Abstract
Four 57Fe-labeled tetrachloroferrates(III) of organic cations (1-butyl-3-methylimidazolium, 1-allyl-3-methylimidazolium, 1-methyl-1-propylpyrrolidinium, tetraphenylphosphonium) were examined by temperature-dependent Mössbauer spectroscopy. The hyperfine and dynamic parameters of the iron(III) site were determined. Single crystal X-ray diffraction data of [Ph4P][FeCl4] were collected at four temperatures (295, 223, 173, and 123 K), and the dynamics of the iron atom inferred from the Mössbauer data and the single crystal Ui,j parameters have been compared.
1. Introduction
Magnetic ionic liquids (MILs) are low vapor pressure homogeneous phases (liquids or low-melting solids) which show a noticeable attraction to strong magnets, and have attracted considerable attention in the recent literature [1–5]. Typically, they consist of an organic cation and a tetrahalogenoferrate(III) anion [6], but other transition metal-based anions have also been reported [7,8]. A detailed Raman spectroscopic and calculational study of FeCl3/1-butyl-3-methylimidazolium chloride mixtures has shown that the predominant metal species is the FeCl4 − anion [9], a conclusion supported by visible absorption spectroscopy [10,11]. Resonant gamma ray fluorescence (Mössbauer effect, ME) and AC susceptibility data of 4-piperidinylpyridinium FeCl4 − were examined at low temperatures and confirmed a Néel temperature of ~2.5 K in this system [12]. The ME spectra showed a sharp transition from a rapidly relaxing paramagnetic resonance line to a six-line hyperfine spectrum between 2.50 and 2.25 K. Below 2 K, the spectra exhibited magnetic saturation. Tetraethylammonium tetrachloro-ferrate(III) was investigated by temperature-dependent Mössbauer spectroscopy and became antiferromagnetic with a Néel temperature of 3.0 K [13,14]. A survey of 164 crystal structures included in the November 2003 Cambridge Structural Database (CSD) revealed that the tetrachloroferrate ions have distorted tetrahedral structure with averaged Fe-Cl bond lengths and Cl-Fe-Cl bond angles of 2.204 Å and 109.32°, respectively [15]. It is somewhat surprising to note that there is very little reference in the ME literature to alkali metal tetrachloroferrates, and in particular to the temperature dependencies of the hyperfine and lattice dynamical parameters which have not been reported in detail.
2. Results and Discussion
The magnetic liquids investigated in this study showed no observable evidence of particulate matter, and no fines could be separated by centrifugation. We have examined the hyperfine parameters and metal atom dynamics of four magnetic liquids and report the related parameters of these materials. The cations associated with the tetrachloroferrate anion are listed graphically in Figure 1. The small scale synthesis of 57Fe-labeled compounds 1–4 is also described.
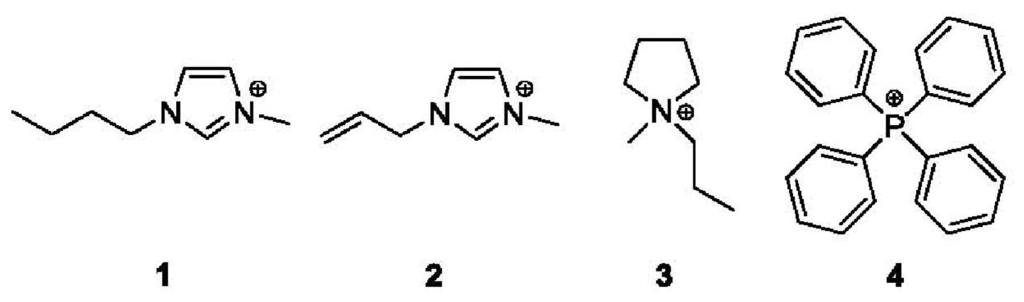
Figure 1.
Organic cations of 57FeCl4 salts 1–4.
The ME spectra in the temperature interval 5 K < T < 320 K consist of an asymmetrically broadened absorption line, characteristic of a relaxation-broadened transition. A representative spectrum of compound 1 is shown in Figure 2.
