Innate Immune Response to Tick-Borne Pathogens: Cellular and Molecular Mechanisms Induced in the Hosts
Abstract
1. Introduction
2. Inflammasomes as Key Multimolecular Mechanisms Reacting to Infections
3. Innate Immune Response to Tick-Borne Pathogens as the First and, in Many Cases, Resolutive Mechanisms of Protection
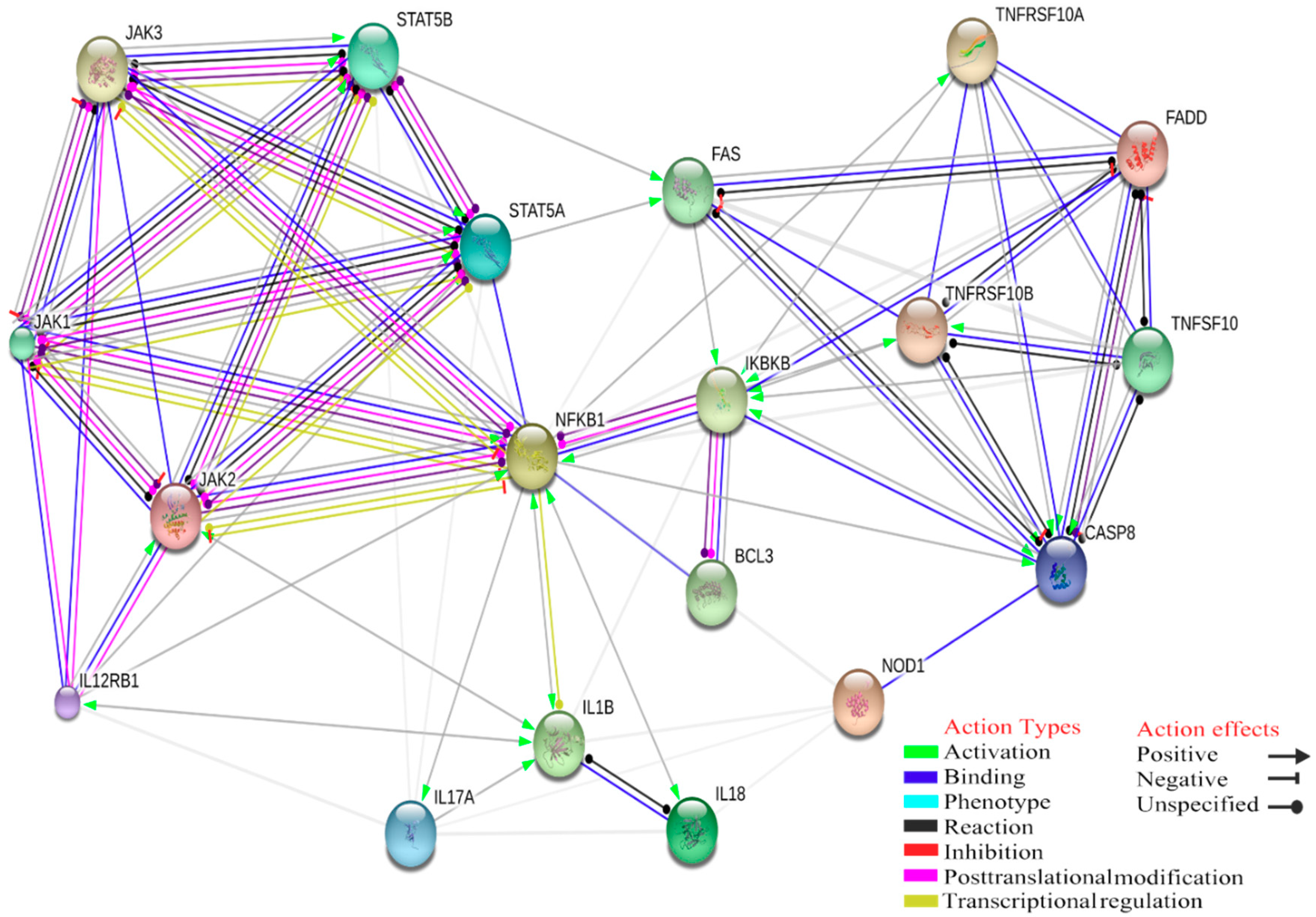
4. Gene Ontology Analysis of Interactions between Innate Immune Response and Tick-Borne Pathogens
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rizzoli, A.; Silaghi, C.; Obiegala, A.; Rudolf, I.; Hubálek, Z.; Földvári, G.; Plantard, O.; Vayssier-Taussat, M.; Bonnet, S.; Spitalská, E.; et al. Ixodes Ricinus and Its Transmitted Pathogens in Urban and Peri-Urban Areas in Europe: New Hazards and Relevance for Public Health. Front. Public Health 2014, 2, 251. [Google Scholar] [CrossRef] [PubMed]
- Scherler, A.; Jacquier, N.; Greub, G. Chlamydiales, Anaplasma and Bartonella: Persistence and Immune Escape of Intracellular Bacteria. Microbes Infect. 2018, 20, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Stuen, S.; Granquist, E.G.; Silaghi, C. Anaplasma Phagocytophilum-a Widespread Multi-Host Pathogen with Highly Adaptive Strategies. Front. Cell. Infect. Microbiol. 2013, 3, UNSP 31. [Google Scholar] [CrossRef]
- Battilani, M.; De Arcangeli, S.; Balboni, A.; Dondi, F. Genetic diversity and molecular epidemiology of Anaplasma. Infect. Genet. Evol. 2017, 49, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Olano, J.R.; Masters, E.; Hogrefe, W.; Walker, D.H. Human Monocytotropic Ehrlichiosis, Missouri. Emerg. Infect. Dis. 2003, 9, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Buller, R.; Arens, M.; Hmiel, S.P.; Paddock, C.D.; Sumner, J.W.; Rikihisa, Y.; Unver, A.; Gaudreault-Keener, M.; Manian, F.A.; Liddell, A.M.; et al. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N. Engl. J. Med. 1999, 341, 148–155. [Google Scholar] [CrossRef]
- Allsopp, M.T.; Allsopp, B.A. Extensive genetic recombination occurs in the field between different genotypes of Ehrlichia ruminantium. Vet. Microbiol. 2007, 124, 58–65. [Google Scholar] [CrossRef]
- Perez, M.; Bodor, M.; Zhang, C.; Xiong, Q.; Rikihisa, Y. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann. N. Y. Acad. Sci. 2006, 1078, 110–117. [Google Scholar] [CrossRef]
- Ismail, N.; McBride, J.W. Tick-Borne Emerging Infections: Ehrlichiosis and Anaplasmosis. Clin Lab. Med. 2017, 37, 317–340. [Google Scholar] [CrossRef]
- Ganguly, S.; Mukhopadhayay, S.K. Tick-borne ehrlichiosis infection in human beings. J. Vector Borne Dis. 2008, 45, 273–280. [Google Scholar]
- Lina, T.T.; Farris, T.; Luo, T.; Mitra, S.; Zhu, B.; McBride, J.W. Hacker within! Ehrlichia Chaffeensis Effector Driven Phagocyte Reprogramming Strategy. Front. Cell. Infect. Microbiol. 2016, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.B.; Walker, D.H. Ehrlichioses: An Important One Health Opportunity. Vet. Sci 2016, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-Borne Rickettsioses around the World: Emerging Diseases Challenging Old Concepts. Clin. Microbiol. Rev. 2005, 18, 719. [Google Scholar] [CrossRef] [PubMed]
- Rovery, C.; Brouqui, P.; Raoult, D. Questions on Mediterranean spotted fever a century after its discovery. Emerg. Infect. Dis. 2008, 14, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Schnittger, L.; Rodriguez, A.E.; Florin-Christensen, M.; Morrison, D.A. Babesia: A World Emerging. Infect. Genet. Evol. 2012, 12, 1788–1809. [Google Scholar] [CrossRef] [PubMed]
- Jalovecka, M.; Hajdusek, O.; Sojka, D.; Kopacek, P.; Malandrin, L. The Complexity of Piroplasms Life Cycles. Front. Cell Infect. Microbiol. 2018, 8, 248. [Google Scholar] [CrossRef]
- Schreeg, M.E.; Marr, H.S.; Tarigo, J.L.; Cohn, L.A.; Bird, D.M.; Scholl, E.H.; Levy, M.G.; Wiegmann, B.M.; Birkenheuer, A.J. Mitochondrial Genome Sequences and Structures Aid in the Resolution of Piroplasmida phylogeny. PLoS ONE 2016, 11, e0165702. [Google Scholar] [CrossRef]
- Bishop, R.; Musoke, A.; Morzaria, S.; Gardner, M.; Nene, V. Theileria: Intracellular Protozoan Parasites of Wild and Domestic Ruminants Transmitted by Ixodid Ticks. Parasitology 2004, 129, S271–S283. [Google Scholar] [CrossRef]
