Ginsenoside Rh2 Ameliorates Atopic Dermatitis in NC/Nga Mice by Suppressing NF-kappaB-Mediated Thymic Stromal Lymphopoietin Expression and T Helper Type 2 Differentiation
Abstract
1. Introduction
2. Results
2.1. Rh2 Attenuated Inflammatory Cytokines in Stimulated NHKs
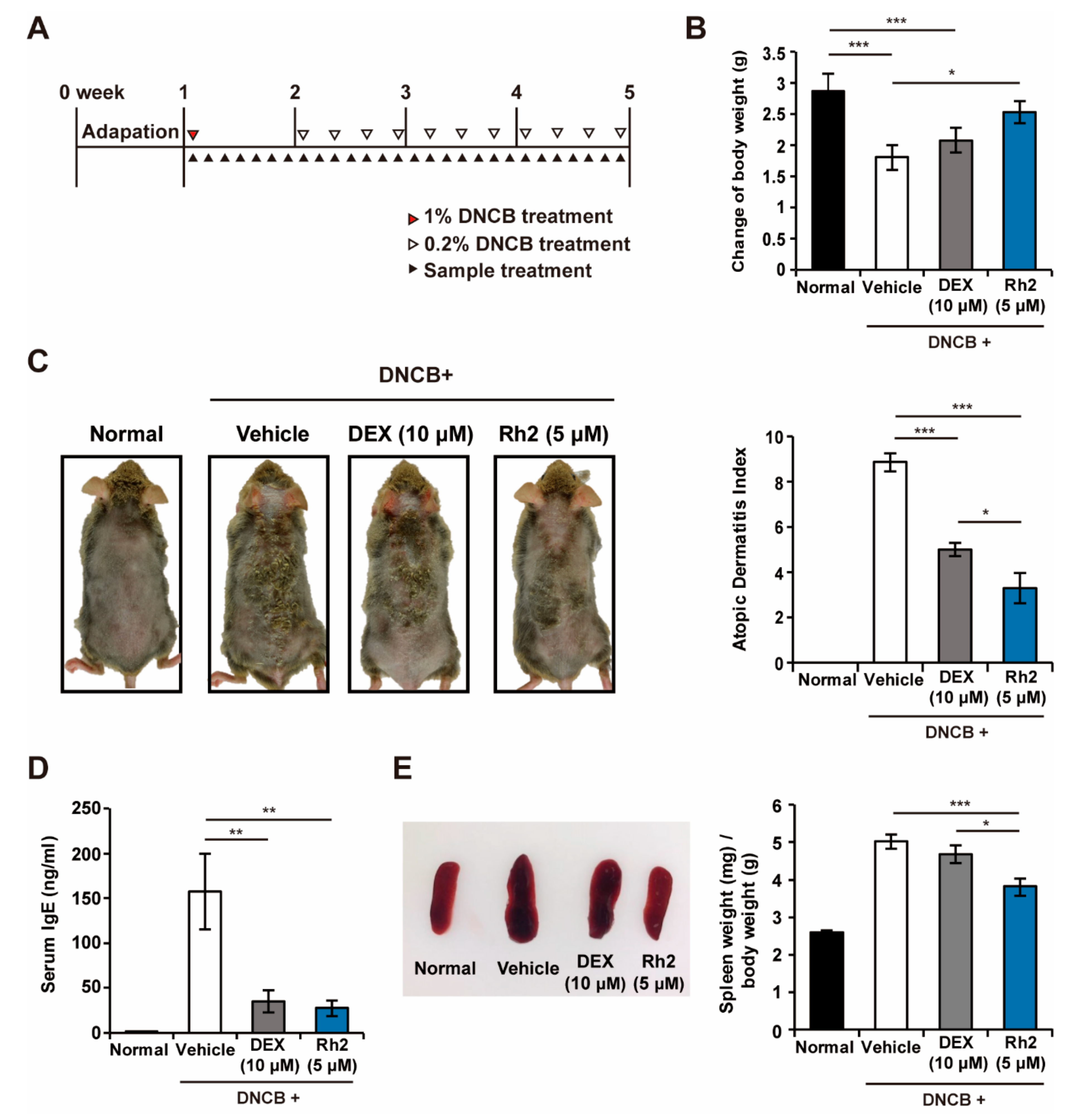
2.2. Rh2 Safely Ameliorated DNCB-Induced AD-Like Skin Inflammation
2.3. Rh2 Suppressed Epidermis Thickening and Mast Cell and Eosinophil Infiltration
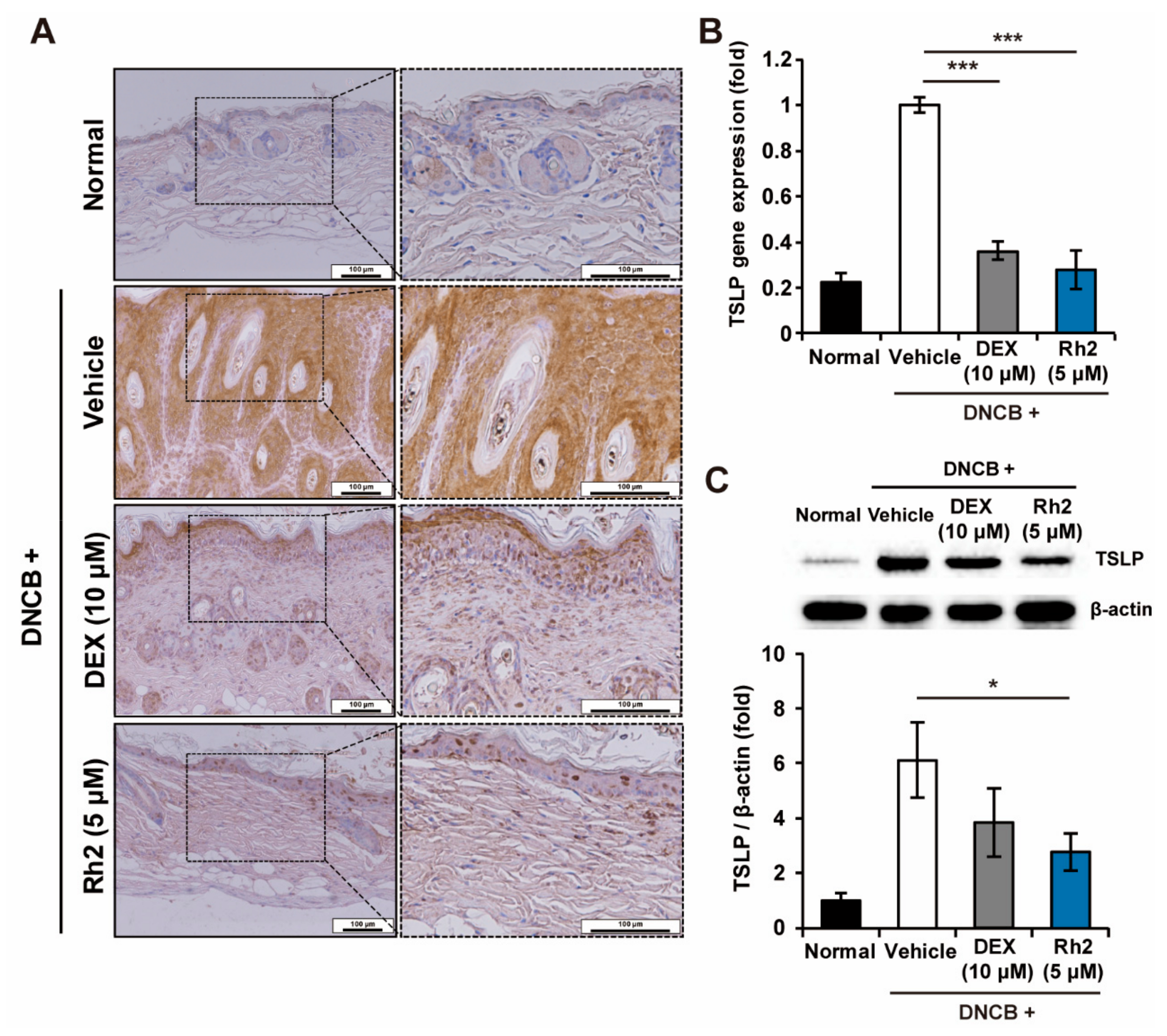
2.4. Rh2 Inhibited the Expression of TSLP in Keratinocytes of an AD Mouse Model
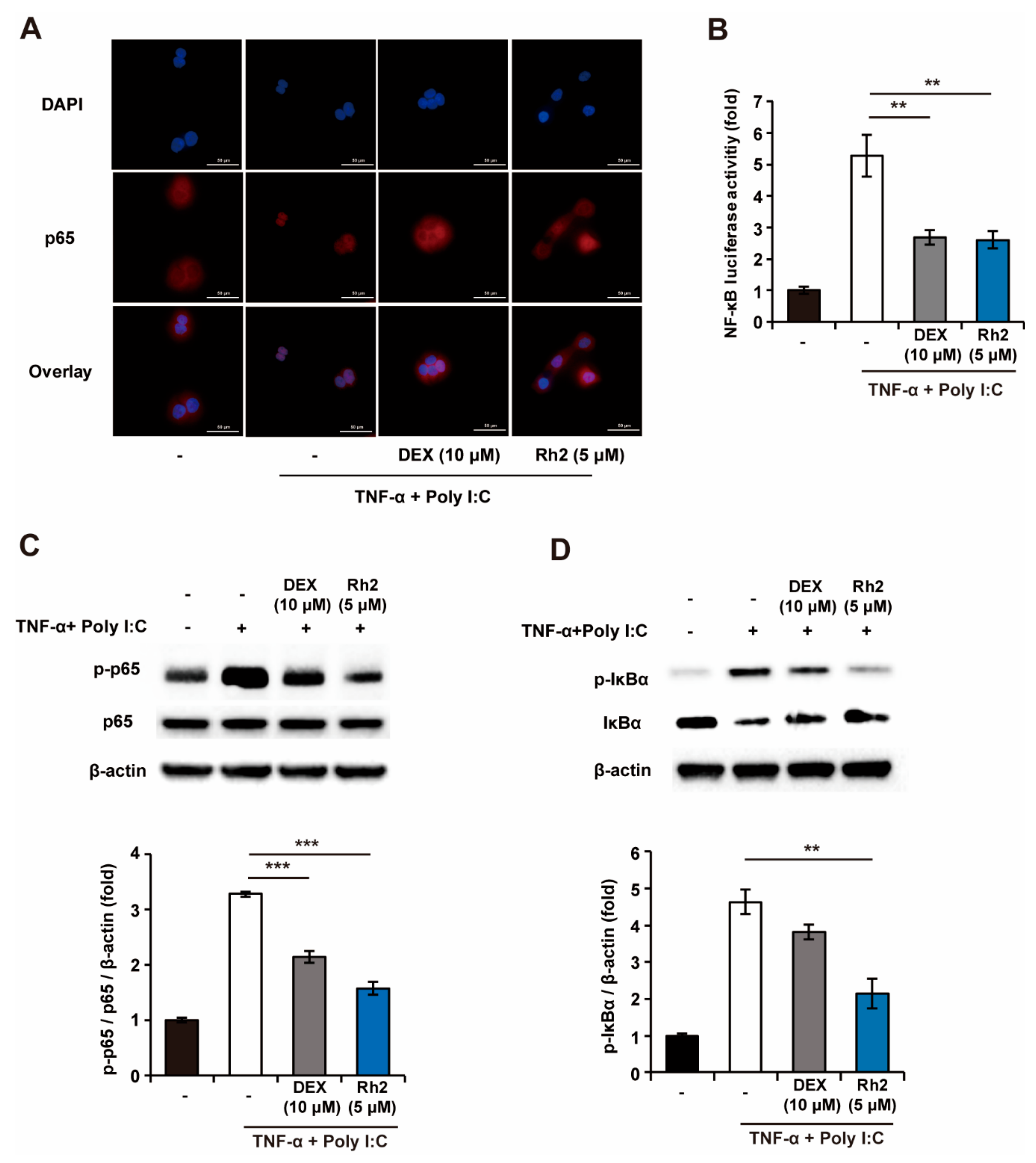
2.5. Rh2 Blocked the NF-κB Pathway in Stimulated NHKs
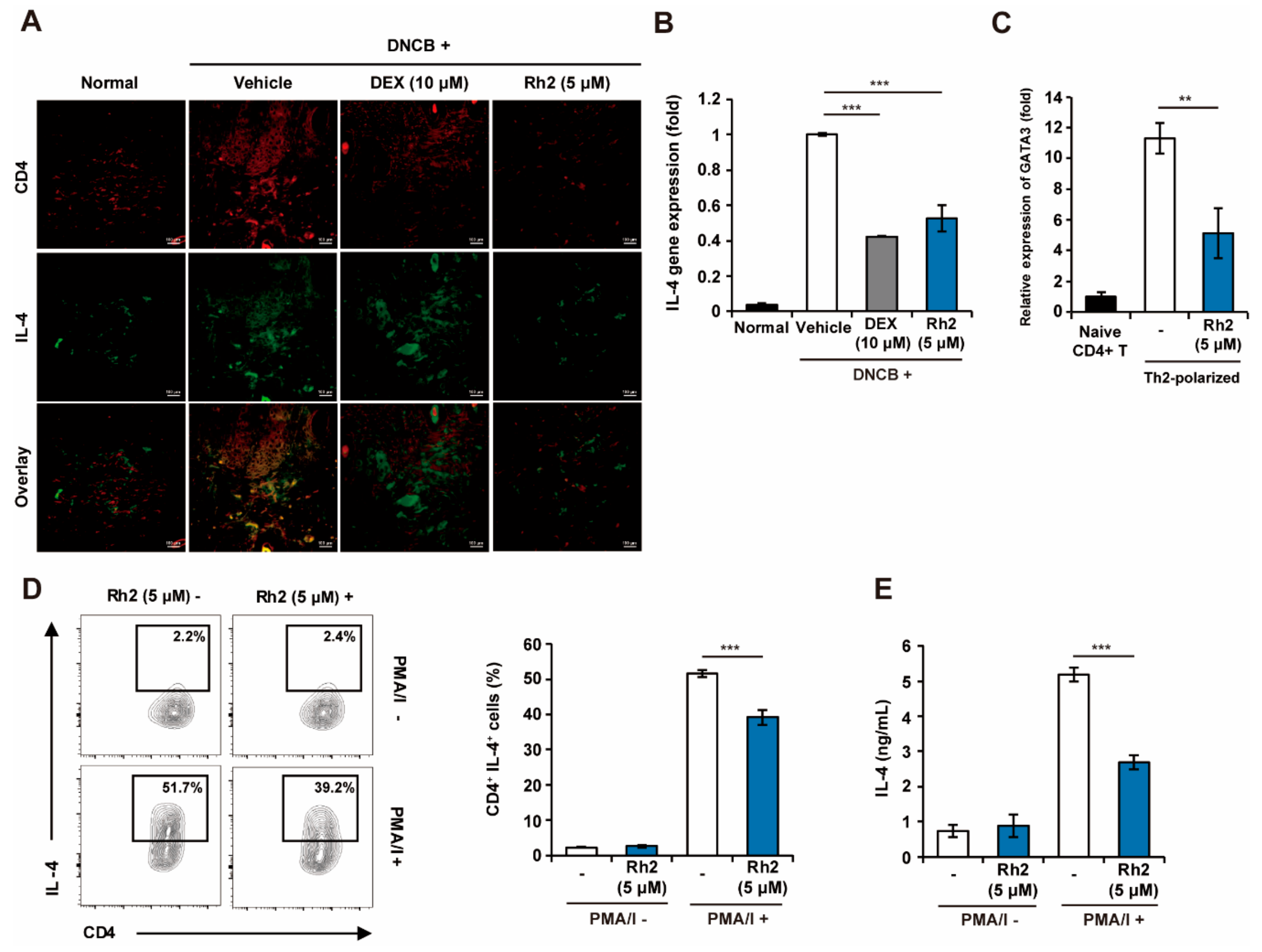
2.6. Rh2 Inhibited Th2 Differentiation and Effector Function
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Cell Viability Assay
4.3. Quantitative Real-Time Reverse Transcription PCR
4.4. Proteome Profiler Array
4.5. Western Blot Analysis
4.6. Animal Models and Evaluation of AD Index
4.7. Histological Analysis
4.8. Immunohistochemistry and Immunofluorescence
4.9. Immunocytochemistry
4.10. Luciferase Assay
4.11. T-cell Differentiation
4.12. ELISA
4.13. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| TSLP | Thymic stromal lymphopoietin |
| DEX | Dexamethasone |
| ELISA | Enzyme-linked immunosorbent assay |
| qRT-PCR | Quantitative real-time reverse transcription PCR |
| NHKs | Normal human keratinocytes |
| DNCB | 2,4-dinitrochlorobenzene |
| DMSO | Dimethylsulfoxide |
| TNF-a | Tumor necrosis factor-alpha |
