Surface-Modified Poly(l-lactide-co-glycolide) Scaffolds for the Treatment of Osteochondral Critical Size Defects—In Vivo Studies on Rabbits
Abstract
1. Introduction
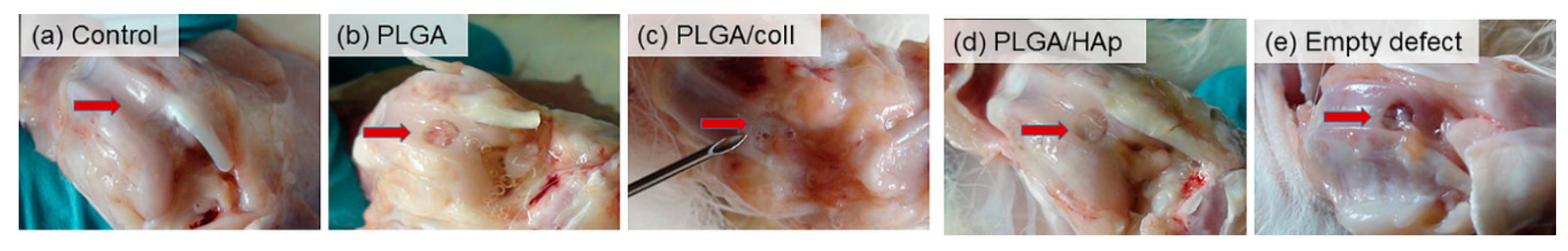
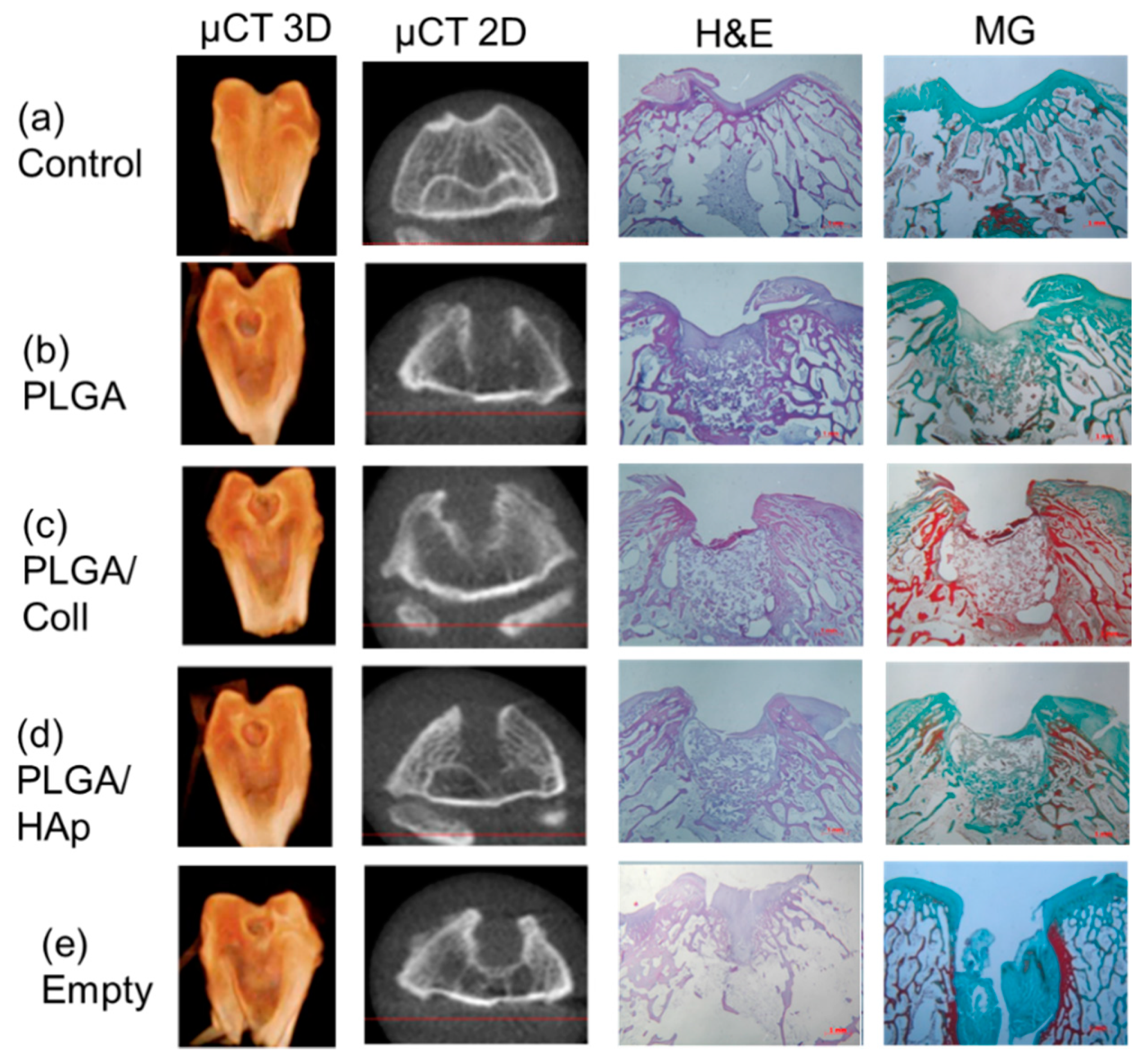
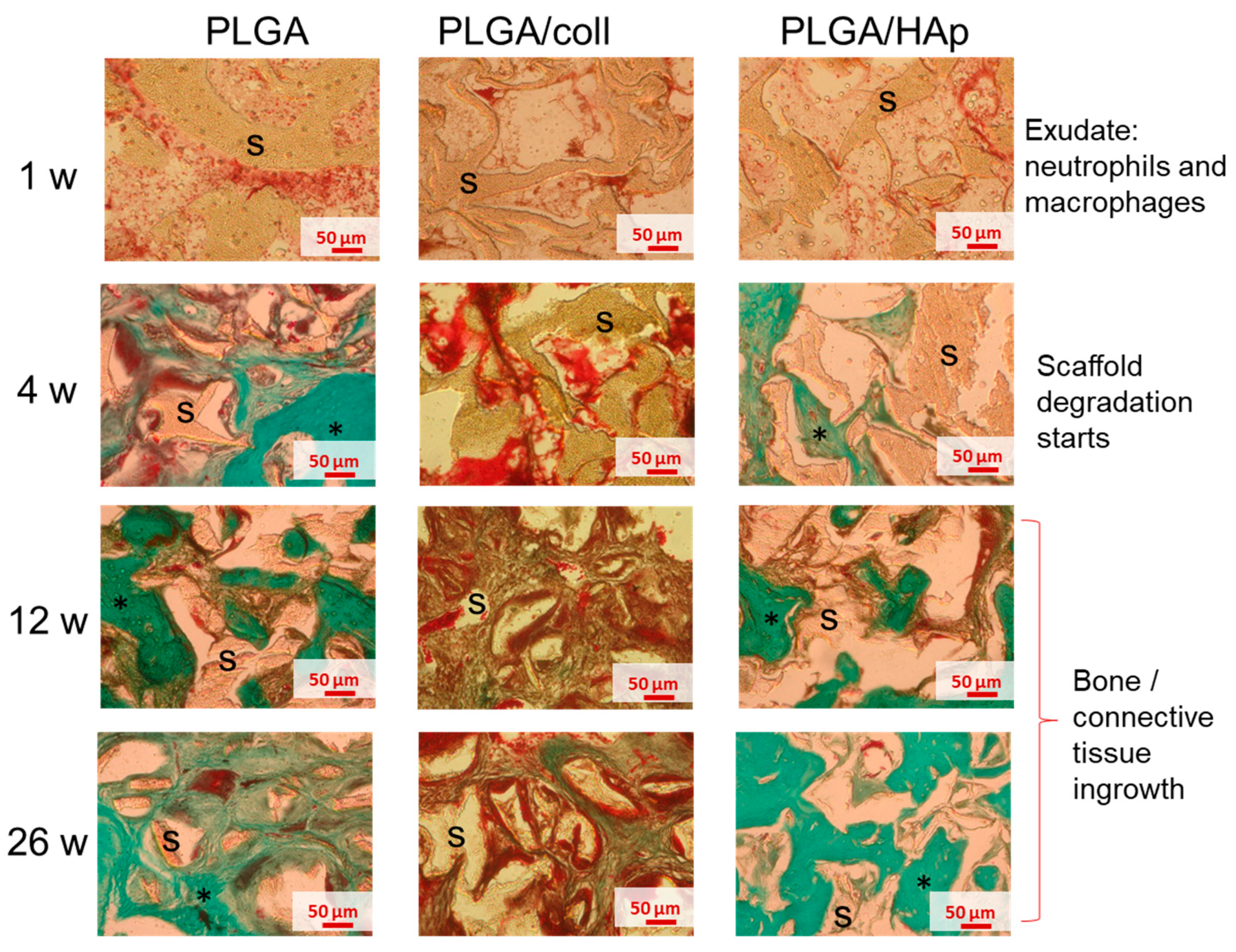
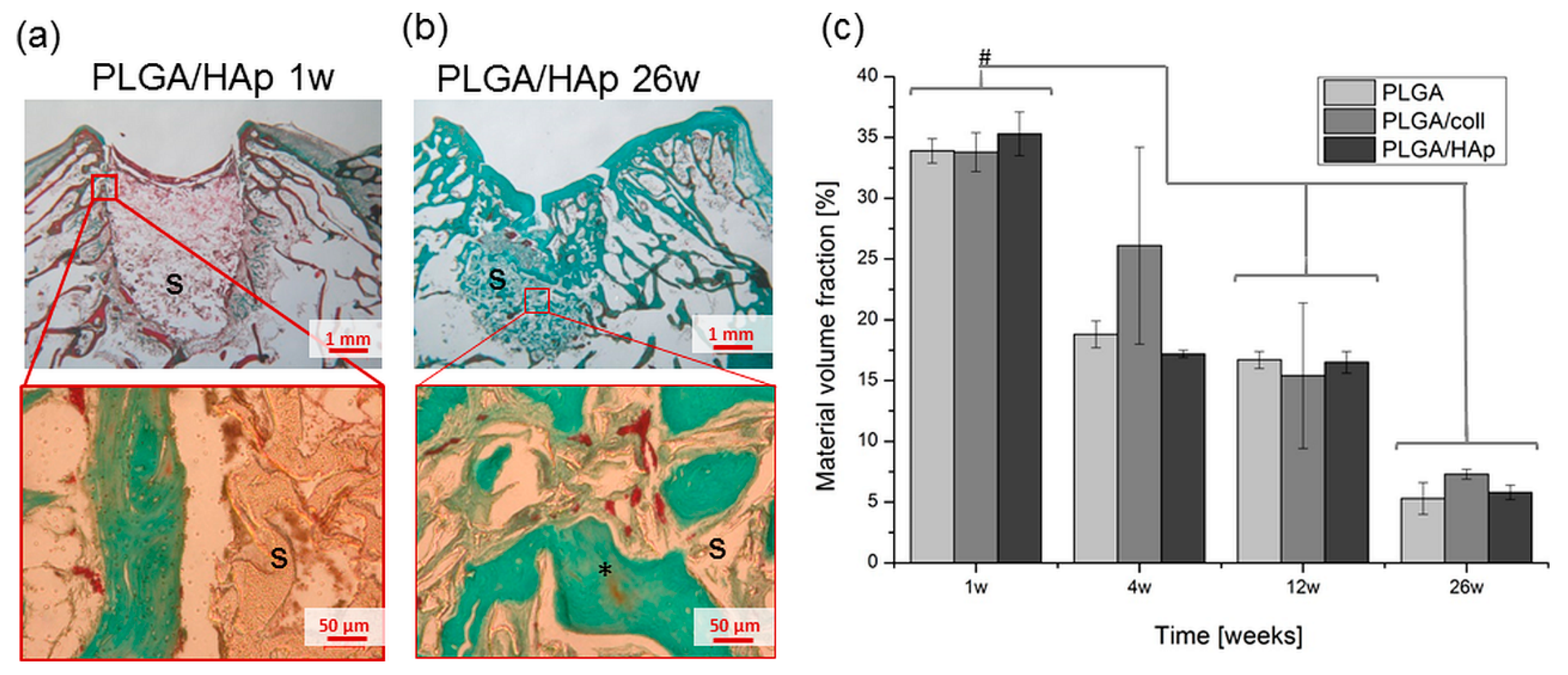
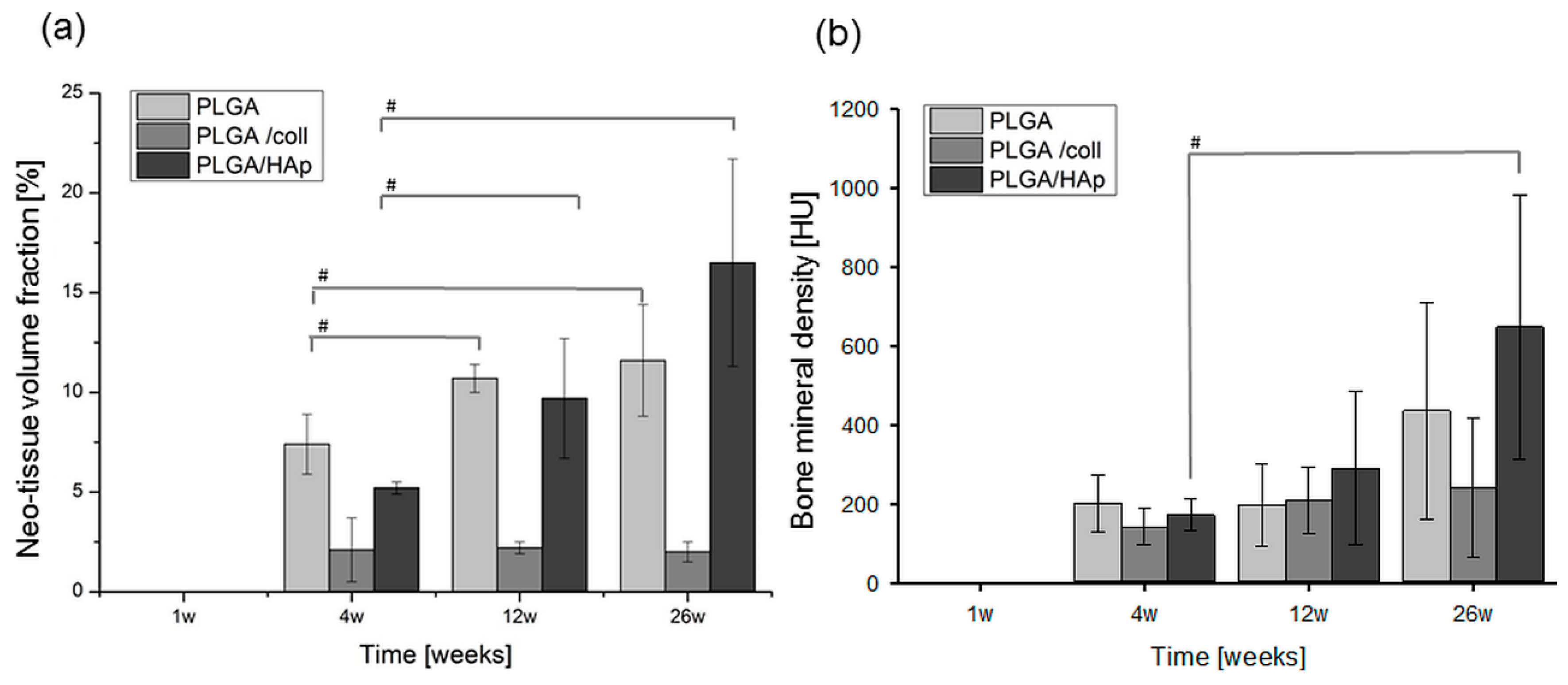
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals and Polymer Synthesis, Scaffolds Manufacturing and Modification
4.2. Scaffold Characterization
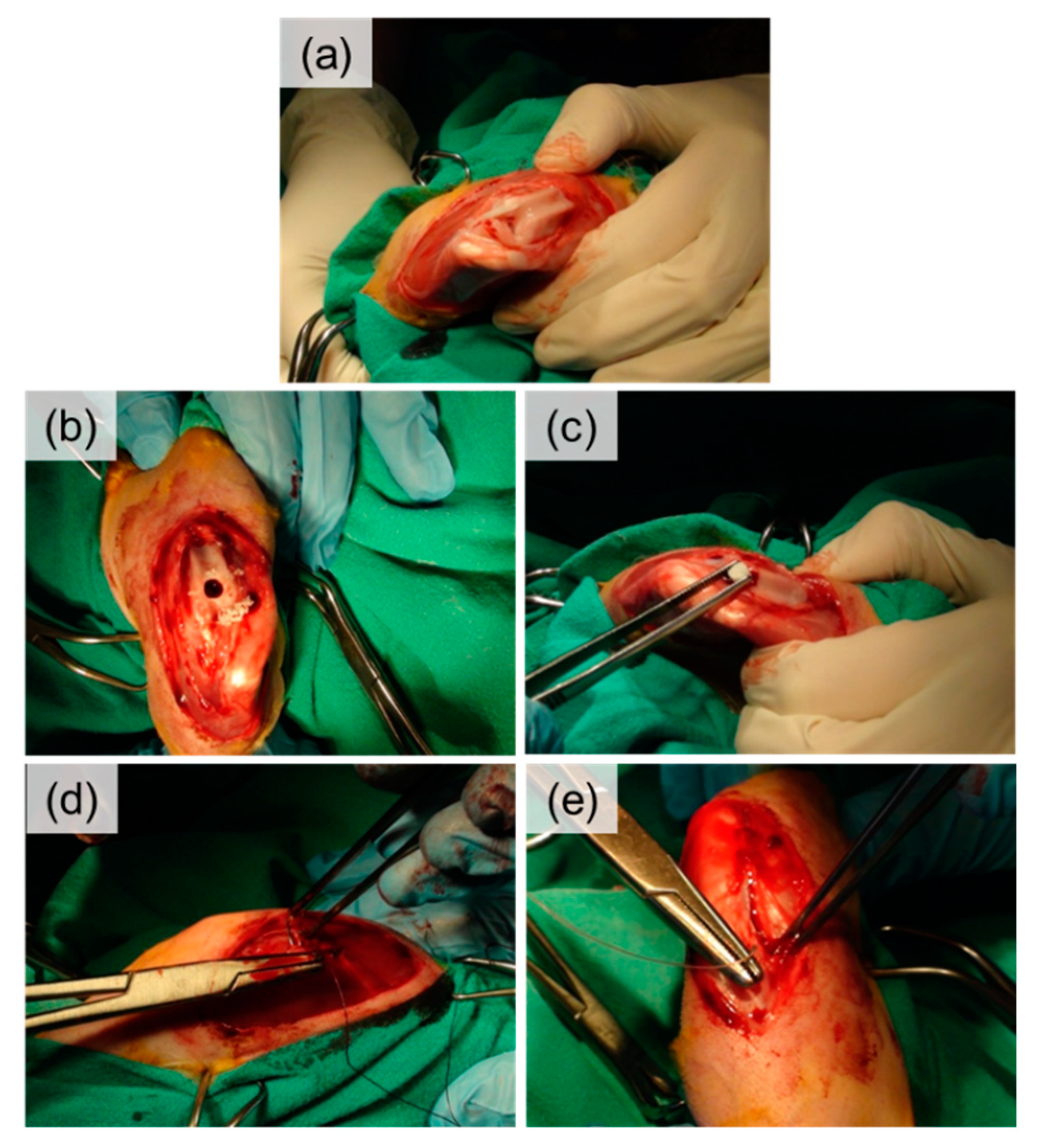
4.3. In Vivo Evaluation in a New Zealand Rabbit Model
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| bCaP | Biomimetic calcium phosphate |
| BMD | Bone mineral density |
| BMP-2 | Bone morphogenetic protein-2 |
| BTE | Bone tissue engineering |
| coll | Collagen type I |
| DCM | Dichloromethane |
| ECM | Extracellular matrix |
| EDX | X-ray spectroscopy |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| FGF-2 | Fibroblast growth factor-2 |
| FTIR | Fourier transform infrared spectroscopy |
| HAp | Hydroxyapatite |
| HU | Hounsfield units |
| µCT | Micro-computed tomography |
| PLGA | Poly(l-lactide-co-glycolide) |
| SBF | Simulated body fluid |
| SEM | Scanning electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray powder diffractometry |
References
- Volkov, A.V.; Muraev, A.A.; Zharkova, I.I.; Voinova, V.V.; Akoulina, E.A.; Zhuikov, V.A.; Khaydapova, D.D.; Chesnokova, D.V.; Menshikh, K.A.; Dudun, A.A.; et al. Poly(3-hydroxybutyrate)/hydroxyapatite/alginate scaffolds seeded with mesenchymal stem cells enhance the regeneration of critical-sized bone defect. Mater. Sci. Eng. C 2020, 114, 110991. [Google Scholar] [CrossRef] [PubMed]
