A Novel Promising Frontier for Human Health: The Beneficial Effects of Nutraceuticals in Cardiovascular Diseases
Abstract
1. Introduction
2. Nutraceuticals in Hypertension
2.1. Resveratrol
2.2. Cocoa
2.3. Quercetin
2.4. Curcumin
2.5. Berberine
2.6. Brassica
2.7. Spirulina Platensis
3. Nutraceuticals in Atherosclerosis
3.1. Resveratrol
3.2. Cocoa
3.3. Quercetin
3.4. Curcumin
3.5. Berberine
3.6. Brassica
3.7. Spirulina Platensis
4. Nutraceuticals in Heart Failure
4.1. Resveratrol
4.2. Cocoa
4.3. Quercetin
4.4. Curcumin
4.5. Berberine
4.6. Brassica
4.7. Spirulina Platensis
5. Nutraceuticals in Diabetes
5.1. Resveratrol
5.2. Cocoa
5.3. Quercetin
5.4. Curcumin
5.5. Berberine
5.6. Brassica
5.7. Spirulina Platensis
6. Discussion
Funding
Conflicts of Interest
Abbreviations
| CVD | cardiovascular diseases |
| DASH | dietary approaches to stop hypertension |
| ESC | European Society of Cardiology |
| ESH | European Society of Hypertension |
| LDL | low density lipoprotein |
| ox-LDL | oxidized-LDL |
| HDL | high-density lipoprotein |
| VLDL | very low-density lipoprotein |
| TG | triglycerides |
| BP | blood pressure |
| SP | systolic pressure |
| DP | diastolic pressure |
| RES | resveratrol |
| NO | nitric oxide |
| eNOS | endothelial NO synthetase |
| AMPK | adenosine monophosphate activated protein kinase |
| MAPK | mitogen-activated protein kinase |
| SIRT | sirtuins |
| NRF | nuclear erythroid factor |
| I-CAM | intracellular adhesion molecule |
| VCAM | vascular CAM |
| ANGIO | angiotensin |
| IL | interleukin |
| TMAO | trimethylamine-N-oxide |
| TNF | tumor necrosis factor |
| ENaC | epithelial sodium channel |
| LV | left ventricle |
| AT1R | angiotensin II type 1 receptor |
| ACE | angiotensin-converting enzyme |
| ACE-I | angiotensin-converting enzyme inhibitor |
| PDGF | platelet derived growth factor |
| CD | cluster of differentiation |
| EPC | endothelial progenitor cell |
| PMD | flow mediated dilatator |
| PPAR | peroxisome proliferator-activated receptor gamma |
| PPARGC1 | PPARG coactivated 1 alpha |
| UCP | uncoupling protein |
| SHR | spontaneous hypertensive rat |
| SHRSP | SHR stroke-prone |
| GST | glutathione S-transferase |
| GID | gastrointestinal digestion |
| PI3K | phosphatidyl Inositol 3-kinsa |
| HMG-CoA | 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase |
| KFKB | nuclear factor kappa light-chain-enhancer of activated B cells |
| Fox03a | forkhead box 03a |
| MCP | monocyte chemotactic protein |
| NADPH | nicotinamide adenine dinucleotide phosphate oxidase |
| ApoE | apolipoprotein E |
| HFD | high-fat diet |
| LPS | lipopolysaccharides |
| ACS | acute coronary syndrome |
| SRA | scavenger receptor A |
| BMI | body mass index |
| HF | heart failure |
| CHF | congestive HF |
| EF | ejection fraction |
| LV | left ventricle |
| LVEF | left ventricle ejection fraction |
| LVAD | left ventricle assist device |
| RAAS | renin angiotensin aldosterone system |
| ACC | American College of Cardiology |
| AHA | American Heart Association |
| IABP | intra-aortic balloon pump |
| CRT | cardiac resynchronization therapy |
| ICD | implanted cardioverter defibrillator |
| SOD | superoxide dismutase |
| SERCA | sarco-endoplasmic reticulum Ca+-ATPase |
| MI | myocardial infarction |
| HAT | histone acetyltransferase |
| CABG | coronary artery bypass graft |
| CRO | C-reactive protein |
| BNP | brain natriuretic peptide |
| AchE | acetylcholine esterase |
| DM | diabetes mellitus |
| T2DM | type 2 DM |
| MDA | malondialdehyde |
| Hb1Ac | glycated hemoglobin |
| GLUT | glucose transporter |
| HOMA | homeostatic model assessment |
| HOMA-IR | HOMA insulin resistance |
| PEPCK | phosphoenolpyruvate carboxy kinase |
| GPX | glutathione peroxidase |
| JNK | c-Jun N-terminal kinases |
| IRS | insulin receptor substrate |
| LKB1 | liver kinase B1 |
References
- Deaton, C.; Froelicher, E.S.; Wu, L.H.; Ho, C.; Shishani, K.; Jaarsma, T. The global burden of cardiovascular disease. Eur. J. Cardiovasc. Nurs. 2011, 10 (Suppl. 2), S5–S13. [Google Scholar] [CrossRef]
- Castellano, J.M.; Narula, J.; Castillo, J.; Fuster, V. Promoting cardiovascular health worldwide: Strategies, challenges, and opportunities. Rev. Esp. Cardiol. (Engl. Ed.) 2014, 67, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.D. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat. Rev. Cardiol. 2014, 11, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Tzoulaki, I.; Elliott, P.; Kontis, V.; Ezzati, M. Worldwide Exposures to Cardiovascular Risk Factors and Associated Health Effects: Current Knowledge and Data Gaps. Circulation 2016, 133, 2314–2333. [Google Scholar] [CrossRef] [PubMed]
- Ravera, A.; Carubelli, V.; Sciatti, E.; Bonadei, I.; Gorga, E.; Cani, D.; Vizzardi, E.; Metra, M.; Lombardi, C. Nutrition and Cardiovascular Disease: Finding the Perfect Recipe for Cardiovascular Health. Nutrients 2016, 8, 363. [Google Scholar] [CrossRef]
- Pletcher, M.J.; Moran, A.E. Cardiovascular Risk Assessment. Med. Clin. N. Am. 2017, 101, 673–688. [Google Scholar] [CrossRef]
- Costantino, S.; Paneni, F.; Cosentino, F. Ageing, metabolism and cardiovascular disease. J. Physiol. 2016, 594, 2061–2073. [Google Scholar] [CrossRef]
- Labarthe, D.R. Prevention of cardiovascular risk factors in the first place. Prev. Med. 1999, 29 Pt 2, S72–S78. [Google Scholar] [CrossRef]
- Houston, M. The role of noninvasive cardiovascular testing, applied clinical nutrition and nutritional supplements in the prevention and treatment of coronary heart disease. Ther. Adv. Cardiovasc. Dis. 2018, 12, 85–108. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursac Kovacevic, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food. Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Roberfroid, M.B. A European consensus of scientific concepts of functional foods. Nutrition 2000, 16, 689–691. [Google Scholar] [CrossRef]
- Sunkara, A.; Raizner, A. Supplemental Vitamins and Minerals for Cardiovascular Disease Prevention and Treatment. Methodist Debakey Cardiovasc. J. 2019, 15, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Fortmann, S.P.; Burda, B.U.; Senger, C.A.; Lin, J.S.; Whitlock, E.P. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 159, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Saneei, P.; Salehi-Abargouei, A.; Esmaillzadeh, A.; Azadbakht, L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: A systematic review and meta-analysis on randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Huang, T.; Bergholdt, H.K.; Nordestgaard, B.G.; Ellervik, C.; Qi, L.; Consortium, C. Dairy consumption, systolic blood pressure, and risk of hypertension: Mendelian randomization study. BMJ 2017, 356, j1000. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.E.; Greenwood, D.C.; Threapleton, D.E.; Gale, C.P.; Cleghorn, C.L.; Burley, V.J. Glycemic index, glycemic load, and blood pressure: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 105, 1176–1190. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Arnoldi, A.; Cicero, A.F. Nutraceuticals for blood pressure control. Ann. Med. 2015, 47, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, B.; Santolamazza, C.; Simonelli, F.; Rubattu, S. Phytochemical Compounds and Protection from Cardiovascular Diseases: A State of the Art. BioMed Res. Int. 2015, 2015, 918069. [Google Scholar] [CrossRef]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cifkova, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Primers 2018, 4, 18014. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018, 33, 3021–3104. [Google Scholar] [CrossRef]
- Bakris, G.; Ali, W.; Parati, G. ACC/AHA Versus ESC/ESH on Hypertension Guidelines: JACC Guideline Comparison. J. Am. Coll. Cardiol. 2019, 73, 3018–3026. [Google Scholar] [CrossRef]
- Wang, C.; Chiang, C.; Yatsuya, H.; Hilawe, E.H.; Ikerdeu, E.; Honjo, K.; Mita, T.; Cui, R.; Hirakawa, Y.; Madraisau, S.; et al. Descriptive Epidemiology of Hypertension and Its Association With Obesity: Based on the WHO STEPwise Approach to Surveillance in Palau. Asia-Pac. J. Public Health 2017. [Google Scholar] [CrossRef]
- Weber, T.; Lang, I.; Zweiker, R.; Horn, S.; Wenzel, R.R.; Watschinger, B.; Slany, J.; Eber, B.; Roithinger, F.X.; Metzler, B. Hypertension and coronary artery disease: Epidemiology, physiology, effects of treatment, and recommendations: A joint scientific statement from the Austrian Society of Cardiology and the Austrian Society of Hypertension. Wien. Klin. Wochenschr. 2016, 128, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.L.; Shrestha, P.A.; Vivo, R.P. Epidemiology of comorbidities in patients with hypertension. Curr. Opin. Cardiol. 2016, 31, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Georgianos, P.I.; Agarwal, R. Epidemiology, diagnosis and management of hypertension among patients on chronic dialysis. Nat. Rev. Nephrol. 2016, 12, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.; Singh, N.P. Epidemiology and Genetics of Hypertension. J. Assoc. Physicians India 2015, 63, 61–98. [Google Scholar]
- Wong, N.D.; Franklin, S.S. Epidemiology of hypertension. J. Am. Soc. Hypertens. 2014, 8, 760–763. [Google Scholar] [CrossRef]
- Qaseem, A.; Wilt, T.J.; Rich, R.; Humphrey, L.; Frost, J.; Forciea, M.A. Treatment of Hypertension in Adults over Age 60 to Higher Vs Lower Targets: A Clinical Practice Guideline from the American College of Physicians and the American Academy of Family Physicians. Ann. Fam. Med. 2017, 15, 185–186. [Google Scholar] [CrossRef]
- Randel, A. AHA/ACC/ASH Release Guideline on the Treatment of Hypertension and CAD. Am. Fam. Physician 2015, 92, 1023–1030. [Google Scholar]
- Ogihara, T. Antihypertensive treatment guideline for elderly hypertension. Nihon Rinsho 2001, 59, 919–926. [Google Scholar] [PubMed]
- Persson, M.; Bohlin, J.; Eklund, P. Development and maintenance of guideline-based decision support for pharmacological treatment of hypertension. Comput. Methods Programs Biomed. 2000, 61, 209–219. [Google Scholar] [CrossRef]
- Frohlich, E.D. Treatment guideline in the USA: Hypertension in the elderly. Br. J. Urol. 1998, 81 (Suppl. 1), 26–28. [Google Scholar] [CrossRef]
- Delmas, D.; Jannin, B.; Latruffe, N. Resveratrol: Preventing properties against vascular alterations and ageing. Mol. Nutr. Food Res. 2005, 49, 377–395. [Google Scholar] [CrossRef] [PubMed]