| Poly I:C | Polyinosinic:polycytidylic acid |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| Th2 | T helper type 2 |
| PMA/I | Phorbol-myristate acetate and inomycin |
References
- Zheng, T.; Yu, J.; Oh, M.H.; Zhu, Z. The atopic march: Progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol. Res. 2011, 3, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Bantz, S.K.; Zhu, Z.; Zheng, T. The Atopic March: Prgression from AD to Allergic Rhinitis and Asthma. J. Clin. Cell Immunol. 2014, 5, 202. [Google Scholar] [PubMed]
- Esche, C.; de Benedetto, A.; Beck, L.A. Keratinocytes in Atopic dermatitis: Inflammatory signals. Curr. Allergy Asthma Rep. 2004, 4, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C.; Madonna, S.; Gisondi, P.; Girolomoni, G. The Interplay Between Keratinocytes and Immune Cells in the Pathogenesis of Psoriasis. Front. Immunol. 2018, 9, 1549. [Google Scholar] [CrossRef]
- Lee, C.-G.; Kwon, H.-K.; Kang, H.; Kim, Y.; Nam, J.H.; Won, Y.H.; Park, S.; Kim, T.; Kang, K.; Rudra, D.; et al. Ets1 suppresses atopic dermatitis by suppressing pathogenic T cell responses. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Asahina, R.; Maeda, S. A review of the roles of keratinocyte-derived cytokines and chemokines in the pathogenesis of atopic dermatitis in humans and dogs. Vet. Dermatol. 2017, 28, 16-e5. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, B. Functions of thymic stromal lymphopoietin in immunity and disease. Immunol. Res. 2012, 52, 211–223. [Google Scholar] [CrossRef]
- Leung, D.; Boguniewicz, M.; Howell, M.; Nomura, I.; Hamid, Q. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef]