- Bhumiratana, S.; Vunjak-Novakovic, G. Concise review: Personalized human bone grafts for reconstructing head and face. Stem Cells Transl. Med. 2012, 1, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Rumian, Ł.; Wolf-Brandstetter, C.; Rößler, S.; Reczyńska, K.; Tiainen, H.; Haugen, H.J.; Scharnweber, D.; Pamuła, E. Sodium alendronate loaded poly (l-lactide-co-glycolide) microparticles immobilized on ceramic scaffolds for local treatment of bone defects. Regen. Biomater. 2020, 7, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, D.; Cui, C.; Weber, F.; Petersen, F.C.; Tiainen, H. Antibacterial surface coating for bone scaffolds based on the dark catalytic effect of titanium dioxide. ACS Appl. Mater. Interfaces 2018, 10, 35784–35793. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, L.; Li, K.; Zhu, L.; Chen, J.; Hao, Y. Pore functionally graded Ti6Al4V scaffolds for bone tissue engineering application. Mater. Des. 2019, 168, 107643. [Google Scholar] [CrossRef]
- Aragón, J.; Salerno, S.; De Bartolo, L.; Irusta, S.; Mendoza, G. Polymeric electrospun scaffolds for bone morphogenetic protein 2 delivery in bone tissue engineering. J. Colloid Interface Sci. 2018, 531, 126–137. [Google Scholar] [CrossRef]
- Ju, J.; Peng, X.; Huang, K.; Li, L.; Liu, X.; Chitrakar, C.; Chang, L.; Gu, Z.; Kuang, T. High-performance porous PLLA-based scaffolds for bone tissue engineering: Preparation, characterization, and in vitro and in vivo evaluation. Polymer 2019, 180, 121707. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Limmahakhun, S.; Oloyede, A.; Sitthiseripratip, K.; Xiao, Y.; Yan, C. 3D-printed cellular structures for bone biomimetic implants. Addit. Manuf. 2017, 15, 93–101. [Google Scholar] [CrossRef]
- Jafari, M.; Paknejad, Z.; Rad, M.R.; Motamedian, S.R.; Eghbal, M.J.; Nadjmi, N.; Khojasteh, A. Polymeric scaffolds in tissue engineering: A literature review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 431–459. [Google Scholar] [CrossRef]
