Targeting Ocular Biofilms with Plant-Derived Antimicrobials in the Era of Antibiotic Resistance
Abstract
1. Introduction
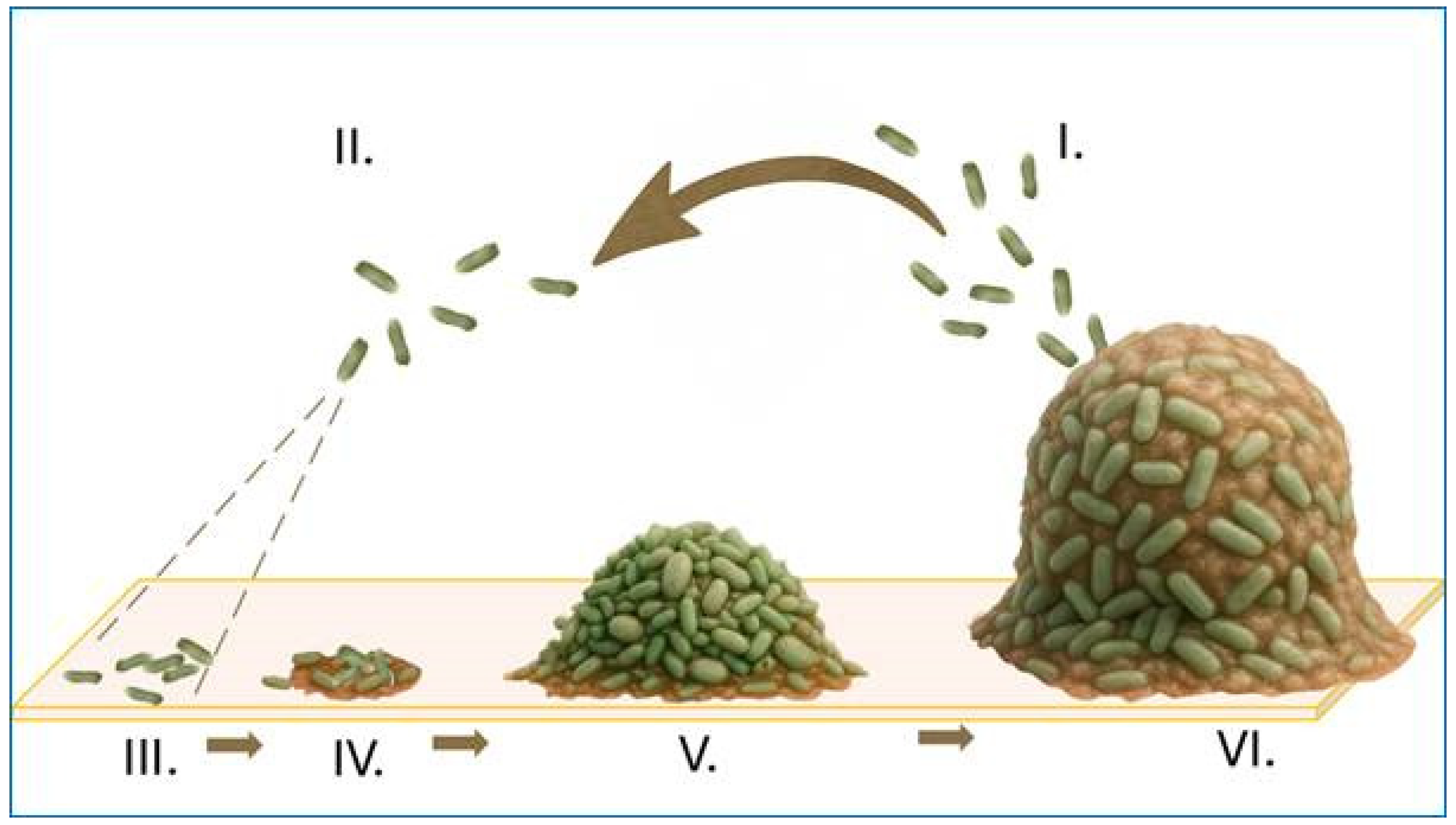
2. Bacterial Biofilms in Ophthalmic Infections
3. Biofilms in Device-Associated Ocular Infections
4. Conventional Antibiotic Therapy in Ocular Infections
5. Challenges of Local Treatment in Ophthalmic Infections
6. Emerging Strategies in Ophthalmic Infection Treatment
7. Plant-Derived Antimicrobials in Ocular Infection Management
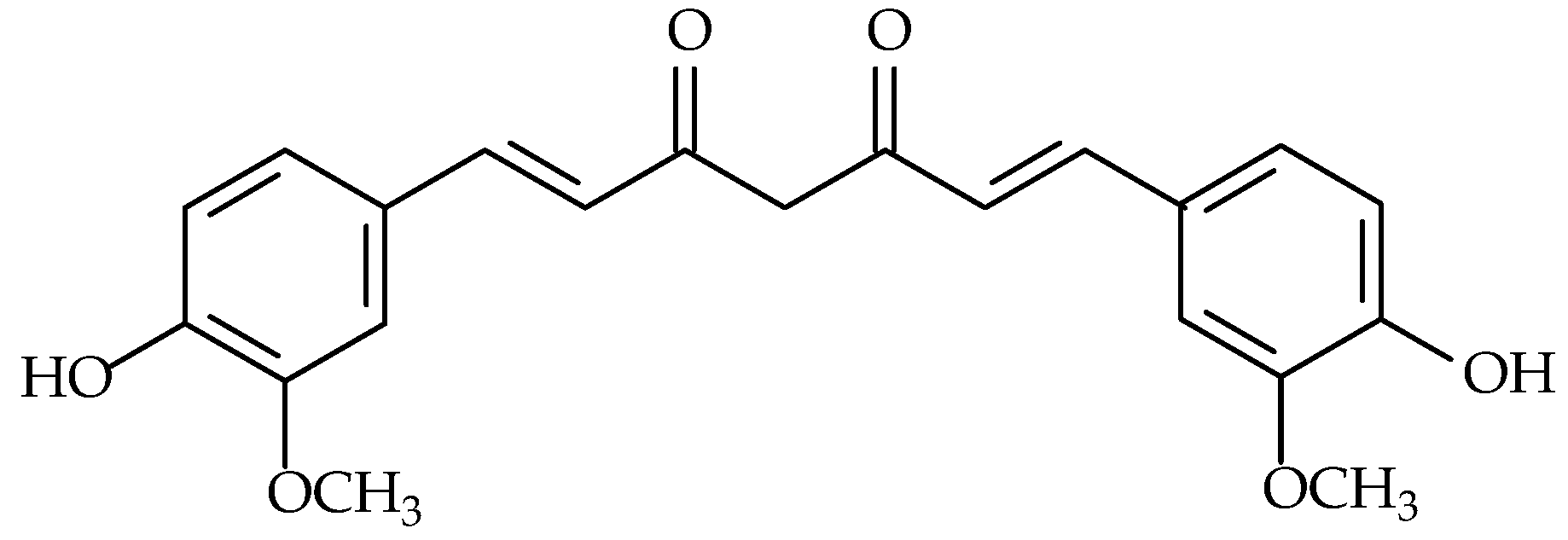
7.1. Resveratrol
7.2. Curcumin
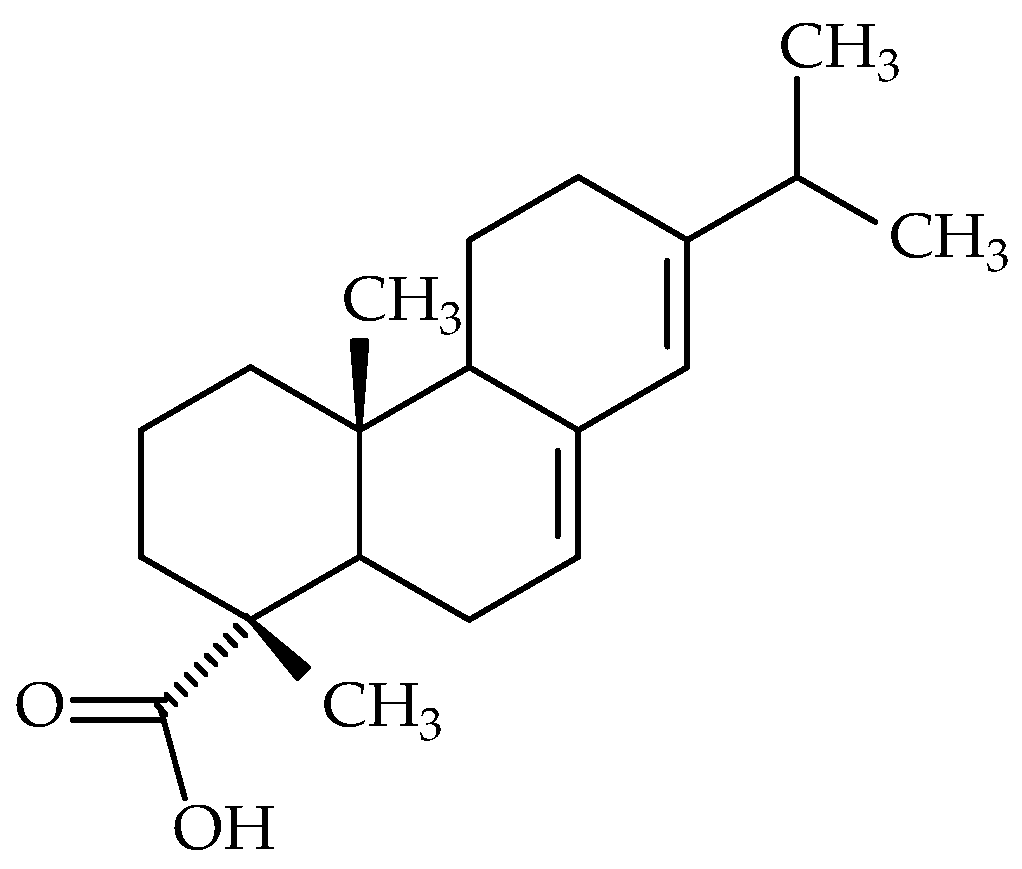
7.3. Abietic Acid
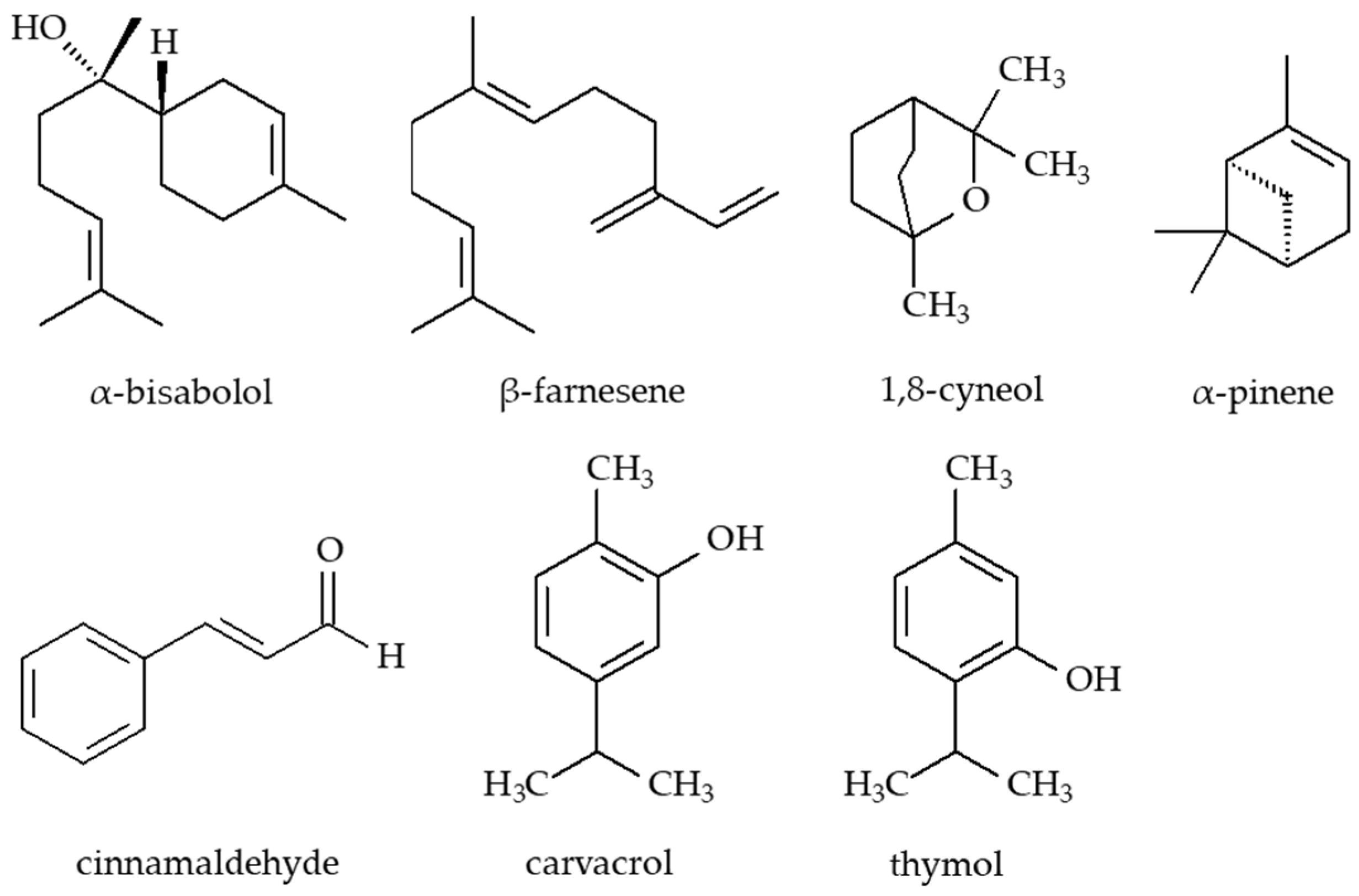
7.4. Essential Oils and Their Active Constituents
8. Limitations: A Critical Analysis
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teweldemedhin, M.; Saravanan, M.; Gebreyesus, A.; Gebreegziabiher, D. Ocular bacterial infections at Quiha Ophthalmic Hospital, Northern Ethiopia: An evaluation according to the risk factors and the antimicrobial susceptibility of bacterial isolates. BMC Infect. Dis. 2017, 17, 207. [Google Scholar] [CrossRef]
- Teweldemedhin, M.; Gebreyesus, H.; Atsbaha, A.H.; Asgedom, S.W.; Saravanan, M. Bacterial profile of ocular infections: A systematic review. BMC Ophthalmol. 2017, 17, 212. [Google Scholar] [CrossRef] [PubMed]
- Grandi, G.; Bianco, G.; Boattini, M.; Scalabrin, S.; Iannaccone, M.; Fea, A.; Cavallo, R.; Costa, C. Bacterial etiology and antimicrobial resistance trends in ocular infections: A 30-Year Study, Turin Area, Italy. Eur. J. Ophthalmol. 2021, 31, 405–414. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Ali, M.M.; Zenebe, M.H. Bacterial etiology of ocular and periocular infections, antimicrobial susceptibility profile and associated factors among patients attending eye unit of Shashemene comprehensive specialized hospital, Shashemene, Ethiopia. BMC Ophthalmol. 2020, 20, 124. [Google Scholar] [CrossRef]
- Perween, N.; Bisht, D.; Aggarwal, P. Bacterial conjunctivitis: Microbiological profile, antimicrobial susceptibility patterns and recommendations for treatment. J. Commun. Dis. 2016, 48, 18–22. [Google Scholar]
- Deepthi, K.G.; Prabagaran, S.R. Ocular bacterial infections: Pathogenesis and diagnosis. Microb. Pathog. 2020, 145, 104206. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, M.; Zeppieri, M.; Rammohan, G. Blepharitis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bharathi, M.J.; Ramakrishnan, R.; Maneksha, V.; Shivakumar, C.; Nithya, V.; Mittal, S. Comparative bacteriology of acute and chronic dacryocystitis. Eye 2008, 22, 953–960. [Google Scholar] [CrossRef]
- Prost, M.; Filipek, B. Kliniczna Farmakologia Okulistyczna. In Leki Stosowane w Leczeniu Chorób Infekcyjnych; Jachowicz, R., Nowak, J.Z., Eds.; Elsevier: Wrocław, Poland, 2013. [Google Scholar]
- Ta, C.N.; Chang, R.T.; Singh, K.; Egbert, P.R.; Shriver, E.M.; Blumenkranz, M.S.; de Kaspar, H.M. Antibiotic resistance patterns of ocular bacterial flora. Ophthalmology 2003, 110, 1946–1951. [Google Scholar] [CrossRef]
- Weiss, A.; Brinser, J.H.; Nazar-Stewart, V. Acute conjunctivitis in childhood. J. Pediatr. 1993, 122, 10–14. [Google Scholar] [CrossRef]