- Ziegler, S.F.; Roan, F.; Bell, B.D.; Stoklasek, T.A.; Kitajima, M.; Han, H. The biology of thymic stromal lymphopoietin (TSLP). Adv. Pharmacol. 2013, 66, 129–155. [Google Scholar]
- Biedermann, T.; Skabytska, Y.; Kaesler, S.; Volz, T. Regulation of T Cell Immunity in Atopic Dermatitis by Microbes: The Yin and Yang of Cutaneous Inflammation. Front. Immunol. 2015, 6, 353. [Google Scholar] [CrossRef]
- Sharma, G.; Im, S.-H. Probiotics as a Potential Immunomodulating Pharmabiotics in Allergic Diseases: Current Status and Future Prospects. Allergy Asthma Immunol. Res. 2018, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Thé, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G. Effects of Saponins on Lipid Metabolism: A Review of Potential Health Benefits in the Treatment of Obesity. Molecules 2016, 21, 1404. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef]
- Leung, K.; Wong, A. Pharmacology of ginsenosides: A literature review. Chin. Med. 2010, 5, 20. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef]
- Kwon, H.-J.; Lee, H.; Choi, G.-E.; Kwon, S.J.; Song, A.Y.; Kim, S.J.; Choi, W.S.; Hwang, S.-H.; Kim, S.C.; Kim, H.S. Ginsenoside F1 Promotes Cytotoxic Activity of NK Cells via Insulin-Like Growth Factor-1-Dependent Mechanism. Front. Immunol. 2018, 9, 2785. [Google Scholar] [CrossRef]
- Jia, L.; Zhao, Y. Current Evaluation of the Millennium Phytomedicine- Ginseng (I): Etymology, Pharmacognosy, Phytochemistry, Market and Regulations. Curr. Med. Chem. 2009, 16, 2475–2484. [Google Scholar] [CrossRef]
