Abstract
The synthesis of 2β-(heterocyclylthiomethyl)penam derivatives on solid support has been developed. Compounds are obtained in good to high yields (based on loading of the original resin). The key step is the solid-phase double rearrangement of the corresponding penicillin sulfoxide.
Introduction
The impact of combinatorial chemistry of small molecules on the drug discovery process is now widely recognized by the scientific community [1]. Solid-phase organic synthesis (SPOS) is a valuable tool for the generation of structurally diverse compounds for combinatorial libraries.
In our work dealing with the solid-phase synthesis of biologically interested compounds, we have developed methodologies for tethering funtionalized polystyrene resins to penicillin derivatives. Our research has also established a new, mild and efficient procedure for the removal of sensitive molecules from Merrifield and Wang resins, using aluminum chloride (AlCl3) [2].
Results and discussion
Heterocyclic thio substituents have been identified as pharmacophores in β−lactam chemistry, particularly with activity against methicillin-resistant staphylococcus aureus (MRSA) [3]. Thus, we considered the solid-phase synthesis of 2β-(heterocyclylthio)methyl substituted penicillins as a rapid and efficient method for the generation of combinatorial libraries.
The key step of this synthesis of the double rearrangement of sulfoxide 1 (Scheme 1). The thermal rearrangement of 1 generates the sulfenic acid which is trapped by the corresponding heterocyclic thiol (Het-SH) to give the disulfide intermediate 2. Then, a new rearrangement rebuilds the thiazolidine ring to obtain the 2β-(heterocyclylthio)methyl penams (3).
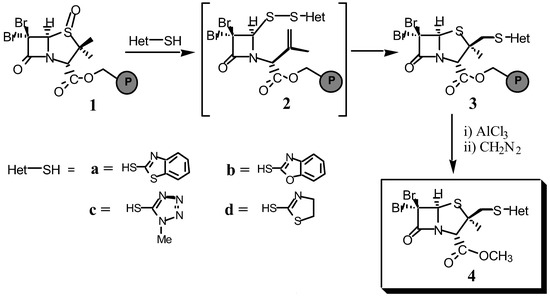
Scheme 1.
This work began with the immobilization of penam derivative onto Merrifield resin and oxidation with m-chloroperbenzoic acid (MCPBA, 1.4 equiv.) to obtain the resin-bound sulfoxide 1. These reactions were monitored by FT-IR. In the case of the reaction of sulfoxide 1 with 2-mercaptobenzothiazole (2-MBT) (a) in the presence of catalytic amounts of p-toluenesulfonic acid, the resin-bound 2β−(benzothiazol-2-yl)thiomethyl derivative (3a) was obtained. After cleavage with AlCl3 and esterification with diazomethane, compound 4a was obtained with an overall yield of 45% (based on initial loading of the Merrifield resin).
The versatility of this methodology has been demonstrated by the synthesis of different 2β-(heterocyclylthio)methyl penams. For example, using 2-mercaptobenzoxazole (b), the Merrifield resinbound 2β−(benzoxazol-2-yl)thiomethyl derivative (3b) was obtained. After cleavage and esterification, compound 3b was transformed into the ester 4b (overall yield: 50%). Similarly, a series of closely related derivatives have been prepared with overall yields ranging from 45 to 55%.
Acknowledgments
Financial support from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina; The Royal Society of Chemistry (U.K.); Agencia de Cooperación Iberoamericana (Spain); Fundación Antorchas (Argentina); Universidad Nacional de Rosario (Argentina) and Asociación Prociencia de Rosario (Argentina) is gratefully acknowledged.
References and Notes
- A Practical Guide to Combinatorial Chemistry; DeWitt, S.H.; Czarnik, A.W. (Eds.) ACS Books: Washington, 1997. Bunin, B.A. The Combinatorial Index; Academic Press: San Diego, 1998. [Google Scholar]
- Mata, E.G. Tetrahedron Lett. 1997, 38, 6335. [CrossRef]
- Hecker, S.J. Journal of Antibiot. 1998, 51, 722. [CrossRef]