- Irvin, A.D.; Morrison, W.I. Immunopathology, immunology and immunoprophylaxis of Theileria infections. In Immune Responses in Parasitic Infections: Immunology, Immunopathology and Immunoprophylaxis; Soulsby, E.J.L., Ed.; CRC Press: Boca Raton, FL, USA, 1987; pp. 223–274. [Google Scholar]
- Sonenshine, D.E.; Macaluso, K.R. Microbial Invasion vs. Tick Immune Regulation. Front. Cell Infect. Microbiol. 2017, 7, 390. [Google Scholar] [CrossRef]
- Chapes, S.K.; Ganta, R.R. Defining the Immune Response to Ehrlichia Species Using Murine Models. Vet. Parasitol. 2008, 158, 344–359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Habib, S.; El Andaloussi, A.; Hisham, A.; Ismail, N. NK Cell-Mediated Regulation of Protective Memory Responses against Intracellular Ehrlichial Pathogens. PLoS ONE 2016, 11, e0153223. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.G.; Johnson, W.C.; Mwangi, W.; Brown, W.C.; Goff, W.L. Bovine NK Cells Acquire Cytotoxic Activity and Produce IFN-Gamma after Stimulation by Mycobacterium Bovis BCG- or Babesia Bovis-Exposed Splenic Dendritic Cells. Vet. Immunol. Immunopathol. 2008, 124, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Goff, W.L.; Storset, A.K.; Johnson, W.C.; Brown, W.C. Bovine Splenic NK Cells Synthesize IFN-Gamma in Response to IL-12-Containing Supernatants from Babesia Bovis-Exposed Monocyte Cultures. Parasite Immunol. 2006, 28, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.; MacHugh, N.D.; Morrison, W.I. Theileria Annulata-Transformed Cell Lines Are Efficient Antigen-Presenting Cells for in Vitro Analysis of CD8 T Cell Responses to Bovine Herpesvirus-1. Vet. Res. 2011, 42, 119. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, S.W.; Bonham, K.S.; Zanoni, I.; Kagan, J.C. Innate Immune Pattern Recognition: A Cell Biological Perspective. In Annual Review of Immunology; Littman, D.R., Yokoyama, W.M., Eds.; Annual Reviews: Palo Alto, CA, USA, 2015; Volume 33, pp. 257–290. [Google Scholar] [CrossRef]
- Shaw, D.K.; McClure, E.E.; Wang, X.; Pedra, J.H.F. Deviant Behavior: Tick-Borne Pathogens and Inflammasome Signaling. Vet. Sci. 2016, 3, 27. [Google Scholar] [CrossRef]
- Caruso, R.; Warner, N.; Inohara, N.; Nunez, G. NOD1 and NOD2: Signaling, Host Defense, and Inflammatory Disease. Immunity 2014, 41, 898–908. [Google Scholar] [CrossRef]
- Kang, S.; Fernandes-Alnemri, T.; Rogers, C.; Mayes, C.; Wang, Y.; Dillon, C.; Roback, L.; Kaiser, W.; Oberst, A.; Sagara, J.; et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat. Commun. 2015, 6, 7515. [Google Scholar] [CrossRef]
- Hayward, J.A.; Mathur, A.; Ngo, C.; Man, S.M. Cytosolic Recognition of Microbes and Pathogens: Inflammasomes in Action. Microbiol. Mol. Biol. Rev. 2018, 82, e00015-18. [Google Scholar] [CrossRef]
- Scorpio, D.G.; Choi, K.-S.; Dumler, J.S. Anaplasma Phagocytophilum-Related Defects in CD8, NKT, and NK Lymphocyte Cytotoxicity. Front. Immunol. 2018, 9, 710. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Xue, Y.; Enosi Tuipulotu, D.; Tan, W.H.; Kay, C.; Man, S.M. Emerging Activators and Regulators of Inflammasomes and Pyroptosis. Trends Immunol. 2019, 40, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Stowe, I.B.; Lee, B.L.; O’Rourke, K.; Anderson, K.; Warming, S.; Cuellar, T.L.; Haley, B.; Roose-Girma, M.; Phung, Q.; et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015, 526, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Fox, D.; Man, S.M. Mechanisms of Gasdermin Family Members in Inflammasome Signaling and Cell Death. J. Mol. Biol. 2018, 430, 3068–3080. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- He, Y.; Hara, H.; Núñez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Behl, B.; Mendonca, M.; Shrivastava, G.; Russo, A.J.; Menoret, A.; Ghosh, A.; Vella, A.T.; Vanaja, S.K.; Sarkar, S.N.; et al. Gasdermin D Restrains Type I Interferon Response to Cytosolic DNA by Disrupting Ionic Homeostasis. Immunity 2018, 49, 413–426.e5. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Walle, L.V.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef]
- Kayagaki, N.; Wong, M.T.; Stowe, I.B.; Ramani, S.R.; Gonzalez, L.C.; Akashi-Takamura, S.; Miyake, K.; Zhang, J.; Lee, W.P.; Muszyński, A.; et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 2013, 341, 1246–1249. [Google Scholar] [CrossRef]