- Sohn, E.-H.; Jang, S.-A.; Lee, C.-H.; Jang, K.-H.; Kang, S.-C.; Park, H.-J.; Pyo, S.-N. Effects of Korean Red Ginseng Extract for the Treatment of Atopic Dermatitis-Like Skin Lesions in Mice. J. Ginseng Res. 2011, 35, 479–486. [Google Scholar] [CrossRef]
- Kee, J.-Y.; Jeon, Y.-D.; Kim, D.-S.; Han, Y.-H.; Park, J.; Youn, D.-H.; Kim, S.-J.; Ahn, K.S.; Um, J.-Y.; Hong, S.-H. Korean Red Ginseng improves atopic dermatitis-like skin lesions by suppressing expression of proinflammatory cytokines and chemokines in vivo and in vitro. J. Ginseng Res. 2017, 41, 134. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.-A.; Han, M.J.; Shin, Y.-W.; Kim, D.-H. Inhibitory Effects of Korean Red Ginseng and Its Genuine Constituents Ginsenosides Rg3, Rf, and Rh2 in Mouse Passive Cutaneous Anaphylaxis Reaction and Contact Dermatitis Models. Biol. Pharm. Bull. 2006, 29, 1862–1867. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Siddiqi, M.H.; Aceituno, V.C.; Simu, S.Y.; Zhang, J.; Jimenez Perez, Z.E.; Kim, Y.-J.; Yang, D.-C. Ginsenoside Rg5:Rk1 attenuates TNF-α/IFN-γ-induced production of thymus- and activation-regulated chemokine (TARC/CCL17) and LPS-induced NO production via downregulation of NF-κB/p38 MAPK/STAT1 signaling in human keratinocytes and macrophages. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef]
- Castex-Rizzi, N.; Galliano, M.F.; Aries, M.F.; Hernandez-Pigeon, H.; Vaissiere, C.; Delga, H.; Caruana, A.; Carrasco, C.; Lévêque, M.; Duplan, H.; et al. In vitro approaches to pharmacological screening in the field of atopic dermatitis. Br. J. Dermatol. 2014, 170, 12–18. [Google Scholar] [CrossRef]