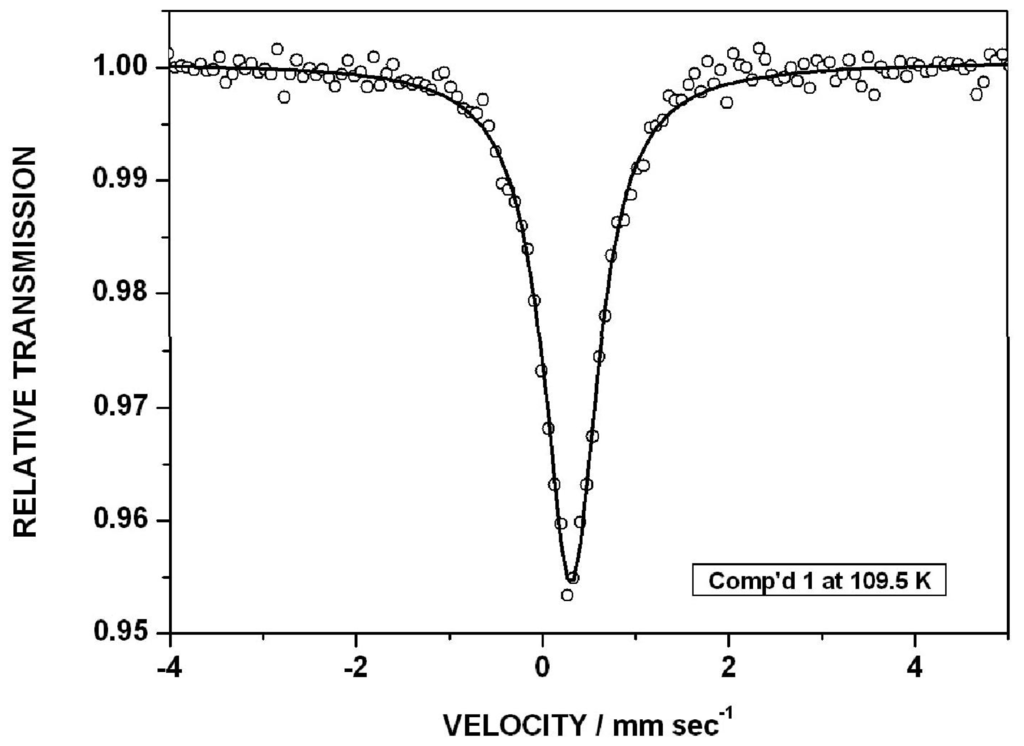
Figure 2.
57Fe Mössbauer spectrum of 1 at 109.5 K. The velocity scale is with respect to the centroid of a room temperature spectrum of α-Fe. The data have been fitted to a doublet with a line width of 0.62 mm s−1 and a quadrupole splitting of 0.25 mm s−1 at 109 K.
The hyperfine parameters at 90 K (isomer shift, IS, and quadrupole splitting, QS) as well as the derived dynamical parameters [16] (effective vibrating mass, Meff, and Mössbauer lattice temperature, θM) are summarized in Table 1.

Table 1.
Hyperfine Parameters of 1–4 at 90 K.
The line width at half maximum is generally temperature-dependent, and in several cases can be understood in terms of a fast relaxation process. In none of these spectra was it possible to extract magnetic ordering information, and it is presumed that magnetically ordered particles, when present, are sufficiently small so that the 5 K minimum temperature used in this study exceeds the blocking temperature of these particles. However, attraction of the liquid samples to a strong magnet was clearly demonstrable even above room temperature. Even molten salts 3 and 4 also exhibited attraction to a strong magnet.
2.1. (1-Butyl-3-Methylimidazolium) (57FeCl4) (1)
The hyperfine parameters (isomer shift (IS) and quadrupole splitting (QS) at 90 K are included in Table 1. The line widths of the ME spectra fitted with a relaxation spectrum program are essentially temperature-independent with an average value of 0.63 ± 0.03 mm s−1 suggestive of a relaxation process which is fast compared to the characteristic ME time scale. The IS decreases with increasing temperature, but is not well fit by a linear correlation leading to an approximate Meff of 102 ± 13 Da and a ME lattice temperature of ~67 ± 4 K. The temperature dependence of the logarithm of the recoil-free fraction, −d(lnf)/dT, is [16.8(4) × 10−3 K−1] with a correlation coefficient of 0.95 for 9 data points.
2.2. (1-Allyl-3-Methylimidazolium) (57FeCl4) (2)
As above, the ME spectra consist of a single broad resonance absorption line. The IS and QS parameters are included in Table 1. The temperature dependencies of both the IS and recoil-free fraction are well fitted by a linear regression, leading to a value of Meff = 87 ± 7 Da and a θM = 90 ± 4 K. The line width is effectively temperature independent arising from the same fast relaxation as above.