- Baker, P.J.; Boucher, D.; Bierschenk, D.; Tebartz, C.; Whitney, P.G.; D’Silva, D.B.; Tanzer, M.C.; Monteleone, M.; Robertson, A.A.B.; Cooper, M.A.; et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur. J. Immunol. 2015, 45, 2918–2926. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Fitzgerald, K.A. Inflammasome Complexes: Emerging Mechanisms and Effector Functions. Cell 2016, 165, 792–800. [Google Scholar] [CrossRef]
- Schmid-Burgk, J.L.; Gaidt, M.M.; Schmidt, T.; Ebert, T.S.; Bartok, E.; Hornung, V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur. J. Immunol. 2015, 45, 2911–2917. [Google Scholar] [CrossRef] [PubMed]
- Wandel, M.P.; Kim, B.-H.; Park, E.-S.; Boyle, K.B.; Nayak, K.; Lagrange, B.; Herod, A.; Henry, T.; Zilbauer, M.; Rohde, J.; et al. Guanylate-binding proteins convert cytosolic bacteria into caspase-4 signaling platforms. Nat. Immunol. 2020, 1–12. [Google Scholar] [CrossRef]
- Santos, J.C.; Boucher, D.; Schneider, L.K.; Demarco, B.; Dilucca, M.; Shkarina, K.; Heilig, R.; Chen, K.W.; Lim, R.Y.H.; Broz, P. Human GBP1 binds LPS to initiate assembly of a caspase-4 activating platform on cytosolic bacteria. Nat. Commun. 2020, 11, 3276. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Q.; Rikihisa, Y. Ehrlichia Chaffeensis and Anaplasma Phagocytophilum Lack Genes for Lipid a Biosynthesis and Incorporate Cholesterol for Their Survival. Infect. Immun. 2003, 71, 5324–5331. [Google Scholar] [CrossRef] [PubMed]
- Amano, K.; Tamura, A.; Ohashi, N.; Urakami, H.; Kaya, S.; Fukushi, K. Deficiency of Peptidoglycan and Lipopolysaccharide Components in Rickettsia-Tsutsugamushi. Infect. Immun. 1987, 55, 2290–2292. [Google Scholar] [CrossRef]
- Min, C.-K.; Yang, J.-S.; Kim, S.; Choi, M.-S.; Kim, I.-S.; Cho, N.-H. Genome-Based Construction of the Metabolic Pathways of Orientia Tsutsugamushi and Comparative Analysis within the Rickettsiales Order. Compar. Funct. Genom. 2008, 2008, 623145. [Google Scholar] [CrossRef]
- Johns, J.L.; MacNamara, K.C.; Walker, N.J.; Winslow, G.M.; Borjesson, D.L. Infection with Anaplasma Phagocytophilum Induces Multilineage Alterations in Hematopoietic Progenitor Cells and Peripheral Blood Cells. Infect. Immun. 2009, 77, 4070–4080. [Google Scholar] [CrossRef]
- Rikihisa, Y. Molecular Events Involved in Cellular Invasion by Ehrlichia Chaffeensis and Anaplasma Phagocytophilum. Vet. Parasitol. 2010, 167, 155–166. [Google Scholar] [CrossRef]
- Herron, M.J.; Ericson, M.E.; Kurtti, T.J.; Munderloh, U.G. The Interactions of Anaplasma Phagocytophilum, Endothelial Cells, and Human Neutrophils. In Rickettsioses: From Genome to Proteome, Pathobiology, and Rickettsiae as an International Threat; Hechemy, K.E., Oteo, J.A., Raoult, D.A., Silverman, D.J., Blanco, J.R., Eds.; New York Acad Sciences: New York, NY, USA, 2005; Volume 1063, pp. 374–382. [Google Scholar] [CrossRef]
- Behrens, E.M.; Canna, S.W.; Slade, K.; Rao, S.; Kreiger, P.A.; Paessler, M.; Kambayashi, T.; Koretzky, G.A. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J. Clin. Investig. 2011, 121, 2264–2277. [Google Scholar] [CrossRef]
- Munderloh, U.G.; Lynch, M.J.; Herron, M.J.; Palmer, A.T.; Kurtti, T.J.; Nelson, R.D.; Goodman, J.L. Infection of Endothelial Cells with Anaplasma Marginale and A-Phagocytophilum. Vet. Microbiol. 2004, 101, 53–64. [Google Scholar] [CrossRef]
- Seidman, D.; Hebert, K.S.; Truchan, H.K.; Miller, D.P.; Tegels, B.K.; Marconi, R.T.; Carlyon, J.A. Essential Domains of Anaplasma Phagocytophilum Invasins Utilized to Infect Mammalian Host Cells. PLoS Pathog. 2015, 11, UNSP e1004669. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Sinha, M.; Luxon, B.A.; Yu, X.J. Survival Strategy of Obligately Intracellular Ehrlichia Chaffeensis: Novel Modulation of Immune Response and Host Cell Cycles. Infect. Immun. 2004, 72, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Moumene, A.; Meyer, D.F. Ehrlichia’s Molecular Tricks to Manipulate Their Host Cells. Microbes Infect. 2016, 18, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Stevenson, H.L.; Scott, M.J.; Ismail, N. Type I Interferon Contributes to Noncanonical Inflammasome Activation, Mediates Immunopathology, and Impairs Protective Immunity during Fatal Infection with Lipopolysaccharide-Negative Ehrlichiae. Am. J. Pathol. 