1. Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disorder in the world and it has specific features, such as progressiveness and unremitting advancement, which result in a progressive decline of cognitive functions [
1]. One common characteristic of this disease with most of other neurodegenerative diseases is the formation of amyloidogenic aggregates. In AD, the proteins that are involved in the pathogenesis are the amyloid-β (Aβ) peptide and Tau protein. The aggregation of these proteins culminates in the development of extracellular senile plaques, composed of Aβ peptides, and intracellular neurofibrillary tangles (NFTs), composed of the hyperphosphorylated Tau protein [
2]. These species and/or the on-pathway species are cytotoxic and they lead to neuronal death [
3]. In the past decades, there was great effort to alter AD pathology by targeting Aβ; however, this approach has been largely unsuccessful. Thus, interest in Tau-targeting strategies and studies is greatly increasing [
4].
Metal ions have a major role in the physiological functioning of the brain and their homeostatic dysfunction is reported in several neurodegenerative diseases, including AD [
5]. The main metal ions that are involved in neuronal physiological activity are iron (Fe
2+/Fe
3+), copper (Cu
2+), zinc (Zn
2+), and also calcium (Ca
2+), whose homeostasis is also particularly important during aging [
6,
7]. All of these metal ions must be finely maintained under physiological levels, given their importance in neuronal physiology. However, in AD pathology, the observation of a significant imbalance in metal ion concentration and localization has prompted researchers to propose AD to also be considered a metallopathy [
8]. In fact, with aging and AD progressiveness, there is an alteration in the localization and levels of neuronal metals and a marked accumulation within protein deposits. Moreover, the described cross-talk between the metal ions and AD pathological proteins suggests a close relationship between protein misfolding, aggregation, and metal ion homeostasis [
9]. Zn
2+ is particularly relevant in AD, since ex vivo observations indicate that senile plaques are highly enriched in this metal ion. Moreover, recent evidence indicates a complex interplay between Aβ and Zn
2+ [
10].
Several recent studies focused on the effect of zinc on Tau physiology and aggregation. The impact of zinc ions on Tau was investigated in the absence of heparin and it was found that zinc can induce a temperature-dependent reversible oligomerization of Tau [
11]. However, these oligomers are amyloidogenic, and instantly dissociated following zinc chelation or temperature decrease. Moreover, isothermal titration calorimetry measurements have led to the hypothesis that zinc binds Tau to one high affinity binding site and three low affinity sites [
11]. There are also findings that indicate that Zn
2+ binds Tau and promotes its hyperphosphorylation through Zn
2+-induced inactivation of protein phosphatase 2A [
12] and the activation of Tau phosphorylation kinases [
13]. Nevertheless, recent preclinical findings suggest a possible phosphorylation-independent pathway, in which Zn
2+ might modulate Tau pathology, by direct-binding to Tau, and consequently promoting its aggregation [
14]. Moreover, it was observed in in vitro studies that low concentrations of Zn
2+ result in the acceleration of Tau fibril formation [
15]. Additionally, it was demonstrated for Tau constructs and mutants with aggregation enhanced properties that, in the presence of zinc ions, Tau aggregation is further promoted with toxicity effects on neuroblastoma cell lines [
16,
17,
18]. There are also studies that suggest what seems to be a tightly regulated Ca
2+-dependent process of maintaining the state of Tau phosphorylation between physiological levels [
19].
In the present study, we investigate the interaction of full-length human Tau with calcium and zinc ions while combining multiple biophysical and mass spectrometry approaches, providing evidence for multiple zinc binding sites, kinetic analysis, and changes in oligomer distribution. Our results lead to a conceptual model that accounts for the diverse reported effects of zinc binding to Tau aggregation.
3. Discussion
Metal ion binding to aggregating proteins is a well-known regulatory mechanism of in vivo protein aggregation that is coupled to amyloid-forming age-related dementias. In the case of AD, it is established that metal dyshomeostasis is an altered cellular process that evolves along the pathology and neuronal protein inclusions in diseased human brains and in animal models accumulate metal ions, such as copper, iron, and zinc [
23,
24]. Several studies have focused on the effects of metal ion to binding Tau and its fragments, including zinc, while also advancing knowledge in the field; these studies have nevertheless opened questions that remain unaddressed [
11,
14,
15,
16,
17,
18]. Eventually, the understanding of the diverse consequences of zinc binding to Tau that has been reported to result in both stoichiometry-dependent effects on the kinetics of fibril formation and its cytotoxicity, as well as in amorphous aggregates, is the most interesting aspect to investigate. Here, contributed a new approach combining multiple biophysical and analytical methods, including native mass spectrometry, to study the effects of zinc and calcium interactions with full length human Tau on aggregation kinetics and the analysis of formed Tau conformers.