2.3. (1-Methyl-1-Propylpyrrolidinium) (57FeCl4) (3)
The ME spectra of this sample consisted not only of the broad central line associated with a paramagnetic compound, but in addition, to two minor Fe resonances presumably due to traces of impurities. These two impurities accounted for 15 and 5% of the total area under the resonance curve at 94.5 K. The influence of these impurities was corrected for in the subsequent data analysis. As in the case of the preceding compounds, the line widths of the major (paramagnetic) resonances are essentially temperature independent, again suggesting that the relaxation rate even at 92 K is fast compared to the ME time scale.
2.4. (Tetraphenylphosphonium) (57FeCl4) (4)
This new compound, which is not an IL, was intended as a crystallographic reference with high symmetry and only weak interactions between the ions. It is in contrast to the preceding ones in that the paramagnetic FeCl4 − complex is ion-paired to a phosphonium cation rather than a quaternary nitrogen atom, but except for a somewhat larger QS hyperfine parameter, the ME derived values are very similar to those of the salts with nitrogen containing cations. Only for this sample, it was possible to archive the Mössbauer spectra over a significantly larger range (5 < T < 319 K) than for the other samples studied. This made it possible to elucidate the spin relaxation mechanism in this case in detail. In addition, single crystal X-ray data were acquired at 4 different temperatures to allow a comparison with the vibrational data derived from the Mössbauer experiments. The temperature-dependent IS and QS parameters, as well as the lnf values, are well fitted by linear regressions (corr. coeff. = 0.999, 0.954 and 0.992 for 14 data points, respectively). The temperature-dependence of the IS and lnf parameters lead to an effective vibrating mass of 67 ± 1 Da. As has been detailed previously [17], the lnf data can be expressed in terms of
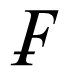 = k2<xave 2>, where k is the wave vector of the gamma radiation and <xave 2> is the mean square amplitude of vibration of the metal atom. This latter quantity can similarly be calculated from the Uij values of the X-ray diffraction data, and thus
= k2<xave 2>, where k is the wave vector of the gamma radiation and <xave 2> is the mean square amplitude of vibration of the metal atom. This latter quantity can similarly be calculated from the Uij values of the X-ray diffraction data, and thus
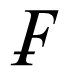 can be compared between the two techniques. This comparison is shown in Figure 3 in which the open points are those derived from the Mössbauer experiments (
can be compared between the two techniques. This comparison is shown in Figure 3 in which the open points are those derived from the Mössbauer experiments (
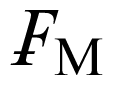 ), while the filled data points refer to the X-ray data (
), while the filled data points refer to the X-ray data (
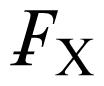 ). As will be seen from the figure, the
). As will be seen from the figure, the
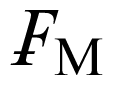 and
and
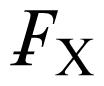 data are in quite reasonable agreement. The departure from linearity in the high temperature regime arises from the population of low frequency vibrational (or librational) motions in the solid. The starred data point is the k2<xave 2> value when the high T data are extrapolated to T = 0 K, making the assumption that f → 1 in the low T limit.
data are in quite reasonable agreement. The departure from linearity in the high temperature regime arises from the population of low frequency vibrational (or librational) motions in the solid. The starred data point is the k2<xave 2> value when the high T data are extrapolated to T = 0 K, making the assumption that f → 1 in the low T limit.
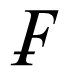 = k2<xave 2>, where k is the wave vector of the gamma radiation and <xave 2> is the mean square amplitude of vibration of the metal atom. This latter quantity can similarly be calculated from the Uij values of the X-ray diffraction data, and thus
= k2<xave 2>, where k is the wave vector of the gamma radiation and <xave 2> is the mean square amplitude of vibration of the metal atom. This latter quantity can similarly be calculated from the Uij values of the X-ray diffraction data, and thus
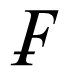 can be compared between the two techniques. This comparison is shown in Figure 3 in which the open points are those derived from the Mössbauer experiments (
can be compared between the two techniques. This comparison is shown in Figure 3 in which the open points are those derived from the Mössbauer experiments (
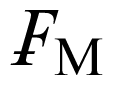 ), while the filled data points refer to the X-ray data (
), while the filled data points refer to the X-ray data (
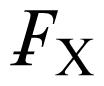 ). As will be seen from the figure, the
). As will be seen from the figure, the
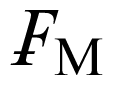 and
and
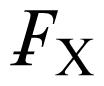 data are in quite reasonable agreement. The departure from linearity in the high temperature regime arises from the population of low frequency vibrational (or librational) motions in the solid. The starred data point is the k2<xave 2> value when the high T data are extrapolated to T = 0 K, making the assumption that f → 1 in the low T limit.