- Diamant, J.I.; Hwang, D.G. Therapy for bacterial conjunctivitis. Ophthalmol. Clin. 1999, 12, 15–20. [Google Scholar] [CrossRef]
- Yannof, M. Ophthalmology. In Disorders of the Conjunctiva and Limbus; Duker, J.S., Ed.; Mosby: Maryland Heights, MO, USA, 2004. [Google Scholar]
- Epling, J.; Smucny, J. Bacterial conjunctivitis. Clin. Evid. 2005, 14, 756–761. [Google Scholar]
- Golde, K.T.; Gardiner, M.F. Bacterial conjunctivitis in children. Int. Ophthalmol. Clin. 2011, 51, 85–92. [Google Scholar] [CrossRef]
- Morrow, G.L.; Abbott, R.L. Conjunctivitis. Am. Fam. Physician 1998, 57, 735–746. [Google Scholar]
- Ohnsman, C.M. Exclusion of students with conjunctivitis from school: Policies of state departments of health. J. Pediatr. Ophthalmol. Strabismus 2007, 44, 101–105. [Google Scholar] [CrossRef]
- Sheikh, A.; Hurwitz, B. Topical antibiotics for acute bacterial conjunctivitis: A systematic review. Br. J. Gen. Pract. 2001, 51, 473–477. [Google Scholar]
- Dydak, K.; Oleksy, M.; Bartoszewicz, M. Eradykacja szczepów MRSA u pacjentów hospitalizowanych. Forum Zakażeń 2018, 9, 143–148. [Google Scholar] [CrossRef]
- Galentine, P.G.; Cohen, E.J.; Laibson, P.R.; Adams, C.P.; Michaud, R.; Arentsen, J.J. Corneal ulcers associated with contact lens wear. Arch. Ophthalmol. 1984, 102, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Fulcher, T.P.; Dart, J.K.G.; McLaughlin-Borlace, L.; Howes, R.; Matheson, M.; Cree, I. Demonstration of biofilm in infectious crystalline keratopathy using ruthenium red and electron microscopy. Ophthalmology 2001, 108, 1088–1092. [Google Scholar] [CrossRef]
- Kumar, D.A.; Agarwal, A. Glued intraocular lens. Curr. Opin. Ophthalmol. 2013, 24, 21–29. [Google Scholar] [CrossRef]
- Taban, M. Acute endophthalmitis following cataract surgery. Arch. Ophthalmol. 2005, 123, 613. [Google Scholar] [CrossRef] [PubMed]
- Vafidis, G.C.; Marsh, R.J.; Stacey, A.R. Bacterial contamination of intraocular lens surgery. Br. J. Ophthalmol. 1984, 68, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, H. Biocompability of intraocular lens materials. Chin. J. Tissue Eng. Res. 2013, 17, 4745–4750. [Google Scholar] [CrossRef]
- West, E.S.; Behrens, A.; McDonnell, P.J.; Tielsch, J.M.; Schein, O.D. The incidence of endophthalmitis after cataract surgery among the U.S. medicare population increased between 1994 and 2001. Ophthalmology 2005, 112, 1388–1394. [Google Scholar] [CrossRef]
- Fritsch, L.N.; Dias, A.L.T.; Silva, N.C.; Fernandes, G.J.M.; Ribeiro, F.B.d.A.O. Comparative analysis of biofilm formation by Candida albicans and Candida krusei in different types of contact lenses. Arq. Bras. Oftalmol. 2022, 85, 235–239. [Google Scholar] [CrossRef]
- Brown, L.; Leck, A.K.; Gichangi, M.; Burton, M.J.; Denning, D.W. The global incidence and diagnosis of fungal keratitis. Lancet Infect. Dis. 2021, 21, e49–e57. [Google Scholar] [CrossRef]
- Danielescu, C.; Stanca, H.T.; Iorga, R.E.; Darabus, D.M.; Potop, V. The diagnosis and treatment of fungal endophthalmitis: An update. Diagnostics 2022, 12, 679. [Google Scholar] [CrossRef]