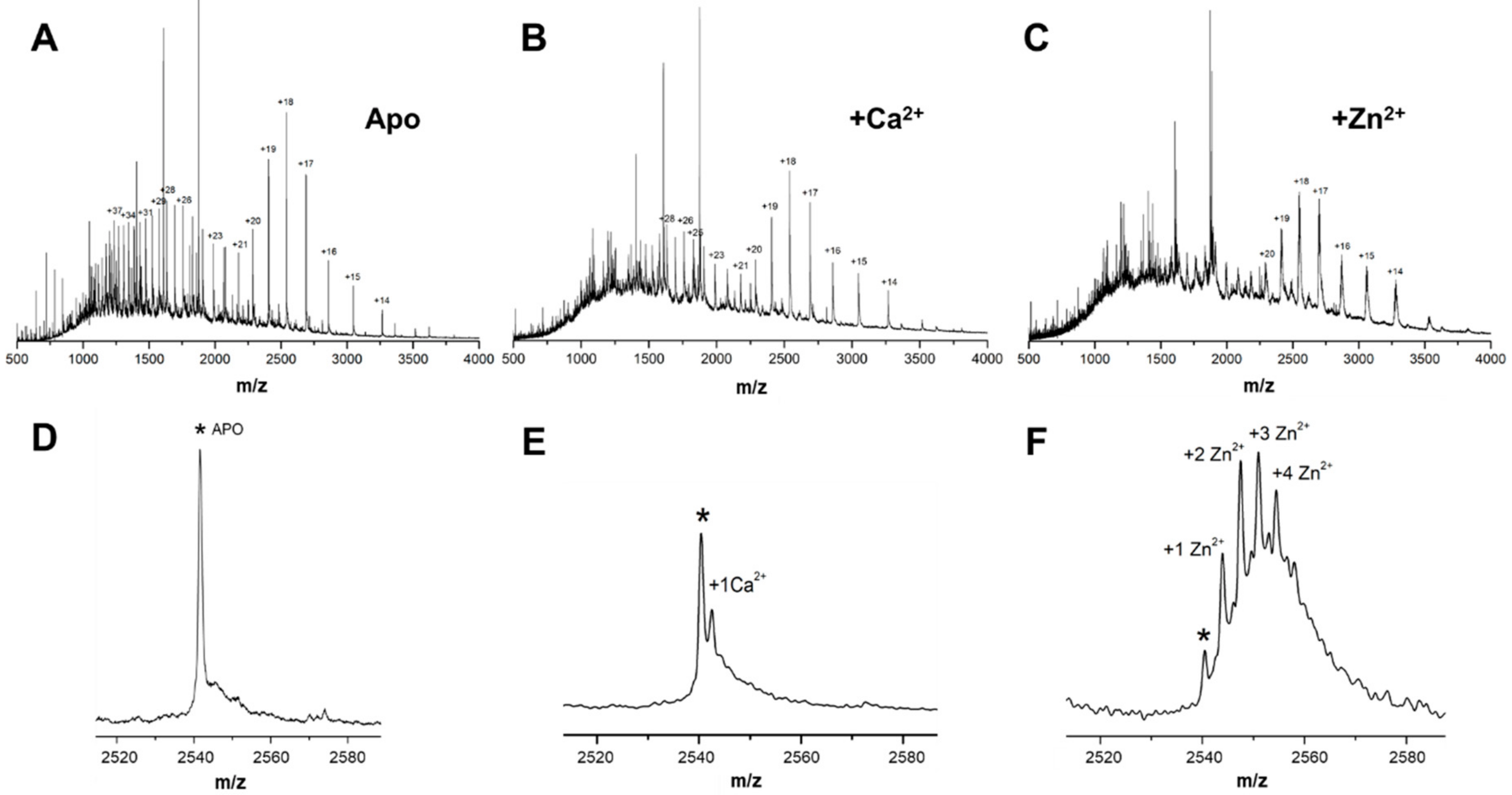
Our investigations of zinc binding to Tau at pathological (µM) zinc concentrations, similar to those found upon traumatic brain injury and AD neurodegeneration [
25], elicited multiple stable zinc binding sites that are, so far, unclear. We determined four Zn
2+ binding sites in the protein while using monomer-enriched full-length human Tau and ESI-native mass spectrometry analysis (
Figure 1). While binding of one zinc to Tau at micromolar affinities has been established in the R1/R3/R4-containing K19 construct and the R3 fragments [
17], as well as in full length Tau [
16] while using isothermal titration calorimetry, the possibility that Tau might accommodate multiple zinc binding sites has also been recognized [
16]. Indeed, more recent work also based on calorimetric analysis of hTau441 titrated with zinc at high temperature has put forward the possibility that three low affinity and one high affinity zinc binding site exist in the protein [
11]. However, the results reported here clearly show that such zinc binding moieties in Tau are also accessible at room temperature. The fact that we employed high resolution structural mass spectrometry here justifies the observation of multiple zinc binding sites, which have so far remained undetected by calorimetric methods at room temperature. Zinc binding to Tau has been previously assigned to Cys-291 and Cys-322 within the R2 repeat [
15], and this is likely to be the major high-affinity zinc binding site. All Zn
2+-Tau conformers present in solution are detected, thus providing solid evidence that Tau properties are amenable to regulation through binding of multiple zinc ions, since native MS approach does not discriminate between sites with distinct affinities or occupancies. A similar approach was undertaken to investigate calcium binding to Tau and it clearly evidenced a single binding site. Frequently Ca
2+-binding sites in polypeptides result from the gathering of surface carbonyl and carboxylate centers that act as major donor groups. The identification of the sites for calcium binding sites within Tau is out of the scope of the present work, but the fact that, similarly to zinc, calcium also accelerates Tau aggregation suggests that its binding might also interfere with an association of Tau repeat regions.