data are in quite reasonable agreement. The departure from linearity in the high temperature regime arises from the population of low frequency vibrational (or librational) motions in the solid. The starred data point is the k2<xave 2> value when the high T data are extrapolated to T = 0 K, making the assumption that f → 1 in the low T limit.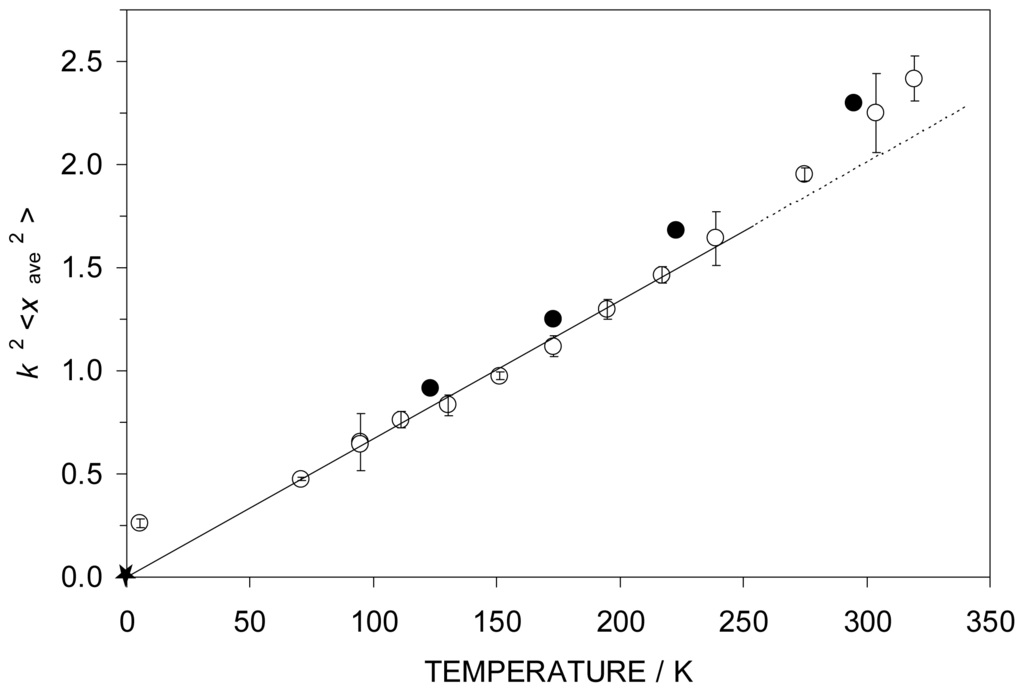
Figure 3.
The k2<xave 2> parameter for 4 extracted from the Mössbauer data (open data points) and the single crystal X-ray data (filled data points). The T = 0 K point extrapolated from the linear portion of the data is indicated by the starred point as discussed in the text.
As noted above, the full width at half maximum of the ME resonance line is temperature-dependent, decreasing with increasing temperature and is indicative of a relaxation mechanism involving the metal atom spin. These temperature-dependent line widths have been analyzed using the Wickmann-Wertheim formalism [18] to yield a value of the relaxation rate, making the assumption that the effective hyperfine field is that of an S = 5/2 state. This relaxation behavior is summarized graphically in Figure 4 and has been analyzed in terms of a power law in T. The T dependence is of the form T5.0 ± 0.7, indicative of a Raman type spin-lattice relaxation mechanism [19].
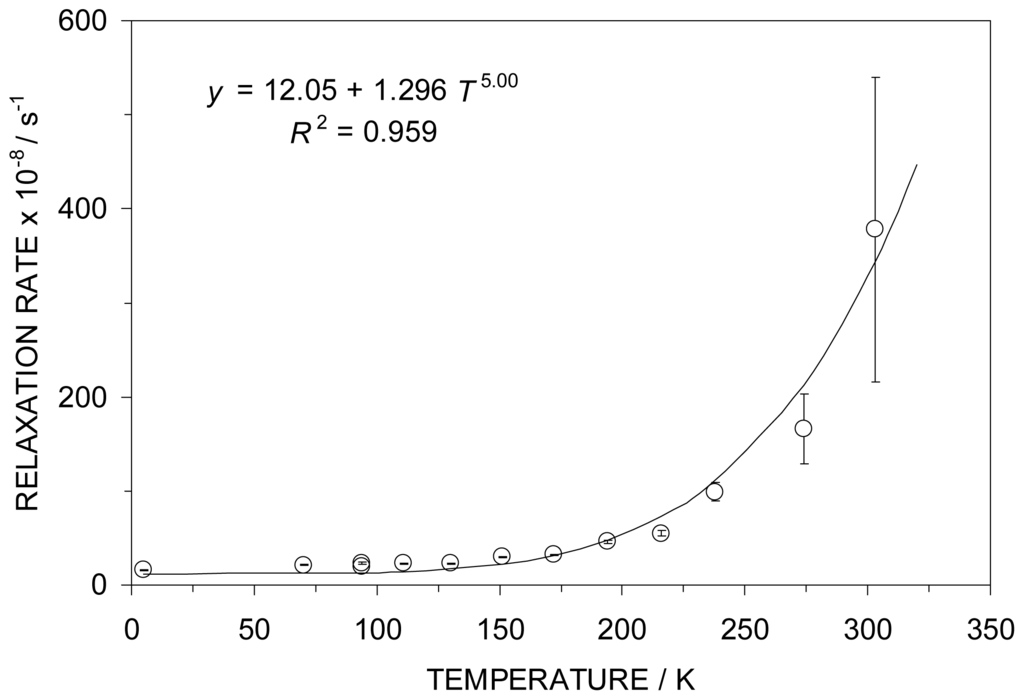
Figure 4.