- Junka, A.; Żywicka, A.; Chodaczek, G.; Dziadas, M.; Czajkowska, J.; Duda-Madej, A.; Bartoszewicz, M.; Mikołajewicz, K.; Krasowski, G.; Szymczyk, P.; et al. Potential of biocellulose carrier impregnated with essential oils to fight against biofilms formed on hydroxyapatite. Sci. Rep. 2019, 9, 1256. [Google Scholar] [CrossRef]
- Cabrera-Aguas, M.; Chidi-Egboka, N.; Kandel, H.; Watson, S.L. Antimicrobial resistance in ocular infection: A review. Clin. Exp. Ophthalmol. 2024, 52, 258–275. [Google Scholar] [CrossRef]
- Solano, C.; Echeverz, M.; Lasa, I. Biofilm dispersion and quorum sensing. Curr. Opin. Microbiol. 2014, 18, 96–104. [Google Scholar] [CrossRef]
- Bartoszewicz, M.; Rygiel, A. Biofilm jako podstawowy mechanizm zakażenia miejsca operowanego—Metody prewencji w leczeniu miejscowym. Chir. Pol. 2006, 8, 171–178. [Google Scholar]
- Lasa, I.; Penadés, J.R. Bap: A family of surface proteins involved in biofilm formation. Res. Microbiol. 2006, 157, 99–107. [Google Scholar] [CrossRef]
- Pepose, J.S.; Wilhelmus, K.R. Divergent approaches to the management of corneal ulcers. Am. J. Ophthalmol. 1992, 114, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, G.M.; Bison, A.L. Infections associated with indwelling devices: Infections related to extravascular devices. Antimicrob. Agents Chemother. 1989, 33, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D.P.; Harmis, N.; Cowell, B.A.; Williams, T.; Holden, B.A. Bacterial interactions with contact lenses; effects of lens material, lens wear and microbial physiology. Biomaterials 2001, 22, 3235–3247. [Google Scholar] [CrossRef]
- Yi, X.; Wang, Y.; Yu, F.S. Corneal epithelial tight junctions and their response to lipopolysaccharide challenge. Investig. Ophthalmol. Vis. Sci. 2000, 41, 4093–4100. [Google Scholar]
- Zegans, M.E.; Shanks, R.M.Q.; O’toole, G.A. Bacterial biofilms and ocular infections. Ocul. Surf. 2005, 3, 73–80. [Google Scholar] [CrossRef]
- Hammersmith, K.M. Diagnosis and management of Acanthamoeba keratitis. Curr. Opin. Ophthalmol. 2006, 17, 327–331. [Google Scholar] [CrossRef]
- Keay, L.J.; Gower, E.W.; Iovieno, A.; Oechsler, R.A.; Alfonso, E.C.; Matoba, A.; Colby, K.; Tuli, S.S.; Hammersmith, K.; Cavanagh, D.; et al. Clinical and microbiological characteristics of fungal keratitis in the united states, 2001–2007: A multicenter study. Ophthalmology 2011, 118, 920–926. [Google Scholar] [CrossRef]
- Oechsler, R.A.; Feilmeier, M.R.; Miller, D.; Shi, W.; Hofling-Lima, A.L.; Alfonso, E.C. Fusarium keratitis: Genotyping, in vitro susceptibility and clinical outcomes. Cornea 2013, 32, 667–673. [Google Scholar] [CrossRef]
- Ritterband, D.C.; Seedor, J.A.; Shah, M.K.; Koplin, R.S.; McCormick, S.A. Fungal keratitis at the new york eye and ear infirmary. Cornea 2006, 25, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Meisler, D.M.; Langston, R.H.S.; Naab, T.J.; Aaby, A.A.; McMahon, J.T.; Tubbs, R.R. Infectious crystalline keratopathy. Am. J. Ophthalmol. 1984, 97, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Yaguchi, C.; Miyai, T.; Miyata, K.; Mineo, S.; Nakamura, M.; Amano, S. Detection of Streptococcus species by polymerase chain reaction in infectious crystalline keratopathy. Cornea 2006, 25, 1227–1230. [Google Scholar] [CrossRef]