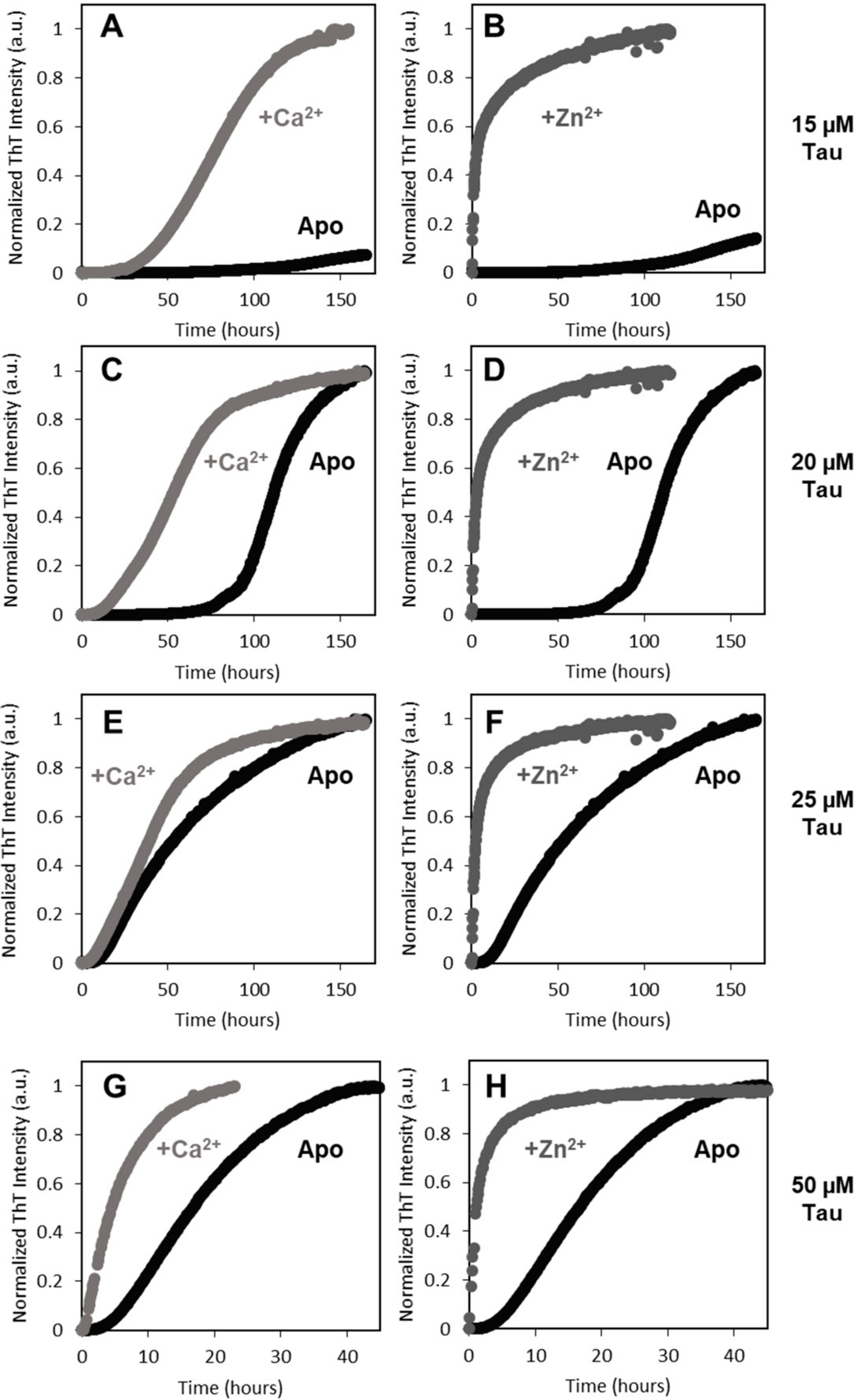
Indeed, calcium and zinc both strongly enhance Tau aggregation. As biophysical and spectroscopic analysis did not evidence metal-induced secondary structure changes and only induced mild differences on the protein hydrodynamic radius, the enhancement of aggregation must result from subtle conformational changes that promote aggregation. Indeed, previous work [
17] has suggested that zinc binding to the R3 repeat of Tau weakens the aggregation-inhibitory interaction between R3 and R4, therefore resulting in faster Tau aggregation, and a similar mechanism might be in place for the Ca
2+-Tau conformers. However, zinc has a much more dramatic effect on Tau rate. The aggregation of full length Zn
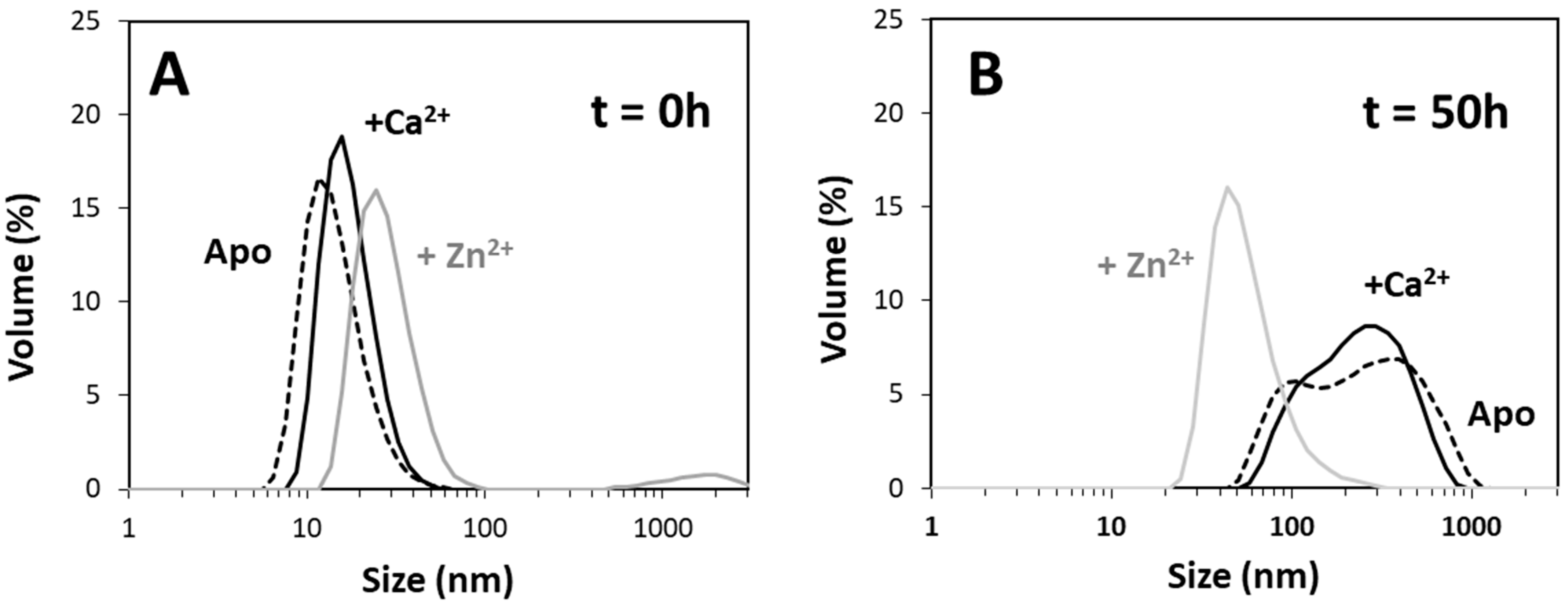
2+-Tau proceeds promptly without lag phase, with the formation of aggregates visible at the naked eye; however, it should be noted that, in this study, we monitored the formation of amyloid fibrils using ThT fluorescence, unlike previous reports that followed the kinetics of hTau441 aggregation from turbidity measurements. We analyzed the type of particles in solution at the plateau stage of the aggregation reaction to investigate the morphology of the formed species. DLS analysis of soluble conformers (after centrifugation) after 50 h of incubation evidenced, in the case of apo and Ca
