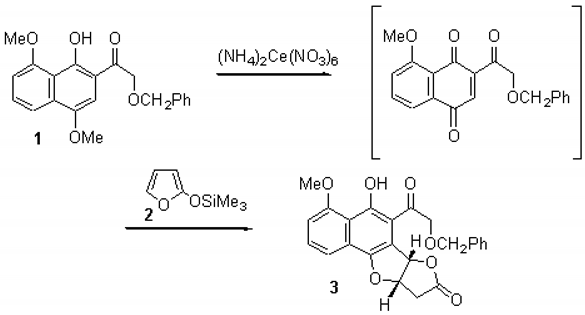
A solution of ceric ammonium nitrate (37 mg, 0.067 mmol) in water was added dropwise to a vigorously stirred solution of naphthol 1 (13 mg, 0.036 mmol) [1] in acetonitrile (2.2 mL) at room temperature and stirred for 15 min. Anhydrous magnesium sulfate was added and the resultant suspension immediately cooled to 0°C. After 1min., a solution of 2-trimethylsilyloxyfuran 2 (0.012 mL, 0.071 mmol) in acetonitrile (0.2 mL) was added dropwise and the resultant solution stirred at 0°C for 30 min. The reaction mixture was diluted with dichloromethane (5 mL), washed with water (2 × 3 mL) and dried over magnesium sulfate. The solvent was removed under reduced pressure to give an orange oil, which was then purified by flash chromatography using light petroleum-ethyl acetate (4:1) as eluent to afford the title compound 3 (9 mg, 60%) as a yellow oil.
IR (cm−1, neat): 3320w, 1785s, 1731, 1077.
1H NMR (200 MHz, CDCl3): 3.10–3.12 (2H, m, H9), 4.12 (3H, s, OMe), 4.67 (1H, d, Jgem 12.0 Hz, OCHAPh), 4.77 (1H, d, Jgem 18.1 Hz, COCHA), 4.88 (1H, d, Jgem 12.0 Hz, OCHBPh), 4.95 (1H, d, Jgem 18.1 Hz, COCHB), 5.43–5.49 (1H, m, H9a), 6.80 (1H, d, J6b,9a 6.1 Hz, H6b), 6.98 (1H, J3,2 7.0 and J3,1 2.0 Hz, H3), 7.28–7.60 (7H, m, H1, H2, Ph), 10.46 (1H, s, OH).
EI-MS: 420 (M+, 2%), 299 (M-CH2OBn, 25), 269 (M-C9H11O2, 13), 149 (COCH2OBn, 15), 91 (C7H7, 100), 57 (CH3CH2CO, 67), 43 (CH3CO, 54).
Anal. calc. for C24H20O7 MH+ (CI, NH3), 421.1286; found MH+, 421.1287.
Supplementary Materials
Reference
- Brimble, M.A.; Oppen, E. Synth. Commun. 1997, 27, 989–1007. [CrossRef]
Sample Availability: Available from the authors. |
© 2000 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/.