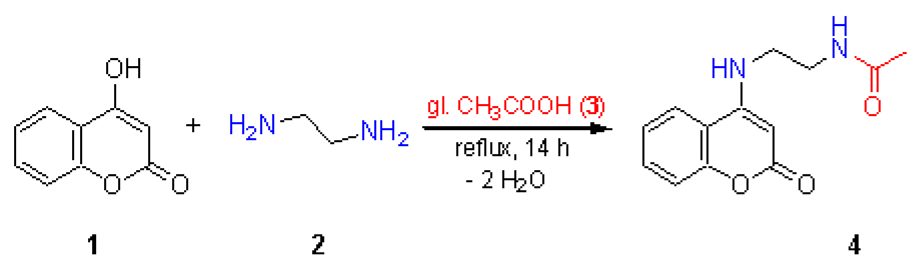
The direct synthesis of 4-(monoalkylamino)coumarins from primary amines [1] was developed on the basis of the reaction of 4-hydroxycoumarin with ammonium acetate in acetic acid as reported by Joshi et al. [2]. The title compound 4 was synthesized in a similar way from 4-hydroxycoumarin (1) and ethylene diamine (2) in boiling glacial acetic acid (3). A simultaneous N-acetylation of the second amino group took place unexpectedly. Compound 4 has not been previously reported and we give here its detailed characterization.
To 4-hydroxycoumarin (1, 1.62 g, 10 mmol) in glacial acetic acid (3, 30 ml, 0.53 mol), ethylene diamine (2, 12.0 g, 0.2 mol) was added under stirring, the mixture was heated at reflux for 14 h and then poured under stirring into 75 ml of water. The resulting precipitate was filtered and washed with hot water (2 x 10 ml). The solid was stirred with ether (20 ml) for 10 min, filtered, washed with little ether and dried at 90-100 °C to yield 1.85 g (75 %) of chromatographically homogeneous 4 as almost colorless crystals with m.p. 261-262 °C (TLC: silica gel 60 F254 Merck aluminium sheets, elution by chloroform/acetone/methanol 6:4:1, vol. parts).
After recrystallization from ethanol: colorless needles, m.p. 262-263 °C.
FT-IR (Nujol, Shimadzu): 3333 (NH), 3271 (NH), 1684 (C=O), 1653 (C=O), 1609, 1557, 1327, 1262, 1223, 1196, 1150, 1080, 1040, 938, 797, 764, 752, 722, 695 cm-1.
1H-NMR (DMSO-d6, 250 MHz): 1.82 (s, 3H, COCH3), 3.25-3.31 (m, 4H, NCH2CH2N), 5.21 (s, 1H,
3-H), 7.31 (m, 2H, 6-H,8-H arom.), 7.58 (m, 1H, 7-H arom.), 7.75 (broad t, 1H, 4-NH), 7.94 (m, 1H, 5-H arom.), 8.11 (broad t, 1H, NH-CO).
13C-NMR (DMSO-d6, 62.5 MHz): 22.6 (CH3), 36.9 (CH2), 42.2 (CH2), 81.3 (C-3), 114.3 (C-4a), 116.9
(C-8), 122.2 (C-5), 123.3 (C-6), 131.9 (C-7), 153.0 (C-8a or C-4), 153.1 (C-4 or C-8a), 161.5 (C-2), 170.1 (NH-CO).
EI-MS (70 eV, m/z (%)): 246 (51, M+), 188 (9), 187 (67), 186 (26), 175 (18), 174 (100), 162 (46), 159 (28), 146 (24), 145 (10), 118 (12), 107 (14), 91 (10), 89 (14), 73 (11), 43 (19), 30 (22).
HR-MS: Mol. mass calcd. for C13H14N2O3 246.10044; Found 246.1006.
Anal. Calcd. for C13H14N2O3 (246.26): C 63.40, H 5.73, N 11.38; Found: C 63.42, H 5.77, N 11.41.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/xxx/s1.
References
- Ivanov, I. C.; Karagiosov, S. K.; Manolov, I. Arch. Pharm. (Weinheim, Germany) 1991, 324, 61–62.
- Joshi, S. D.; Sakhardande, V. D.; Seshadri, S. Indian J. Chem. Sect. B 1984, 23 (3), 206–208.
Sample Availability: Available from MDPI. |
© 1999 MDPI. All rights reserved. Molecules website www.mdpi.org/molecules/.