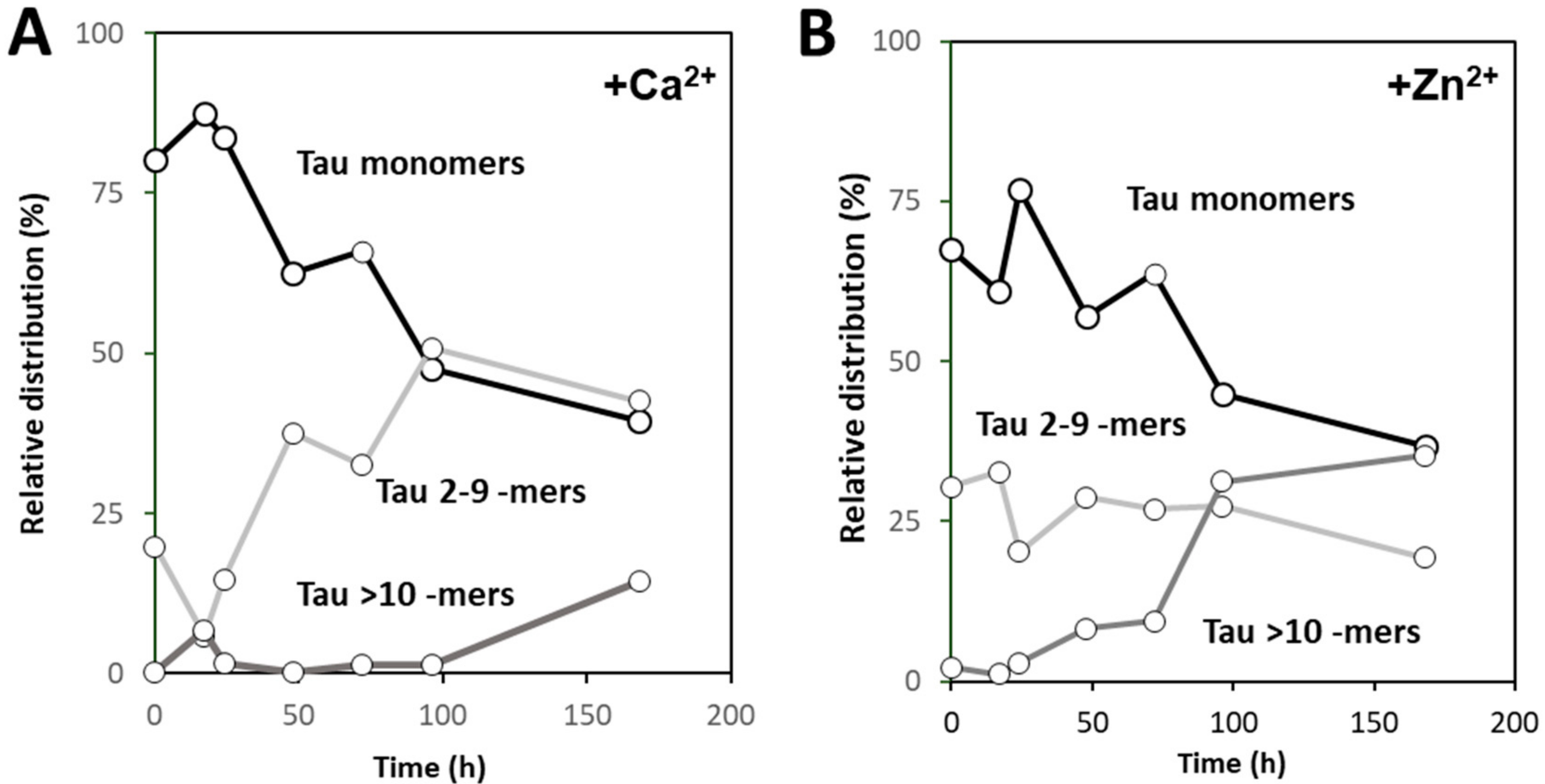
2+-Tau, a broad distribution of species with dimensions in the 100–40 nm range, in agreement with the formation of large oligomeric species and fibrils. AFM characterization of total species indeed corroborates this analysis, as mostly fibrils are observed. However, in the case of Zn
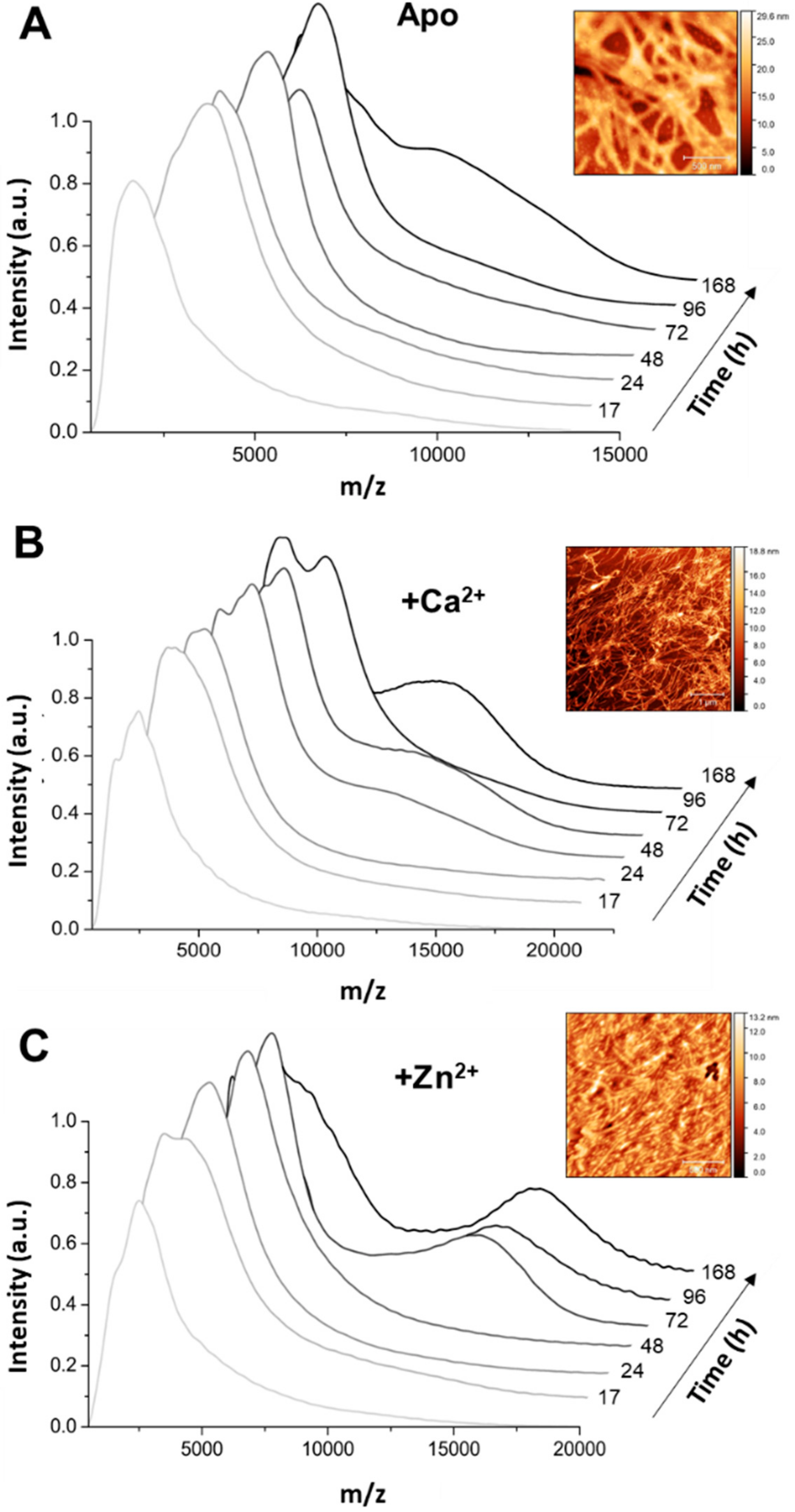
2+-Tau, a substantially different scenario emerged as only a predominant soluble species with a radius ≈44 nm was observed, which suggests the formation of smaller oligomers. AFM analysis of soluble and insoluble species evidence the existence of amorphous aggregates that coated the mica together with oligomers and a mixed population of shorter and longer fibrils.
Using native MS and deconvolution of species along the reaction allowed for discriminating the different populations of oligomers evolving during the reaction, and establishing that zinc and calcium binding differently influence the aggregation pathway of hTau441. Calcium enhances aggregation by speeding the interconversion of monomers into intermediate aggregates (Tau 2–9 -mers), with the formation of large oligomer at the plateau stage of the ThT kinetics, corresponding to fibril formation, similarly to what is observed for apo hTau441. However, Zn2+-Tau evidences the instantaneous formation of intermediate aggregates (~30% of total