2015, 185, 446–461. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A.; Kuriakose, J.A.; McBride, J.W. An Ehrlichia Chaffeensis Tandem Repeat Protein Interacts with Multiple Host Targets Involved in Cell Signaling, Transcriptional Regulation, and Vesicle Trafficking. Infect. Immun. 2009, 77, 1734–1745. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, X.; Wakeel, A.; Popov, V.L.; McBride, J.W. A variable-length PCR target protein of Ehrlichia chaffeensis contains major species-specific antibody epitopes in acidic serine-rich tandem repeats. Infect. Immun. 2008, 76, 1572–1580. [Google Scholar] [CrossRef][Green Version]
- Luo, T.; Zhang, X.; Nicholson, W.L.; Zhu, B.; McBride, J.W. Molecular characterization of antibody epitopes of Ehrlichia chaffeensis ankyrin protein 200 and tandem repeat protein 47 and evaluation of synthetic immunodeterminants for serodiagnosis of human monocytotropic ehrlichiosis. Clin. Vaccine Immunol. 2010, 17, 87–97. [Google Scholar] [CrossRef][Green Version]
- McBride, J.W.; Zhang, X.; Wakeel, A.; Kuriakose, J.A. Tyrosine-phosphorylated Ehrlichia chaffeensis and Ehrlichia canis tandem repeat orthologs contain a major continuous cross-reactive antibody epitope in lysine-rich repeats. Infect. Immun. 2011, 79, 3178–3187. [Google Scholar] [CrossRef]
- Kuriakose, J.A.; Zhang, X.; Luo, T.; McBride, J.W. Molecular basis of antibody mediated immunity against Ehrlichia chaffeensis involves species-specific linear epitopes in tandem repeat proteins. Microbes Infect. 2012, 14, 1054–1063. [Google Scholar] [CrossRef]
- Hackstadt, T. The Biology of Rickettsiae. Infect. Agents Dis.-Rev. Issues Comment. 1996, 5, 127–143. [Google Scholar]
- Clifton, D.R.; Rydkina, E.; Huyck, H.; Pryhuber, G.; Freeman, R.S.; Silverman, D.J.; Sahni, S.K. Expression and secretion of chemotactic cytokines IL-8 and MCP-1 by human endothelial cells after Rickettsia rickettsii infection: Regulation by nuclear transcription factor NF-kappaB. Int. J. Med. 2005, 295, 267–278. [Google Scholar]
- Groom, J.R.; Luster, A.D. CXCR3 Ligands: Redundant, Collaborative and Antagonistic Functions. Immunol. Cell Biol. 2011, 89, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.Y.; Hou, R.H.; Boyson, J.E.; Means, T.K.; Hess, C.; Olson, D.P.; Strominger, J.L.; Brenner, M.B.; Gumperz, J.E.; Wilson, S.B.; et al. CD1d-Restricted NKT Cells Express a Chemokine Receptor Profile Indicative of Th1-Type Inflammatory Homing Cells. J. Immunol. 2003, 171, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.K.; Rydkina, E. Host-Cell Interactions with Pathogenic Rickettsia Species. Future Microbiol. 2009, 4, 323–339. [Google Scholar] [CrossRef]
- Mazurier, F.; Fontanellas, A.; Salesse, S. A novel immunodeficient mouse model–RAG2 × common cytokine receptor gamma chain double mutants–requiring exogenous cytokine administration for human hematopoietic stem cell engraftment. J. Interferon Cytokine Res. 1999, 19, 533–541. [Google Scholar] [CrossRef]
- Turco, J.; Winkler, H.H. Gamma-Interferon-Induced Inhibition of the Growth of Rickettsia Prowazekii in Fibroblasts Cannot Be Explained by the Degradation of Tryptophan or Other Amino Acids. Infect. Immun. 1986, 53, 38–46. [Google Scholar] [CrossRef]
- Feng, H.M.; Walker, D.H. Mechanisms of Intracellular Killing of Rickettsia Conorii in Infected Human Endothelial Cells, Hepatocytes, and Macrophages. Infect. Immun. 2000, 68, 6729–6736. [Google Scholar] [CrossRef]
- Jordan, J.M.; Woods, M.E.; Soong, L.; Walker, D.H. Rickettsiae Stimulate Dendritic Cells through Toll-Like Receptor 4, Leading to Enhanced NK Cell Activation In Vivo. J. Infect. Dis. 2009, 199, 236–242. [Google Scholar] [CrossRef]
- Fang, R.; Ismail, N.; Walker, D.H. Contribution of NK Cells to the Innate Phase of Host Protection Against an Intracellular Bacterium Targeting Systemic Endothelium. Am. J. Pathol. 2012, 181, 185–195. [Google Scholar] [CrossRef]
- Osterloh, A.; Papp, S.; Moderzynski, K.; Kuehl, S.; Richardt, U.; Fleischer, B. Persisting Rickettsia Typhi Causes Fatal Central Nervous System Inflammation. Infect. Immun. 2016, 84, 1615–1632. [Google Scholar] [CrossRef]