- Smoliga, J.M.; Baur, J.A.; Hausenblas, H.A. Resveratrol and health--a comprehensive review of human clinical trials. Mol. Nutr. Food Res. 2011, 55, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.Y.; Ito, A.; Asagami, T.; Tsao, P.S.; Adimoolam, S.; Kimoto, M.; Tsuji, H.; Reaven, G.M.; Cooke, J.P. Impaired nitric oxide synthase pathway in diabetes mellitus: Role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation 2002, 106, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 2015, 1852, 1155–1177. [Google Scholar] [CrossRef]
- Rivera, L.; Moron, R.; Zarzuelo, A.; Galisteo, M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem. Pharmacol. 2009, 77, 1053–1063. [Google Scholar] [CrossRef]
- Dolinsky, V.W.; Chakrabarti, S.; Pereira, T.J.; Oka, T.; Levasseur, J.; Beker, D.; Zordoky, B.N.; Morton, J.S.; Nagendran, J.; Lopaschuk, G.D.; et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim. Biophys. Acta 2013, 1832, 1723–1733. [Google Scholar] [CrossRef]
- Chan, V.; Fenning, A.; Iyer, A.; Hoey, A.; Brown, L. Resveratrol improves cardiovascular function in DOCA-salt hypertensive rats. Curr. Pharm. Biotechnol. 2011, 12, 429–436. [Google Scholar] [CrossRef]
- Rimbaud, S.; Ruiz, M.; Piquereau, J.; Mateo, P.; Fortin, D.; Veksler, V.; Garnier, A.; Ventura-Clapier, R. Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PLoS ONE 2011, 6, e26391. [Google Scholar] [CrossRef]
- Hollenberg, N.K. Red wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cells. Curr. Hypertens. Rep. 2003, 5, 287–288. [Google Scholar] [PubMed]
- Arunachalam, G.; Yao, H.; Sundar, I.K.; Caito, S.; Rahman, I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem. Biophys. Res. Commun. 2010, 393, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Luo, T.; Luo, X.; Tang, Z. Resveratrol prevents AngII-induced hypertension via AMPK activation and RhoA/ROCK suppression in mice. Hypertens. Res. 2014, 37, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, B.; Campen, M.J.; Channell, M.M.; Wherry, S.J.; Varamini, B.; Davis, J.G.; Baur, J.A.; Smoliga, J.M. Resveratrol for primary prevention of atherosclerosis: Clinical trial evidence for improved gene expression in vascular endothelium. Int. J. Cardiol. 2013, 166, 246–248. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Effects of Grape Pomace Polyphenolic Extract (Taurisolo((R))) in Reducing TMAO Serum Levels in Humans: Preliminary Results from a Randomized, Placebo-Controlled, Cross-Over Study. Nutrients 2019, 11, 139. [Google Scholar] [CrossRef]
- Tome-Carneiro, J.; Larrosa, M.; Gonzalez-Sarrias, A.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef]
- Poulsen, M.M.; Vestergaard, P.F.; Clasen, B.F.; Radko, Y.; Christensen, L.P.; Stodkilde-Jorgensen, H.; Moller, N.; Jessen, N.; Pedersen, S.B.; Jorgensen, J.O. High-dose resveratrol supplementation in obese men: An investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes 2013, 62, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Serban, C.; Ursoniu, S.; Wong, N.D.; Muntner, P.; Graham, I.M.; Mikhailidis, D.P.; Rizzo, M.; Rysz, J.; Sperling, L.S.; et al. Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors--Results from a systematic review and meta-analysis of randomized controlled trials. Int. J. Cardiol. 2015, 189, 47–55. [Google Scholar] [CrossRef]
- Marques, B.; Trindade, M.; Aquino, J.C.F.; Cunha, A.R.; Gismondi, R.O.; Neves, M.F.; Oigman, W. Beneficial effects of acute trans-resveratrol supplementation in treated hypertensive patients with endothelial dysfunction. Clin. Exp. Hypertens. 2018, 40, 218–223. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, W.; Zhang, P.; He, S.; Huang, D. Effect of resveratrol on blood pressure: A meta-analysis of randomized controlled trials. Clin. Nutr. 2015, 34, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Puca, A.; Damato, A.; Marino, M.; Franco, E.; Pompeo, F.; Traficante, A.; Civitillo, F.; Santini, L.; Trimarco, V.; et al. Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension 2013, 62, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Theodotou, M.; Fokianos, K.; Mouzouridou, A.; Konstantinou, C.; Aristotelous, A.; Prodromou, D.; Chrysikou, A. The effect of resveratrol on hypertension: A clinical trial. Exp. Ther. Med. 2017, 13, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Movahed, A.; Ostovar, A.; Iranpour, D.; Thandapilly, S.J.; Raj, P.; Louis, X.L.; Smoliga, J.M.; Netticadan, T. The efficacy of resveratrol in controlling hypertension: Study protocol for a randomized, crossover, double-blinded, placebo-controlled trial. Trials 2016, 17, 296. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G. Cocoa, diabetes, and hypertension: Should we eat more chocolate? Am. J. Clin. Nutr. 2005, 81, 541–542. [Google Scholar] [CrossRef]
- Cohen, D.L.; Townsend, R.R. Cocoa ingestion and hypertension-another cup please? J. Clin. Hypertens. (Greenwich) 2007, 9, 647–648. [Google Scholar] [CrossRef]
- Rabadan-Chavez, G.M.; Reyes-Maldonado, E.; Quevedo-Corona, L.; Paniagua-Castro, N.; Escalona-Cardoso, G.; Jaramillo-Flores, M.E. The prothrombotic state associated with obesity-induced hypertension is reduced by cocoa and its main flavanols. Food Funct. 2016, 7, 4880–4888. [Google Scholar] [CrossRef]
- Rostami, A.; Khalili, M.; Haghighat, N.; Eghtesadi, S.; Shidfar, F.; Heidari, I.; Ebrahimpour-Koujan, S.; Eghtesadi, M. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015, 11, 21–29. [Google Scholar]
- Fisher, N.D.; Hughes, M.; Gerhard-Herman, M.; Hollenberg, N.K. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J. Hypertens. 2003, 21, 2281–2286. [Google Scholar] [CrossRef]
- Nogueira Lde, P.; Knibel, M.P.; Torres, M.R.; Nogueira Neto, J.F.; Sanjuliani, A.F. Consumption of high-polyphenol dark chocolate improves endothelial function in individuals with stage 1 hypertension and excess body weight. Int. J. Hypertens. 2012, 2012, 147321. [Google Scholar] [CrossRef]
- Engler, M.B.; Engler, M.M.; Chen, C.Y.; Malloy, M.J.; Browne, A.; Chiu, E.Y.; Kwak, H.K.; Milbury, P.; Paul, S.M.; Blumberg, J.; et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J. Am. Coll. Nutr. 2004, 23, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Dejam, A.; Kleinbongard, P.; Schewe, T.; Sies, H.; Kelm, M. Vascular effects of cocoa rich in flavan-3-ols. JAMA 2003, 290, 1030–1031. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Necozione, S.; Lippi, C.; Croce, G.; Valeri, L.; Pasqualetti, P.; Desideri, G.; Blumberg, J.B.; Ferri, C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 2005, 46, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Balzer, J.; Rassaf, T.; Heiss, C.; Kleinbongard, P.; Lauer, T.; Merx, M.; Heussen, N.; Gross, H.B.; Keen, C.L.; Schroeter, H.; et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. J. Am. Coll. Cardiol. 2008, 51, 2141–2149. [Google Scholar] [CrossRef]
- Flammer, A.J.; Hermann, F.; Sudano, I.; Spieker, L.; Hermann, M.; Cooper, K.A.; Serafini, M.; Luscher, T.F.; Ruschitzka, F.; Noll, G.; et al. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation 2007, 116, 2376–2382. [Google Scholar] [CrossRef]
- Shiina, Y.; Funabashi, N.; Lee, K.; Murayama, T.; Nakamura, K.; Wakatsuki, Y.; Daimon, M.; Komuro, I. Acute effect of oral flavonoid-rich dark chocolate intake on coronary circulation, as compared with non-flavonoid white chocolate, by transthoracic Doppler echocardiography in healthy adults. Int. J. Cardiol. 2009, 131, 424–429. [Google Scholar] [CrossRef]
- Heiss, C.; Kleinbongard, P.; Dejam, A.; Perre, S.; Schroeter, H.; Sies, H.; Kelm, M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J. Am. Coll. Cardiol. 2005, 46, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Endale, M.; Park, S.C.; Kim, S.; Kim, S.H.; Yang, Y.; Cho, J.Y.; Rhee, M.H. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, and inhibits MAPK/AP-1 and IKK/NF-kappaB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology 2013, 218, 1452–1467. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Hwang, M.K.; Lee, D.E.; Lee, K.W.; Lee, H.J. Protective effect of quercetin against arsenite-induced COX-2 expression by targeting PI3K in rat liver epithelial cells. J. Agric. Food Chem. 2010, 58, 5815–5820. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Mani, I.; Iversen, L.; Ziboh, V.A. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot. Essent. Fatty Acids 1998, 58, 17–24. [Google Scholar] [CrossRef]
- Bureau, G.; Longpre, F.; Martinoli, M.G. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J. Neurosci. Res. 2008, 86, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Madhappan, B.; Christodoulou, S.; Boucher, W.; Cao, J.; Papadopoulou, N.; Cetrulo, C.L.; Theoharides, T.C. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 2005, 145, 934–944. [Google Scholar] [CrossRef]
- Nakajima, K.I.; Marunaka, Y. Intracellular chloride ion concentration in differentiating neuronal cell and its role in growing neurite. Biochem. Biophys. Res. Commun. 2016, 479, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Asano, J.; Niisato, N.; Nakajima, K.; Miyazaki, H.; Yasuda, M.; Iwasaki, Y.; Hama, T.; Dejima, K.; Hisa, Y.; Marunaka, Y. Quercetin stimulates Na+/K+/2Cl- cotransport via PTK-dependent mechanisms in human airway epithelium. Am. J. Respir. Cell Mol. Biol. 2009, 41, 688–695. [Google Scholar] [CrossRef]
- Marunaka, Y.; Marunaka, R.; Sun, H.; Yamamoto, T.; Kanamura, N.; Inui, T.; Taruno, A. Actions of Quercetin, a Polyphenol, on Blood Pressure. Molecules 2017, 22, 209. [Google Scholar] [CrossRef]
- Edwards, R.L.; Lyon, T.; Litwin, S.E.; Rabovsky, A.; Symons, J.D.; Jalili, T. Quercetin reduces blood pressure in hypertensive subjects. J. Nutr. 2007, 137, 2405–2411. [Google Scholar] [CrossRef]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kurbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef]
- Zahedi, M.; Ghiasvand, R.; Feizi, A.; Asgari, G.; Darvish, L. Does Quercetin Improve Cardiovascular Risk factors and Inflammatory Biomarkers in Women with Type 2 Diabetes: A Double-blind Randomized Controlled Clinical Trial. Int. J. Prev. Med. 2013, 4, 777–785. [Google Scholar]
- Conquer, J.A.; Maiani, G.; Azzini, E.; Raguzzini, A.; Holub, B.J. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J. Nutr. 1998, 128, 593–597. [Google Scholar] [CrossRef]