- Reiss, G.R.; Campbell, R.J.; Bourne, W.M. Infectious crystalline keratopathy. Surv. Ophthalmol. 1986, 31, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Elder, M.J.; Matheson, M.; Stapleton, F.; Dart, J.K.G. Biofilm formation in infectious crystalline keratopathy due to Candida albicans. Cornea 1996, 15, 301–304. [Google Scholar] [CrossRef]
- Cheng, K.H.; Leung, S.L.; Hoekman, H.W.; Beekhuis, W.H.; Mulder, P.G.; Geerards, A.J.; Kijlstra, A. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet 1999, 354, 181–185. [Google Scholar] [CrossRef]
- Schein, O.D.; McNally, J.J.; Katz, J.; Chalmers, R.L.; Tielsch, J.M.; Alfonso, E.; Bullimore, M.; O’Day, D.; Shovlin, J. The incidence of microbial keratitis among wearers of a 30-day silicone hydrogel extended-wear contact lens. Ophthalmology 2005, 112, 2172–2179. [Google Scholar] [CrossRef]
- Fazly Bazzaz, B.S.; Jalalzadeh, M.; Sanati, M.; Zarei-Ghanavati, S.; Khameneh, B. Biofilm formation by Staphylococcus epidermidis on foldable and rigid intraocular lenses. Jundishapur. J. Microbiol. 2014, 7, e10020. [Google Scholar] [CrossRef]
- Pathengay, A.; Flynn, H.W.; Isom, R.F.; Miller, D. Endophthalmitis outbreaks following cataract surgery: Causative organisms, etiologies, and visual acuity outcomes. J. Cataract Refract. Surg. 2012, 38, 1278–1282. [Google Scholar] [CrossRef]
- Perkins, R.E.; Kundsin, R.B.; Pratt, M.V.; Abrahamsen, I.; Leibowitz, H.M. Bacteriology of normal and infected conjunctiva. J. Clin. Microbiol. 1975, 1, 147–149. [Google Scholar] [CrossRef]
- Prost, M. Kliniczna Farmakologia Okulistyczna, 1st ed.; Jachowicz, R., Nowak, J.Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Prost, M.; Oleszczańska-Prost, E. Okulistyka Dziecięca, 1st ed.; Medical Education: Warszawa, Poland, 2019. [Google Scholar]
- Sanfilippo, C.M.; Morrissey, I.; Janes, R.; Morris, T.W. Surveillance of the activity of aminoglycosides and fluoroquinolones against ophthalmic pathogens from Europe in 2010–2011. Curr. Eye Res. 2015, 41, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, G.; Danesh-Meyer, H.; Goldberg, I.; Kampik, A. Chirurgia Okulistyczna, 4th ed.; Szaflik, J., Kampik, A., Eds.; Edra Urban & Partner: Wrocław, Poland, 2016. [Google Scholar]
- Wong, C.A.; Galvis, V.; Tello, A.; Villareal, D.; Rey, J.J. Susceptibilidad antibiótica in vitro a fluoroquinolonas. Arch. Soc. Esp. Oftalmol. 2012, 87, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.M.; Figueiras, A.; Veiga, F. Improvements in topical ocular drug delivery systems: Hydrogels and contact lenses. J. Pharm. Pharm. Sci. 2015, 18, 683–695. [Google Scholar] [CrossRef]
- Real, D.A.; Bolaños, K.; Priotti, J.; Yutronic, N.; Kogan, M.J.; Sierpe, R.; Donoso-González, O. Cyclodextrin-modified nanomaterials for drug delivery: Classification and advances in controlled release and bioavailability. Pharmaceutics 2021, 13, 2131. [Google Scholar] [CrossRef]
- Harris, A. Leki Generyczne w Okulistyce; WMW Górnicki: Wrocław, Poland, 2013. [Google Scholar]