- Kang, S.-J.; Jin, H.-M.; Cho, Y.-N.; Kim, S.E.; Kim, U.J.; Park, K.-H.; Jang, H.-C.; Jung, S.-I.; Kee, S.-J.; Park, Y.-W. Increased Level and Interferon-Gamma Production of Circulating Natural Killer Cells in Patients with Scrub Typhus. PLoS Negl. Trop. Dis. 2017, 11, e0005815. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.A.; Hauptmann, M.; Kolbaum, J.; Gharaibeh, M.H.; Neumann, M.; Glatzel, M.; Fleischer, B. Dissemination of Orientia tsutsugamushi and inflammatory responses in a murine model of scrub typhus. PLoS Negl. Trop. Dis. 2014, 8, e3064. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.I.; Stankovic, S.; Baxter, A.G. Raising the NKT Cell Family. Nat. Immunol. 2010, 11, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Paget, C.; Mallevaey, T.; Speak, A.O.; Torres, D.; Fontaine, J.; Sheehan, K.C.F.; Capron, M.; Ryffel, B.; Faveeuw, C.; de Moraes, M.L.; et al. Activation of Invariant NKT Cells by Toll-like Receptor 9-Stimulated Dendritic Cells Requires Type I Interferon and Charged Glycosphingolipids. Immunity 2007, 27, 597–609. [Google Scholar] [CrossRef] [PubMed]
- La Manna, M.P.; Torina, A.; Agnone, A.; Blanda, V.; Caracappa, S.; Alongi, A.; Di Marco, V.; Giudice, E.; Dieli, F.; Sireci, G. Detection of Natural Killer T Cells in Mice Infected with Rickettsia Conorii. Transbound. Emerg. Dis. 2013, 60, 80–85. [Google Scholar] [CrossRef]
- Radulovic, S.; Price, P.W.; Beier, M.S.; Gaywee, J.; Macaluso, J.A.; Azad, A. Rickettsia-Macrophage Interactions: Host Cell Responses to Rickettsia Akari and Rickettsia Typhi. Infect. Immun. 2002, 70, 2576–2582. [Google Scholar] [CrossRef][Green Version]
- Jordan, J.M.; Woods, M.E.; Olano, J.; Walker, D.H. The Absence of Toll-Like Receptor 4 Signaling in C3H/HeJ Mice Predisposes Them to Overwhelming Rickettsial Infection and Decreased Protective Th1 Responses. Infect. Immun. 2008, 76, 3717–3724. [Google Scholar] [CrossRef]
- Bechelli, J.; Smalley, C.; Zhao, X.; Judy, B.; Valdes, P.; Walker, D.H.; Fang, R. MyD88 Mediates Instructive Signaling in Dendritic Cells and Protective Inflammatory Response during Rickettsial Infection. Infect. Immun. 2016, 84, 883–893. [Google Scholar] [CrossRef]
- Papp, S.; Moderzynski, K.; Rauch, J.; Heine, L.; Kuehl, S.; Richardt, U.; Mueller, H.; Fleischer, B.; Osterloh, A. Liver Necrosis and Lethal Systemic Inflammation in a Murine Model of Rickettsia Typhi Infection: Role of Neutrophils, Macrophages and NK Cells. PLoS Negl. Trop. Dis. 2016, 10, e0004935. [Google Scholar] [CrossRef]
- Fang, R.; Ismail, N.; Soong, L.; Popov, V.L.; Whitworth, T.; Bouyer, D.H.; Walker, D.H. Differential Interaction of Dendritic Cells with Rickettsia Conorii: Impact on Host Susceptibility to Murine Spotted Fever Rickettsiosis. Infect. Immun. 2007, 75, 3112–3123. [Google Scholar] [CrossRef]
- Richert-Spuhler, L.E.; Lund, J.M. The Immune Fulcrum: Regulatory T Cells Tip the Balance Between Pro- and Anti-Inflammatory Outcomes upon Infection. In Regulatory T Cells in Health and Disease; Liston, A., Ed.; Elsevier Academic Press Inc: San Diego, CA, USA, 2015; Volume 136, pp. 217–243. [Google Scholar] [CrossRef]
- Shoda, L.K.; Palmer, G.H.; Florin-Christensen, J.; Florin-Christensen, M.; Godson, D.L.; Brown, W.C. Babesia bovis-stimulated macrophages express interleukin-1beta, interleukin-12, tumor necrosis factor alpha, and nitric oxide and inhibit parasite replication in vitro. Infect. Immun. 2000, 68, 5139–5145. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, G.; Magalhaes, K.G.; Belaunzaran, M.L.; Poncini, C.V.; Lammel, E.M.; Gonzalez Cappa, S.M.; Bozza, P.T.; Isola, E.L.D. Lipids from Attenuated and Virulent Babesia Bovis Strains Induce Differential TLR2-Mediated Macrophage Activation. Mol. Immunol. 2010, 47, 747–755. [Google Scholar] [CrossRef]