- Serban, M.C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Kondratiuk, V.E.; Synytsia, Y.P. Effect of quercetin on the echocardiographic parameters of left ventricular diastolic function in patients with gout and essential hypertension. Wiad. Lek. 2018, 71, 1554–1559. [Google Scholar] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Satoskar, R.R.; Shah, S.J.; Shenoy, S.G. Evaluation of anti-inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986, 24, 651–654. [Google Scholar]
- Li, H.L.; Liu, C.; de Couto, G.; Ouzounian, M.; Sun, M.; Wang, A.B.; Huang, Y.; He, C.W.; Shi, Y.; Chen, X.; et al. Curcumin prevents and reverses murine cardiac hypertrophy. J. Clin. Investig. 2008, 118, 879–893. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, W.; Li, M.; Ren, H.; Chen, C.; Wang, J.; Wang, W.E.; Yang, J.; Zeng, C. Curcumin Exerts its Anti-hypertensive Effect by Down-regulating the AT1 Receptor in Vascular Smooth Muscle Cells. Sci. Rep. 2016, 6, 25579. [Google Scholar] [CrossRef]
- Tapia, E.; Soto, V.; Ortiz-Vega, K.M.; Zarco-Marquez, G.; Molina-Jijon, E.; Cristobal-Garcia, M.; Santamaria, J.; Garcia-Nino, W.R.; Correa, F.; Zazueta, C.; et al. Curcumin induces Nrf2 nuclear translocation and prevents glomerular hypertension, hyperfiltration, oxidant stress, and the decrease in antioxidant enzymes in 5/6 nephrectomized rats. Oxid. Med. Cell. Longev. 2012, 2012, 269039. [Google Scholar] [CrossRef]
- Santos-Parker, J.R.; Strahler, T.R.; Bassett, C.J.; Bispham, N.Z.; Chonchol, M.B.; Seals, D.R. Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging (Albany NY) 2017, 9, 187–208. [Google Scholar] [CrossRef]
- Kang, D.G.; Sohn, E.J.; Kwon, E.K.; Han, J.H.; Oh, H.; Lee, H.S. Effects of berberine on angiotensin-converting enzyme and NO/cGMP system in vessels. Vascul. Pharmacol. 2002, 39, 281–286. [Google Scholar] [CrossRef]
- Olmez, E.; Ilhan, M. Evaluation of the alpha-adrenoceptor antagonistic action of berberine in isolated organs. Arzneimittelforschung 1992, 42, 1095–1097. [Google Scholar]
- Chiou, W.F.; Yen, M.H.; Chen, C.F. Mechanism of vasodilatory effect of berberine in rat mesenteric artery. Eur. J. Pharmacol. 1991, 204, 35–40. [Google Scholar] [PubMed]
- Ko, W.H.; Yao, X.Q.; Lau, C.W.; Law, W.I.; Chen, Z.Y.; Kwok, W.; Ho, K.; Huang, Y. Vasorelaxant and antiproliferative effects of berberine. Eur. J. Pharmacol. 2000, 399, 187–196. [Google Scholar] [CrossRef]
- Lan, J.; Zhao, Y.; Dong, F.; Yan, Z.; Zheng, W.; Fan, J.; Sun, G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 2015, 161, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.W.; Yin, S.C.; Ting, C.T.; Lin, S.J.; Hsueh, C.M.; Chen, C.Y.; Hsu, S.L. Berberine inhibits platelet-derived growth factor-induced growth and migration partly through an AMPK-dependent pathway in vascular smooth muscle cells. Eur. J. Pharmacol. 2008, 590, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wang, Y.; Li, J.; Su, C.; Wu, F.; Xia, W.H.; Yang, Z.; Yu, B.B.; Qiu, Y.X.; Tao, J. Berberine improves endothelial function by reducing endothelial microparticles-mediated oxidative stress in humans. Int. J. Cardiol. 2013, 167, 936–942. [Google Scholar] [CrossRef]
- Xu, M.G.; Wang, J.M.; Chen, L.; Wang, Y.; Yang, Z.; Tao, J. Berberine-induced upregulation of circulating endothelial progenitor cells is related to nitric oxide production in healthy subjects. Cardiology 2009, 112, 279–286. [Google Scholar] [CrossRef]
- Affuso, F.; Ruvolo, A.; Micillo, F.; Sacca, L.; Fazio, S. Effects of a nutraceutical combination (berberine, red yeast rice and policosanols) on lipid levels and endothelial function randomized, double-blind, placebo-controlled study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 656–661. [Google Scholar] [CrossRef]
- Izzo, R.; de Simone, G.; Giudice, R.; Chinali, M.; Trimarco, V.; De Luca, N.; Trimarco, B. Effects of nutraceuticals on prevalence of metabolic syndrome and on calculated Framingham Risk Score in individuals with dyslipidemia. J. Hypertens. 2010, 28, 1482–1487. [Google Scholar] [CrossRef]
- Han, J.H.; Lee, H.J.; Kim, T.S.; Kang, M.H. The effect of glutathione S-transferase M1 and T1 polymorphisms on blood pressure, blood glucose, and lipid profiles following the supplementation of kale (Brassica oleracea acephala) juice in South Korean subclinical hypertensive patients. Nutr. Res. Pract. 2015, 9, 49–56. [Google Scholar] [CrossRef]
- Wu, L.; Noyan Ashraf, M.H.; Facci, M.; Wang, R.; Paterson, P.G.; Ferrie, A.; Juurlink, B.H. Dietary approach to attenuate oxidative stress, hypertension, and inflammation in the cardiovascular system. Proc. Natl. Acad. Sci. USA 2004, 101, 7094–7099. [Google Scholar] [CrossRef]
- Rubattu, S.; Di Castro, S.; Cotugno, M.; Bianchi, F.; Mattioli, R.; Baima, S.; Stanzione, R.; Madonna, M.; Bozzao, C.; Marchitti, S.; et al. Protective effects of Brassica oleracea sprouts extract toward renal damage in high-salt-fed SHRSP: Role of AMPK/PPARalpha/UCP2 axis. J. Hypertens. 2015, 33, 1465–1479. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, B.; Bellostas Muguerza, N.; Petersen, A.M.; Kveiborg, B.; Madsen, C.R.; Thomas, H.; Ihlemann, N.; Sorensen, J.C.; Kober, L.; Sorensen, H.; et al. Ingestion of broccoli sprouts does not improve endothelial function in humans with hypertension. PLoS ONE 2010, 5, e12461. [Google Scholar] [CrossRef]
- Kulshreshtha, A.; Zacharia, A.J.; Jarouliya, U.; Bhadauriya, P.; Prasad, G.B.; Bisen, P.S. Spirulina in health care management. Curr. Pharm. Biotechnol. 2008, 9, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Conte, G.M.; Salviati, E.; Pepe, G.; Bertamino, A.; Ostacolo, C.; Sansone, F.; Prete, F.D.; Aquino, R.P.; Campiglia, P. Fast Profiling of Natural Pigments in Different Spirulina (Arthrospira platensis) Dietary Supplements by DI-FT-ICR and Evaluation of their Antioxidant Potential by Pre-Column DPPH-UHPLC Assay. Molecules 2018, 23, 1152. [Google Scholar] [CrossRef]
- Lu, J.; Ren, D.F.; Xue, Y.L.; Sawano, Y.; Miyakawa, T.; Tanokura, M. Isolation of an antihypertensive peptide from alcalase digest of Spirulina platensis. J. Agric. Food Chem. 2010, 58, 7166–7171. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; She, X.; Wu, H.; Ma, J.; Ren, D.; Lu, J. Long-Term Regulation of the Local Renin-Angiotensin System in the Myocardium of Spontaneously Hypertensive Rats by Feeding Bioactive Peptides Derived from Spirulina platensis. J. Agric. Food Chem. 2015, 63, 7765–7774. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.Y.; Ko, S.C.; Kim, C.S.; Oh, G.W.; Ryu, B.; Qian, Z.J.; Kim, G.; Park, W.S.; Choi, I.W.; Phan, T.T.; et al. A heptameric peptide purified from Spirulina sp. gastrointestinal hydrolysate inhibits angiotensin I-converting enzyme- and angiotensin II-induced vascular dysfunction in human endothelial cells. Int. J. Mol. Med. 2017, 39, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Carrizzo, A.; Conte, G.M.; Sommella, E.; Damato, A.; Ambrosio, M.; Sala, M.; Scala, M.C.; Aquino, R.P.; De Lucia, M.; Madonna, M.; et al. Novel Potent Decameric Peptide of Spirulina platensis Reduces Blood Pressure Levels Through a PI3K/AKT/eNOS-Dependent Mechanism. Hypertension 2019, 73, 449–457. [Google Scholar] [CrossRef]
- Miczke, A.; Szulinska, M.; Hansdorfer-Korzon, R.; Kregielska-Narozna, M.; Suliburska, J.; Walkowiak, J.; Bogdanski, P. Effects of spirulina consumption on body weight, blood pressure, and endothelial function in overweight hypertensive Caucasians: A double-blind, placebo-controlled, randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 150–156. [Google Scholar]
- Martinez-Samano, J.; Torres-Montes de Oca, A.; Luqueno-Bocardo, O.I.; Torres-Duran, P.V.; Juarez-Oropeza, M.A. Spirulina maxima Decreases Endothelial Damage and Oxidative Stress Indicators in Patients with Systemic Arterial Hypertension: Results from Exploratory Controlled Clinical Trial. Mar. Drugs 2018, 16, 496. [Google Scholar] [CrossRef]
- Huang, H.; Liao, D.; Pu, R.; Cui, Y. Quantifying the effects of spirulina supplementation on plasma lipid and glucose concentrations, body weight, and blood pressure. Diabetes Metab. Syndr. Obes. 2018, 11, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Oropeza, M.A.; Mascher, D.; Torres-Duran, P.V.; Farias, J.M.; Paredes-Carbajal, M.C. Effects of dietary Spirulina on vascular reactivity. J. Med. Food 2009, 12, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Torres-Duran, P.V.; Ferreira-Hermosillo, A.; Juarez-Oropeza, M.A. Antihyperlipemic and antihypertensive effects of Spirulina maxima in an open sample of Mexican population: A preliminary report. Lipids Health Dis. 2007, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Insull, W., Jr. The pathology of atherosclerosis: Plaque development and plaque responses to medical treatment. Am. J. Med. 2009, 122 (Suppl. 1), S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Titov, V.N.; Shiriaeva Iu, K. Arteriolosclerosis and atherosclerosis. Pathology of the distal and proximal arterial bed. Pathogenesis of diabetic microangiopathy. Klin. Lab. Diagn. 2011, 4, 3–14. [Google Scholar]
- Kolodgie, F.D.; Nakazawa, G.; Sangiorgi, G.; Ladich, E.; Burke, A.P.; Virmani, R. Pathology of atherosclerosis and stenting. Neuroimaging Clin. N. Am. 2007, 17, 285–301. [Google Scholar] [CrossRef]
- Faust, O.; Acharya, U.R.; Sudarshan, V.K.; Tan, R.S.; Yeong, C.H.; Molinari, F.; Ng, K.H. Computer aided diagnosis of Coronary Artery Disease, Myocardial Infarction and carotid atherosclerosis using ultrasound images: A review. Phys. Med. 2017, 33, 1–15. [Google Scholar] [CrossRef]
- Adams, A.; Bojara, W.; Schunk, K. Early Diagnosis and Treatment of Coronary Heart Disease in Symptomatic Subjects With Advanced Vascular Atherosclerosis of the Carotid Artery (Type III and IV b Findings Using Ultrasound). Cardiol. Res. 2017, 8, 7–12. [Google Scholar] [CrossRef]
- Teramoto, T.; Sasaki, J.; Ishibashi, S.; Birou, S.; Daida, H.; Dohi, S.; Egusa, G.; Hiro, T.; Hirobe, K.; Iida, M.; et al. Diagnosis of atherosclerosis. Executive Summary of the Japan Atherosclerosis Society (JAS) Guidelines for the Diagnosis and Prevention of Atherosclerotic Cardiovascular Diseases in Japan-2012 Version. J. Atheroscler. Thromb. 2014, 21, 296–298. [Google Scholar] [CrossRef]
- Mitevska, I.P.; Baneva, N.; Bosevski, M.; Kostovska, E.S. Prevalence of risk factors and asymptomatic carotid atherosclerosis in diabetic patients screened for silent myocardial ischemia by SPECT myocardial imaging. Nucl. Med. Rev. Cent. East. Eur. 2017, 20, 3–9. [Google Scholar] [CrossRef][Green Version]
