How Periodontal Disease and Presence of Nitric Oxide Reducing Oral Bacteria Can Affect Blood Pressure
Abstract
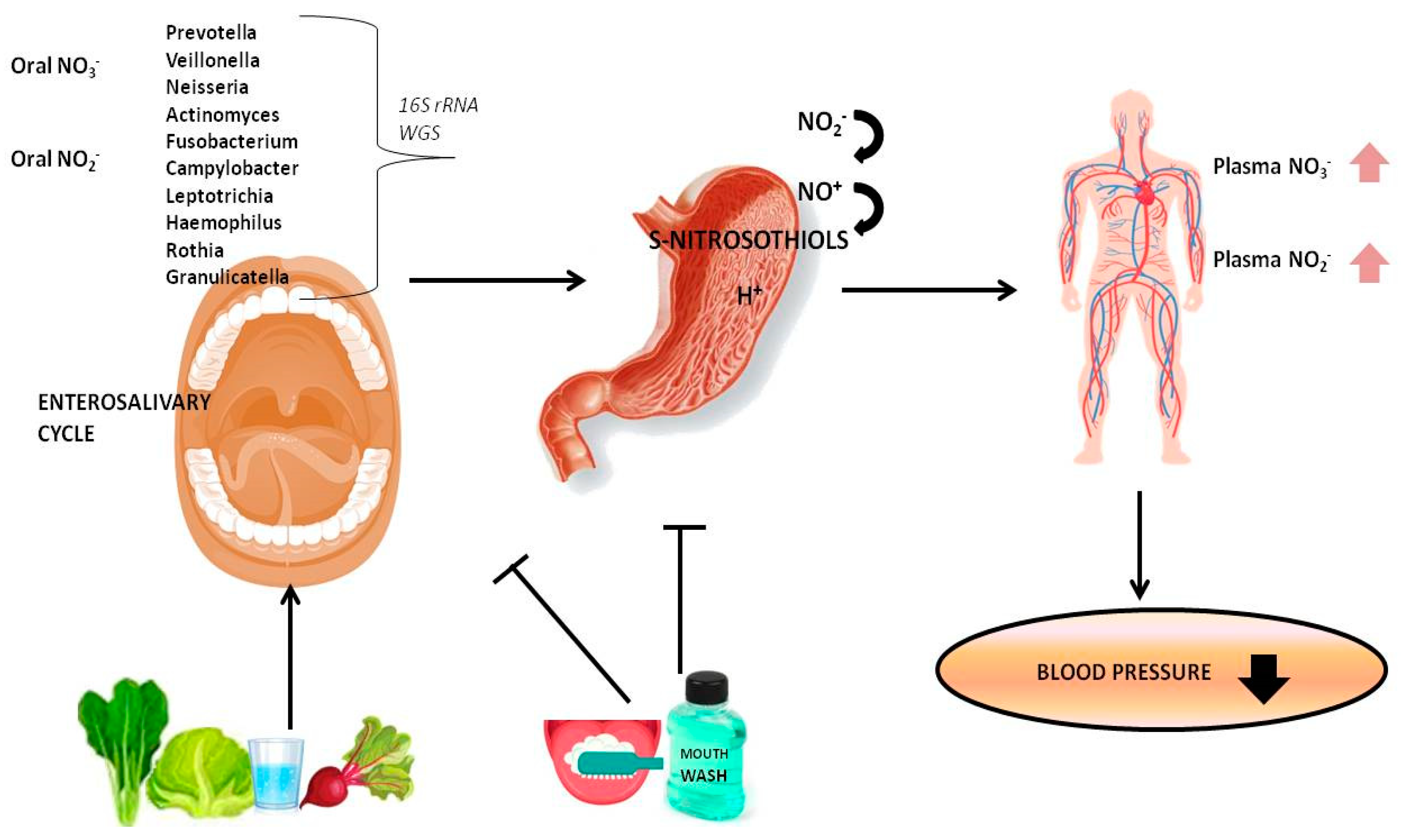
1. Introduction
2. NO3-NO2-NO Reduction Pathway
2.1. Endogenous NO and NOS
2.2. Dietary Nitrate
3. The Role of Oral Microbiome in NO Homeostasis
3.1. Oral Nitrate-Nitrite Reducing Bacteria
3.2. Interaction among Oral Microbiota, Dietary Nitrate, and NO Homeostasis
4. Effects of Antiseptic Mouthwash on Oral Microbiota and BP
5. Endothelial Disfunction, NO Bioavailability, and BP
6. Periodontitis Affects Hypertension (HT)
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BP | blood pressure |
| CVD | cardiovascular disease |
| cGMP | cyclic GMP |
| EDRF | endothelium derived relaxing factor |
| HT | hypertension |
| HOP | hypertension-associated oral pathogens |
| NAR | nitrate reductases |
| BR | nitrate-rich beetroot juice |
| NO | nitric oxide |
| NOR | nitric oxide reductases |
| NOSs | NO synthases |
| PWV | pulse-wave velocity |
| SFR-Q | Salivary Flow Rate Questionnaires |
| SBP | systolic blood pressure |
| WGS | whole genome sequencing |
References
- Sampaio-Maia, B.; Caldas, I.M.; Pereira, M.L.; Pérez-Mongiovi, D.; Araujo, R. The Oral Microbiome in Health and Its Implication in Oral and Systemic Diseases. Adv. Appl. Microbiol. 2016, 97, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Tsioufis, C.; Kasiakogias, A.; Thomopoulos, C.; Stefanadis, C. Periodontitis and blood pressure: The concept of dental hypertension. Atherosclerosis 2011, 219, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Weitzberg, E.; Lundberg, J.O.; Ahluwalia, A. Clinical evidence demonstrating the utility of inorganic nitrate in cardiovascular health. Nitric Oxide 2014, 38, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Bryan, N.S.; Tribble, G.; Angelov, N. Oral Microbiome and Nitric Oxide: The Missing Link in the Management of Blood Pressure. Curr. Hypertens. Rep. 2017, 19, 33. [Google Scholar] [CrossRef]
- Lind, M.; Hayes, A.; Caprnda, M.; Petrovic, D.; Rodrigo, L.; Kruzliak, P.; Zulli, A. Inducible nitric oxide synthase: Good or bad? Biomed. Pharmacother. 2017, 93, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.; Michel, L.Y.M.; Balligand, J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Cole, J.A.; Benjamin, N. Nitrate, bacteria and human health. Nat. Rev. Microbiol. 2004, 2, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W.S. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2019, 99, 311–379. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Fernandes, T.; Gomes-Gatto, C.V.; Pereira, N.P.; Alayafi, Y.R.; das Neves, V.J.; Oliveira, E.M. NO Signaling in the Cardiovascular System and Exercise. Adv. Exp. Med. Biol. 2017, 1000, 211–245. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Si, X.; Gong, Y.; Yan, G.; Wang, D.; Qiao, Y.; Liu, B.; Hou, J.; Tang, C. An association between the endothelial nitric oxide synthase gene G894T polymorphism and premature coronary artery disease: A meta-analysis. Oncotarget 2017, 8, 77990–77998. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef]