- Terukina, T.; Saito, H.; Tomita, Y.; Hattori, Y.; Otsuka, M. Development and effect of a sustainable and controllable simvastatin-releasing device based on PLGA microspheres/carbonate apatite cement composite: In vitro evaluation for use as a drug delivery system from bone-like biomaterial. J. Drug Deliv. Sci. Technol. 2017, 37, 74–80. [Google Scholar] [CrossRef]
- Nafea, E.H.; El-Massik, M.A.; El-Khordagui, L.K.; Marei, M.K.; Khalafallah, N.M. Alendronate PLGA microspheres with high loading efficiency for dental applications. J. Microencapsul. 2007, 24, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.T.C.R.; Cardoso, B.C.O.; e Silva, M.E.S.R.; Freitas, R.F.S.; Sousa, R.G. Synthesis, characterization, and study of PLGA copolymer in vitro degradation. J. Biomater. Nanobiotechnol. 2015, 6, 8. [Google Scholar] [CrossRef]
- Czajkowska, B.; Dobrzynski, P.; Bero, M. Interaction of cells with l-lactide/glycolide copolymers synthesized with the use of tin or zirconium compounds. J. Biomed. Mater. Res. Part A 2005, 74, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled Ring-Opening Polymerization of Lactide and Glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef]
- Garric, X.; Molès, J.-P.; Garreau, H.; Braud, C.; Guilhou, J.-J.; Vert, M. Growth of various cell types in the presence of lactic and glycolic acids: The adverse effect of glycolic acid released from PLAGA copolymer on keratinocyte proliferation. J. Biomater. Sci. Polym. Ed. 2002, 13, 1189–1201. [Google Scholar] [CrossRef]
- Krucińska, I.; Żywicka, B.; Komisarczyk, A.; Szymonowicz, M.; Kowalska, S.; Zaczyńska, E.; Struszczyk, M.; Czarny, A.; Jadczyk, P.; Umińska-Wasiluk, B. Biological properties of low-toxicity PLGA and PLGA/PHB fibrous nanocomposite implants for osseous tissue regeneration. Part I: Evaluation of potential biotoxicity. Molecules 2017, 22, 2092. [Google Scholar]
- Pamula, E.; Blazewicz, M.; Czajkowska, B.; Dobrzynski, P.; Bero, M.; Kasperczyk, J. Elaboration and Characterization of Biodegradable Scaffolds from poly (l-lactide-co-glycolide) synthesized with Low-Toxic Zirconium Acetylacetonate. Med. Sci. Monit. 2004, 9, 64–67. [Google Scholar]