species), which slightly decrease until ≈170 h incubation. Such species likely provide nuclei that promote the formation of larger oligomers at the expense of monomers, in secondary nucleation-like processes.
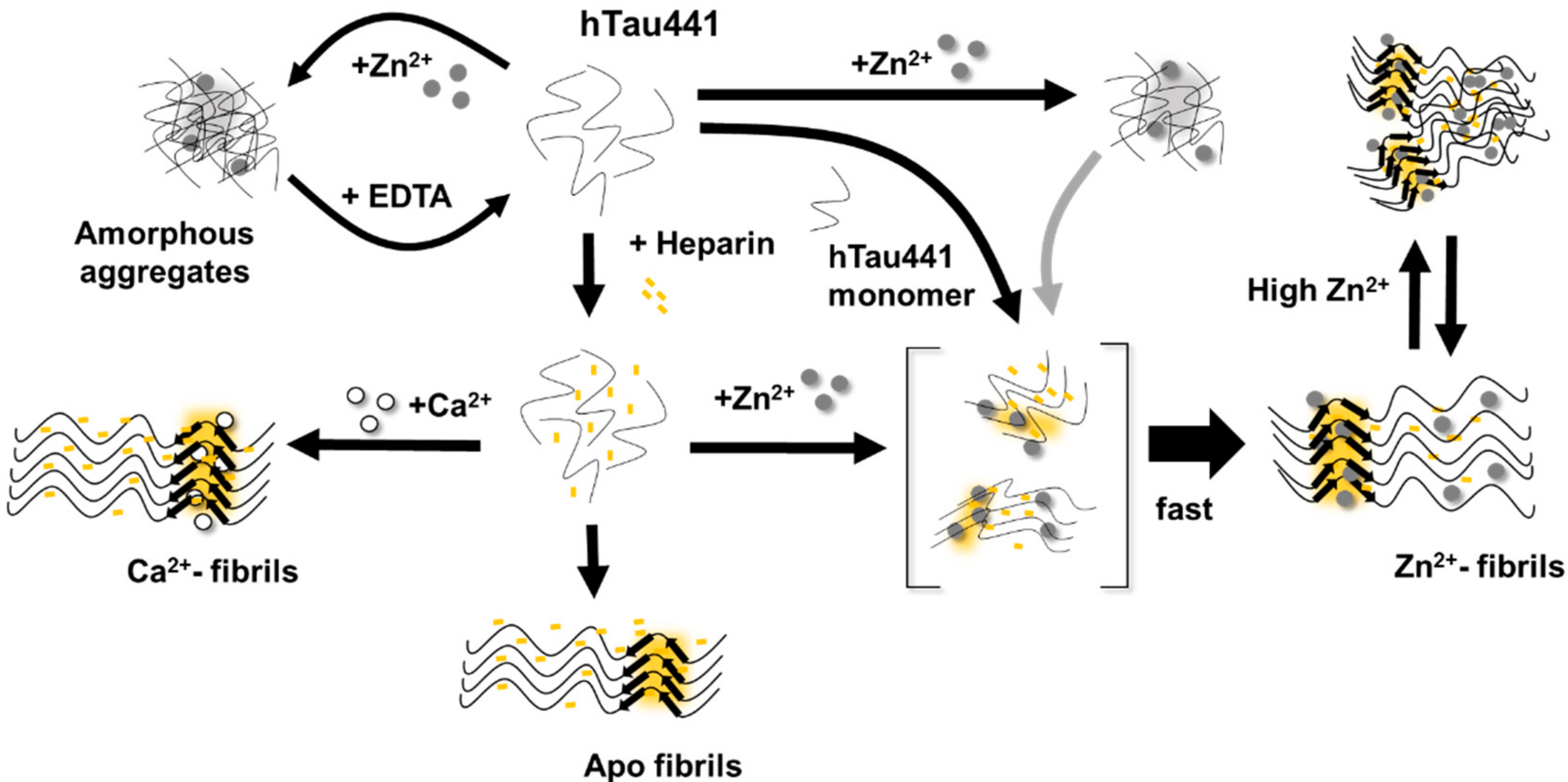
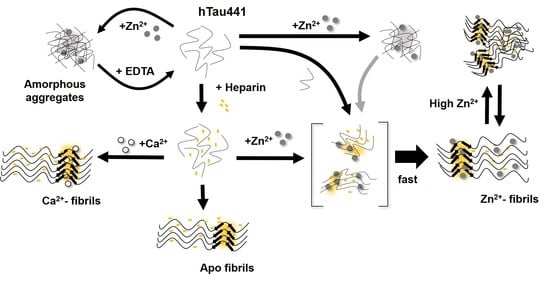
All considered, we propose a general mechanism here, prompted by the observations reported, which reconciles the different findings of the effects of metals, and, in particular, zinc, in Tau aggregation (
Figure 6). Zinc binding to residues within the R3/R4 regions are clearly responsible for faster ThT-reactive fibrillation. However, the effect of amorphous aggregations might result from lower affinity interactions of zinc to binding moieties elsewhere in the polypeptide. We speculate that this might involve binding of zinc to residues within the fuzzy coat regions, which contribute to the formation of unordered and amorphous aggregates, which are not observed in the presence of calcium, and that might also interfere with ordered stacking of residues in the repeat regions that will lead to amyloid cross-beta formation. Higher levels of zinc may promote some disorganization of fibrils, further contributing to sample heterogeneity.