- Jeziorkowska, R.; Sysa-Jędrzejowska, A.; Samochocki, Z. Topical steroid therapy in atopic dermatitis in theory and practice. Postep. Derm.i Alergol. 2015, 32, 162–166. [Google Scholar] [CrossRef][Green Version]
- Shin, J.H.; Chung, M.J.; Seo, J.G. A multistrain probiotic formulation attenuates skin symptoms of atopic dermatitis in a mouse model through the generation of CD4+Foxp3+T cells. Food Nutr. Res. 2016, 60. [Google Scholar] [CrossRef]
- Vu, A.T.; Chen, X.; Xie, Y.; Kamijo, S.; Ushio, H.; Kawasaki, J.; Hara, M.; Ikeda, S.; Okumura, K.; Ogawa, H.; et al. Extracellular Double-Stranded RNA Induces TSLP via an Endosomal Acidification- and NF- j B-Dependent Pathway in Human Keratinocytes. J. Invest. Dermatol. 2011, 131, 2205–2212. [Google Scholar] [CrossRef]
- Jang, Y.; Jeong, S.H.; Park, Y.-H.; Bae, H.C.; Lee, H.; Ryu, W.-I.; Park, G.H.; Son, S.W. UVB Induces HIF-1α-Dependent TSLP Expression via the JNK and ERK Pathways. J. Invest. Dermatol. 2013, 133, 2601–2608. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Leyva-Castillo, J.M.; Hener, P.; Jiang, H.; Li, M. TSLP Produced by Keratinocytes Promotes Allergen Sensitization through Skin and Thereby Triggers Atopic March in Mice. J. Invest. Dermatol. 2013, 133, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yamane, H.; Cote-Sierra, J.; Guo, L.; Paul, W.E. GATA-3 promotes Th2 responses through three different mechanisms: Induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006, 16, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hulse, K.E.; Reefer, A.J.; Engelhard, V.H.; Patrie, J.T.; Ziegler, S.F.; Chapman, M.D.; Woodfolk, J.A. Targeting allergen to FcgammaRI reveals a novel T(H)2 regulatory pathway linked to thymic stromal lymphopoietin receptor. J. Allergy Clin. Immunol. 