- Dobrzynski, P.; Kasperczyk, J.; Janeczek, H.; Bero, M. Synthesis of biodegradable copolymers with the use of low toxic zirconium compounds. 1. Copolymerization of glycolide with l-lactide initiated by Zr (Acac) 4. Macromolecules 2001, 34, 5090–5098. [Google Scholar] [CrossRef]
- Pamula, E.; Menaszek, E. In vitro and in vivo degradation of poly (l-lactide-co-glycolide) films and scaffolds. J. Mater. Sci. Mater. Med. 2008, 19, 2063–2070. [Google Scholar] [CrossRef]
- Krok-Borkowicz, M.; Filova, E.; Chlupac, J.; Klepetar, J.; Bacakova, L.; Pamuła, E. Influence of pore size and hydroxyapatite deposition in poly (l-lactide-co-glycolide) scaffolds on osteoblast-like cells cultured in static and dynamic conditions. Mater. Lett. 2019, 241, 1–5. [Google Scholar] [CrossRef]
- Wojak-Ćwik, I.M.; Rumian, Ł.; Krok-Borkowicz, M.; Hess, R.; Bernhardt, R.; Dobrzyński, P.; Möller, S.; Schnabelrauch, M.; Hintze, V.; Scharnweber, D.; et al. Synergistic effect of bimodal pore distribution and artificial extracellular matrices in polymeric scaffolds on osteogenic differentiation of human mesenchymal stem cells. Mater. Sci. Eng. C 2019, 97, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, M.; Wang, T.; Guo, S. Fabrication of porous micro-nanostructured networks on Ti6Al4V for faster hydroxyapatite deposition in SBF. Mater. Lett. 2019, 256, 126571. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Candidato, R.T., Jr.; Lusvarghi, L.; Bolelli, G.; Pawlowski, L.; Candiani, G.; Altomare, L.; De Nardo, L.; Cannillo, V. Bioactive Zn-doped hydroxyapatite coatings and their antibacterial efficacy against Escherichia coli and Staphylococcus aureus. Surf. Coat. Technol. 2018, 352, 84–91. [Google Scholar] [CrossRef]