- Niemirowicz, K.; Car, H. Nanonośniki jako nowoczesne transportery w kontrolowanym dostarczaniu leków. Chemik 2012, 66, 868–881. [Google Scholar]
- Asbell, P.A.; Sanfilippo, C.M.; Sahm, D.F.; DeCory, H.H. Trends in antibiotic resistance among ocular microorganisms in the United States from 2009 to 2018. JAMA Ophthalmol. 2020, 138, 439. [Google Scholar] [CrossRef]
- Maurice, N.M.; Bedi, B.; Sadikot, R.T. Pseudomonas aeruginosa biofilms: Host response and clinical implications in lung infections. Am. J. Respir. Cell Mol. Biol. 2018, 58, 428–439. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Mohanta, Y.K.; Pohl, P.; Jaradat, N.; Aboul-Soud, M.A.M.; Zengin, G. Variation of phytochemical constituents, antioxidant, antibacterial, antifungal, and anti-inflammatory properties of Grantia aucheri (Boiss.) at different growth stages. Microb. Pathog. 2022, 172, 105805. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Epifano, F.; Fiorito, S.; Álvarez-Suarez, J.M. Phytochemical analysis and biological investigation of Nepeta juncea Benth. different extracts. Plants 2020, 9, 646. [Google Scholar] [CrossRef]
- Mun, S.H.; Joung, D.K.; Kim, Y.S.; Kang, O.H.; Kim, S.B.; Seo, Y.S.; Kim, Y.C.; Lee, D.S.; Shin, D.W.; Kweon, K.T.; et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef]
- Teow, S.Y.; Ali, S.A. Synergistic antibacterial activity of curcumin with antibiotics against Staphylococcus aureus. Pak. J. Pharm. Sci. 2015, 28, 2109–2114. [Google Scholar]
- Jaiswal, S.; Mishra, P. Antimicrobial and antibiofilm activity of curcumin-silver nanoparticles with improved stability and selective toxicity to bacteria over mammalian cells. Med. Microbiol. Immunol. 2018, 207, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Betts, J.W.; Sharili, A.S.; La Ragione, R.M.; Wareham, D.W. In vitro antibacterial activity of curcumin–polymyxin b combinations against multidrug-resistant bacteria associated with traumatic wound infections. J. Nat. Prod. 2016, 79, 1702–1706. [Google Scholar] [CrossRef]
- Cho, H.S.; Lee, J.H.; Ryu, S.Y.; Joo, S.W.; Cho, M.H.; Lee, J. Inhibition of Pseudomonas aeruginosa and Escherichia coli o157:h7 biofilm formation by plant metabolite ε-viniferin. J. Agric. Food Chem. 2013, 61, 7120–7126. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, A.H.; Ahmed, A.A.E.; Bashir, M.; Abdalgadir, H.; Khalid, A.; Gul, S. The use of medicinal plants in common ophthalmic disorders: A systematic review with meta-analysis. Heliyon 2023, 9, e15340. [Google Scholar] [CrossRef]
- Gumus, T.; Demirci, A.S.; Sagdic, O.; Arici, M. Inhibition of heat resistant molds: Aspergillus fumigatus and Paecilomyces variotii by some plant essential oils. Food Sci. Biotechnol. 2010, 19, 1241–1244. [Google Scholar] [CrossRef]
- Manandhar, S.; Luitel, S.; Dahal, R.K. In Vitro antimicrobial activity of some medicinal plants against human pathogenic bacteria. J. Trop. Med. 2019, 2019, 1895340. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Setzer, W.N. Aromatic herbs, medicinal plant-derived essential oils, and phytochemical extracts as potential therapies for coronaviruses: Future perspectives. Plants 2020, 9, 800. [Google Scholar] [CrossRef]