In conclusion, the binding of the two metals to Tau results in substantially different effects on the protein aggregation that open new clues into their implication in disease pathomechanisms. This is especially relevant when considering recent evidence implicating zinc binding to Tau to the generation of toxic aggregates, independently of phosphorylation [
14].
4. Materials and Methods
4.1. hTau441 Expression and Purification
All of the reagents were of the highest grade commercially available. ThT was obtained from Sigma. A Chelex resin (Bio-Rad) was used to remove contaminant trace metals from all solutions. The expression and purification of recombinant full-length human Tau (hTau441) was adapted from that described in [
26].
E. coli (BL21 DE3) cells were transformed with the plasmid pET15b-Tau provided by I. Landrieu to obtain the overexpression of proteins (University of Lille, Lille, France), encoding the longest isoform of human Tau (441 amino acid residues). The transformed cells were plated in Luria-Bertani (LB) solid medium containing ampicillin (Nzytech, Lisbon, Portugal). A single colony was used to inoculate LB medium, being further incubated overnight at 37 °C. Bacterial cells were then grown in M9 medium and then induced with IPTG. The cells were harvested after 3h by centrifugation. The cell pellet was and resuspended in buffer A (50 mM Tris-HCl pH 6.5 and 1 mM EDTA (Sigma-Aldrich, St. Louis, MO, USA)), DNAse (PanReac, Applichem, Darmstadt, Germany), 1 mM phenylmethanesulfonyl