The temperature-dependence of the relaxation rate (in arbitrary units) for the paramagnetic Fe atom in 4 obeys a power law which is 5th order in T, indicative of a Raman spin-lattice relaxation process.
As expected, X-ray crystallography of (unlabeled) 4 revealed a highly symmetrical tetragonal crystal lattice without specific interionic contacts. The ions are arranged along 4-fold rotoinversion axes in the direction of the crystallographic c axis. The crystallographic data are listed in Table 2. A view of the crystal packing at 123 K is depicted in Figure 5. Finally, a new survey of the CSD (June 2011) resulted in 366 FeCl4 − ions in 278 crystal structures, after coordinating or disordered anions were excluded, and gave an average Fe-Cl bond length of 2.184 Å and a Cl-Fe-Cl angle of 109.457° in more or less distorted tetrahedral geometries.

Table 2.
Crystal data and refinement details of 4 at different temperatures.
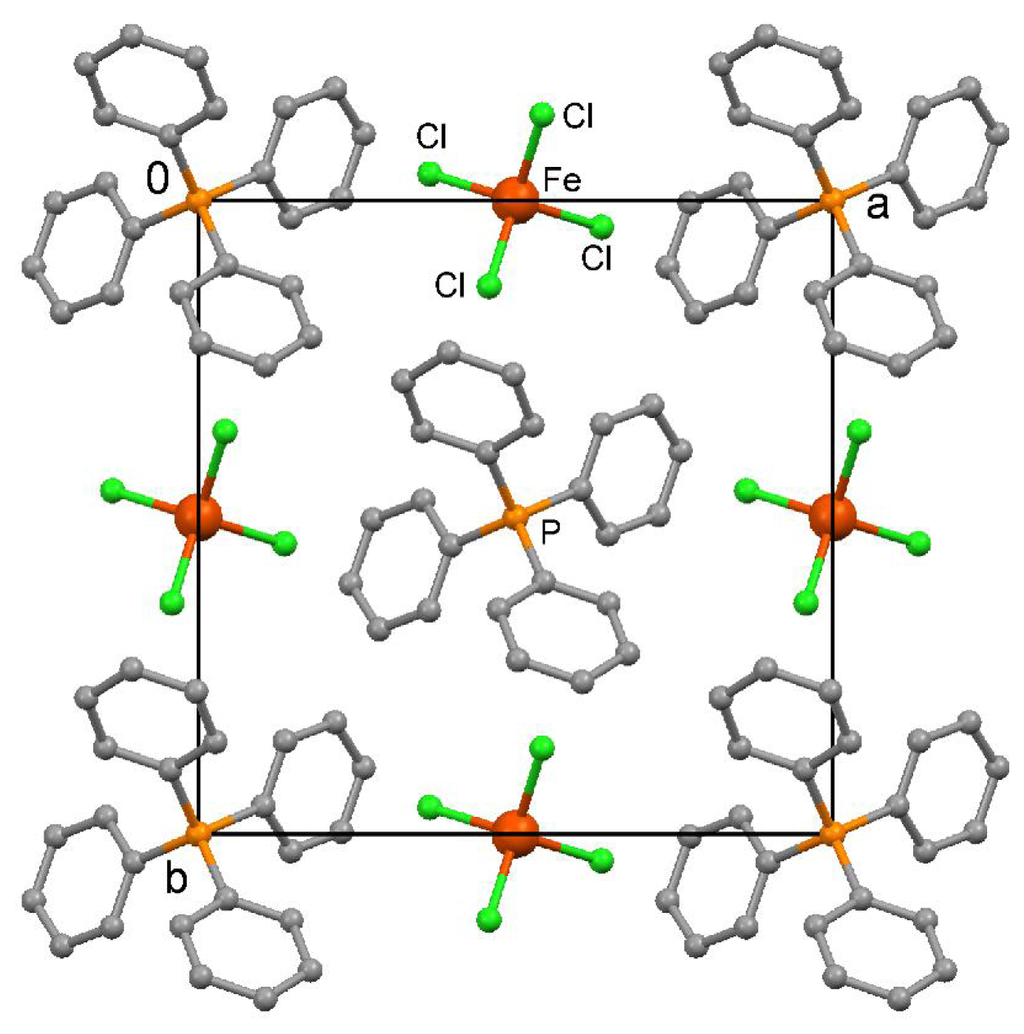
Figure 5.