- You, T.H.; Lu, Y.Q.; Tian, Z.J.; Zhou, Y.L.; Wang, T. Correlation between myocardial ischemia and carotid atherosclerosis in hypertensive patients. Nan Fang Yi Ke Da Xue Xue Bao 2010, 30, 311–314. [Google Scholar] [PubMed]
- Yahagi, K.; Kolodgie, F.D.; Lutter, C.; Mori, H.; Romero, M.E.; Finn, A.V.; Virmani, R. Pathology of Human Coronary and Carotid Artery Atherosclerosis and Vascular Calcification in Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, F.; Yasuda, S.; Noguchi, T.; Ishibashi-Ueda, H. Pathology of coronary atherosclerosis and thrombosis. Cardiovasc. Diagn. Ther. 2016, 6, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Herrington, W.; Lacey, B.; Sherliker, P.; Armitage, J.; Lewington, S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ. Res. 2016, 118, 535–546. [Google Scholar] [CrossRef]
- Matsushita, K.; Selvin, E.; Bash, L.D.; Astor, B.C.; Coresh, J. Risk implications of the new CKD Epidemiology Collaboration (CKD-EPI) equation compared with the MDRD Study equation for estimated GFR: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Kidney Dis. 2010, 55, 648–659. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Santos, R.D. Subclinical carotid vascular disease and risk factors for atherosclerosis in type 1 and type 2 diabetes. Arch. Endocrinol. Metab. 2017, 61, 105–107. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Bai, L.; Shi, M.; Lu, H.; Wu, Y.; Tu, J.; Ni, J.; Wang, J.; Cao, L.; Lei, P.; et al. Features and risk factors of carotid atherosclerosis in a population with high stroke incidence in China. Oncotarget 2017. [Google Scholar] [CrossRef]
- Bittner, D.O.; Klinghammer, L.; Marwan, M.; Schmid, J.; Layritz, C.; Hoffmann, U.; Achenbach, S.; Pflederer, T. Influence of Cardiovascular Risk Factors on the Prevalence of Coronary Atherosclerosis in Patients with Angiographically Normal Coronary Arteries. Acad. Radiol. 2017, 24, 580–586. [Google Scholar] [CrossRef]
- Foteinos, G.; Hu, Y.; Xiao, Q.; Metzler, B.; Xu, Q. Rapid endothelial turnover in atherosclerosis-prone areas coincides with stem cell repair in apolipoprotein E-deficient mice. Circulation 2008, 117, 1856–1863. [Google Scholar] [CrossRef]
- Guo, F.X.; Hu, Y.W.; Zheng, L.; Wang, Q. Shear Stress in Autophagy and Its Possible Mechanisms in the Process of Atherosclerosis. DNA Cell Biol. 2017, 36, 335–346. [Google Scholar] [CrossRef]
- Thondapu, V.; Bourantas, C.V.; Foin, N.; Jang, I.K.; Serruys, P.W.; Barlis, P. Biomechanical stress in coronary atherosclerosis: Emerging insights from computational modelling. Eur. Heart J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, E.; Friess, U. Oxidative stress, AGE, and atherosclerosis. Kidney Int. 2007, 72, S17–S26. [Google Scholar] [CrossRef] [PubMed]
- Villa, F.; Malovini, A.; Carrizzo, A.; Spinelli, C.C.; Ferrario, A.; Maciag, A.; Madonna, M.; Bellazzi, R.; Milanesi, L.; Vecchione, C.; et al. Serum BPIFB4 levels classify health status in long-living individuals. Immun. Ageing 2015, 12, 27. [Google Scholar] [CrossRef]
- von Hundelshausen, P.; Weber, C. Chronic inflammation and atherosclerosis. Dtsch. Med. Wochenschr. 2013, 138, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Titov, V.N. Atherosclerosis as pathology of polyene fatty acids. Vestn. Ross. Akad. Med. Nauk 2001, 5, 48–53. [Google Scholar]
- Woolf, N. Pathology of atherosclerosis. Br. Med. Bull. 1990, 46, 960–985. [Google Scholar] [CrossRef]
- Klingenberg, R.; Matter, C.M.; Luscher, T.F. Immune cells in atherosclerosis—Good or bad? Praxis (Bern 1994) 2016, 105, 437–444. [Google Scholar] [CrossRef]
- Schaftenaar, F.; Frodermann, V.; Kuiper, J.; Lutgens, E. Atherosclerosis: The interplay between lipids and immune cells. Curr. Opin. Lipidol. 2016, 27, 209–215. [Google Scholar] [CrossRef]
- Tada, H.; Kawashiri, M.A.; Nohara, A.; Inazu, A.; Kobayashi, J.; Yasuda, K.; Mabuchi, H.; Yamagishi, M.; Hayashi, K. Lipid Management in a Japanese Community:Attainment Rate of Target Set by the Japan Atherosclerosis Society Guidelines for the Prevention of Atherosclerotic Cardiovascular Diseases 2012. J. Atheroscler. Thromb. 2017, 24, 338–345. [Google Scholar] [CrossRef][Green Version]
- Authors/Task Force, M.; Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016, 253, 281–344. [Google Scholar] [CrossRef]
- Martinez-Abundis, E.; Mendez-Del Villar, M.; Perez-Rubio, K.G.; Zuniga, L.Y.; Cortez-Navarrete, M.; Ramirez-Rodriguez, A.; Gonzalez-Ortiz, M. Novel nutraceutic therapies for the treatment of metabolic syndrome. World J. Diabetes 2016, 7, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Zhang, L.; Jiang, S.; Bai, Y. Beneficial effects of resveratrol on atherosclerosis. J. Med. Food 2008, 11, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.J.; Ahn, J.Y.; Kim, S.; Choi, M.S.; Ha, T.Y. Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem. Biophys. Res. Commun. 2008, 367, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Voloshyna, I.; Hussaini, S.M.; Reiss, A.B. Resveratrol in cholesterol metabolism and atherosclerosis. J. Med. Food 2012, 15, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, T.; Nanmoku, M.; Shimizu, M.; Inoue, J.; Sato, R. Resveratrol increases the expression and activity of the low density lipoprotein receptor in hepatocytes by the proteolytic activation of the sterol regulatory element-binding proteins. Atherosclerosis 2012, 220, 369–374. [Google Scholar] [CrossRef]
- Gnoni, G.V.; Paglialonga, G. Resveratrol inhibits fatty acid and triacylglycerol synthesis in rat hepatocytes. Eur. J. Clin. Investig. 2009, 39, 211–218. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, Z.; Wu, W.; Song, C.; Meng, S.; Fan, L.; Bing, X.; Chen, J. Effects of dietary resveratrol supplementation on hepatic and serum pro-/anti-inflammatory activity in juvenile GIFT tilapia, Oreochromis niloticus. Dev. Comp. Immunol. 2017, 73, 220–228. [Google Scholar] [CrossRef]
- Wang, G.; Hu, Z.; Song, X.; Cui, Q.; Fu, Q.; Jia, R.; Zou, Y.; Li, L.; Yin, Z. Analgesic and Anti-Inflammatory Activities of Resveratrol through Classic Models in Mice and Rats. Evid. Based Complement. Alternat. Med. 2017, 2017, 5197567. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Du, Y.; Li, G.; Wang, L.; Zhou, F. Resveratrol Ameliorated Vascular Calcification by Regulating Sirt-1 and Nrf2. Transplant. Proc. 2016, 48, 3378–3386. [Google Scholar] [CrossRef]
- Peredo-Escarcega, A.E.; Guarner-Lans, V.; Perez-Torres, I.; Ortega-Ocampo, S.; Carreon-Torres, E.; Castrejon-Tellez, V.; Diaz-Diaz, E.; Rubio-Ruiz, M.E. The Combination of Resveratrol and Quercetin Attenuates Metabolic Syndrome in Rats by Modifying the Serum Fatty Acid Composition and by Upregulating SIRT 1 and SIRT 2 Expression in White Adipose Tissue. Evid. Based Complement. Alternat. Med. 2015, 2015, 474032. [Google Scholar] [CrossRef]
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 2011, 286, 11492–11505. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Li, W.Q.; Dou, J.L.; Li, L.; Hu, Y.J.; Guo, J.Z.; Lu, D.; Zhang, G.; Sun, L. Resveratrol reduces inflammatory cytokines via inhibiting nuclear factor-kappaB and mitogen-activated protein kinase signal pathway in a rabbit atherosclerosis model. Zhonghua Xin Xue Guan Bing Za Zhi 2013, 41, 866–869. [Google Scholar] [PubMed]
- Novaes, R.D.; Peluzio Mdo, C.; Maldonado, I.R. Resveratrol causes antiatherogenic effects in an animal model of atherosclerosis. Arq. Bras. Cardiol. 2012, 98, 571–572. [Google Scholar] [CrossRef] [PubMed]
- Buttari, B.; Profumo, E.; Segoni, L.; D’Arcangelo, D.; Rossi, S.; Facchiano, F.; Saso, L.; Businaro, R.; Iuliano, L.; Rigano, R. Resveratrol counteracts inflammation in human M1 and M2 macrophages upon challenge with 7-oxo-cholesterol: Potential therapeutic implications in atherosclerosis. Oxid. Med. Cell. Longev. 2014, 2014, 257543. [Google Scholar] [CrossRef]
- Prasad, K. Resveratrol, wine, and atherosclerosis. Int. J. Angiol. 2012, 21, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Rocha, K.K.; Souza, G.A.; Seiva, F.R.; Ebaid, G.X.; Novelli, E.L. Weekend ethanol consumption and high-sucrose diet: Resveratrol effects on energy expenditure, substrate oxidation, lipid profile, oxidative stress and hepatic energy metabolism. Alcohol. Alcohol. 2011, 46, 10–16. [Google Scholar] [CrossRef]
- Sahebkar, A. Effects of resveratrol supplementation on plasma lipids: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2013, 71, 822–835. [Google Scholar] [CrossRef]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol supplementation improves glycemic control in type 2 diabetes mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Bo, S.; Ciccone, G.; Castiglione, A.; Gambino, R.; De Michieli, F.; Villois, P.; Durazzo, M.; Cavallo-Perin, P.; Cassader, M. Anti-inflammatory and antioxidant effects of resveratrol in healthy smokers a randomized, double-blind, placebo-controlled, cross-over trial. Curr. Med. Chem. 2013, 20, 1323–1331. [Google Scholar] [CrossRef]
- Tome-Carneiro, J.; Gonzalvez, M.; Larrosa, M.; Garcia-Almagro, F.J.; Aviles-Plaza, F.; Parra, S.; Yanez-Gascon, M.J.; Ruiz-Ros, J.A.; Garcia-Conesa, M.T.; Tomas-Barberan, F.A.; et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 2012, 56, 810–821. [Google Scholar] [CrossRef]
- Kurosawa, T.; Itoh, F.; Nozaki, A.; Nakano, Y.; Katsuda, S.; Osakabe, N.; Tsubone, H.; Kondo, K.; Itakura, H. Suppressive effect of cocoa powder on atherosclerosis in Kurosawa and Kusanagi-hypercholesterolemic rabbits. J. Atheroscler. Thromb. 2005, 12, 20–28. [Google Scholar] [CrossRef]
- Paramo, J.A. A new favourable effect of cocoa on atherosclerosis? Cardiovasc. Res. 2008, 79, 3–4. [Google Scholar] [CrossRef]
- Guan, H.; Lin, Y.; Bai, L.; An, Y.; Shang, J.; Wang, Z.; Zhao, S.; Fan, J.; Liu, E. Dietary Cocoa Powder Improves Hyperlipidemia and Reduces Atherosclerosis in apoE Deficient Mice through the Inhibition of Hepatic Endoplasmic Reticulum Stress. Mediators Inflamm. 2016, 2016, 1937572. [Google Scholar] [CrossRef]
- Pelaez-Soto, A.; Fernandez-Espinar, M.T.; Roig, P.; Gil, J.V. Evaluation of the Ability of Polyphenol Extracts of Cocoa and Red Grape to Promote the Antioxidant Response in Yeast Using a Rapid Multiwell Assay. J. Food Sci. 2017, 82, 324–332. [Google Scholar] [CrossRef]
- Yasuda, A.; Natsume, M.; Sasaki, K.; Baba, S.; Nakamura, Y.; Kanegae, M.; Nagaoka, S. Cacao procyanidins reduce plasma cholesterol and increase fecal steroid excretion in rats fed a high-cholesterol diet. Biofactors 2008, 33, 211–223. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Derr, J.A.; Mustad, V.A.; Seligson, F.H.; Pearson, T.A. Effects of a milk chocolate bar per day substituted for a high-carbohydrate snack in young men on an NCEP/AHA Step 1 Diet. Am. J. Clin. Nutr. 1994, 60 (Suppl. 6), 1037S–1042S. [Google Scholar] [CrossRef]
