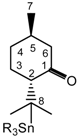
Abstract
Trialkyltin menthones of type 2 are obtained selectively by 1,4-addition of trialkylstannyl lithium to (-)-pulegone. Reduction of 2 with borane in THF using as catalyst the reagent prepared from borane and (S)-valinol gave a mixture of the corresponding trialkyltin alcohols 3 (Me: 84%; n-Bu: 90,6%) and 4 (Me: 16% and n-Bu: 9,4%).
Introduction
Taking into account the excellent results obtained with the (-)-8-phenylmenthyl group as a chiral auxiliary, we considered of interest the synthesis of some organotin analogues. The 8-triorganotinmenthyl moiety might affect the stereoselectivity due to its bulk and also to electronic effects. The stereoselective synthesis of these compounds was carried out according to Scheme 1 and Scheme 2.
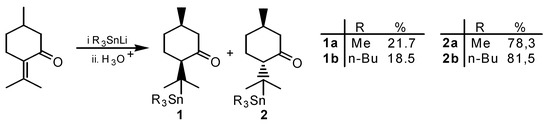
Scheme 1.
1,4-Addition of trialkylstannyl lithium to (-)-pulegone.
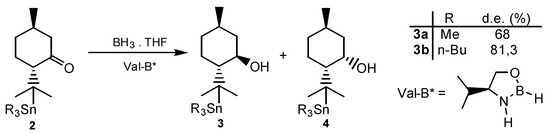
Scheme 2.
Stereoselective reduction of trialkylstannylmenthones of type 2.
Experimental
The 1,4-addition of trimethyl- and tri-n-butyl lithium to (-)-pulegone led to menthones of type 1 and 2 with an average yield of 72% following standard techniques [1]. Compounds 1 and 2 were separated by column chromatography (silica gel 60). The reduction of type 2 ketones with borane in THF using (S)-valinol as a catalyst was carried out according to known procedures [2].
Results and Discussion
The reduction of (-)-menthone carried out with the reagent prepared from borane and (S)-valinol in THF in order to determine the degree of asymmetric induction which can be achieved with this reagent, yielded quantitatively a mixture of (-)-menthol (80%) and (+)-neo-menthol (20%), i.e., 60% of diastereoisomeric excess (d.e.).
Under the same reaction conditions, the reduction of 2a (d.e. 68%) and 2b (d.e. 81,3%) led to the corresponding 8-trialkylstannylmenthols with better diastereoisomeric excesses.
Acknowledgements:
This work was supported by CONICET (Buenos Aires), CIC (Provincia de Buenos Aires) and Universidad Nacional del Sur (Bahía Blanca, Argentina).
References and Notes
- Radivoy, G.E. Doctor in Chemistry Thesis, Universidad Nacional del Sur, 1997.
- Itsuno, S.; Nakano, M.; Miyazaky, K; Masuda, H.; Ito, K.; Hirao, A; Nakahama, S. Asymmetric Synthesis Using Chirally Modified Borohydrides. Part 3. Enantioselective Reduction of Ketones and Oxime Ethers with Reagents Prepared from Borane and Chiral Amino Alcohols. J. Chem. Soc. Perkin Trans. I 1985, 2039. [Google Scholar] [CrossRef]