View of the crystal packing in (Ph4P) (FeCl4) in the direction of the crystallographic c axis at 123 K. Fe-Cl bond length 2.1939(5) Å, Cl-Fe-Cl angles 105.51(2) and 113.47(3)°. All hydrogen atoms were omitted for clarity.
3. Experimental Section
57Fe powder (>95%) was purchased from Advanced Materials Technologies, Singapore. NMR spectra were recorded with a Varian Unity 500 spectrometer. Very broad signals were observed as expected from paramagnetic compounds. IR spectra were obtained with a Nicolet 5700 FT spectrometer; intensities are indicated as w = weak, m = medium, s = strong. HR MS were recorded with a Finnigan MAT 95 instrument.
3.1. General Procedure for the Synthesis of 1–4
Hydrochloric acid (0.55 mL, 12 M) was added to 57Fe powder (9.97 mg, 175 μmol). Hydrogen evolution started immediately, and the mixture was stirred for 48 hours at room temperature in contact with air. Then, the corresponding chloride salt of 1–4 (1 equation) was added to the solution, and the product separated (1, 2 as liquid; 3, 4 as solid). The mixture was stirred for another 15 minutes, and the product was extracted with dichloromethane. The solution was dried over MgSO4, filtered, and the solvent evaporated. Volatiles were removed in vacuum to give the hygroscopic products which were stored in Schlenk tubes under nitrogen.
3.2. (1-Butyl-3-Methylimidazolium) 57FeCl4 (1)
Yield: 43 mg (63%) as a brown liquid. 1H NMR (CD2Cl2, 500 MHz): δ 1.27, 1.84, 2.87, 3.81, 5.49, 7.47, 9.31. IR (neat): 3148 w, 3116 w, 3101 w, 2960 w, 2934 w, 2873 w, 1591 w, 1563 m, 1460 m, 1382 w, 1162 s, 1101 w, 830 m, 740 s, 648 w, 620 s cm−1. FAB MS [M+] m/z = 139.15 (th. 139.12). FAB MS [M−] m/z = 196.76, 198.76, 200.75, 202.75 (th. 196.81, 198.81, 200.81, 202.80).
3.3. (1-Allyl-3-Methylimidazolium) 57FeCl4 (2)
Yield: 46 mg (81%) of a yellow-brown oily liquid. 1H NMR (CD2Cl2, 500 MHz): δ 6.06, 6.73, 7.80, 9.07. IR (neat): ν = 3152 w, 3114 w, 3095 w, 1592 w, 1562 m, 1446 w, 1421 w, 1159 s, 1105 w, 990 w, 947 m, 830 m, 741 m, 674 w, 620 s cm−1.
3.4. (1-Methyl-1-Propylpyrrolidinium) 57FeCl4 (3)
Yield: 47 mg (82%) of a yellow solid. M.p. 97–104 °C. 1H NMR (CD2Cl2, 500 MHz): δ 2.95, 4.27. IR (neat): ν = 2971 m, 2939 w, 2879 w, 1458 s, 1303 w, 1261 w, 1038 w, 1002 m, 970 w, 936 m, 883 w, 817 w, 756 m cm−1. FAB MS [M+] m/z = 128.19 (th. 128.14).
3.5. (Tetraphenylphosphonium) 57FeCl4 (4)
Yield: 93 mg (98%) of a yellow solid. M.p. 200–208 °C. 1H NMR (CD2Cl2, 500 MHz): δ 8.02. IR (neat): ν = 1591 w, 1485 m, 1437 m, 1340 w, 1170 w, 1106 s, 996 m, 924 w, 742 w, 721 s, 683 s cm−1. FAB MS [M+] m/z = 339.11 (th. 339.13). FAB MS [M−] m/z = 196.77, 198.77, 200.76, 202.74 (th. 196.81, 198.81, 200.81, 202.80).