- Kondo, K.; Hirano, R.; Matsumoto, A.; Igarashi, O.; Itakura, H. Inhibition of LDL oxidation by cocoa. Lancet 1996, 348, 1514. [Google Scholar] [CrossRef]
- Mursu, J.; Voutilainen, S.; Nurmi, T.; Rissanen, T.H.; Virtanen, J.K.; Kaikkonen, J.; Nyyssonen, K.; Salonen, J.T. Dark chocolate consumption increases HDL cholesterol concentration and chocolate fatty acids may inhibit lipid peroxidation in healthy humans. Free Radic. Biol. Med. 2004, 37, 1351–1359. [Google Scholar] [CrossRef]
- Fraga, C.G.; Actis-Goretta, L.; Ottaviani, J.I.; Carrasquedo, F.; Lotito, S.B.; Lazarus, S.; Schmitz, H.H.; Keen, C.L. Regular consumption of a flavanol-rich chocolate can improve oxidant stress in young soccer players. Clin. Dev. Immunol. 2005, 12, 11–17. [Google Scholar] [CrossRef]
- Ludovici, V.; Barthelmes, J.; Nagele, M.P.; Enseleit, F.; Ferri, C.; Flammer, A.J.; Ruschitzka, F.; Sudano, I. Cocoa, Blood Pressure, and Vascular Function. Front. Nutr. 2017, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Judd, J.T.; Baer, D.J.; Clevidence, B.A.; Paul, D.R.; Edwards, A.J.; Wiseman, S.A.; Muesing, R.A.; Chen, S.C. Black tea consumption reduces total and LDL cholesterol in mildly hypercholesterolemic adults. J. Nutr. 2003, 133, 3298S–3302S. [Google Scholar] [CrossRef] [PubMed]
- Zomer, E.; Owen, A.; Magliano, D.J.; Liew, D.; Reid, C.M. The effectiveness and cost effectiveness of dark chocolate consumption as prevention therapy in people at high risk of cardiovascular disease: Best case scenario analysis using a Markov model. BMJ 2012, 344, e3657. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Bhaskar, S.; Kumar, K.S.; Krishnan, K.; Antony, H. Quercetin alleviates hypercholesterolemic diet induced inflammation during progression and regression of atherosclerosis in rabbits. Nutrition 2013, 29, 219–229. [Google Scholar] [CrossRef]
- Schulz, H.U.; Schurer, M.; Bassler, D.; Weiser, D. Investigation of pharmacokinetic data of hypericin, pseudohypericin, hyperforin and the flavonoids quercetin and isorhamnetin revealed from single and multiple oral dose studies with a hypericum extract containing tablet in healthy male volunteers. Arzneimittelforschung 2005, 55, 561–568. [Google Scholar] [CrossRef]
- Xue, F.; Nie, X.; Shi, J.; Liu, Q.; Wang, Z.; Li, X.; Zhou, J.; Su, J.; Xue, M.; Chen, W.D.; et al. Quercetin Inhibits LPS-Induced Inflammation and ox-LDL-Induced Lipid Deposition. Front. Pharmacol. 2017, 8, 40. [Google Scholar] [CrossRef]
- Mbikay, M.; Sirois, F.; Simoes, S.; Mayne, J.; Chretien, M. Quercetin-3-glucoside increases low-density lipoprotein receptor (LDLR) expression, attenuates proprotein convertase subtilisin/kexin 9 (PCSK9) secretion, and stimulates LDL uptake by Huh7 human hepatocytes in culture. FEBS Open Bio 2014, 4, 755–762. [Google Scholar] [CrossRef]
- Kaur, G.; Meena, C. Amelioration of obesity, glucose intolerance, and oxidative stress in high-fat diet and low-dose streptozotocin-induced diabetic rats by combination consisting of “curcumin with piperine and quercetin”. ISRN Pharmacol. 2012, 2012, 957283. [Google Scholar] [CrossRef]
- Lu, X.L.; Zhao, C.H.; Yao, X.L.; Zhang, H. Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway. Biomed. Pharmacother. 2017, 85, 658–671. [Google Scholar] [CrossRef]
- Kleemann, R.; Verschuren, L.; Morrison, M.; Zadelaar, S.; van Erk, M.J.; Wielinga, P.Y.; Kooistra, T. Anti-inflammatory, anti-proliferative and anti-atherosclerotic effects of quercetin in human in vitro and in vivo models. Atherosclerosis 2011, 218, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.H.; Chan, S.H.; Chu, P.M.; Tsai, K.L. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Mol. Nutr. Food Res. 2015, 59, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, L.; Guo, X.; Zhang, S.; Wang, J.; Zhou, F.; Liu, L.; Tang, Y.; Yao, P. Quercetin attenuates high fat diet-induced atherosclerosis in apolipoprotein E knockout mice: A critical role of NADPH oxidase. Food Chem. Toxicol. 2017, 105, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Talirevic, E.; Jelena, S. Quercetin in the treatment of dyslipidemia. Med. Arh. 2012, 66, 87–88. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. Effects of quercetin supplementation on lipid profile: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2017, 57, 666–676. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Pawar, Y.B.; Munjal, B.; Arora, S.; Karwa, M.; Kohli, G.; Paliwal, J.K.; Bansal, A.K. Bioavailability of a lipidic formulation of curcumin in healthy human volunteers. Pharmaceutics 2012, 4, 517–530. [Google Scholar] [CrossRef]
- Cox, K.H.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015, 29, 642–651. [Google Scholar] [CrossRef]
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1411–1417. [Google Scholar] [CrossRef]
- Strimpakos, A.S.; Sharma, R.A. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox Signal. 2008, 10, 511–545. [Google Scholar] [CrossRef]
- Kumar, A.; Cannon, C.P. Acute coronary syndromes: Diagnosis and management, part I. Mayo Clin. Proc. 2009, 84, 917–938. [Google Scholar] [CrossRef]
- Alwi, I.; Santoso, T.; Suyono, S.; Sutrisna, B.; Suyatna, F.D.; Kresno, S.B.; Ernie, S. The effect of curcumin on lipid level in patients with acute coronary syndrome. Acta Med. Indones. 2008, 40, 201–210. [Google Scholar]
- Azhdari, M.; Karandish, M.; Mansoori, A. Metabolic benefits of curcumin supplementation in patients with metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of curcumin on cardiovascular risk factors in obese and overweight adolescent girls: A randomized clinical trial. Sao Paulo Med. J. 2019, 137, 414–422. [Google Scholar] [CrossRef]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Sundar Dhilip Kumar, S.; Houreld, N.N.; Abrahamse, H. Therapeutic Potential and Recent Advances of Curcumin in the Treatment of Aging-Associated Diseases. Molecules 2018, 23, 835. [Google Scholar] [CrossRef]
- Simental-Mendia, L.E.; Pirro, M.; Gotto, A.M., Jr.; Banach, M.; Atkin, S.L.; Majeed, M.; Sahebkar, A. Lipid-modifying activity of curcuminoids: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 1178–1187. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Wu, M.; Wang, J. Advance on study in anti-atherosclerosis mechanism of berberine. Zhongguo Zhong Yao Za Zhi 2008, 33, 2013–2016. [Google Scholar] [CrossRef]
- Wu, M.; Wang, J.; Liu, L.T. Advance of studies on anti-atherosclerosis mechanism of berberine. Chin. J. Integr. Med. 2010, 16, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Zou, Z.; Zhou, X.; Hu, Y.; Ma, H.; Xiao, Y.; Li, X.; Ye, X. Comparative effect of berberine and its derivative 8-cetylberberine on attenuating atherosclerosis in ApoE-/- mice. Int. Immunopharmacol. 2017, 43, 195–202. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, M.; Liang, B.; Shirwany, N.; Zhu, Y.; Zou, M.H. Activation of AMP-activated protein kinase is required for berberine-induced reduction of atherosclerosis in mice: The role of uncoupling protein 2. PLoS ONE 2011, 6, e25436. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yao, W.; Zheng, X.; Liao, K. Berberine promotes the development of atherosclerosis and foam cell formation by inducing scavenger receptor A expression in macrophage. Cell Res. 2009, 19, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Rovati, L.C.; Setnikar, I. Eulipidemic effects of berberine administered alone or in combination with other natural cholesterol-lowering agents. A single-blind clinical investigation. Arzneimittelforschung 2007, 57, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.J.; Wei, J.; Zuo, Z.Y.; Wang, Y.M.; Song, D.Q.; You, X.F.; Zhao, L.X.; Pan, H.N.; Jiang, J.D. Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism 2008, 57, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef]
- Zhao, W.; Xue, R.; Zhou, Z.X.; Kong, W.J.; Jiang, J.D. Reduction of blood lipid by berberine in hyperlipidemic patients with chronic hepatitis or liver cirrhosis. Biomed. Pharmacother. 2008, 62, 730–731. [Google Scholar] [CrossRef]
- Persico, M.; Masarone, M.; Damato, A.; Ambrosio, M.; Federico, A.; Rosato, V.; Bucci, T.; Carrizzo, A.; Vecchione, C. Non alcoholic fatty liver disease and eNOS dysfunction in humans. BMC Gastroenterol. 2017, 17, 35. [Google Scholar] [CrossRef]
- An, S.; Han, J.I.; Kim, M.J.; Park, J.S.; Han, J.M.; Baek, N.I.; Chung, H.G.; Choi, M.S.; Lee, K.T.; Jeong, T.S. Ethanolic extracts of Brassica campestris spp. rapa roots prevent high-fat diet-induced obesity via beta(3)-adrenergic regulation of white adipocyte lipolytic activity. J. Med. Food 2010, 13, 406–414. [Google Scholar] [CrossRef]
- Palomaki, A.; Pohjantahti-Maaroos, H.; Wallenius, M.; Kankkunen, P.; Aro, H.; Husgafvel, S.; Pihlava, J.M.; Oksanen, K. Effects of dietary cold-pressed turnip rapeseed oil and butter on serum lipids, oxidized LDL and arterial elasticity in men with metabolic syndrome. Lipids Health Dis. 2010, 9, 137. [Google Scholar] [CrossRef]
- Vo, T.S.; Kim, S.K. Down-regulation of histamine-induced endothelial cell activation as potential anti-atherosclerotic activity of peptides from Spirulina maxima. Eur. J. Pharm. Sci. 2013, 50, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.H.; Kim, M.Y.; Sok, D.E.; Hwang, S.Y.; Kim, J.H.; Kim, H.R.; Lee, J.H.; Kim, Y.B.; Kim, M.R. Spirulina prevents atherosclerosis by reducing hypercholesterolemia in rabbits fed a high-cholesterol diet. J. Nutr. Sci. Vitaminol. (Tokyo) 2010, 56, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Koley, H.; Dutta, S.; Bhowal, J. Hypocholesterolemic effect of Spirulina platensis (SP) fortified functional soy yogurts on diet-induced hypercholesterolemia. J. Funct. Foods 2018, 48, 54–64. [Google Scholar] [CrossRef]
- Szulinska, M.; Gibas-Dorna, M.; Miller-Kasprzak, E.; Suliburska, J.; Miczke, A.; Walczak-Galezewska, M.; Stelmach-Mardas, M.; Walkowiak, J.; Bogdanski, P. Spirulina maxima improves insulin sensitivity, lipid profile, and total antioxidant status in obese patients with well-treated hypertension: A randomized double-blind placebo-controlled study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2473–2481. [Google Scholar] [PubMed]