- Li, J.; Zheng, H.; Feng, C. Deciphering mechanism of conformationally controlled electron transfer in nitric oxide synthases. Front. Biosci. 2018, 23, 1803–1821. [Google Scholar]
- Omar, S.A.; Webb, A.J. Nitrite reduction and cardiovascular protection. J. Mol. Cell. Cardiol. 2014, 73, 57–69. [Google Scholar] [CrossRef]
- Premont, R.T.; Reynolds, J.D.; Zhang, R.; Stamler, J.S. Role of Nitric Oxide Carried by Hemoglobin in Cardiovascular Physiology: Developments on a Three-Gas Respiratory Cycle. Circ. Res. 2020, 126, 129–158. [Google Scholar] [CrossRef]
- Rajapakse, N.W.; Giam, B.; Kuruppu, S.; Head, G.A.; Kaye, D.M. Impaired l-arginine-nitric oxide pathway contributes to the pathogenesis of resistant hypertension. Clin. Sci. 2019, 133, 2061–2067. [Google Scholar] [CrossRef]
- Belzer, V.; Hanani, M. Nitric oxide as a messenger between neurons and satellite glial cells in dorsal root ganglia. Glia 2019, 67, 1296–1307. [Google Scholar] [CrossRef]
- Young, R.; Bush, S.J.; Lefevre, L.; McCulloch, M.E.B.; Lisowski, Z.M.; Muriuki, C.; Waddell, L.A.; Sauter, K.A.; Pridans, C.; Clark, E.L.; et al. Species-Specific Transcriptional Regulation of Genes Involved in Nitric Oxide Production and Arginine Metabolism in Macrophages. Immunohorizons 2018, 2, 27–37. [Google Scholar] [CrossRef]
- Luo, W.; Wang, Y.; Yang, H.; Dai, C.; Hong, H.; Li, J.; Liu, Z.; Guo, Z.; Chen, X.; He, P.; et al. Heme oxygenase-1 ameliorates oxidative stress-induced endothelial senescence via regulating endothelial nitric oxide synthase activation and coupling. Aging 2018, 10, 1722–1744. [Google Scholar] [CrossRef] [PubMed]
- Zweier, J.L.; Wang, P.; Samouilov, A.; Kuppusamy, P. Enzyme-independent formation of nitric oxide in biological tissues. Nat. Med. 1995, 1, 804–809. [Google Scholar] [CrossRef]
- Shiva, S.; Wang, X.; Ringwood, L.A.; Xu, X.; Yuditskaya, S.; Annavajjhala, V.; Miyajima, H.; Hogg, N.; Harris, Z.L.; Gladwin, M.T. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat. Chem. Biol. 2006, 2, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Paula, G.H.; Lacchini, R.; Tanus-Santos, J.E. Endothelial nitric oxide synthase: From biochemistry and gene structure to clinical implications of NOS3 polymorphisms. Gene 2016, 575, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Paula, G.H.; Lacchini, R.; Tanus-Santos, J.E. Clinical and pharmacogenetic impact of endothelial nitric oxide synthase polymorphisms on cardiovascular diseases. Nitric Oxide 2017, 63, 39–51. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Mónica, F.Z.; Bian, K.; Murad, F. The Endothelium-Dependent Nitric Oxide-cGMP Pathway. Adv. Pharmacol. 2016, 77, 1–27. [Google Scholar] [CrossRef]
- Pinheiro, L.C.; Ferreira, G.C.; Amaral, J.H.; Portella, R.L.; Tella, S.D.O.C.; Passos, M.A.; Tanus-Santos, J.E. Oral nitrite circumvents antiseptic mouthwash-induced disruption of enterosalivary circuit of nitrate and promotes nitrosation and blood pressure lowering effect. Free Radic. Biol. Med. 2016, 101, 226–235. [Google Scholar] [CrossRef]
- Petersson, J.; Jädert, C.; Phillipson, M.; Borniquel, S.; Lundberg, J.O.; Holm, L. Physiological recycling of endogenous nitrate by oral bacteria regulates gastric mucus thickness. Free Radic. Biol. Med. 2015, 89, 241–247. [Google Scholar] [CrossRef]