3.6. X-ray Crystallography
X-ray diffraction data of (unlabeled) 4 were acquired with an Oxford Diffraction Gemini-R Ultra diffractometer using ω scans at four temperatures (295, 223, 173, and 123 K). Intensity data were recorded using graphite-monochromated MoKα radiation (λ = 0.71073 Å) and refined on F2. Standard strategies were employed for data collection, cell refinement and data processing. Hydrogen atoms were placed in calculated positions. CCDC 792734-792737 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre [20].
3.7. Mössbauer Spectroscopy
Temperature-dependent Mössbauer experiments were carried out as previously described [21], using a 57Co(Rh) source in transmission geometry. Spectrometer calibration was effected using a 20 mg cm−2 α-Fe absorber at room temperature, and all isomer shifts are referred to the centroid of such spectra. Sample preparation depended on the nature of the sample. The liquid samples (1 and 2), which were supplied as small droplets resulting from the enriched 57Fe synthesis, were taken up in a few drops of CH2Cl2, transferred to a Perspex sample holder, and the excess solvent was removed by evaporation in a vacuum dessicator. These samples, consisting of thin films, were then rapidly cooled in liquid nitrogen and transferred cold into the cryostat. Solid samples (3 and 4) were mixed with a small amount of BN and transferred, as is, to Perspex sample holders. The temperature of the cryostat was monitored over the data collection intervals (up to 24 hours per temperature point) using the Daswin software [22]. The transmission counting rate was monitored before and after each temperature point to assure no sample loss. For compound 4, low temperature spectra at 5, 70, and 94 K were acquired using a closed cycle cryostat (Janis Model SHI-850-5) in transmission geometry.
4. Conclusions
In this study, four “magnetic ionic liquids” and related solids have been examined by temperature-dependent Mössbauer spectroscopy. In all cases, the common anionic constituent is the 57FeCl4 − tetrahedral structure, and the hyperfine and dynamical properties of this moiety have been elucidated over a significant temperature range. The spectra consist of a broad absorption which is characteristic of a paramagnetic iron site relaxing by spin-spin or spin-lattice processes. The IS values at 90 K fall into a narrow range (0.31–0.33 mm s−1) characteristic of high spin Fe(III). Similarly, the QS values occupy a narrow range and are comparable to values reported in the literature [12,23]. Neither of these two parameters appears particularly sensitive to the structure of the cationic part of the salts which evidently involve only weak interactions between the anion and the organic cation. The lattice dynamics of the metal center in tetraphenylphosphonium tetrachloroferrate(III) (5) have been determined over a wide temperature range, and the
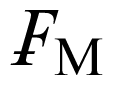 values have been found to be in excellent agreement with the
values have been found to be in excellent agreement with the
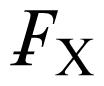 data extracted from single crystal X-ray data. The iron atom in this compound is found to relax by a Raman process, the relaxation rate depending on T~5.
data extracted from single crystal X-ray data. The iron atom in this compound is found to relax by a Raman process, the relaxation rate depending on T~5.
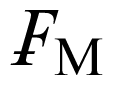 values have been found to be in excellent agreement with the
values have been found to be in excellent agreement with the
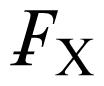 data extracted from single crystal X-ray data. The iron atom in this compound is found to relax by a Raman process, the relaxation rate depending on T~5.
data extracted from single crystal X-ray data. The iron atom in this compound is found to relax by a Raman process, the relaxation rate depending on T~5.Acknowledgments
We are grateful to T. Müller for the HR MS.