- Hamedifard, Z.; Milajerdi, A.; Reiner, Z.; Taghizadeh, M.; Kolahdooz, F.; Asemi, Z. The effects of spirulina on glycemic control and serum lipoproteins in patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 2609–2621. [Google Scholar] [CrossRef]
- Hernandez-Lepe, M.A.; Wall-Medrano, A.; Lopez-Diaz, J.A.; Juarez-Oropeza, M.A.; Hernandez-Torres, R.P.; Ramos-Jimenez, A. Hypolipidemic Effect of Arthrospira (Spirulina) maxima Supplementation and a Systematic Physical Exercise Program in Overweight and Obese Men: A Double-Blind, Randomized, and Crossover Controlled Trial. Mar. Drugs 2019, 17, 270. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Lepe, M.A.; Olivas-Aguirre, F.J.; Gomez-Miranda, L.M.; Hernandez-Torres, R.P.; Manriquez-Torres, J.J.; Ramos-Jimenez, A. Systematic Physical Exercise and Spirulina maxima Supplementation Improve Body Composition, Cardiorespiratory Fitness, and Blood Lipid Profile: Correlations of a Randomized Double-Blind Controlled Trial. Antioxidants 2019, 8, 507. [Google Scholar] [CrossRef]
- Serban, M.C.; Sahebkar, A.; Dragan, S.; Stoichescu-Hogea, G.; Ursoniu, S.; Andrica, F.; Banach, M. A systematic review and meta-analysis of the impact of Spirulina supplementation on plasma lipid concentrations. Clin. Nutr. 2016, 35, 842–851. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, Y.J.; Ryu, H.K.; Kim, M.H.; Chung, H.W.; Kim, W.Y. A randomized double-blind, placebo-controlled study to establish the effects of spirulina in elderly Koreans. Ann. Nutr. Metab. 2008, 52, 322–328. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Starakis, I.K.; Papadomanolaki, M.G.; Mavroeidi, N.G.; Ganotakis, E.S. The hypolipidaemic effects of Spirulina (Arthrospira platensis) supplementation in a Cretan population: A prospective study. J. Sci. Food Agric. 2014, 94, 432–437. [Google Scholar] [CrossRef]
- Wasson, S.; Fleming, D.; Reddy, H.K. Pathology, prognosis, and prevention of heart failure. Mo. Med. 2005, 102, 480–484. [Google Scholar] [PubMed]
- Braam, R.L.; Gaillard, C.A. Heart failure: Chapter 3. Underlying pathology in heart failure ‘Failure of the circulation versus failure of the heart’. Neth. Heart J. 2004, 12, 303–307. [Google Scholar] [PubMed]
- Izzo, C.; Carrizzo, A.; Alfano, A.; Virtuoso, N.; Capunzo, M.; Calabrese, M.; De Simone, E.; Sciarretta, S.; Frati, G.; Oliveti, M.; et al. The Impact of Aging on Cardio and Cerebrovascular Diseases. Int. J. Mol. Sci. 2018, 19, 481. [Google Scholar] [CrossRef] [PubMed]
- Kajita, A. Pathology of heart failure. Kyobu Geka 1971, 24, 100–106. [Google Scholar]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Card. Fail. 2017. [Google Scholar] [CrossRef]
- Swedberg, K. Guidelines for the treatment of chronic heart failure. Trends Cardiovasc. Med. 2017. [Google Scholar] [CrossRef]
- Wang, G.Y.; Wang, Y.M.; Zhang, L.N.; Li, Q.; Yue, H.; Song, C.M.; Feng, J.K.; Wang, N. Effect of resveratrol on heart function of rats with adriamycin-induced heart failure. Zhongguo Zhong Yao Za Zhi 2007, 32, 1563–1565. [Google Scholar]
- Xuan, W.; Wu, B.; Chen, C.; Chen, B.; Zhang, W.; Xu, D.; Bin, J.; Liao, Y. Resveratrol improves myocardial ischemia and ischemic heart failure in mice by antagonizing the detrimental effects of fractalkine*. Crit. Care Med. 2012, 40, 3026–3033. [Google Scholar] [CrossRef]
- Gu, X.S.; Wang, Z.B.; Ye, Z.; Lei, J.P.; Li, L.; Su, D.F.; Zheng, X. Resveratrol, an activator of SIRT1, upregulates AMPK and improves cardiac function in heart failure. Genet. Mol. Res. 2014, 13, 323–335. [Google Scholar] [CrossRef]
- Gupta, P.K.; DiPette, D.J.; Supowit, S.C. Protective effect of resveratrol against pressure overload-induced heart failure. Food Sci. Nutr. 2014, 2, 218–229. [Google Scholar] [CrossRef]
- Ahmet, I.; Tae, H.J.; Lakatta, E.G.; Talan, M. Long-term low dose dietary resveratrol supplement reduces cardiovascular structural and functional deterioration in chronic heart failure in rats. Can. J. Physiol. Pharmacol. 2017, 95, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.M.; Byrne, N.J.; Robertson, I.M.; Kim, T.T.; Samokhvalov, V.; Levasseur, J.; Soltys, C.L.; Fung, D.; Tyreman, N.; Denou, E.; et al. Resveratrol improves exercise performance and skeletal muscle oxidative capacity in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H842–H853. [Google Scholar] [CrossRef] [PubMed]
- Riba, A.; Deres, L.; Sumegi, B.; Toth, K.; Szabados, E.; Halmosi, R. Cardioprotective Effect of Resveratrol in a Postinfarction Heart Failure Model. Oxid. Med. Cell. Longev. 2017, 2017, 6819281. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.; Louis, X.L.; Thandapilly, S.J.; Movahed, A.; Zieroth, S.; Netticadan, T. Potential of resveratrol in the treatment of heart failure. Life Sci. 2014, 95, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Sung, M.M.; Dyck, J.R. Therapeutic potential of resveratrol in heart failure. Ann. N. Y. Acad. Sci. 2015, 1348, 32–45. [Google Scholar] [CrossRef]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Militaru, C.; Donoiu, I.; Craciun, A.; Scorei, I.D.; Bulearca, A.M.; Scorei, R.I. Oral resveratrol and calcium fructoborate supplementation in subjects with stable angina pectoris: Effects on lipid profiles, inflammation markers, and quality of life. Nutrition 2013, 29, 178–183. [Google Scholar] [CrossRef]
- Taub, P.R.; Ramirez-Sanchez, I.; Ciaraldi, T.P.; Perkins, G.; Murphy, A.N.; Naviaux, R.; Hogan, M.; Maisel, A.S.; Henry, R.R.; Ceballos, G.; et al. Alterations in skeletal muscle indicators of mitochondrial structure and biogenesis in patients with type 2 diabetes and heart failure: Effects of epicatechin rich cocoa. Clin. Transl. Sci. 2012, 5, 43–47. [Google Scholar] [CrossRef]
- Taub, P.R.; Ramirez-Sanchez, I.; Ciaraldi, T.P.; Gonzalez-Basurto, S.; Coral-Vazquez, R.; Perkins, G.; Hogan, M.; Maisel, A.S.; Henry, R.R.; Ceballos, G.; et al. Perturbations in skeletal muscle sarcomere structure in patients with heart failure and type 2 diabetes: Restorative effects of (-)-epicatechin-rich cocoa. Clin. Sci. 2013, 125, 383–389. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, I.; Taub, P.R.; Ciaraldi, T.P.; Nogueira, L.; Coe, T.; Perkins, G.; Hogan, M.; Maisel, A.S.; Henry, R.R.; Ceballos, G.; et al. (-)-Epicatechin rich cocoa mediated modulation of oxidative stress regulators in skeletal muscle of heart failure and type 2 diabetes patients. Int. J. Cardiol. 2013, 168, 3982–3990. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar] [CrossRef]
- De Palma, R.; Sotto, I.; Wood, E.G.; Khan, N.Q.; Butler, J.; Johnston, A.; Rothman, M.T.; Corder, R. Cocoa flavanols reduce N-terminal pro-B-type natriuretic peptide in patients with chronic heart failure. ESC Heart Fail. 2016, 3, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Yao, S.; Wan, J.; Gan, X. Chocolate Consumption and Risk of Heart Failure: A Meta-Analysis of Prospective Studies. Nutrients 2017, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.J.; Symons, J.D.; Jalili, T. Therapeutic potential of quercetin to decrease blood pressure: Review of efficacy and mechanisms. Adv. Nutr. 2012, 3, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Huxley, R.R.; Neil, H.A. The relation between dietary flavonol intake and coronary heart disease mortality: A meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2003, 57, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Sunagawa, Y.; Kawamura, T.; Takaya, T.; Wada, H.; Nagasawa, A.; Komeda, M.; Fujita, M.; Shimatsu, A.; Kita, T.; et al. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J. Clin. Investig. 2008, 118, 868–878. [Google Scholar] [CrossRef]
- Tang, Y.H.; Bao, M.W.; Yang, B.; Zhang, Y.; Zhang, B.S.; Zhou, Q.; Chen, J.L.; Huang, C.X. Curcumin attenuates left ventricular dysfunction and remodeling in rabbits with chronic heart failure. Zhonghua Xin Xue Guan Bing Za Zhi 2009, 37, 262–267. [Google Scholar]
- Wongcharoen, W.; Jai-Aue, S.; Phrommintikul, A.; Nawarawong, W.; Woragidpoonpol, S.; Tepsuwan, T.; Sukonthasarn, A.; Apaijai, N.; Chattipakorn, N. Effects of curcuminoids on frequency of acute myocardial infarction after coronary artery bypass grafting. Am. J. Cardiol. 2012, 110, 40–44. [Google Scholar] [CrossRef]
- Campbell, M.S.; Ouyang, A.; Krishnakumar, I.M.; Charnigo, R.J.; Westgate, P.M.; Fleenor, B.S. Influence of enhanced bioavailable curcumin on obesity-associated cardiovascular disease risk factors and arterial function: A double-blinded, randomized, controlled trial. Nutrition 2019, 62, 135–139. [Google Scholar] [CrossRef]
- Dastani, M.; Bigdelu, L.; Hoseinzadeh, M.; Rahimi, H.R.; Karimani, A.; Hooshang Mohammadpour, A.; Salari, M. The effects of curcumin on the prevention of atrial and ventricular arrhythmias and heart failure in patients with unstable angina: A randomized clinical trial. Avicenna J. Phytomed. 2019, 9, 1–9. [Google Scholar] [PubMed]
- Li, Y.; Chen, X.; Liu, H.; Luo, F.; Li, G. Effects of ginseng total saponins with berberine on plasma brain natriuretic peptide and Ca2+ concentration in experimental rats with chronic congestive heart failure. Zhongguo Zhong Yao Za Zhi 2009, 34, 324–327. [Google Scholar] [PubMed]
- Zhang, X.D.; Ren, H.M.; Liu, L. Effects of different dose berberine on hemodynamic parameters and [Ca2+]i of cardiac myocytes of diastolic heart failure rat model. Zhongguo Zhong Yao Za Zhi 2008, 33, 818–821. [Google Scholar] [PubMed]
- Marin-Neto, J.A.; Maciel, B.C.; Secches, A.L.; Gallo Junior, L. Cardiovascular effects of berberine in patients with severe congestive heart failure. Clin. Cardiol. 1988, 11, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.H.; Zeng, X.J.; Li, Y.Y. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2003, 92, 173–176. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, J.; Lan, T. Protective effect of berberine on isolated perfused heart in heart failure. Hua Xi Yi Ke Da Xue Xue Bao 2001, 32, 417–418. [Google Scholar]
- Zeng, X.; Zeng, X. Relationship between the clinical effects of berberine on severe congestive heart failure and its concentration in plasma studied by HPLC. Biomed. Chromatogr. 1999, 13, 442–444. [Google Scholar] [CrossRef]
- Khan, M.; Shobha, J.C.; Mohan, I.K.; Naidu, M.U.; Sundaram, C.; Singh, S.; Kuppusamy, P.; Kutala, V.K. Protective effect of Spirulina against doxorubicin-induced cardiotoxicity. Phytother. Res. 2005, 19, 1030–1037. [Google Scholar] [CrossRef]