- Raizada, P.; Gautam, S.; Priya, B.; Singh, P. Preparation and photocatalytic activity of hydroxyapatite supported BiOCl nanocomposite for oxytetracyline removal. Adv. Mater. Lett 2016, 7, 312–318. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhu, R.; Wang, Y.; Li, B.; Ma, Y.; Yin, Y. Synthesis and characterization of serial random and block-copolymers based on lactide and glycolide. Polym. Sci. Ser. B 2016, 58, 720–729. [Google Scholar] [CrossRef]
- Prasad, A.; Sankar, M.R.; Katiyar, V. State of art on solvent casting particulate leaching method for orthopedic scaffoldsfabrication. Mater. Today Proc. 2017, 4, 898–907. [Google Scholar] [CrossRef]
- Pamula, E.; Filová, E.; Bačáková, L.; Lisá, V.; Adamczyk, D. Resorbable polymeric scaffolds for bone tissue engineering: The influence of their microstructure on the growth of human osteoblast-like MG 63 cells. J. Biomed. Mater. Res. Part A 2009, 89A, 432–443. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef]
- Ding, X.; Li, X.; Li, C.; Qi, M.; Zhang, Z.; Sun, X.; Wang, L.; Zhou, Y. Chitosan/dextran hydrogel constructs containing strontium-doped hydroxyapatite with enhanced osteogenic potential in rat cranium. ACS Biomater. Sci. Eng. 2019, 5, 4574–4586. [Google Scholar] [CrossRef]
- Cho, Y.; Hong, J.; Ryoo, H.; Kim, D.; Park, J.; Han, J. Osteogenic responses to zirconia with hydroxyapatite coating by aerosol deposition. J. Dent. Res. 2015, 94, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Seong, Y.-J.; Song, E.-H.; Park, C.; Lee, H.; Kang, I.-G.; Kim, H.-E.; Jeong, S.-H. Porous calcium phosphate–collagen composite microspheres for effective growth factor delivery and bone tissue regeneration. Mater. Sci. Eng. C 2020, 109, 110480. [Google Scholar]
- Lee, I.C.; Lee, Y.T.; Yu, B.Y.; Lai, J.Y.; Young, T.H. The behavior of mesenchymal stem cells on micropatterned PLLA membranes. J. Biomed. Mater. Res. Part A 2009, 91, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.D.; Singh, R.K.; Lee, J.-H.; Kim, H.-W. Electrophoretic coatings of hydroxyapatite with various nanocrystal shapes. Mater. Lett. 2019, 234, 148–154. [Google Scholar] [CrossRef]
