.; Salvatori, L.; Russo, M.A.; Petrangeli, E. Distinct Phenotypes of Human Prostate Cancer Cells Associate with Different Adaptation to Hypoxia and Pro-Inflammatory Gene Expression. PLoS ONE 2014, 9, e96250. [Google Scholar] [CrossRef]
- Corsini, E.; Galbiati, V.; Nikitovic, D.; Tsatsakis, A.M. Role of oxidative stress in chemical allergens induced skin cells activation. Food Chem. Toxicol. 2013, 61, 74–81. [Google Scholar] [CrossRef]
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Engelmann, U.; Walther, C.; Bondarenko, B.; Funk, P.; Schläfke, S. Efficacy and safety of a combination of sabal and urtica extract in lower urinary tract symptoms. A randomized, double-blind study versus tamsulosin. Arzneimittelforschung 2006, 56, 222–229. [Google Scholar] [CrossRef]
- Lopatkin, N.; Sivkov, A.; Schläfke, S.; Funk, P.; Medvedev, A.; Engelmann, U. Efficacy and safety of a combination of Sabal and Urtica extract in lower urinary tract symptoms--long-term follow-up of a placebo-controlled, double-blind, multicenter trial. Int. Urol. Nephrol. 2007, 39, 1137–1146. [Google Scholar] [CrossRef]
- Ibishev, K.S.; Krainy, P.A.; Mitusov, V.V.; Sizyakin, D.V.; Magomedov, G.A. A comparative analysis of the effectiveness of serenoa repens and serenoa repens in combination with urtica dioiccus for lower urinary symptoms suggestive of benign prostatic hyperplasia associated with chronic inflammation in prostate tissue. Urologiia 2019, 40–46. [Google Scholar] [CrossRef]
- Pigat, N.; Reyes-Gomez, E.; Boutillon, F.; Palea, S.; Barry Delongchamps, N.; Koch, E.; Goffin, V. Combined Sabal and Urtica Extracts (WS® 1541) Exert Anti-proliferative and Anti-inflammatory Effects in a Mouse Model of Benign Prostate Hyperplasia. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Ishiyama, M.; Tominaga, H.; Shiga, M.; Sasamoto, K.; Ohkura, Y.; Ueno, K. A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol. Pharm. Bull. 1996, 19, 1518–1520. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saponaro, M.; Giacomini, I.; Morandin, G.; Cocetta, V.; Ragazzi, E.; Orso, G.; Carnevali, I.; Berretta, M.; Mancini, M.; Pagano, F.; et al. Serenoa repens and Urtica dioica Fixed Combination: In-Vitro Validation of a Therapy for Benign Prostatic Hyperplasia (BPH). Int. J. Mol. Sci. 2020, 21, 9178. https://doi.org/10.3390/ijms21239178
Saponaro M, Giacomini I, Morandin G, Cocetta V, Ragazzi E, Orso G, Carnevali I, Berretta M, Mancini M, Pagano F, et al. Serenoa repens and Urtica dioica Fixed Combination: In-Vitro Validation of a Therapy for Benign Prostatic Hyperplasia (BPH). International Journal of Molecular Sciences. 2020; 21(23):9178. https://doi.org/10.3390/ijms21239178
Chicago/Turabian StyleSaponaro, Miriam, Isabella Giacomini, Giulia Morandin, Veronica Cocetta, Eugenio Ragazzi, Genny Orso, Ilaria Carnevali, Massimiliano Berretta, Mariangela Mancini, Francesco Pagano, and et al. 2020. "Serenoa repens and Urtica dioica Fixed Combination: In-Vitro Validation of a Therapy for Benign Prostatic Hyperplasia (BPH)" International Journal of Molecular Sciences 21, no. 23: 9178. https://doi.org/10.3390/ijms21239178
APA StyleSaponaro, M., Giacomini, I., Morandin, G., Cocetta, V., Ragazzi, E., Orso, G., Carnevali, I., Berretta, M., Mancini, M., Pagano, F., & Montopoli, M. (2020). Serenoa repens and Urtica dioica Fixed Combination: In-Vitro Validation of a Therapy for Benign Prostatic Hyperplasia (BPH). International Journal of Molecular Sciences, 21(23), 9178. https://doi.org/10.3390/ijms21239178