- Ionov, V.A.; Basova, M.M. Use of blue-green micro-seaweed Spirulina platensis for the correction of lipid and hemostatic disturbances in patients with ischemic heart disease. Vopr. Pitan. 2003, 72, 28–31. [Google Scholar]
- Kerner, W.; Bruckel, J.; German Diabetes, A. Definition, classification and diagnosis of diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2014, 122, 384–386. [Google Scholar] [CrossRef]
- American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. 1), S81–S90. [Google Scholar] [CrossRef]
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003, 26 (Suppl. 1), S5–S20. [Google Scholar]
- Ammon, H.P. Molecular pathology of diabetes mellitus. The effects of insulin. Pharm. Unserer Zeit 2001, 30, 21–26. [Google Scholar] [CrossRef]
- Fujii, A. Pathology of diabetes mellitus. Nihon Rinsho 2002, 60 (Suppl. 8), 131–136. [Google Scholar]
- Ametov, A.S.; Kochergina, I.I.; Ulanova, K.A. Effect of insulin therapy on insulin resistance and risk for ischemic heart disease and death from cardiovascular pathology in patients with diabetes mellitus, type 2. Terapevticheskii Arkhiv 2010, 82, 42–46. [Google Scholar] [PubMed]
- Levy, B.I.; Schiffrin, E.L.; Mourad, J.J.; Agostini, D.; Vicaut, E.; Safar, M.E.; Struijker-Boudier, H.A. Impaired tissue perfusion: A pathology common to hypertension, obesity, and diabetes mellitus. Circulation 2008, 118, 968–976. [Google Scholar] [CrossRef]
- Sadzeviciene, R.; Paipaliene, P.; Zekonis, G.; Zilinskas, J. The influence of microvascular complications caused by diabetes mellitus on the inflammatory pathology of periodontal tissues. Stomatologija 2005, 7, 121–124. [Google Scholar] [PubMed]
- Younis, B.B.; Arshad, R.; Yousuf, H.; Salman, F.; Masood, J.; Khurshid, S. Impact of type 2 diabetes mellitus on quality of life in people with diabetespresenting to a specialist diabetes clinic. Turk. J. Med. Sci. 2017, 47, 123–126. [Google Scholar] [CrossRef]
- Teuscher, A.; Richterich, R. New Swiss guide lines for the diagnosis of diabetes mellitus. Schweiz. Med. Wochenschr. 1971, 101, 390. [Google Scholar]
- Martinka, E. Comments on current guidelines of type 2 diabetes mellitus treatment. Vnitr. Lek. 2017, 63, 211–217. [Google Scholar]
- Yang, T.; Wang, L.; Zhu, M.; Zhang, L.; Yan, L. Properties and molecular mechanisms of resveratrol: A review. Pharmazie 2015, 70, 501–506. [Google Scholar] [PubMed]
- Vallianou, N.G.; Evangelopoulos, A.; Kazazis, C. Resveratrol and diabetes. Rev. Diabet. Stud. 2013, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Szkudelska, K.; Szkudelski, T. Resveratrol, obesity and diabetes. Eur. J. Pharmacol. 2010, 635, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.L.; Wang, X.M.; He, B.L. Advance of resveratrol in treating diabetes mellitus. Zhongguo Zhong Xi Yi Jie He Za Zhi 2013, 33, 279–281. [Google Scholar] [PubMed]
- Ren, Z.; Wang, L.; Cui, J.; Huoc, Z.; Xue, J.; Cui, H.; Mao, Q.; Yang, R. Resveratrol inhibits NF-kB signaling through suppression of p65 and IkappaB kinase activities. Pharmazie 2013, 68, 689–694. [Google Scholar]
- Pan, W.; Yu, H.; Huang, S.; Zhu, P. Resveratrol Protects against TNF-alpha-Induced Injury in Human Umbilical Endothelial Cells through Promoting Sirtuin-1-Induced Repression of NF-KB and p38 MAPK. PLoS ONE 2016, 11, e0147034. [Google Scholar] [CrossRef]
- Sadeghi, A.; Seyyed Ebrahimi, S.S.; Golestani, A.; Meshkani, R. Resveratrol Ameliorates Palmitate-Induced Inflammation in Skeletal Muscle Cells by Attenuating Oxidative Stress and JNK/NF-KB Pathway in a SIRT1-Independent Mechanism. J. Cell. Biochem. 2017. [Google Scholar] [CrossRef]
- Wang, X.; Buechler, N.L.; Yoza, B.K.; McCall, C.E.; Vachharajani, V.T. Resveratrol attenuates microvascular inflammation in sepsis via SIRT-1-Induced modulation of adhesion molecules in ob/ob mice. Obesity 2015, 23, 1209–1217. [Google Scholar] [CrossRef]
- Liu, C.W.; Sung, H.C.; Lin, S.R.; Wu, C.W.; Lee, C.W.; Lee, I.T.; Yang, Y.F.; Yu, I.S.; Lin, S.W.; Chiang, M.H.; et al. Resveratrol attenuates ICAM-1 expression and monocyte adhesiveness to TNF-alpha-treated endothelial cells: Evidence for an anti-inflammatory cascade mediated by the miR-221/222/AMPK/p38/NF-kappaB pathway. Sci. Rep. 2017, 7, 44689. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhu, L.; Hong, X.; Wang, Y.T.; Wang, F.; Bao, J.P.; Xie, X.H.; Liu, L.; Wu, X.T. Resveratrol attenuated TNF-alpha-induced MMP-3 expression in human nucleus pulposus cells by activating autophagy via AMPK/SIRT1 signaling pathway. Exp. Biol. Med. 2016, 241, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.Y.; Su, Y.; Gao, P.; Yang, Q.H.; Wang, Z.; Xu, Q. Resveratrol ameliorates high glucose-induced oxidative stress injury in human umbilical vein endothelial cells by activating AMPK. Life Sci. 2015, 136, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Caggiano, R.; Cattaneo, F.; Moltedo, O.; Esposito, G.; Perrino, C.; Trimarco, B.; Ammendola, R.; Faraonio, R. miR-128 Is Implicated in Stress Responses by Targeting MAFG in Skeletal Muscle Cells. Oxid. Med. Cell. Longev. 2017, 2017, 9308310. [Google Scholar] [CrossRef] [PubMed]
- Faraonio, R.; Vergara, P.; Marzo, D.D.; Napolitano, M.; Russo, T.; Cimino, F. Transcription regulation in NIH3T3 cell clones resistant to diethylmaleate-induced oxidative stress and apoptosis. Antioxid. Redox Signal. 2006, 8, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta 2015, 1852, 1145–1154. [Google Scholar] [CrossRef]
- Bo, S.; Ponzo, V.; Evangelista, A.; Ciccone, G.; Goitre, I.; Saba, F.; Procopio, M.; Cassader, M.; Gambino, R. Effects of 6 months of resveratrol versus placebo on pentraxin 3 in patients with type 2 diabetes mellitus: A double-blind randomized controlled trial. Acta Diabetol. 2017, 54, 499–507. [Google Scholar] [CrossRef]
- Timmers, S.; de Ligt, M.; Phielix, E.; van de Weijer, T.; Hansen, J.; Moonen-Kornips, E.; Schaart, G.; Kunz, I.; Hesselink, M.K.; Schrauwen-Hinderling, V.B.; et al. Resveratrol as Add-on Therapy in Subjects With Well-Controlled Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2016, 39, 2211–2217. [Google Scholar] [CrossRef]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Z.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef]
- Movahed, A.; Nabipour, I.; Lieben Louis, X.; Thandapilly, S.J.; Yu, L.; Kalantarhormozi, M.; Rekabpour, S.J.; Netticadan, T. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid. Based Complement. Alternat. Med. 2013, 2013, 851267. [Google Scholar] [CrossRef]
- Brasnyo, P.; Molnar, G.A.; Mohas, M.; Marko, L.; Laczy, B.; Cseh, J.; Mikolas, E.; Szijarto, I.A.; Merei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef]
- van der Made, S.M.; Plat, J.; Mensink, R.P. Resveratrol does not influence metabolic risk markers related to cardiovascular health in overweight and slightly obese subjects: A randomized, placebo-controlled crossover trial. PLoS ONE 2015, 10, e0118393. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, R.; Wang, B.; Mi, M.T. Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2014, 99, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Bashmakov, Y.K.; Assaad-Khalil, S.H.; Abou Seif, M.; Udumyan, R.; Megallaa, M.; Rohoma, K.H.; Zeitoun, M.; Petyaev, I.M. Resveratrol promotes foot ulcer size reduction in type 2 diabetes patients. ISRN Endocrinol. 2014, 2014, 816307. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Sui, B.D.; Liu, N.; Lv, Y.J.; Zheng, C.X.; Lu, Y.B.; Huang, W.T.; Zhou, C.H.; Chen, J.; Pang, D.L.; et al. Anti-aging pharmacology in cutaneous wound healing: Effects of metformin, resveratrol, and rapamycin by local application. Aging Cell 2017, 16, 1083–1093. [Google Scholar] [CrossRef]
- Yaman, I.; Derici, H.; Kara, C.; Kamer, E.; Diniz, G.; Ortac, R.; Sayin, O. Effects of resveratrol on incisional wound healing in rats. Surg. Today 2013, 43, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, J.F.; Leal Vde, O.; Stenvinkel, P.; Carraro-Eduardo, J.C.; Mafra, D. Resveratrol: Why is it a promising therapy for chronic kidney disease patients? Oxid. Med. Cell. Longev. 2013, 2013, 963217. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Mai, F.; Martella, L.; De Feo, M.; Soddu, D.; Fellini, E.; Veneri, M.; Stamerra, C.A.; Ferri, C. Cocoa, glucose tolerance, and insulin signaling: Cardiometabolic protection. J. Agric. Food Chem. 2015, 63, 9919–9926. [Google Scholar] [CrossRef]
- Stote, K.S.; Clevidence, B.A.; Novotny, J.A.; Henderson, T.; Radecki, S.V.; Baer, D.J. Effect of cocoa and green tea on biomarkers of glucose regulation, oxidative stress, inflammation and hemostasis in obese adults at risk for insulin resistance. Eur. J. Clin. Nutr. 2012, 66, 1153–1159. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Martin, M.A.; Goya, L.; Ramos, S. Cocoa flavonoids attenuate high glucose-induced insulin signalling blockade and modulate glucose uptake and production in human HepG2 cells. Food Chem. Toxicol. 2014, 64, 10–19. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Martin, M.A.; Bravo, L.; Goya, L.; Ramos, S. Cocoa flavonoids improve insulin signalling and modulate glucose production via AKT and AMPK in HepG2 cells. Mol. Nutr. Food Res. 2013, 57, 974–985. [Google Scholar] [CrossRef]
- Martin, M.A.; Serrano, A.B.; Ramos, S.; Pulido, M.I.; Bravo, L.; Goya, L. Cocoa flavonoids up-regulate antioxidant enzyme activity via the ERK1/2 pathway to protect against oxidative stress-induced apoptosis in HepG2 cells. J. Nutr. Biochem. 2010, 21, 196–205. [Google Scholar] [CrossRef]
- Grassi, D.; Lippi, C.; Necozione, S.; Desideri, G.; Ferri, C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am. J. Clin. Nutr. 2005, 81, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Almoosawi, S.; Tsang, C.; Ostertag, L.M.; Fyfe, L.; Al-Dujaili, E.A. Differential effect of polyphenol-rich dark chocolate on biomarkers of glucose metabolism and cardiovascular risk factors in healthy, overweight and obese subjects: A randomized clinical trial. Food Funct. 2012, 3, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; Adamson, A.J.; Mathers, J.C. Effects on nutrient intake of a family-based intervention to promote increased consumption of low-fat starchy foods through education, cooking skills and personalised goal setting: The Family Food and Health Project. Br. J. Nutr. 2012, 107, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Curtis, P.J.; Sampson, M.; Potter, J.; Dhatariya, K.; Kroon, P.A.; Cassidy, A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: A 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012, 35, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, N.; Vafa, M.; Eghtesadi, S.; Heidari, I.; Hosseini, A.; Rostami, A. The Effects of Tocotrienols Added to Canola Oil on Microalbuminuria, Inflammation, and Nitrosative Stress in Patients with Type 2 Diabetes: A Randomized, Double-blind, Placebo-controlled Trial. Int. J. Prev. Med. 2014, 5, 617–623. [Google Scholar] [PubMed]