- Nakamura, M.; Hori, N.; Ando, H.; Namba, S.; Toyama, T.; Nishimiya, N.; Yamashita, K. Surface free energy predominates in cell adhesion to hydroxyapatite through wettability. Mater. Sci. Eng. C 2016, 62, 283–292. [Google Scholar] [CrossRef]
- Douglas, T.; Pamula, E.; Hauk, D.; Wiltfang, J.; Sivananthan, S.; Sherry, E.; Warnke, P.H. Porous polymer/hydroxyapatite scaffolds: Characterization and biocompatibility investigations. J. Mater. Sci. Mater. Med. 2009, 20, 1909–1915. [Google Scholar] [CrossRef]
- Widdowson, J.P.; Picton, A.J.; Vince, V.; Wright, C.J.; Mearns-Spragg, A. In vivo comparison of jellyfish and bovine collagen sponges as prototype medical devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1524–1533. [Google Scholar] [CrossRef]
- Lynn, A.; Yannas, I.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. Part B 2004, 71, 343–354. [Google Scholar] [CrossRef]
- Pontz, B.; Meigel, W.; Rauterberg, J.; Kühn, K. Localization of two species specific antigenic determinants on the peptide chains of calf skin collagen. Eur. J. Biochem. 1970, 16, 50–54. [Google Scholar] [CrossRef]
- Michaeli, D.; Martin, G.R.; Kettman, J.; Benjamini, E.; Leung, D.; Blatt, B. Localization of antigenic determinants in the polypeptide chains of collagen. Science 1969, 166, 1522–1523. [Google Scholar]
- Fu, Y.; Therkildsen, M.; Aluko, R.E.; Lametsch, R. Exploration of collagen recovered from animal by-products as a precursor of bioactive peptides: Successes and challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 2011–2027. [Google Scholar] [CrossRef] [PubMed]
- Tauro, J.; Parsons, J.; Ricci, J.; Alexander, H. Comparison of bovine collagen xenografts to autografts in the rabbit. Clin. Orthop. Relat. Res. 1991, 271–284. [Google Scholar] [CrossRef]
- Angele, P.; Abke, J.; Kujat, R.; Faltermeier, H.; Schumann, D.; Nerlich, M.; Kinner, B.; Englert, C.; Ruszczak, Z.; Mehrl, R. Influence of different collagen species on physico-chemical properties of crosslinked collagen matrices. Biomaterials 2004, 25, 2831–2841. [Google Scholar] [CrossRef]