- Benjamin, N.; O’Driscoll, F.; Dougall, H.; Duncan, C.; Smith, L.; Golden, M.; McKenzie, H. Stomach NO synthesis. Nature 1994, 368, 502. [Google Scholar] [CrossRef]
- Rocha, B.S.; Lundberg, J.O.; Radi, R.; Laranjinha, J. Role of nitrite, urate and pepsin in the gastroprotective effects of saliva. Redox Biol. 2016, 8, 407–414. [Google Scholar] [CrossRef]
- Weitzberg, E.; Lundberg, J.O. Novel aspects of dietary nitrate and human health. Annu. Rev. Nutr. 2013, 33, 129–159. [Google Scholar] [CrossRef] [PubMed]
- McKnight, G.M.; Smith, L.M.; Drummond, R.S.; Duncan, C.W.; Golden, M.; Benjamin, N. Chemical synthesis of nitric oxide in the stomach from dietary nitrate in humans. Gut 1997, 40, 211–214. [Google Scholar] [CrossRef]
- Montenegro, M.F.; Sundqvist, M.L.; Larsen, F.J.; Zhuge, Z.; Carlström, M.; Weitzberg, E.; Lundberg, J.O. Blood Pressure-Lowering Effect of Orally Ingested Nitrite Is Abolished by a Proton Pump Inhibitor. Hypertension 2017, 69, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.V.; Staniek, K.; Nohl, H. Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett. 1999, 454, 127–130. [Google Scholar] [CrossRef]
- Castiglione, N.; Rinaldo, S.; Giardina, G.; Stelitano, V.; Cutruzzolà, F. Nitrite and nitrite reductases: From molecular mechanisms to significance in human health and disease. Antioxid. Redox Signal. 2012, 17, 684–716. [Google Scholar] [CrossRef]
- Walters, C.L.; Casselden, R.J.; Taylor, A.M. Nitrite metabolism by skeletal muscle mitochondria in relation to haem pigments. Biochim. Biophys. Acta 1967, 143, 310–318. [Google Scholar] [CrossRef]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef]
- Suzuki, H.; DeLano, F.A.; Parks, D.A.; Jamshidi, N.; Granger, D.N.; Ishii, H.; Suematsu, M.; Zweifach, B.W.; Schmid-Schönbein, G.W. Xanthine oxidase activity associated with arterial blood pressure in spontaneously hypertensive rats. Proc. Natl. Acad. Sci. USA 1998, 95, 4754–4759. [Google Scholar] [CrossRef]
- Landmesser, U.; Spiekermann, S.; Preuss, C.; Sorrentino, S.; Fischer, D.; Manes, C.; Mueller, M.; Drexler, H. Angiotensin II induces endothelial xanthine oxidase activation: Role for endothelial dysfunction in patients with coronary disease. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 943–948. [Google Scholar] [CrossRef]
- Kelley, E.E. A new paradigm for XOR-catalyzed reactive species generation in the endothelium. Pharmacol. Rep. 2015, 67, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Millar, T.M.; Stevens, C.R.; Benjamin, N.; Eisenthal, R.; Harrison, R.; Blake, D.R. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998, 427, 225–228. [Google Scholar] [CrossRef]
- Hyde, E.R.; Luk, B.; Cron, S.; Kusic, L.; McCue, T.; Bauch, T.; Kaplan, H.; Tribble, G.; Petrosino, J.F.; Bryan, N.S. Characterization of the rat oral microbiome and the effects of dietary nitrate. Free Radic. Biol. Med. 2014, 77, 249–257. [Google Scholar] [CrossRef]