2010, 125, 247–256.e1-8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Ito, T.; Wang, Y.-H.; Homey, B.; Watanabe, N.; Martin, R.; Barnes, C.J.; McIntyre, B.W.; Gilliet, M.; Kumar, R.; et al. Maintenance and Polarization of Human TH2 Central Memory T Cells by Thymic Stromal Lymphopoietin-Activated Dendritic Cells. Immunity 2006, 24, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Samukawa, K.; Izumi, Y.; Shiota, M.; Nakao, T.; Osada-Oka, M.; Miura, K.; Iwao, H. Red ginseng inhibits scratching behavior associated with atopic dermatitis in experimental animal models. J. Pharmacol. Sci. 2012, 118, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jin, S.W.; Park, B.H.; Kim, H.G.; Khanal, T.; Han, H.J.; Hwang, Y.P.; Choi, J.M.; Chung, Y.C.; Hwang, S.K.; et al. Cultivated ginseng inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in NC/Nga mice and TNF-α/IFN-γ-induced TARC activation in HaCaT cells. Food Chem. Toxicol. 2013, 56, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Ziegler, S.F. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFκB. Proc. Natl. Acad. Sci. USA 2007, 104, 914–919. [Google Scholar] [CrossRef]
- Pattarini, L.; Trichot, C.; Bogiatzi, S.; Grandclaudon, M.; Meller, S.; Keuylian, Z.; Durand, M.; Volpe, E.; Madonna, S.; Cavani, A.; et al. TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J. Exp. Med. 2017, 214, 1529–1546. [Google Scholar] [CrossRef]