References
- De Pedro, I; Rojas, DP; Albo, J; Luis, P; Irabien, A; Blanco, JA; Fernandez, JR. Long-range magnetic ordering in magnetic ionic liquid: Emim[FeCl4]. J Phys Condens Matter 2010, 22, 296006:1–296006:4. [Google Scholar]
- Okuno, M; Hamaguchi, HO; Hayashi, S. Magnetic manipulation of materials in a magnetic ionic liquid. Appl Phys Lett 2006, 89, 132506:1–132506:2. [Google Scholar]
- Li, M; De Rooy, SL; Bwambok, DK; El-Zahab, B; Di Tusa, JF; Warner, IM. Magnetic chiral ionic liquids derived from amino acids. Chem. Commun 2009, 2009, 6922–6924. [Google Scholar]
- Lee, SH; Ha, SH; Ha, SS; Jin, HB; You, CY; Koo, YM. Magnetic behavior of mixture of magnetic ionic liquid [bmim]FeCl4 and water. J Appl Phys 2007, 101, 09J102:1–09J102:3. [Google Scholar]
- Lee, SH; Ha, SH; You, CY; Koo, YM. Recovery of magnetic ionic liquid [bmim]FeCl4 using electromagnet. Korean J. Chem. Eng 2007, 24, 436–437. [Google Scholar]
- Yoshida, Y; Saito, G. Influence of structural variations in 1-alkyl-3-methylimidazolium cation and tetrahalogenoferrate(III) anion on the physical properties of the paramagnetic ionic liquids. J. Mater. Chem 2006, 16, 1254–1262. [Google Scholar]
- Del Sesto, RE; McCleskey, TM; Burrell, AK; Baker, GA; Thompson, JD; Scott, BL; Wilkes, JS; Williams, P. Structure and magnetic behavior of transition metal based ionic liquids. Chem. Commun 2008, 4, 447–449. [Google Scholar]
- Mallick, B; Balke, B; Felser, C; Mudring, AV. Dysprosium room-temperature ionic liquids with strong luminescence and response to magnetic fields. Angew. Chem. Int. Ed 2008, 47, 7635–7638. [Google Scholar]
- Sitze, MS; Schreiter, ER; Patterson, EV; Freeman, RG. Ionic liquids based on FeCl3 and FeCl2. Raman scattering and ab initio calculations. Inorg. Chem 2001, 40, 2298–2304. [Google Scholar]
- Hayashi, S; Hamaguchi, H-O. Discovery of a magnetic ionic liquid [bmim]FeCl4. Chem. Lett 2004, 33, 1590–1591. [Google Scholar]
- Hayashi, S; Saha, S; Hamaguchi, HO. A new class of magnetic fluids: Bmim[FeCl4] and nbmim[FeCl4] ionic liquids. IEEE Trans. Magn 2006, 42, 12–14. [Google Scholar]
- James, BD; Juraja, SM; Liesegang, J; Reiff, WM; Skelton, BW; White, AH. 4-Piperidinylpyridinium tetrachloroferrate(III): Structure and low-temperature magnetic ordering. Inorg. Chim. Acta 2001, 312, 88–92. [Google Scholar]
- Partiti, CSM; Rechenberg, HR. Lattice and molecular dynamics in (C2H5)4NFeX4 (X = Cl, Br). Hyperfine Interactions 1992, 70, 1075–1078. [Google Scholar]
- Edwards, PR; Johnson, CE. Mössbauer hyperfine interactions in tetrahedral Fe(III) ions. J. Chem. Phys 1968, 49, 211–216. [Google Scholar]
- Wyrzykowski, D; Maniecki, T; Pattek-Janczyk, A; Stanek, J; Warnke, Z. Thermal analysis and spectroscopic characteristics of tetrabutylammonium tetrachloroferrate(III). Thermochim. Acta 2005, 435, 92–98. [Google Scholar]
- Herber, RH. Chemical Mössbauer Spectroscopy; Herber, RH, Ed.; Plenum Press: New York, NY, USA, 1984; p. 199. [Google Scholar]
- Herber, RH; Nowik, I. Hyperfine interactions and metal atom dynamic effects of pentafluorophenyl substituents on ferrocene complexes. J. Nucl. Radiochem. Sci 2008, 9, 33–36. [Google Scholar]
- Wickman, HH; Wertheim, GK. Chemical Applications of Mössbauer Spectroscopy; Goldanskii, VI, Herber, RH, Eds.; Plenum Press: New York, NY, USA, 1968; Volume Chapter 11, pp. 548–621. [Google Scholar]
- Abragam, A; Bleany, B. Electron Paramagnetic Resonance of Transition Ions; Clarendon Press: Oxford, UK, 1970; pp. 560–561. [Google Scholar]
- CCDC CIF Depository Request Form for Data Published from 1994; CCDC: Cambridge, UK. Available online: http://www.ccdc.cam.ac.uk/data_request/cif accessed on 4 August 2011.
- Herber, RH; Nowik, I. Metal atom dynamics in organometallics: Vibrational amplitude determination for bis phosphino ferrocenes including a waxy system. J. Organomet. Chem 2008, 693, 3007–3010. [Google Scholar]
- Data Acquisition Software—No Cost, Universal, and Easy-to-Use; MegaDaq Homepage. Available online: http://www.megadaq.com accessed on 20 September 2011.
- Clausen, CA, III; Good, ML. Mössbauer Effect Methodology; Gruverman, IJ, Ed.; Plenum Press: NY, USA, 1968; Volume 4, pp. 187–200. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).