- Mohamed, M.S.M.; Abdallah, A.A.; Mahran, M.H.; Shalaby, A.M. Potential alternative treatment of ocular bacterial infections by oil derived from Syzygium aromaticum flower (clove). Curr. Eye Res. 2018, 43, 873–881. [Google Scholar] [CrossRef]
- Stan, D.; Enciu, A.M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural compounds with antimicrobial and antiviral effect and nanocarriers used for their transportation. Front. Pharmacol. 2021, 12, 723233. [Google Scholar] [CrossRef] [PubMed]
- Gopal, J.; Muthu, M.; Paul, D.; Kim, D.-H.; Chun, S. Bactericidal activity of green tea extracts: The importance of catechin containing nano particles. Sci. Rep. 2016, 6, 19710. [Google Scholar] [CrossRef]
- Dag, D.; Oztop, M.H. Formation and characterization of green tea extract loaded liposomes. J. Food Sci. 2017, 82, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Lee, J.H.; Cho, M.H.; Lee, J. Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling 2015, 31, 1–11. [Google Scholar] [CrossRef]
- Martins, N.; Ferreira, I.C.F.R.; Barros, L.; Carvalho, A.M.; Henriques, M.; Silva, S. Plants used in folk medicine: The potential of their hydromethanolic extracts against Candida species. Ind. Crops Prod. 2015, 66, 62–67. [Google Scholar] [CrossRef]
- Samadi, F.M.; Suhail, S.; Sonam, M.; Sharma, N.; Singh, S.; Gupta, S.; Dobhal, A.; Pradhan, H. Antifungal efficacy of herbs. J. Oral Biol. Craniofac. Res. 2019, 9, 28–32. [Google Scholar] [CrossRef]
- Ito, Y.; Ito, T.; Yamashiro, K.; Mineshiba, F.; Hirai, K.; Omori, K.; Yamamoto, T.; Takashiba, S. Antimicrobial and antibiofilm effects of abietic acid on cariogenic Streptococcus mutans. Odontology 2020, 108, 57–65. [Google Scholar] [CrossRef]
- Keylor, M.H.; Matsuura, B.S.; Stephenson, C.R.J. Chemistry and biology of resveratrol-derived natural products. Chem. Rev. 2015, 115, 8976–9027. [Google Scholar] [CrossRef]
- Lançon, A.; Frazzi, R.; Latruffe, N. Anti-oxidant, Anti-inflammatory and anti-angiogenic properties of resveratrol in ocular diseases. Molecules 2016, 21, 304. [Google Scholar] [CrossRef]
- Koushki, M.; Amiri-Dashatan, N.; Ahmadi, N.; Abbaszadeh, H.A.; Rezaei-Tavirani, M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci. Nutr. 2018, 6, 2473–2490. [Google Scholar] [CrossRef]
- Radeva, L.; Yoncheva, K. Resveratrol—A promising therapeutic agent with problematic properties. Pharmaceutics 2025, 17, 134. [Google Scholar] [CrossRef]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to improve oral bioavailability and beneficial effects of resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wan, G.; Yan, P.; Qian, C.; Li, F.; Peng, G. Fabrication of resveratrol coated gold nanoparticles and investigation of their effect on diabetic retinopathy in streptozotocin induced diabetic rats. J. Photochem. Photobiol. B 2019, 195, 51–57. [Google Scholar] [CrossRef]
- Chan, M.M.Y. Antimicrobial effect of resveratrol on dermatophytes and bacterial pathogens of the skin. Biochem. Pharmacol. 2002, 63, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Hwang, I.A.; Sung, W.S.; Kang, H.; Kang, B.S.; Seu, Y.B.; Lee, D.G. Fungicidal effect of resveratrol on human infectious fungi. Arch. Pharm. Res. 2005, 28, 557–560. [Google Scholar] [CrossRef]