- Sánchez, G.A.; Miozza, V.A.; Delgado, A.; Busch, L. Total salivary nitrates and nitrites in oral health and periodontal disease. Nitric Oxide 2014, 36, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhang, J. Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol. 2017, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Kitai, T.; Tang, W.H.W. Gut microbiota in cardiovascular disease and heart failure. Clin. Sci. 2018, 132, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Rezaie, P.; Kengne, A.P.; Mobarhan, M.G.; Ferns, G.A. Gut microbiome and metabolic syndrome. Diabetes Metab. Syndr. 2016, 10, S150–S157. [Google Scholar] [CrossRef]
- Kapil, V.; Haydar, S.M.A.; Pearl, V.; Lundberg, J.O.; Weitzberg, E.; Ahluwalia, A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic. Biol. Med. 2013, 55, 93–100. [Google Scholar] [CrossRef]
- Zhurakivska, K.; Troiano, G.; Caponio, V.C.A.; Dioguardi, M.; Laino, L.; Maffione, A.B.; Lo Muzio, L. Do Changes in Oral Microbiota Correlate With Plasma Nitrite Response? A Systematic Review. Front. Physiol. 2019, 10, 1029. [Google Scholar] [CrossRef]
- Duncan, C.; Dougall, H.; Johnston, P.; Green, S.; Brogan, R.; Leifert, C.; Smith, L.; Golden, M.; Benjamin, N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat. Med. 1995, 1, 546–551. [Google Scholar] [CrossRef]
- Mantilla Gómez, S.; Danser, M.M.; Sipos, P.M.; Rowshani, B.; van der Velden, U.; van der Weijden, G.A. Tongue coating and salivary bacterial counts in healthy/gingivitis subjects and periodontitis patients. J. Clin. Periodontol. 2001, 28, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [CrossRef] [PubMed]
- Arank, A.; Syed, S.A.; Kenney, E.B.; Freter, R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl. Microbiol. 1969, 17, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.E.; Holdeman, L.V.; Smibert, R.M.; Hash, D.E.; Burmeister, J.A.; Ranney, R.R. Bacteriology of severe periodontitis in young adult humans. Infect. Immun. 1982, 38, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Wade, W.G. Has the use of molecular methods for the characterization of the human oral microbiome changed our understanding of the role of bacteria in the pathogenesis of periodontal disease? J. Clin. Periodontol. 2011, 38 (Suppl. S11), 7–16. [Google Scholar] [CrossRef]
- Koopman, J.E.; Buijs, M.J.; Brandt, B.W.; Keijser, B.J.F.; Crielaard, W.; Zaura, E. Nitrate and the Origin of Saliva Influence Composition and Short Chain Fatty Acid Production of Oral Microcosms. Microb. Ecol. 2016, 72, 479–492. [Google Scholar] [CrossRef]
- Hyde, E.R.; Andrade, F.; Vaksman, Z.; Parthasarathy, K.; Jiang, H.; Parthasarathy, D.K.; Torregrossa, A.C.; Tribble, G.; Kaplan, H.B.; Petrosino, J.F.; et al. Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: Implications for nitric oxide homeostasis. PLoS ONE 2014, 9, e88645. [Google Scholar] [CrossRef]
- Crane, B.R.; Sudhamsu, J.; Patel, B.A. Bacterial nitric oxide synthases. Annu. Rev. Biochem. 2010, 79, 445–470. [Google Scholar] [CrossRef]
- Kurtz, T.W.; DiCarlo, S.E.; Pravenec, M.; Morris, R.C. Functional foods for augmenting nitric oxide activity and reducing the risk for salt-induced hypertension and cardiovascular disease in Japan. J. Cardiol. 2018, 72, 42–49. [Google Scholar] [CrossRef]