- Cherim, M.; Sirbu, R.; Tomescu, A.; Popa, M.F.; Cadar, E. Comparative Studies on the Physico-chemical Characteristics of Bio-materials with Collagen from Calf and Fish Skins from Black Sea. Mater. Plast. 2019, 56, 179. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodriguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Willard, J.J.; Drexler, J.W.; Das, A.; Roy, S.; Shilo, S.; Shoseyov, O.; Powell, H.M. Plant-derived human collagen scaffolds for skin tissue engineering. Tissue Eng. Part A 2013, 19, 1507–1518. [Google Scholar] [CrossRef]
- Lu, L.; Peter, S.J.; Lyman, M.D.; Lai, H.-L.; Leite, S.M.; Tamada, J.A.; Uyama, S.; Vacanti, J.P.; Langer, R.; Mikos, A.G. In vitro and in vivo degradation of porous poly (DL-lactic-co-glycolic acid) foams. Biomaterials 2000, 21, 1837–1845. [Google Scholar] [CrossRef]
- Tian, B.; Chen, W.; Dong, Y.; Marymont, J.V.; Lei, Y.; Ke, Q.; Guo, Y.; Zhu, Z. Silver nanoparticle-loaded hydroxyapatite coating: Structure, antibacterial properties, and capacity for osteogenic induction in vitro. RSC Adv. 2016, 6, 8549–8562. [Google Scholar] [CrossRef]
- Gronowicz, G.; Jacobs, E.; Peng, T.; Zhu, L.; Hurley, M.; Kuhn, L.T. Calvarial bone regeneration is enhanced by sequential delivery of FGF-2 and BMP-2 from layer-by-layer coatings with a biomimetic calcium phosphate barrier layer. Tissue Eng. Part A 2017, 23, 1490–1501. [Google Scholar] [CrossRef]
- Adamczak, M.; Scislowska-Czarnecka, A.; Genet, M.J.; Dupont-Gillain, C.C.; PAMULA, E. Surface characterization, collagen adsorption and cell behaviour on poly (l-lactide-co-glycolide). Acta Bioeng. Biomech. 2011, 13. [Google Scholar]
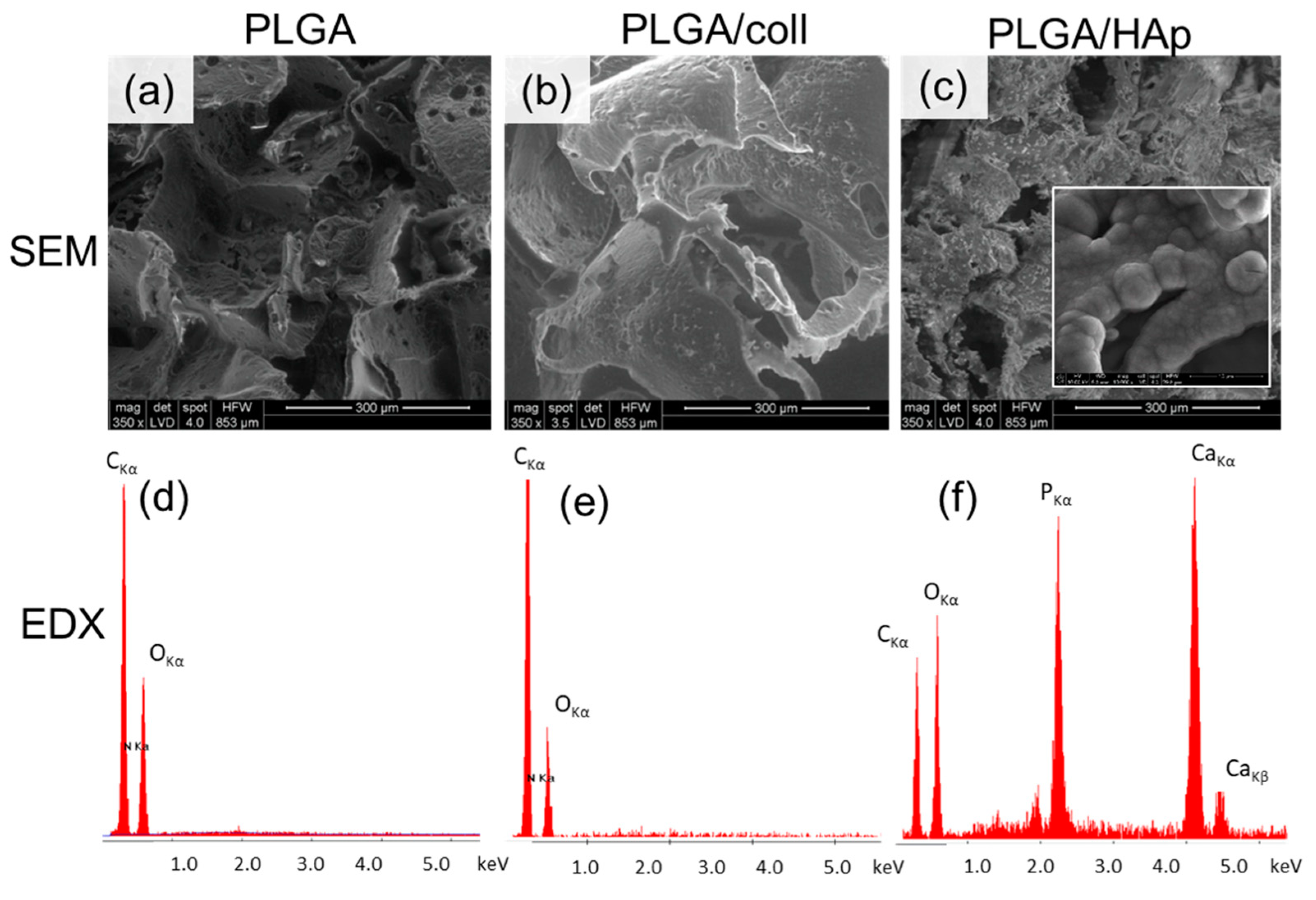
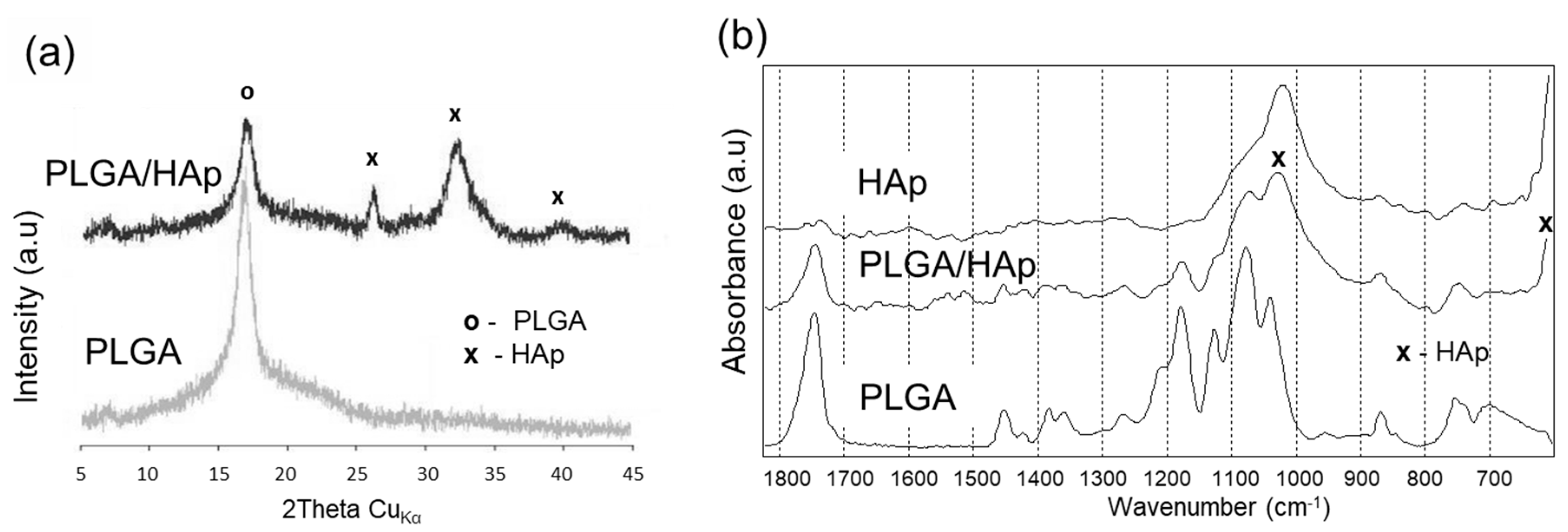
| Scaffold Type | Surface Chemical Composition (at.%) | Water Contact Angle (Deg.) | Strength σ (MPa) | Modulus E (MPa) | ||||