- Docherty, J.J. Resveratrol selectively inhibits Neisseria gonorrhoeae and Neisseria meningitidis. J. Antimicr. Chemother. 2001, 47, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Schulz, B.; Ruhnke, M. Resveratrol and its antifungal activity against Candida species. Mycoses 2011, 54, 30–33. [Google Scholar] [CrossRef]
- Houillé, B.; Papon, N.; Boudesocque, L.; Bourdeaud, E.; Besseau, S.; Courdavault, V.; Enguehard-Gueiffier, C.; Delanoue, G.; Guérin, L.; Bouchara, J.P.; et al. Antifungal activity of resveratrol derivatives against Candida species. J. Nat. Prod. 2014, 77, 1658–1662. [Google Scholar] [CrossRef]
- Paulo, L.; Ferreira, S.; Gallardo, E.; Queiroz, J.A.; Domingues, F. Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World J. Microbiol. Biotechnol. 2010, 26, 1533–1538. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Yuan, W.; Li, S.; Gupta, S.C. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: Identification of novel components of turmeric. Mol. Nutr. Food Res. 2013, 57, 1529–1542. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Nagahama, K.; Utsumi, T.; Kumano, T.; Maekawa, S.; Oyama, N.; Kawakami, J. Discovery of a new function of curcumin which enhances its anticancer therapeutic potency. Sci. Rep. 2016, 6, 30962. [Google Scholar] [CrossRef]
- Yakubu, J.; Pandey, A.V. Innovative delivery systems for curcumin: Exploring nanosized and conventional formulations. Pharmaceutics 2024, 16, 637. [Google Scholar] [CrossRef] [PubMed]
- Kondratyuk, T.P.; Pezzuto, J.M. Natural product polyphenols of relevance to human health. Arch. Physiol. Biochem. 2004, 42, 46–63. [Google Scholar] [CrossRef]
- Moiseev, R.V.; Morrison, P.W.J.; Steele, F.; Khutoryanskiy, V.V. Penetration enhancers in ocular drug delivery. Pharmaceutics 2019, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Sai, N.; Dong, X.; Huang, P.; You, L.; Yang, C.; Liu, Y.; Wang, W.; Wu, H.; Yu, Y.; Du, Y.; et al. A novel gel-forming solution based on PEG-DSPE/Solutol HS 15 mixed micelles and gellan gum for ophthalmic delivery of curcumin. Molecules 2020, 25, 81. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, A.K.; Agrahari, A.K.; Sharma, K.; Singh, A.S.; Gupta, M.K.; Tiwari, V.K.; Prakash, P. Making of water soluble curcumin to potentiate conventional antimicrobials by inducing apoptosis-like phenomena among drug-resistant bacteria. Sci. Rep. 2020, 10, 14204. [Google Scholar] [CrossRef]
- Teow, S.Y.; Liew, K.; Ali, S.A.; Khoo, A.S.B.; Peh, S.C. Antibacterial action of curcumin against Staphylococcus aureus: A brief review. J. Trop. Med. 2016, 2016, 2853045. [Google Scholar] [CrossRef] [PubMed]
- de Lima Silva, M.G.; da Silva, L.Y.S.; de Freitas, T.S.; Rocha, J.E.; Pereira, R.L.S.; Tintino, S.R.; de Oliveira, M.R.C.; Bezerra Martins, A.O.B.P.; Lima, M.C.P.; Alverni da Hora, G.C.; et al. Antibacterial effect and evaluation of the inhibitory effect against efflux pump in Staphylococcus aureus by abietic acid: In vitro and in silico assays. Proc. Biochem. 2022, 122, 363–372. [Google Scholar] [CrossRef]