- Reimer, R.J. SLC17: A functionally diverse family of organic anion transporters. Mol. Asp. Med. 2013, 34, 350–359. [Google Scholar] [CrossRef]
- Burleigh, M.C.; Liddle, L.; Monaghan, C.; Muggeridge, D.J.; Sculthorpe, N.; Butcher, J.P.; Henriquez, F.L.; Allen, J.D.; Easton, C. Salivary nitrite production is elevated in individuals with a higher abundance of oral nitrate-reducing bacteria. Free Radic. Biol. Med. 2018, 120, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Vanhatalo, A.; Blackwell, J.R.; L’Heureux, J.E.; Williams, D.W.; Smith, A.; van der Giezen, M.; Winyard, P.G.; Kelly, J.; Jones, A.M. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic. Biol. Med. 2018, 124, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, S.; Gan, J.M.; Rathod, K.S.; Khambata, R.S.; Ghosh, S.M.; Hartley, A.; Van Eijl, S.; Sagi-Kiss, V.; Chowdhury, T.A.; Curtis, M.; et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: A randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 2016, 103, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.E.; Trinh, P.; Colombo, P.C.; Genkinger, J.M.; Mathema, B.; Uhlemann, A.-C.; LeDuc, C.; Leibel, R.; Rosenbaum, M.; Paster, B.J.; et al. Association Between Nitrate-Reducing Oral Bacteria and Cardiometabolic Outcomes: Results From ORIGINS. J. Am. Heart Assoc. 2019, 8, e013324. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.H.; Kern, T.; Bak, E.G.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.; Pedersen, O. Impact of a vegan diet on the human salivary microbiota. Sci. Rep. 2018, 8, 5847. [Google Scholar] [CrossRef] [PubMed]
- Doel, J.J.; Benjamin, N.; Hector, M.P.; Rogers, M.; Allaker, R.P. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur. J. Oral Sci. 2005, 113, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bondonno, C.P.; Liu, A.H.; Croft, K.D.; Considine, M.J.; Puddey, I.B.; Woodman, R.J.; Hodgson, J.M. Antibacterial mouthwash blunts oral nitrate reduction and increases blood pressure in treated hypertensive men and women. Am. J. Hypertens. 2015, 28, 572–575. [Google Scholar] [CrossRef]
- Govoni, M.; Jansson, E.A.; Weitzberg, E.; Lundberg, J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 2008, 19, 333–337. [Google Scholar] [CrossRef]
- McDonagh, S.T.J.; Wylie, L.J.; Winyard, P.G.; Vanhatalo, A.; Jones, A.M. The Effects of Chronic Nitrate Supplementation and the Use of Strong and Weak Antibacterial Agents on Plasma Nitrite Concentration and Exercise Blood Pressure. Int. J. Sports Med. 2015, 36, 1177–1185. [Google Scholar] [CrossRef]
- Woessner, M.; Smoliga, J.M.; Tarzia, B.; Stabler, T.; Van Bruggen, M.; Allen, J.D. A stepwise reduction in plasma and salivary nitrite with increasing strengths of mouthwash following a dietary nitrate load. Nitric Oxide 2016, 54, 1–7. [Google Scholar] [CrossRef]
- Sundqvist, M.L.; Lundberg, J.O.; Weitzberg, E. Effects of antiseptic mouthwash on resting metabolic rate: A randomized, double-blind, crossover study. Nitric Oxide 2016, 61, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Graudal, N.A.; Hubeck-Graudal, T.; Jurgens, G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst. Rev. 2017, 4, CD004022. [Google Scholar] [CrossRef]