fluoride (PMSF, Roth, Karsruhe, Germany), and freshly supplemented with complete™ EDTA-free protease inhibitor cocktail 1× (1 tablet, Roche). The bacterial cells were disrupted while using high-pressure French Press homogenizer at 20,000 psi, following centrifugation at 48,000×
g and 4 °C for 1 h to remove insoluble materials. The bacterial cell extract was heated for 15 min. at 75 °C in a water bath and then centrifuged again to remove precipitated proteins. Cation-exchange chromatography (CEX) was performed in a Hiprep SP Fast-Flow 16/10 column (GE Healthcare, Chicago, IL, USA) while using a fast protein liquid chromatography (FPLC) ÄKTA purifier UPC 10 system (GE Healthcare, Chicago, IL, USA). Buffer A was used as running buffer and the protein of interested was eluted with buffer A containing 1 M NaCl. The fractions containing Tau were combined and the buffer was switched to buffer A. A protocol optimized for producing hTau441 monomers was developed, which consisted in urea (7.6 M) solubilization of post-chromatography fractions in the presence of 50 mM DTT, prior to passage on gel filtration while using a tricorn Superdex S-200 column. The eluted monomeric Tau samples were then lyophilized and stored at −20 °C. Tau purity was assessed by Mass spectrometry and SDS-PAGE, and the concentration was spectrophotometrically determined using
= 7550 M
−1 cm
−1.