- Terkawi, M.A.; Zhang, G.; Jia, H.; Aboge, G.; Goo, Y.K.; Nishikawa, Y.; Yokoyama, N.; Igarashi, I.; Kawazu, S.I.; Fujisaki, K.; et al. C3 Contributes to the Cross-Protective Immunity Induced by Babesia Gibsoni Phosphoriboprotein P0 against a Lethal B-Rodhaini Infection. Parasite Immunol. 2008, 30, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.G.; Johnson, W.C.; Brown, W.C.; Goff, W.L. Differential Response of Splenic Monocytes and DC from Cattle to Microbial Stimulation with Mycobacterium Bovis BCG and Babesia Bovis Merozoites. Vet. Immunol. Immunopathol. 2007, 115, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.A.; Yan, H.; Bastos, R.G.; Johnson, W.C.; Gavin, P.R.; Allen, A.J.; Barrington, G.M.; Herrmann-Hoesing, L.M.; Knowles, D.P.; Goff, W.L. Dynamics of Bovine Spleen Cell Populations during the Acute Response to Babesia Bovis Infection: An Immunohistological Study. Parasite Immunol. 2011, 33, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Goff, W.L.; Johnson, W.C.; Horn, R.H.; Barrington, G.M.; Knowles, D.P. The Innate Immune Response in Calves to Boophilus Microplus Tick Transmitted Babesia Bovis Involves Type-1 Cytokine Induction and NK-like Cells in the Spleen. Parasite Immunol. 2003, 25, 185–188. [Google Scholar] [CrossRef]
- Morel, N.; Mastropaolo, M.; de Echaide, S.T.; Signorini, M.L.; Mangold, A.J. Risks of cattle babesiosis (Babesia bovis) outbreaks in a semi-arid region of Argentina. Prev. Vet. Med. 2019, 170, 104747. [Google Scholar] [CrossRef]
- Goff, W.L.; Johnson, W.C.; Tuo, W.; Valdez, R.A.; Parish, S.M.; Barrington, G.M.; Davis, W.C. Age-related innate immune response in calves to Babesia bovis involves IL-12 induction and IL-10 modulation. Ann. N. Y. Acad. Sci. 2002, 969, 164–168. [Google Scholar] [CrossRef]
- Ahmed, J.S.; Glass, E.J.; Salih, D.A.; Seitzer, U. Innate immunity to tropical theileriosis. Innate Immun. 2008, 14, 5–12. [Google Scholar] [CrossRef]
- Dobbelaere, D.A.; Rottenberg, S. Theileria-induced leukocyte transformation. Curr. Opin. Microbiol. 2003, 6, 377–382. [Google Scholar] [CrossRef]
- Dobbelaere, D.A.; Küenzi, P. The strategies of the Theileria parasite: A new twist in host-pathogen interactions. Curr. Opin. Immunol. 2004, 16, 524–530. [Google Scholar] [CrossRef]
- Campbell, J.D.M.; Howie, S.E.M.; Odling, K.A.; Glass, E.J. Theileria annulata induces aberrant T cell activation in vitro and in vivo. Clin. Exp. Immunol. 1995, 99, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Glass, E.J.; Preston, P.M.; Springbett, A.; Craigmile, S.; Kirvar, E.; Wilkie, G.; Brown, C.D. Bos taurus and Bos indicus (Sahiwal) calves respond differently to infection with Theileria annulata and produce markedly different levels of acute phase proteins. Int. J. Parasitol. 2005, 35, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Sager, H.; Brunschwiler, C.; Jungi, T.W. Interferon production by Theileria annulata-transformed cell lines is restricted to the beta family. Parasite Immunol. 1998, 20, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.J.; Campbell, J.D.; Russell, G.C.; Hopkins, J.; Glass, E.J. T cell activation by Theileria annulata-infected macrophages correlates with cytokine production. Clin. Exp. Immunol. 1995, 102, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.E.; Abraham, A.; Sakyi, L.J.; Brown, C.G.; Preston, P.M. Nitric oxide inhibits establishment of macroschizont-infected cell lines and is produced by macrophages of calves undergoing bovine tropical theileriosis or East Coast fever. Parasite Immunol. 1995, 17, 91–102. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Dewangan, P.; Panigrahi, M.; Kumar, A.; Saravanan, B.C.; Ghosh, S.; Asaf, V.N.M.; Parida, S.; Gaur, G.K.; Sharma, D.; Bhushan, B. The mRNA expression of immune-related genes in crossbred and Tharparkar cattle in response to in vitro infection with Theileria annulata. Mol. Biol. Rep. 2015, 42, 1247–1255. [Google Scholar] [CrossRef]
- Yin, F.; Liu, J.; Gao, S.; Liu, A.; Zhao, S.; Li, S.; Wang, J.; Li, Y.; Luo, J.; Guan, G.; et al. Exploring the TLR and NLR signaling pathway relevant molecules induced by the Theileria annulata infection in calves. Parasitol. Res. 2018, 117, 3269–3276. [Google Scholar] [CrossRef]
- Vrentas, C.E.; Schaut, R.G.; Boggiatto, P.M.; Olsen, S.C.; Sutterwala, F.S.; Moayeri, M. Inflammasomes in livestock and wildlife: Insights into the intersection of pathogens and natural host species. Vet. Immunol. Immunopathol. 2018, 201, 49–56. [Google Scholar] [CrossRef]
- McCoy, K.D.; Leger, E.; Dietrich, M. Host Specialization in Ticks and Transmission of Tick-Borne Diseases: A Review. Front. Cell. Infect. Microbiol. 2013, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Wikel, S. Ticks and Tick-Borne Pathogens at the Cutaneous Interface: Host Defenses, Tick Countermeasures, and a Suitable Environment for Pathogen Establishment. Front. Microbiol. 2013, 4, 337. [Google Scholar] [CrossRef]