- Mitsui, T.; Harasawa, R. The effects of essential oil, povidone-iodine, and chlorhexidine mouthwash on salivary nitrate/nitrite and nitrate-reducing bacteria. J. Oral Sci. 2017, 59, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Ellepola, A.N.B.; Chandy, R.; Khan, Z.U. In vitro Impact of Limited Exposure to Subtherapeutic Concentrations of Chlorhexidine Gluconate on the Adhesion-Associated Attributes of Oral Candida Species. Med. Princ. Pract. 2016, 25, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Senkus, K.E.; Crowe-White, K.M. Influence of mouth rinse use on the enterosalivary pathway and blood pressure regulation: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tribble, G.D.; Angelov, N.; Weltman, R.; Wang, B.-Y.; Eswaran, S.V.; Gay, I.C.; Parthasarathy, K.; Dao, D.-H.V.; Richardson, K.N.; Ismail, N.M.; et al. Frequency of Tongue Cleaning Impacts the Human Tongue Microbiome Composition and Enterosalivary Circulation of Nitrate. Front. Cell Infect. Microbiol. 2019, 9, 39. [Google Scholar] [CrossRef]
- Dei Zotti, F.; Lobysheva, I.I.; Balligand, J.-L. Nitrosyl-hemoglobin formation in rodent and human venous erythrocytes reflects NO formation from the vasculature in vivo. PLoS ONE 2018, 13, e0200352. [Google Scholar] [CrossRef]
- Yuyun, M.F.; Ng, L.L.; Ng, G.A. Endothelial dysfunction, endothelial nitric oxide bioavailability, tetrahydrobiopterin, and 5-methyltetrahydrofolate in cardiovascular disease. Where are we with therapy? Microvasc. Res. 2018, 119, 7–12. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Ye, Z.-X.; Wang, X.-F.; Chang, J.; Yang, M.-W.; Zhong, H.-H.; Hong, F.-F.; Yang, S.-L. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed. Pharmacother. 2018, 97, 423–428. [Google Scholar] [CrossRef]
- Rassaf, T.; Heiss, C.; Hendgen-Cotta, U.; Balzer, J.; Matern, S.; Kleinbongard, P.; Lee, A.; Lauer, T.; Kelm, M. Plasma nitrite reserve and endothelial function in the human forearm circulation. Free Radic. Biol. Med. 2006, 41, 295–301. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Jax, T.; Kerber, S.; Gharini, P.; Balzer, J.; Zotz, R.B.; Scharf, R.E.; Willers, R.; et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic. Biol. Med. 2006, 40, 295–302. [Google Scholar] [CrossRef]
- Lee, J.; Bae, E.H.; Ma, S.K.; Kim, S.W. Altered Nitric Oxide System in Cardiovascular and Renal Diseases. Chonnam. Med. J. 2016, 52, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kerley, C.P.; Dolan, E.; Cormican, L. Nitrate-rich beetroot juice selectively lowers ambulatory pressures and LDL cholesterol in uncontrolled but not controlled hypertension: A pilot study. Ir. J. Med. Sci. 2017, 186, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Gori, T. Exogenous NO Therapy for the Treatment and Prev.ention of Atherosclerosis. Int. J. Mol. Sci. 2020, 21, 2703. [Google Scholar] [CrossRef]
- Raubenheimer, K.; Hickey, D.; Leveritt, M.; Fassett, R.; Ortiz de Zevallos Munoz, J.; Allen, J.D.; Briskey, D.; Parker, T.J.; Kerr, G.; Peake, J.M.; et al. Acute Effects of Nitrate-Rich Beetroot Juice on Blood Pressure, Hemostasis and Vascular Inflammation Markers in Healthy Older Adults: A Randomized, Placebo-Controlled Crossover Study. Nutrients 2017, 9, 1270. [Google Scholar] [CrossRef] [PubMed]