- Zeng, G.; Nystrom, F.H.; Ravichandran, L.V.; Cong, L.N.; Kirby, M.; Mostowski, H.; Quon, M.J. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 2000, 101, 1539–1545. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, M.J.; Choi, H.N.; Jeong, S.M.; Lee, Y.M.; Kim, J.I. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr. Res. Pract. 2011, 5, 107–111. [Google Scholar] [CrossRef]
- Coskun, O.; Kanter, M.; Korkmaz, A.; Oter, S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol. Res. 2005, 51, 117–123. [Google Scholar] [CrossRef]
- Stewart, L.K.; Wang, Z.; Ribnicky, D.; Soileau, J.L.; Cefalu, W.T.; Gettys, T.W. Failure of dietary quercetin to alter the temporal progression of insulin resistance among tissues of C57BL/6J mice during the development of diet-induced obesity. Diabetologia 2009, 52, 514–523. [Google Scholar] [CrossRef]
- Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P.S. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81. [Google Scholar] [CrossRef]
- Askari, G.; Ghiasvand, R.; Feizi, A.; Ghanadian, S.M.; Karimian, J. The effect of quercetin supplementation on selected markers of inflammation and oxidative stress. J. Res. Med. Sci. 2012, 17, 637–641. [Google Scholar] [PubMed]
- Panchal, S.K.; Poudyal, H.; Brown, L. Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J. Nutr. 2012, 142, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Kumpulainen, J.; Jarvinen, R.; Rissanen, H.; Heliovaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Manson, J.E.; Buring, J.E.; Sesso, H.D.; Liu, S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: A prospective study and cross-sectional analysis. J. Am. Coll. Nutr. 2005, 24, 376–384. [Google Scholar] [CrossRef]
- Hajavi, J.; Abbas Momtazi, A.; Johnston, T.P.; Banach, M.; Majeed, M.; Sahebkar, A. Curcumin: A naturally occurring modulator of adipokines in diabetes. J. Cell. Biochem. 2017. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Thiagarajan, R.; Rastrelli, L.; Daglia, M.; Sobarzo-Sanchez, E.; Alinezhad, H.; Nabavi, S.M. Curcumin: A natural product for diabetes and its complications. Curr. Top. Med. Chem. 2015, 15, 2445–2455. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef]
- Wongeakin, N.; Bhattarakosol, P.; Patumraj, S. Molecular mechanisms of curcumin on diabetes-induced endothelial dysfunctions: Txnip, ICAM-1, and NOX2 expressions. BioMed Res. Int. 2014, 2014, 161346. [Google Scholar] [CrossRef]
- Zhang, D.W.; Fu, M.; Gao, S.H.; Liu, J.L. Curcumin and diabetes: A systematic review. Evid. Based Complement. Alternat. Med. 2013, 2013, 636053. [Google Scholar] [CrossRef]
- Meng, B.; Li, J.; Cao, H. Antioxidant and antiinflammatory activities of curcumin on diabetes mellitus and its complications. Curr. Pharm. Des. 2013, 19, 2101–2113. [Google Scholar]
- Rungseesantivanon, S.; Thenchaisri, N.; Ruangvejvorachai, P.; Patumraj, S. Curcumin supplementation could improve diabetes-induced endothelial dysfunction associated with decreased vascular superoxide production and PKC inhibition. BMC Complement. Altern. Med. 2010, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Leibel, R.; Tortoriello, D.V. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 2008, 149, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Oharomari, L.K.; de Moraes, C.; Navarro, A.M. Exercise Training but not Curcumin Supplementation Decreases Immune Cell Infiltration in the Pancreatic Islets of a Genetically Susceptible Model of Type 1 Diabetes. Sports Med. Open 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Wickenberg, J.; Ingemansson, S.L.; Hlebowicz, J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr. J. 2010, 9, 43. [Google Scholar] [CrossRef]
- Chous, A.P.; Richer, S.P.; Gerson, J.D.; Kowluru, R.A. The Diabetes Visual Function Supplement Study (DiVFuSS). Br. J. Ophthalmol. 2016, 100, 227–234. [Google Scholar] [CrossRef]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: A randomized, double-blind clinical trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Huo, L.; Wang, M.; Hong, J.; Wu, P.; et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559–2565. [Google Scholar] [CrossRef]
- Li, Z.; Geng, Y.N.; Jiang, J.D.; Kong, W.J. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid. Based Complement. Altern. Med. 2014, 2014, 289264. [Google Scholar] [CrossRef]
- Chang, W.; Chen, L.; Hatch, G.M. Berberine as a therapy for type 2 diabetes and its complications: From mechanism of action to clinical studies. Biochem. Cell Biol. 2015, 93, 479–486. [Google Scholar] [CrossRef]
- Chatuphonprasert, W.; Lao-Ong, T.; Jarukamjorn, K. Improvement of superoxide dismutase and catalase in streptozotocin-nicotinamide-induced type 2-diabetes in mice by berberine and glibenclamide. Pharm. Biol. 2013. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Wang, Q.; Li, W.; Feng, Y. Effects of berberine and pomegranate seed oil on plasma phospholipid metabolites associated with risks of type 2 diabetes mellitus by U-HPLC/Q-TOF-MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 1007, 110–120. [Google Scholar] [CrossRef]
- Pang, B.; Zhao, L.H.; Zhou, Q.; Zhao, T.Y.; Wang, H.; Gu, C.J.; Tong, X.L. Application of berberine on treating type 2 diabetes mellitus. Int. J. Endocrinol. 2015, 2015, 905749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Chen, L. Effect of berberine in treating type 2 diabetes mellitus and complications and its relevant mechanisms. Zhongguo Zhong Yao Za Zhi 2015, 40, 1660–1665. [Google Scholar] [PubMed]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, J.; Xue, R.; Wu, J.D.; Zhao, W.; Wang, Z.Z.; Wang, S.K.; Zhou, Z.X.; Song, D.Q.; Wang, Y.M.; et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism 2010, 59, 285–292. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, Y.; Shi, X.; Li, X.; Hong, J.; Chen, J.; Gu, W.; Lu, X.; Xu, G.; Ning, G. Effect of traditional Chinese medicine berberine on type 2 diabetes based on comprehensive metabonomics. Talanta 2010, 81, 766–772. [Google Scholar] [CrossRef]
- Deng, X.W.; Xie, N. Progress of berberine for treatment of type 2 diabetes. Zhongguo Zhong Yao Za Zhi 2014, 39, 1374–1378. [Google Scholar]
- Bahadoran, Z.; Mirmiran, P.; Hosseinpanah, F.; Hedayati, M.; Hosseinpour-Niazi, S.; Azizi, F. Broccoli sprouts reduce oxidative stress in type 2 diabetes: A randomized double-blind clinical trial. Eur. J. Clin. Nutr. 2011, 65, 972–977. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Potential efficacy of broccoli sprouts as a unique supplement for management of type 2 diabetes and its complications. J. Med. Food 2013, 16, 375–382. [Google Scholar] [CrossRef]
- He, R.; Alashi, A.; Malomo, S.A.; Girgih, A.T.; Chao, D.; Ju, X.; Aluko, R.E. Antihypertensive and free radical scavenging properties of enzymatic rapeseed protein hydrolysates. Food Chem. 2013, 141, 153–159. [Google Scholar] [CrossRef]
- Duran, C.G.; Burbank, A.J.; Mills, K.H.; Duckworth, H.R.; Aleman, M.M.; Kesic, M.J.; Peden, D.B.; Pan, Y.; Zhou, H.; Hernandez, M.L. A proof-of-concept clinical study examining the NRF2 activator sulforaphane against neutrophilic airway inflammation. Respir. Res. 2016, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Hosseinpanah, F.; Rajab, A.; Asghari, G.; Azizi, F. Broccoli sprouts powder could improve serum triglyceride and oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Diabetes Res. Clin. Pract. 2012, 96, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Tohidi, M.; Nazeri, P.; Mehran, M.; Azizi, F.; Mirmiran, P. Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: A randomized double-blind clinical trial. Int. J. Food Sci. Nutr. 2012, 63, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Xie, Z.; Cao, D.; Gong, M.; Yang, L.; Zhou, Z.; Ou, Y. C-Phycocyanin inhibits hepatic gluconeogenesis and increases glycogen synthesis via activating Akt and AMPK in insulin resistance hepatocytes. Food Funct. 2018, 9, 2829–2839. [Google Scholar] [CrossRef] [PubMed]
- Sadek, K.M.; Lebda, M.A.; Nasr, S.M.; Shoukry, M. Spirulina platensis prevents hyperglycemia in rats by modulating gluconeogenesis and apoptosis via modification of oxidative stress and MAPK-pathways. Biomed. Pharmacother. 2017, 92, 1085–1094. [Google Scholar] [CrossRef]
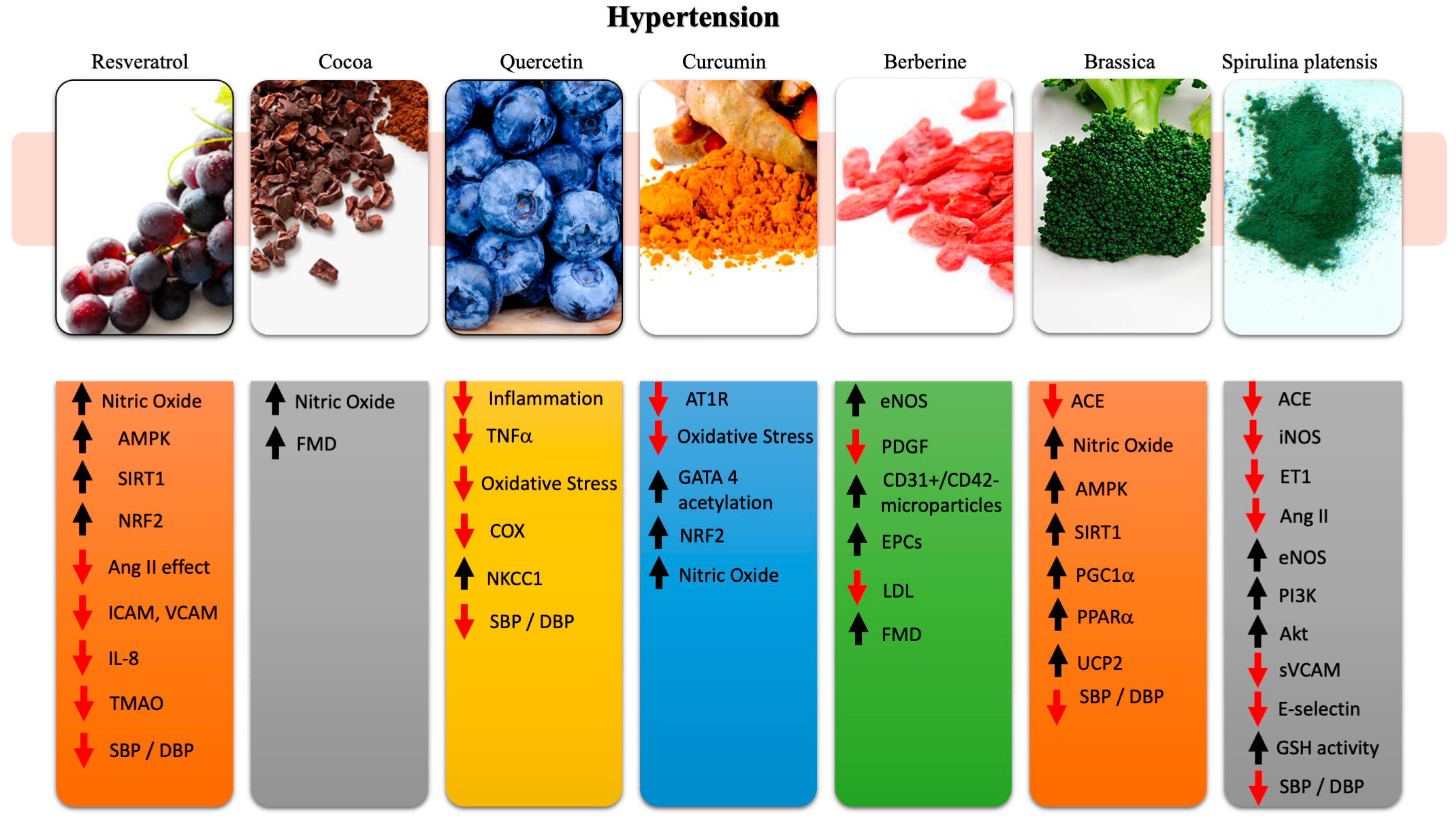
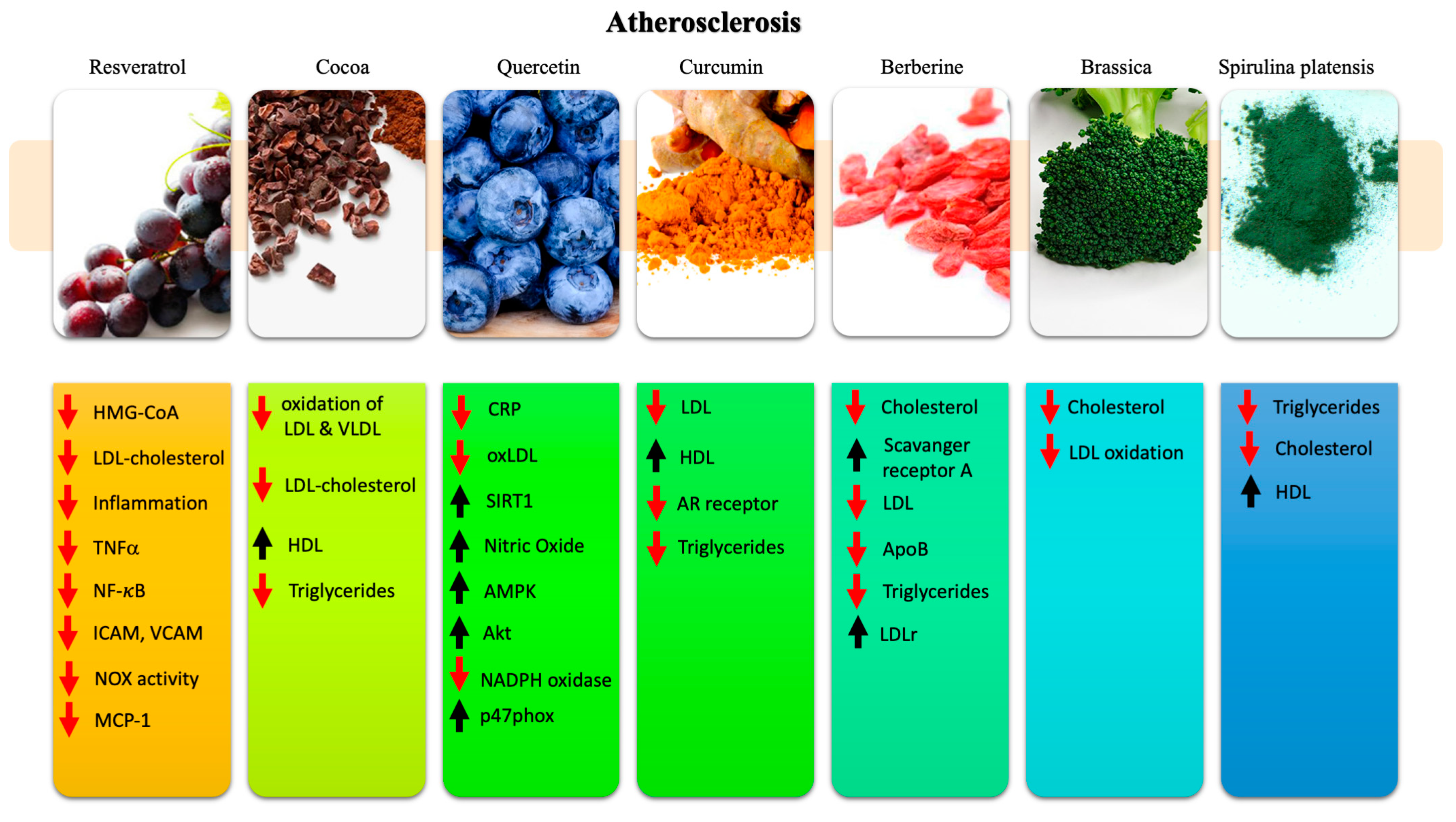
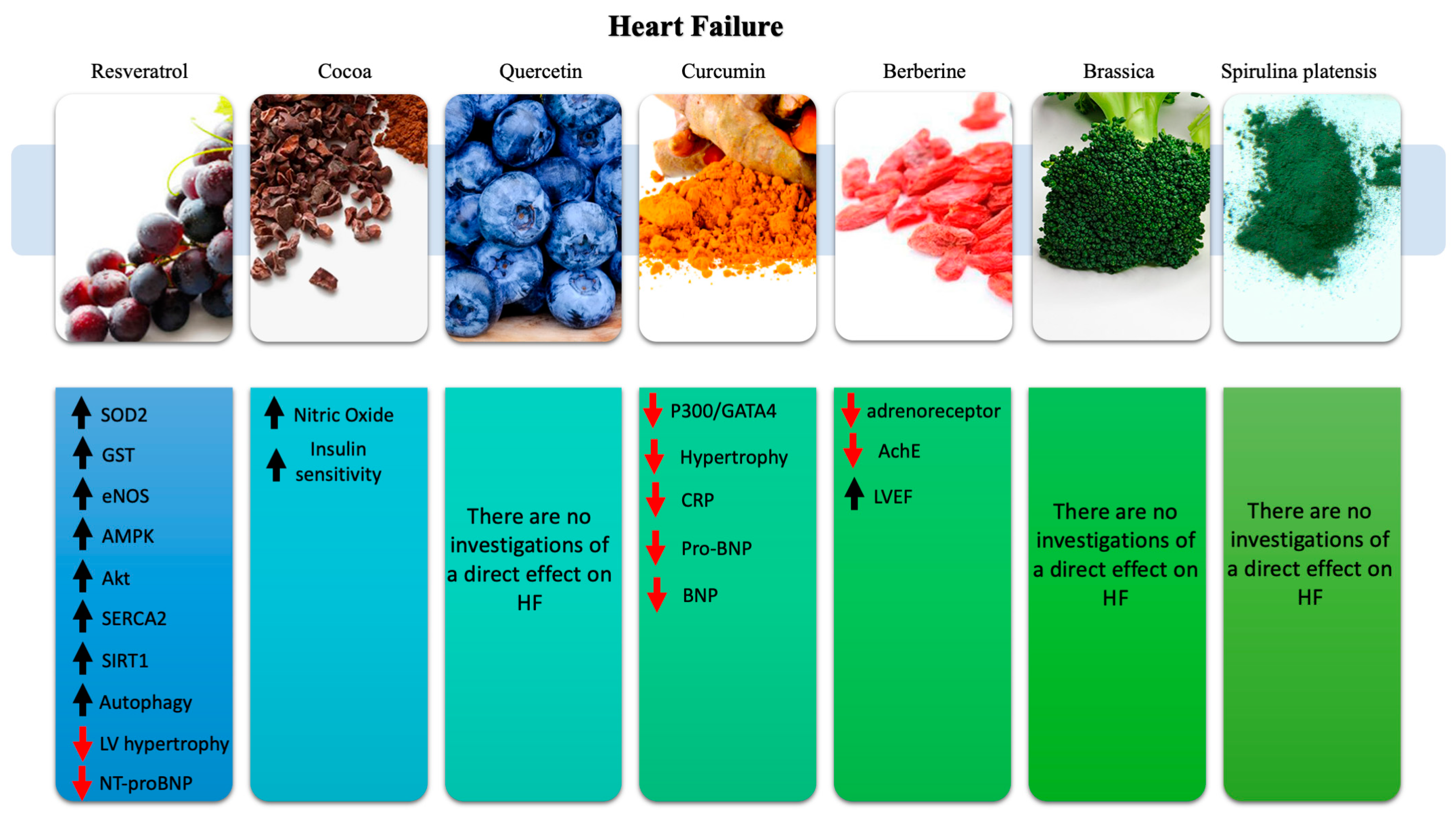
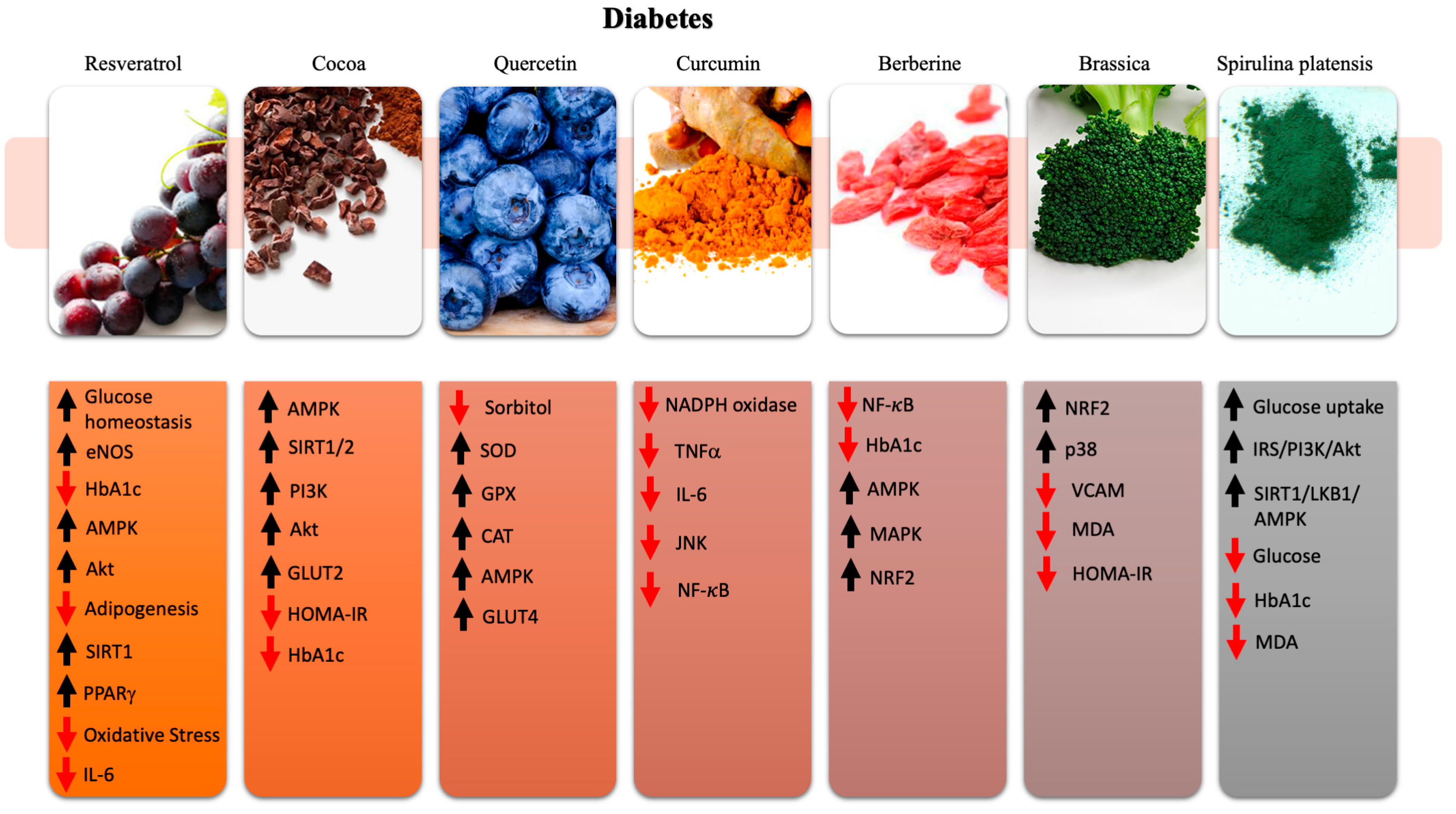
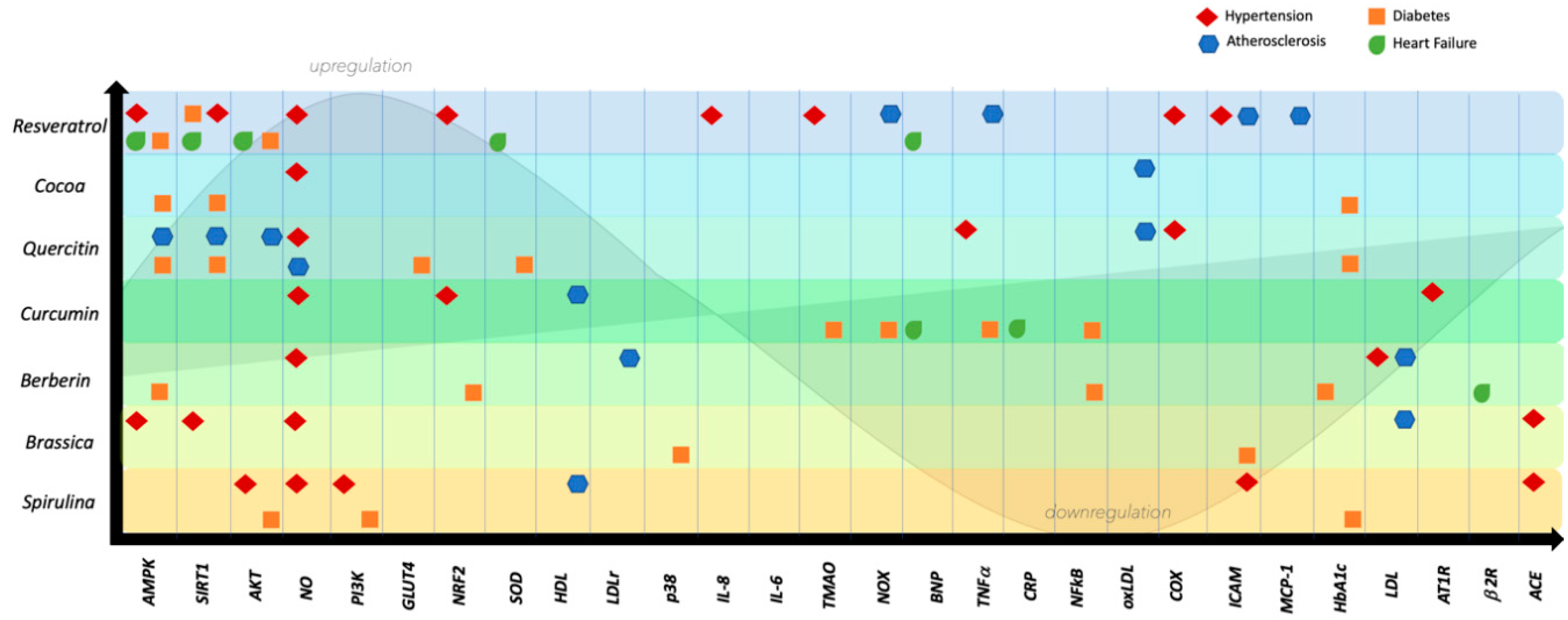
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrizzo, A.; Izzo, C.; Forte, M.; Sommella, E.; Di Pietro, P.; Venturini, E.; Ciccarelli, M.; Galasso, G.; Rubattu, S.; Campiglia, P.; et al. A Novel Promising Frontier for Human Health: The Beneficial Effects of Nutraceuticals in Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 8706. https://doi.org/10.3390/ijms21228706
Carrizzo A, Izzo C, Forte M, Sommella E, Di Pietro P, Venturini E, Ciccarelli M, Galasso G, Rubattu S, Campiglia P, et al. A Novel Promising Frontier for Human Health: The Beneficial Effects of Nutraceuticals in Cardiovascular Diseases. International Journal of Molecular Sciences. 2020; 21(22):8706. https://doi.org/10.3390/ijms21228706
Chicago/Turabian StyleCarrizzo, Albino, Carmine Izzo, Maurizio Forte, Eduardo Sommella, Paola Di Pietro, Eleonora Venturini, Michele Ciccarelli, Gennaro Galasso, Speranza Rubattu, Petro Campiglia, and et al. 2020. "A Novel Promising Frontier for Human Health: The Beneficial Effects of Nutraceuticals in Cardiovascular Diseases" International Journal of Molecular Sciences 21, no. 22: 8706. https://doi.org/10.3390/ijms21228706
APA StyleCarrizzo, A., Izzo, C., Forte, M., Sommella, E., Di Pietro, P., Venturini, E., Ciccarelli, M., Galasso, G., Rubattu, S., Campiglia, P., Sciarretta, S., Frati, G., & Vecchione, C. (2020). A Novel Promising Frontier for Human Health: The Beneficial Effects of Nutraceuticals in Cardiovascular Diseases. International Journal of Molecular Sciences, 21(22), 8706. https://doi.org/10.3390/ijms21228706