4.2. hTau441 Aggregation Kinetics
hTau441 aggregation kinetics were performed by recording the Thioflavin-T (ThT) fluorescence intensity as a function of time in a plate reader (FLUOstar OPTIMA, BMG Labtech, Ortenberg, Germany) with a 440 nm excitation filter and a 480 nm emission filter. The fluorescence was recorded while using bottom optics in half-area 96-well polyethylene glycol-coated black polystyrene microplates with a clear bottom (Corning, ref. 3881, New York, NY, USA). The microplates were sealed with transparent foil to avoid evaporation. The experimental concentrations of hTau441 varied between 15 to 50 µM. The concentration of heparin (heparin sodium salt from porcine) used was 0.5 mg/mL, DTT was 1 mM, NaCl was 50 mM [
27], PMSF was 1 mM, ThT was 75 µM, and metal ions 1.1 mM (CaCl
2 and ZnCl
2). All of the samples were prepared while using 50 mM Tris-HCl pH 7.4. Tau aggregation was performed at 37 °C with an orbital shaking at 600 rpm for 300 s before each measurement.
4.3. Dynamic Light Scattering
DLS measurements were carried out in a Malvern Zetasizer Nano S instrument. hTau441 was analyzed from 50 μM samples in 50 mM Tris-HCl (pH 7.4) in the absence or presence of 1.1 mM CaCl2 or ZnCl2. The samples also contained the reagents at the same concentration than in the kinetics aggregation assays (except ThT). The reagents were filtered or centrifuged before use. Samples that were incubated at 37 °C and 800 rpm were analyzed at time points 0 h and 50 h. The samples were centrifuged for 10 min. at 34,000× g at room temperature and 50 µL aliquots from soluble fraction were collected and measured in a 45 µL quartz cuvette (Hellma) at 25 °C. The operating procedure was set to 17 runs, with each being averaged for eight measurements. The resulting data were analyzed while using DTS (version 7.01) software (Malvern, Worcestershire, UK).
4.4. Circular Dichroism
CD measurements were performed on a Jasco J-1500 spectropolarimeter equipped with a Peltier-controlled thermostated cell support. Far UV-CD spectra (200 to 260 nm) were recorded at 10 μM hTau441 with or without 40 μM CaCl2 or ZnCl2 in 50 mM Tris-HCl (pH 7.4). Individual solutions of Tau with and without metal ions were incubated for 1 h at 4 °C.
4.5. ATR-FTIR
Fourier-transform infrared spectroscopy was performed in a Bruker Tensor FTIR instrument that was equipped with an attenuated total reflection cell (Harrick) (ATR-FTIR) to assess the secondary structure of protein samples. The final volume analyzed was 20 µL with 4 µM hTau441. Metal ions, like CaCl2 and ZnCl2, were used at a final concentration of 16 µM. The samples were incubated at 4 °C for 1 h. The spectra were obtained from 4000 to 900 cm−1, with a 4 cm−1 resolution and 120 sample scans.
4.6. Native Mass Spectroscopy
The samples for mass spectrometry were delivered in concentration of 35 µM, and sample clean-up was performed while using Micro Bio-spin™ 6 columns (Bio-Rad, Hercules, CA, USA). The buffer used for native MS analysis was 100mM ammonium bicarbonate at pH 7.2. Mass spectrometry analysis was performed on a modified Waters QTOF Ultima II instrument with an off-line nano electrospray source. The samples were introduced into the QTOF mass analyzer while using in-house built capillary emitters (Sutter P-1000, BF120-69-10). Mass calibration was completed using 100 mg/mL of CsI in water in range of 1 to 20kDa. Data analysis of obtained mass spectra was accomplished while using MassLynx 4.1, Origin 8 Pro and PeakFIt v 4.12.
4.7. Atomic Force Microscopy
The topography/morphology of the samples was characterized by AFM with a PicoSPM LE (Molecular Imaging) system and Agilent Technologies PicoView 1.14.4 software (Keysight Technologies, Santa Rosa, CA, USA). The images were obtained in air, at room temperature, with HQ:NSC35/Hard/Al BS and HiRes-C14/Cr-Au µmasch cantilevers in dynamic mode. Approximately 10 µL of each Tau solution was deposited in freshly cleaved mica (Agar Scientific, Stansted, UK). The samples were allowed to rest for about 20 min. before rinsing with water and then dried in air before imaging.