- Jonvik, K.L.; Nyakayiru, J.; Pinckaers, P.J.; Senden, J.M.; van Loon, L.J.; Verdijk, L.B. Nitrate-Rich Vegetables Increase Plasma Nitrate and Nitrite Concentrations and Lower Blood Pressure in Healthy Adults. J. Nutr. 2016, 146, 986–993. [Google Scholar] [CrossRef]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: A randomized, phase 2, double-blind, placebo-controlled study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef]
- Shannon, O.M.; Duckworth, L.; Barlow, M.J.; Woods, D.; Lara, J.; Siervo, M.; O’Hara, J.P. Dietary nitrate supplementation enhances high-intensity running performance in moderate normobaric hypoxia, independent of aerobic fitness. Nitric Oxide 2016, 59, 63–70. [Google Scholar] [CrossRef]
- Litwin, N.S.; Van Ark, H.J.; Hartley, S.C.; Michell, K.A.; Vazquez, A.R.; Fischer, E.K.; Melby, C.L.; Weir, T.L.; Wei, Y.; Rao, S.; et al. Impact of Red Beetroot Juice on Vascular Endothelial Function and Cardiometabolic Responses to a High-Fat Meal in Middle-Aged/Older Adults with Overweight and Obesity: A Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. Curr. Dev. Nutr. 2019, 3, nzz113. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Aoyama, N.; Suzuki, J.-I.; Kumagai, H.; Ikeda, Y.; Akazawa, H.; Komuro, I.; Minabe, M.; Izumi, Y.; Isobe, M. Specific periodontopathic bacterial infection affects hypertension in male cardiovascular disease patients. Heart Vessel. 2018, 33, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Pistoia, F.; Sacco, S.; Degan, D.; Tiseo, C.; Ornello, R.; Carolei, A. Hypertension and Stroke: Epidemiological Aspects and Clinical Evaluation. High. Blood Press. Cardiovasc. Prev. 2016, 23, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Boillot, A.; Demmer, R.T.; Mallat, Z.; Sacco, R.L.; Jacobs, D.R.; Benessiano, J.; Tedgui, A.; Rundek, T.; Papapanou, P.N.; Desvarieux, M. Periodontal microbiota and phospholipases: The Oral Infections and Vascular Disease Epidemiology Study (INVEST). Atherosclerosis 2015, 242, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Marchina, S.; Deveau, J.A.; Frayne, C.; Sulmonte, K.; Kumar, S. Risk of Stroke-Associated Pneumonia and Oral Hygiene. Cerebrovasc. Dis. 2016, 41, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, T.; Webb, I.; Stenhouse, L.; Pattni, A.; Ready, D.; Wanyonyi, K.L.; White, S.; Gallagher, J.E. Evidence summary: The relationship between oral and cardiovascular disease. Br. Dent. J. 2017, 222, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; D’Aiuto, F.; Nibali, L.; Donald, A.; Storry, C.; Parkar, M.; Suvan, J.; Hingorani, A.D.; Vallance, P.; Deanfield, J. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 2007, 356, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Desvarieux, M.; Demmer, R.T.; Jacobs, D.R.; Rundek, T.; Boden-Albala, B.; Sacco, R.L.; Papapanou, P.N. Periodontal bacteria and hypertension: The oral infections and vascular disease epidemiology study (INVEST). J. Hypertens. 2010, 28, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, M.J.; Liebeskind, D.S.; Chan, S.-L. The importance of comorbidities in ischemic stroke: Impact of hypertension on the cerebral circulation. J. Cereb. Blood Flow Metab. 2018, 38, 2129–2149. [Google Scholar] [CrossRef] [PubMed]