- De la Fuente, J.; Villar, M.; Cabezas-Cruz, A.; Estrada-Peña, A.; Ayllón, N.; Alberdi, P. Tick-Host-Pathogen Interactions: Conflict and Cooperation. PLoS Pathog. 2016, 12, e1005488. [Google Scholar] [CrossRef] [PubMed]
- Rikihisa, Y. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin. Microbiol. Rev. 2011, 24, 469–489. [Google Scholar] [CrossRef]
- Rejmanek, D.; Foley, P.; Barbet, A.; Foley, J. Evolution of antigen variation in the tick-borne pathogen Anaplasma phagocytophilum. Mol. Biol. Evol. 2012, 29, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Jaarsma, R.I.; Sprong, H.; Takumi, K.; Kazimirova, M.; Silaghi, C.; Mysterud, A.; Rudolf, I.; Beck, R.; Földvári, G.; Tomassone, L.; et al. Anaplasma phagocytophilum evolves in geographical and biotic niches of vertebrates and ticks. Parasit. Vectors 2019, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
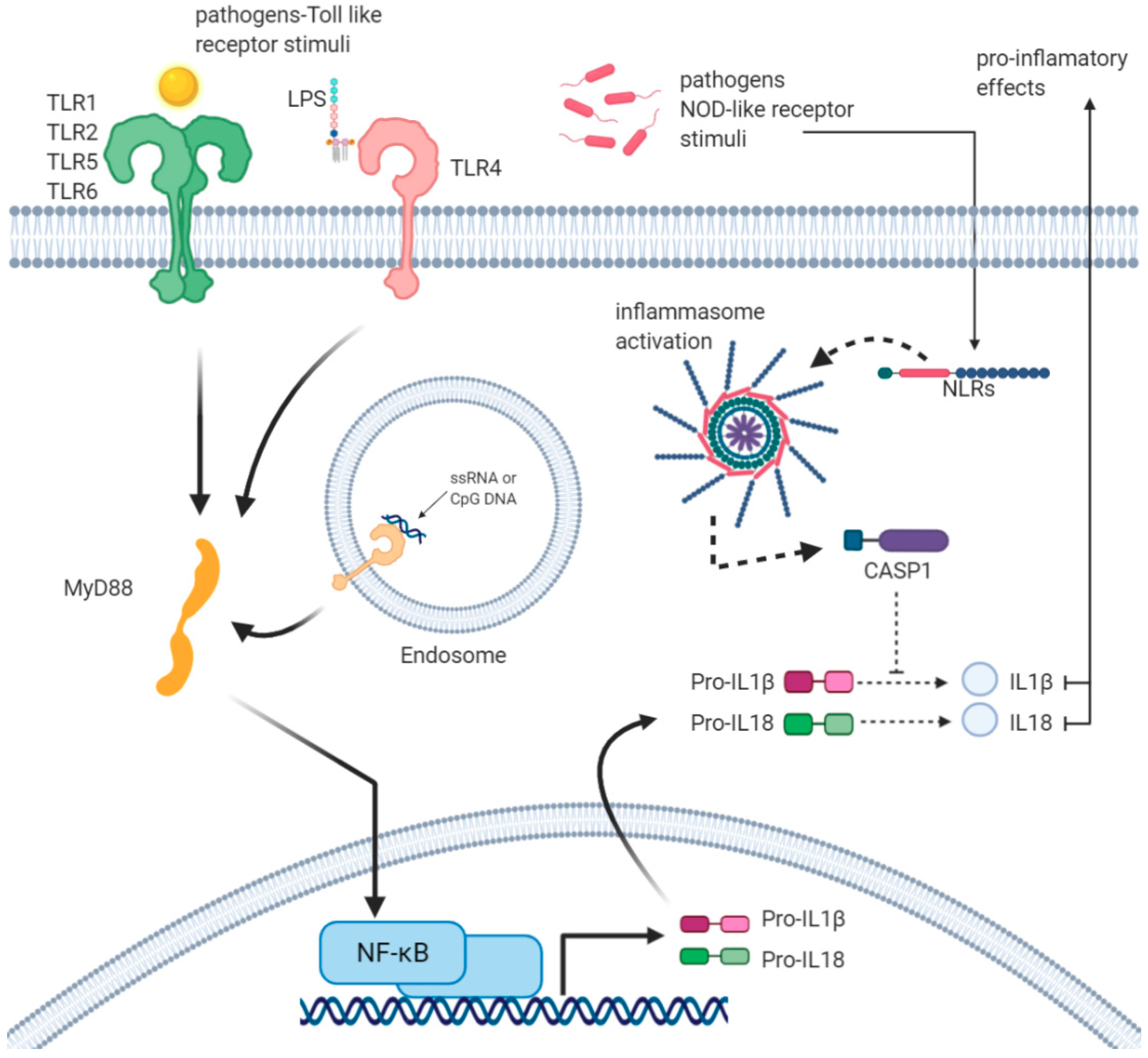
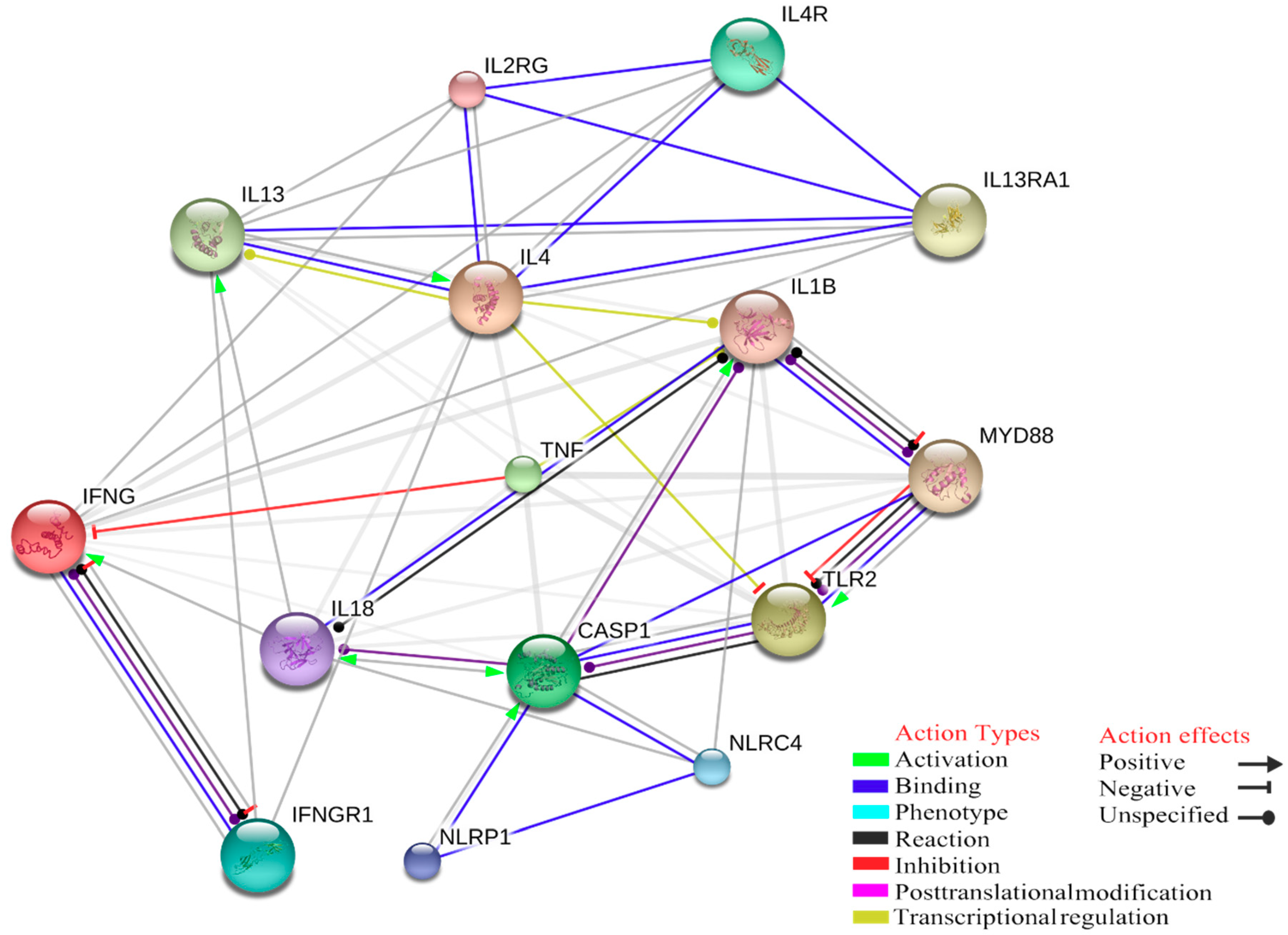
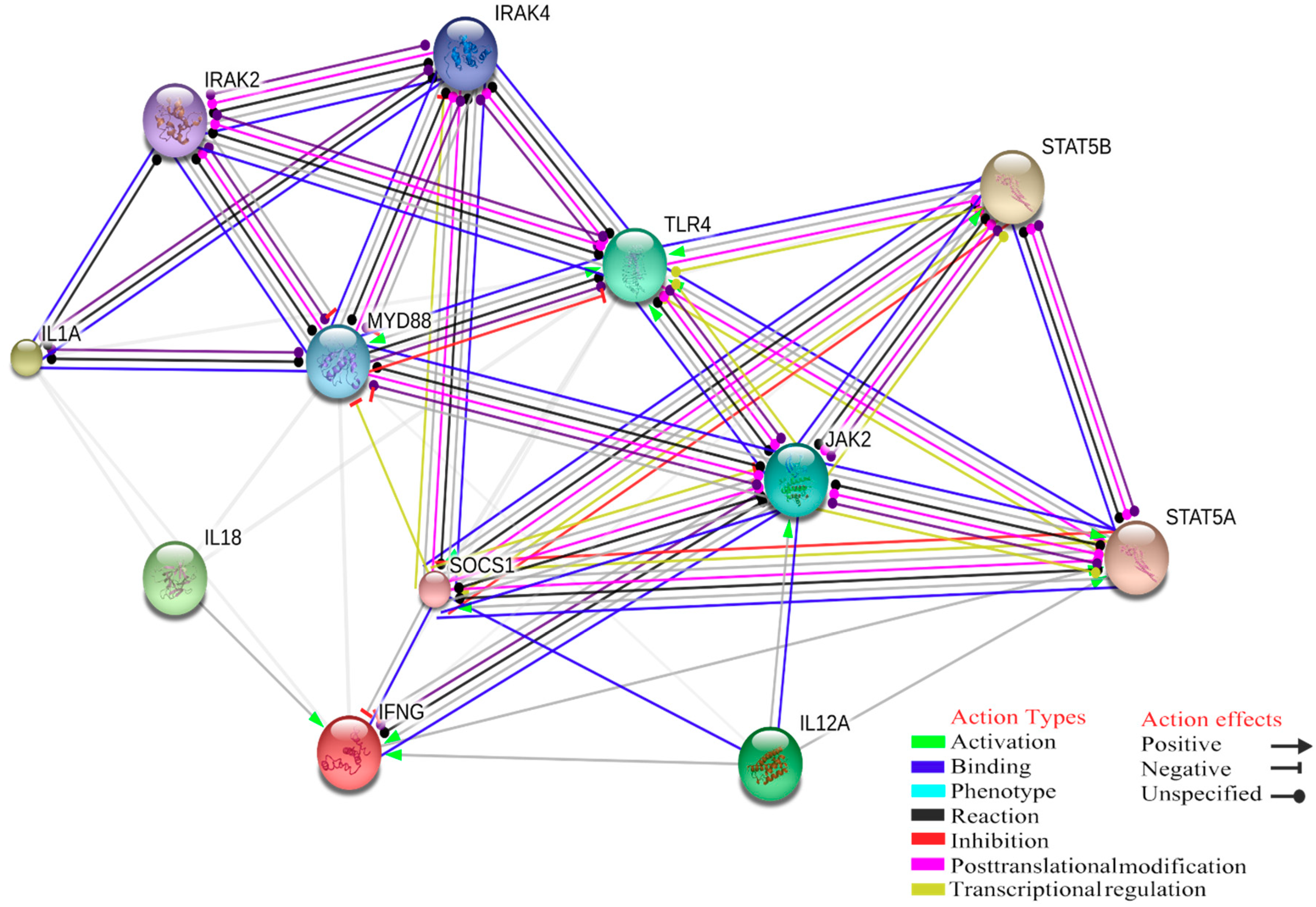
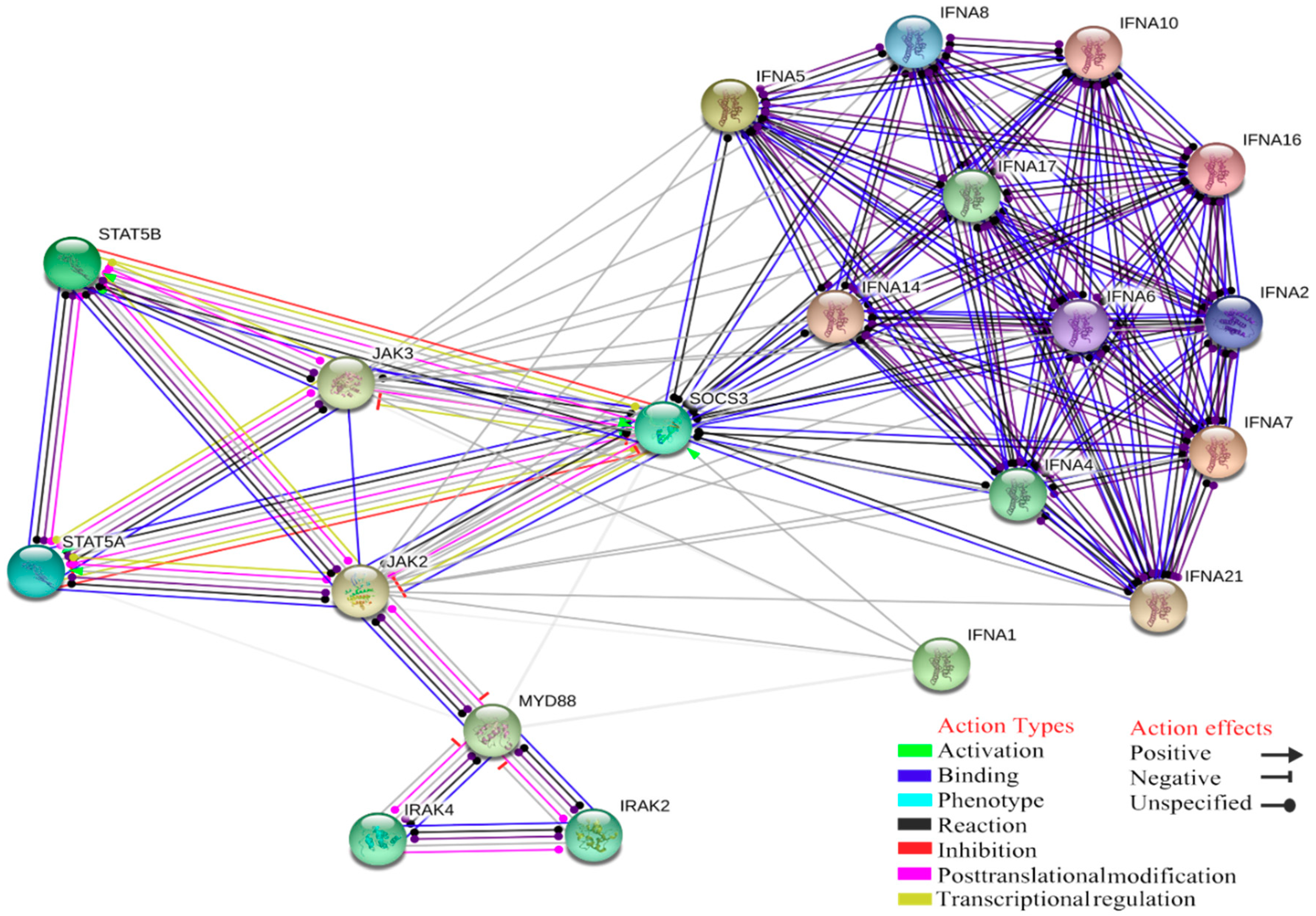
| GO ID | GO Terms | Number of Genes Associated with GO Terms |
|---|---|---|
| GO:0072643 | IFN-γ secretion | 483 |
| GO:0061702 | inflammasome complex | 463 |
| GO:0004998 | transferrin receptor activity | 344 |
| GO:0042116 | macrophage activation | 897 |
| GO:0002224 | toll-like receptor signalling pathway | 1371 |
| Total number in all GO terms | 2388 | |
| GO ID | GO Terms | Number of Gene Products Associated with GO Terms |
|---|---|---|
| GO:0030101 | natural killer cell activation | 122 |
| GO:1902713 | regulation of interferon-gamma secretion | 35 |
| GO:0072643 | interferon-gamma secretion | 44 |
| GO:0051775 | Response to redox state | 32 |
| GO:0006568 | tryptophan metabolic process | 52 |
| GO:0004517 | nitric-oxide synthase activity | 109 |
| GO:1905076 | regulation of interleukin-17 secretion | 24 |
| Total number in all GO terms | 202 | |
| Biological Process | No. of Genes | Genes Involved | Raw p-Value | FDR |
|---|---|---|---|---|
| regulation of toll-like receptor 3 signaling pathway | 9 | TIRAP, F2RL1, WDFY1, FLOT1, PELI1, CAV1, TNFAIP3, Ptpn22, UBQLN1 | 6.14 × 10−05 | 7.16 × 10−04 |
| regulation of interferon-gamma secretion | 12 | Nr1h4, HMHB1, Cd160, Cd2, ZC3H12A, Ptpn22, App, Il36rn, CD244, Rasgrp1, ABL1, Lgals9 | 3.74 × 10−16 | 1.28 × 10−14 |
| response to triacyl bacterial lipopeptide | 3 | Tlr1, TLR2, Cd14 | 6.14 × 10−05 | 7.15 × 10−04 |
| type I interferon production | 5× | Trex1, myd88, Irf3, Irf7, TBK1 | 1.21 × 10−06 | 1.83 × 10−05 |
| MyD88-independent toll-like receptor signaling pathway | 32 | Cd300lf, CD14, TICAM2, PRKCE, BIRC2, TLR3, IKBKB, UBB, TANK, tlr6, Irf3, UBE2D2, CHUK, TRAF3, Irf7, UBC, Tnip3, UBE2D1, CASP8, UBA52, UBE2D3, IKBKE, IKBKG, TLR4, TBK1, RPS27A, FADD, TICAM1, LY96, RIPK1, RAB11FIP2, BIRC3 | 5.93 × 10−42 | 5.69 × 10−40 |
| toll-like receptor 2 signaling pathway | 5 | TLR2, IRAK1, RIPK2, TNIP2, PIK3AP1 | 1.60 × 10−07 | 2.73 × 10−06 |
| positive regulation of antigen processing and presentation of peptide antigen via MHC class II | 2 | TREM2, PYCARD | 1.27 × 10−03 | 1.14 × 10−02 |
| regulation of MyD88-dependent toll-like receptor signaling pathway | 4 | CD300LF, IRF7, CD300A, IRF1 | 1.27 × 10−03 | 1.14 × 10−02 |
| macrophage apoptotic process | 2 | IRF3, CTSL | 1.27 × 10−03 | 1.14 × 10−02 |
| regulation of interleukin-4 biosynthetic process | 2 | Cd86, IRF-4 | 1.27 × 10−03 | 1.14 × 10−02 |
| macrophage activation involved in immune response | 13 | TREM2, TREX1, PRKCE, IL33, TYROBP, Syk, DYSF, GRN, SUCNR1, TICAM1, LBP, SBNO2, HAVCR2 | 1.06 × 10−17 | 3.97 × 10−16 |
| regulation of interleukin-18 production | 5 | TLR9, TLR2, IL10, NLRP9, Cd84 | 4.91 × 10−07 | 7.86 × 10−06 |
| detection of diacyl bacterial lipopeptide | 2 | TLR6, TLR2 | 1.27 × 10−03 | 1.12 × 10−02 |
| interferon-gamma secretion | 9 | TCIRG1, VTCN1, LILRB1, ISG15, TRIM27, BTN3A2, GATA3, F2RL1, BTN3A1 | 1.27 × 10−12 | 3.46 × 10−11 |
| interleukin-10 secretion | 2 | ISG15, F2RL1 | 1.27 × 10−03 | 1.12 × 10−02 |