- Park, S.; Ko, E.; Lee, J.H.; Song, Y.; Cui, C.-H.; Hou, J.; Jeon, B.M.; Kim, H.S.; Kim, S.C. Gypenoside LXXV Promotes Cutaneous Wound Healing In Vivo by Enhancing Connective Tissue Growth Factor Levels Via the Glucocorticoid Receptor Pathway. Molecules 2019, 24, 1595. [Google Scholar] [CrossRef]
- Cui, C.H.; Kim, D.J.; Jung, S.C.; Kim, S.C.; Im, W.T. Enhanced production of gypenoside LXXV using a novel ginsenoside-transforming β-glucosidase from ginseng-cultivating soil bacteria and its anti-cancer property. Molecules 2017, 22, 844. [Google Scholar] [CrossRef]
- Tomimori, Y.; Tanaka, Y.; Goto, M.; Fukuda, Y. Repeated Topical Challenge with Chemical Antigen Elicits Sustained Dermatitis in NC/Nga Mice in Specific-Pathogen-Free Condition. J. Invest. Dermatol. 2005, 124, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Sohn, E.J.; Kim, D.W.; Jeong, H.J.; Kim, M.J.; Kang, H.W.; Shin, M.J.; Ahn, E.H.; Kwon, S.W.; Kim, Y.N.; et al. Transduced PEP-1-FK506BP ameliorates atopic dermatitis in NC/Nga mice. J. Invest. Dermatol. 2011, 131, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Oranje, A.P.; Glazenburg, E.J.; Wolkerstorfer, A.; de Waard-van der Spek, F.B. Practical issues on interpretation of scoring atopic dermatitis: The SCORAD index, objective SCORAD and the three-item severity score. Br. J. Dermatol. 2007, 157, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Gläser, R.; Harder, J.; Dressel, S.; Wittersheim, M.; Cordes, J.; Meyer-Hoffert, U.; Mrowietz, U.; Fölster-Holst, R.; Proksch, E.; Schröder, J.M.; et al. Enhanced expression and secretion of antimicrobial peptides in atopic dermatitis and after superficial skin injury. J. Invest. Dermatol. 