- Pietropaoli, D.; Del Pinto, R.; Ferri, C.; Ortu, E.; Monaco, A. Definition of hypertension-associated oral pathogens in NHANES. J. Periodontol. 2019, 90, 866–876. [Google Scholar] [CrossRef]
- Kannosh, I.; Staletovic, D.; Toljic, B.; Radunovic, M.; Pucar, A.; Matic Petrovic, S.; Grubisa, I.; Lazarevic, M.; Brkic, Z.; Knezevic Vukcevic, J.; et al. The presence of periopathogenic bacteria in subgingival and atherosclerotic plaques-An age related comparative analysis. J. Infect. Dev. Ctries. 2018, 12, 1088–1095. [Google Scholar] [CrossRef]
- Brun, A.; Rangé, H.; Prouvost, B.; Mazighi, M.; Kapila, Y.; Bouchard, P.; Michel, J.B. Innovative application of nested PCR for detection of Porphyromonas gingivalis in human highly calcified atherothrombotic plaques. J. Oral Microbiol. 2020, 12, 1742523. [Google Scholar] [CrossRef] [PubMed]
- Agita, A.; Alsagaff, M.T. Inflammation, Immunity, and Hypertension. Acta Med. Indones 2017, 49, 158–165. [Google Scholar] [PubMed]
- Bruno, A.S.; Lopes, P.D.D.; de Oliveira, K.C.M.; de Oliveira, A.K.; de Assis, C.S.B. Vascular Inflammation in Hypertension: Targeting Lipid Mediators Unbalance and Nitrosative Stress. Curr. Hypertens. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.H.; LaMonte, M.J.; Genco, R.J.; Zhao, J.; Cimato, T.R.; Hovey, K.M.; Wactawski-Wende, J. Association of clinical measures of periodontal disease with blood pressure and hypertension among postmenopausal women. J. Periodontol. 2018, 89, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.H.; LaMonte, M.J.; Genco, R.J.; Zhao, J.; Li, L.; Hovey, K.M.; Tsompana, M.; Buck, M.J.; Andrews, C.A.; Mcskimming, D.I.; et al. Is the Oral Microbiome Associated with Blood Pressure in Older Women? High. Blood Press. Cardiovasc. Prev. 2019, 26, 217–225. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pignatelli, P.; Fabietti, G.; Ricci, A.; Piattelli, A.; Curia, M.C. How Periodontal Disease and Presence of Nitric Oxide Reducing Oral Bacteria Can Affect Blood Pressure. Int. J. Mol. Sci. 2020, 21, 7538. https://doi.org/10.3390/ijms21207538
Pignatelli P, Fabietti G, Ricci A, Piattelli A, Curia MC. How Periodontal Disease and Presence of Nitric Oxide Reducing Oral Bacteria Can Affect Blood Pressure. International Journal of Molecular Sciences. 2020; 21(20):7538. https://doi.org/10.3390/ijms21207538
Chicago/Turabian StylePignatelli, Pamela, Giulia Fabietti, Annalisa Ricci, Adriano Piattelli, and Maria Cristina Curia. 2020. "How Periodontal Disease and Presence of Nitric Oxide Reducing Oral Bacteria Can Affect Blood Pressure" International Journal of Molecular Sciences 21, no. 20: 7538. https://doi.org/10.3390/ijms21207538
APA StylePignatelli, P., Fabietti, G., Ricci, A., Piattelli, A., & Curia, M. C. (2020). How Periodontal Disease and Presence of Nitric Oxide Reducing Oral Bacteria Can Affect Blood Pressure. International Journal of Molecular Sciences, 21(20), 7538. https://doi.org/10.3390/ijms21207538