|---|---|---|---|---|---|---|---|---|
| C | O | Ca | P | N | ||||
| PLGA | 61.0 | 39.0 | bdl | bdl | bdl | 126.7 ± 9.3 | 0.58 ± 0.10 | 0.63 ± 0.12 |
| PLGA/coll | 56.0 | 40.2 | bdl | bdl | 2.8 | 103.4 ± 6.8 * | 0.61 ± 0.09 | 0.66 ± 0.15 |
| PLGA/HaAp | 17.0 | 66 | 10.6 | 6.4 | bdl | nd | 1.14 ± 0.31 * | 1.72 ± 0.37 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krok-Borkowicz, M.; Reczyńska, K.; Rumian, Ł.; Menaszek, E.; Orzelski, M.; Malisz, P.; Silmanowicz, P.; Dobrzyński, P.; Pamuła, E. Surface-Modified Poly(l-lactide-co-glycolide) Scaffolds for the Treatment of Osteochondral Critical Size Defects—In Vivo Studies on Rabbits. Int. J. Mol. Sci. 2020, 21, 7541. https://doi.org/10.3390/ijms21207541
Krok-Borkowicz M, Reczyńska K, Rumian Ł, Menaszek E, Orzelski M, Malisz P, Silmanowicz P, Dobrzyński P, Pamuła E. Surface-Modified Poly(l-lactide-co-glycolide) Scaffolds for the Treatment of Osteochondral Critical Size Defects—In Vivo Studies on Rabbits. International Journal of Molecular Sciences. 2020; 21(20):7541. https://doi.org/10.3390/ijms21207541
Chicago/Turabian StyleKrok-Borkowicz, Małgorzata, Katarzyna Reczyńska, Łucja Rumian, Elżbieta Menaszek, Maciej Orzelski, Piotr Malisz, Piotr Silmanowicz, Piotr Dobrzyński, and Elżbieta Pamuła. 2020. "Surface-Modified Poly(l-lactide-co-glycolide) Scaffolds for the Treatment of Osteochondral Critical Size Defects—In Vivo Studies on Rabbits" International Journal of Molecular Sciences 21, no. 20: 7541. https://doi.org/10.3390/ijms21207541
APA StyleKrok-Borkowicz, M., Reczyńska, K., Rumian, Ł., Menaszek, E., Orzelski, M., Malisz, P., Silmanowicz, P., Dobrzyński, P., & Pamuła, E. (2020). Surface-Modified Poly(l-lactide-co-glycolide) Scaffolds for the Treatment of Osteochondral Critical Size Defects—In Vivo Studies on Rabbits. International Journal of Molecular Sciences, 21(20), 7541. https://doi.org/10.3390/ijms21207541