2010, 130, 1355–1364. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, E.; Park, S.; Lee, J.H.; Cui, C.-H.; Hou, J.; Kim, M.-h.; Kim, S.C. Ginsenoside Rh2 Ameliorates Atopic Dermatitis in NC/Nga Mice by Suppressing NF-kappaB-Mediated Thymic Stromal Lymphopoietin Expression and T Helper Type 2 Differentiation. Int. J. Mol. Sci. 2019, 20, 6111. https://doi.org/10.3390/ijms20246111
Ko E, Park S, Lee JH, Cui C-H, Hou J, Kim M-h, Kim SC. Ginsenoside Rh2 Ameliorates Atopic Dermatitis in NC/Nga Mice by Suppressing NF-kappaB-Mediated Thymic Stromal Lymphopoietin Expression and T Helper Type 2 Differentiation. International Journal of Molecular Sciences. 2019; 20(24):6111. https://doi.org/10.3390/ijms20246111
Chicago/Turabian StyleKo, Eunsu, Sungjoo Park, Jun Hyoung Lee, Chang-Hao Cui, Jingang Hou, Myung-ho Kim, and Sun Chang Kim. 2019. "Ginsenoside Rh2 Ameliorates Atopic Dermatitis in NC/Nga Mice by Suppressing NF-kappaB-Mediated Thymic Stromal Lymphopoietin Expression and T Helper Type 2 Differentiation" International Journal of Molecular Sciences 20, no. 24: 6111. https://doi.org/10.3390/ijms20246111
APA StyleKo, E., Park, S., Lee, J. H., Cui, C.-H., Hou, J., Kim, M.-h., & Kim, S. C. (2019). Ginsenoside Rh2 Ameliorates Atopic Dermatitis in NC/Nga Mice by Suppressing NF-kappaB-Mediated Thymic Stromal Lymphopoietin Expression and T Helper Type 2 Differentiation. International Journal of Molecular Sciences, 20(24), 6111. https://doi.org/10.3390/ijms20246111