| regulation of interleukin-1 beta biosynthetic process | 6 | JAK2, AGER, IFNG, App, TYROBP, AZU1 | 1.54 × 10−08 | 2.97 × 10−07 |
| interleukin-1 beta secretion | 7 | TLR6, AIM2, CD36, TLR4, NLRC4, F2RL1, TMEM106A | 1.45 × 10−09 | 3.09 × 10−08 |
| positive regulation of interleukin-18 production | 3 | TLR9, TLR2, NLRP9 | 1.06 × 10−04 | 1.19 × 10−03 |
| regulation of interleukin-12 production | 27 | ARRB2, TLR9, AGER, FOXP1, TLR3, IRAK3, IFNG, CD36, LILRB1, TLR2, TRAF6, IL12B, SYK, RIPK2, HSPD1, THBS1, SCIMP, TLR4, RELA, IL10, cd40, HMGB1, IRF1, TLR8, Lgals9, SLAMF1, TIRAP | 4.05 × 10−29 | 2.62 × 10−27 |
| positive regulation of granzyme B production | 2 | PTPN22, CD244 | 2.10 × 10−03 | 1.75 × 10−02 |
| regulation of gamma-delta T cell differentiation | 2 | SYK, PTPRC | 3.13 × 10−03 | 2.45 × 10−02 |
| iron ion transport | 7 | TCIRG1, TFR2, TFRC, ATP6V1G2, LTF, TF, SLC11A1 | 1.53 × 10−04 | 1.66 × 10−03 |
| regulation of inflammatory response to wounding | 2 | AGER, Grn | 3.13 × 10−03 | 2.43 × 10−02 |
| positive regulation of type I interferon-mediated signaling pathway | 6 | IRF3, IRF7, WNT5A, IKBKE, TBK1, FADD | 2.26 × 10−07 | 3.79 × 10−06 |
| regulation of epithelial cell apoptotic process | 17 | ARRB2, JAK2, IL6, Il4, CD160, PDPK1, FGB, IL13, Tnf, TNFAIP3, FGA, THBS1, cd40, GATA3, TNIP2, FGG, ABL1 | 6.20 × 10−14 | 1.88 × 10−12 |
| negative regulation of inflammatory response to antigenic stimulus | 4 | FCGR2B, IL12B, NLRP6, IL10 | 9.48 × 10−05 | 1.07 × 10−03 |
| negative regulation of interferon-beta production | 4 | PYCARD, LILRB1, CACTIN, PTPRS | 1.20 × 10−04 | 1.33 × 10−03 |
| cellular response to interferon-beta | 6 | TREX1, TLR3, AIM2, IFNB, IRF1, ACOD1 | 2.80 × 10−06 | 4.02 × 10−05 |
| interleukin-12-mediated signaling pathway | 6 | JAK2, IFNG, IL12B, MIF, IL10, IL12A | 1.13 × 10−04 | 1.24 × 10−03 |
| interferon-gamma-mediated signaling pathway | 8 | JAK2, IFNGR1, IRF4, IFNG, IRF3, Ifngr2, b2m, IRF1 | 3.02 × 10−06 | 4.30 × 10−05 |
| macrophage activation involved in immune response | 50 | Csf2, SNCA, JAK2, AGER, TREM2, TREX1, ITGB2, PRKCE, FOXP1, TLR3, C1QA, TRPV1, MAPT, IFNGR1, JUN, IL33, ITGAM, IFNG, TLR6, CX3CL1, TLR2, TLR7, CRTC3, CLU, IL13, App, SLC7A2, CD93, TYROBP, Tnf, SYK, DYSF, EDN2, FPR2, SUCNR1, AIF1, JMJD6, GRN, NAMPT, TLR4, AZU1, TICAM1, Ifngr2, TLR1, LBP, TLR8, TMEM106A, SLC11A1, SBNO2, HAVCR2, C5AR1 | 1.06 × 10−17 | 3.97 × 10−16 |
| regulation of macrophage cytokine production | 7 | Irak3, CD36, LILRB1, WNT5A, CD74, TLR4, RTN4 | 6.92 × 10−08 | 1.23 × 10−06 |
| macrophage chemotaxis | 5 | CX3CL1, SFTPD, AZU1, EDN2, CCL3 | 6.62 × 10−06 | 8.87 × 10−05 |
| negative regulation of macrophage apoptotic process | 2 | CLU, LDLR | 5.72 × 10−03 | 4.13 × 10−02 |
| Total genes | 166 | |||
| immune system process | 28 | |||
| regulation of immune system process | 18 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torina, A.; Villari, S.; Blanda, V.; Vullo, S.; La Manna, M.P.; Shekarkar Azgomi, M.; Di Liberto, D.; de la Fuente, J.; Sireci, G. Innate Immune Response to Tick-Borne Pathogens: Cellular and Molecular Mechanisms Induced in the Hosts. Int. J. Mol. Sci. 2020, 21, 5437. https://doi.org/10.3390/ijms21155437
Torina A, Villari S, Blanda V, Vullo S, La Manna MP, Shekarkar Azgomi M, Di Liberto D, de la Fuente J, Sireci G. Innate Immune Response to Tick-Borne Pathogens: Cellular and Molecular Mechanisms Induced in the Hosts. International Journal of Molecular Sciences. 2020; 21(15):5437. https://doi.org/10.3390/ijms21155437
Chicago/Turabian StyleTorina, Alessandra, Sara Villari, Valeria Blanda, Stefano Vullo, Marco Pio La Manna, Mojtaba Shekarkar Azgomi, Diana Di Liberto, José de la Fuente, and Guido Sireci. 2020. "Innate Immune Response to Tick-Borne Pathogens: Cellular and Molecular Mechanisms Induced in the Hosts" International Journal of Molecular Sciences 21, no. 15: 5437. https://doi.org/10.3390/ijms21155437
APA StyleTorina, A., Villari, S., Blanda, V., Vullo, S., La Manna, M. P., Shekarkar Azgomi, M., Di Liberto, D., de la Fuente, J., & Sireci, G. (2020). Innate Immune Response to Tick-Borne Pathogens: Cellular and Molecular Mechanisms Induced in the Hosts. International Journal of Molecular Sciences, 21(15), 5437. https://doi.org/10.3390/ijms21155437