- de Lima Silva, M.G.; de Lima, L.F.; Alencar Fonseca, V.J.; Santos da Silva, L.Y.; Calixto Donelardy, A.C.; de Almeida, R.S.; de Morais Oliveira-Tintino, C.D.; Pereira Bezerra Martins, A.O.B.; Ribeiro-Filho, J.; Bezerra Morais-Braga, M.F.; et al. Enhancing the antifungal efficacy of fluconazole with a diterpene: Abietic acid as a promising adjuvant to combat antifungal resistance in Candida spp. Antibiotics 2023, 12, 1565. [Google Scholar] [CrossRef] [PubMed]
- Dzięgielewska, M.; Bartoszewicz, M.; Książczyk, M.; Dudek, B.; Brożyna, M.; Szymczyk-Ziółkowska, P.; Gruber, P.; Pawlak, J.; Kozłowska, W.; Zielińska, S.; et al. Abietic acid as a novel agent against ocular biofilms: An in vitro and preliminary in vivo investigation. Int. J. Mol. Sci. 2024, 25, 1528. [Google Scholar] [CrossRef]
- Novy, P.; Davidova, H.; Serrano-Rojero, C.S.; Rondevaldova, J.; Pulkrabek, J.; Kokoska, L. Composition and antimicrobial activity of Euphrasia rostkoviana Hayne essential oil. Evid. Based Complement. Alter. Med. 2015, 2015, 734101. [Google Scholar] [CrossRef] [PubMed]
- Sivamani, P. Ocular infection bacterial isolates and their sensitivity to essential oils of selected herbals. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 311–321. [Google Scholar]
- El-Badry, A.S.; Ali, S.S. Essential oils: A promising remedy against fungal and bacterial human keratitis. Egypt. J. Bot. 2015, 1, 403–408. [Google Scholar]
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Antimicrobial effects of selected plant essential oils on the growth of a Pseudomonas putida strain isolated from meat. Meat Sci. 2006, 3, 236–244. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Gong, X.; Wu, X.; Liu, L.; Chi, F. Rosemary and tea tree essential oils exert antibiofilm activities in vitro against Staphylococcus aureus and Escherichia coli. J. Food Prot. 2020, 83, 1261–1267. [Google Scholar] [CrossRef]
- Bowbe, K.H.; Salah, K.B.H.; Moumni, S.; Ashkan, M.F.; Merghni, A. Anti-staphylococcal activities of Rosmarinus officinalis and Myrtus communis essential oils through ROS-mediated oxidative stress. Antibiotics 2023, 12, 266. [Google Scholar] [CrossRef]
- de Oliveira, J.R.; Camargo, S.E.A.; de Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils-present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.; Noreña, C.P.Z.; Barros, H.L.; Smaoui, S.; Lima, P.S.; de Oliveira, M.M.; Oliveira, J.M. Rheological and structural trends on encapsulation of bioactive compounds of essential oils: A global systematic review of recent research. Food Hydrocoll. 2022, 129, 107628. [Google Scholar] [CrossRef]
- Ojeda-Sana, A.M.; van Baren, C.M.; Elechosa, M.A.; Juárez, M.A.; Moreno, S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control 2013, 31, 189–195. [Google Scholar] [CrossRef]
- Nair, S.; Vanathi, M.; Tandon, R. Biological topicals in ocular surface disorders. Indian J. Ophthalmol. 2025, 73, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Prakash, J.; Srivastava, S. Validation of traditional claim of Tulsi, Ocimum sanctum Linn. as a medicinal plant. Ind. J. Exp. Biol. 2002, 40, 765–773. [Google Scholar]
- Ng, J.Y.; Kim, M.; Suri, A. Exploration of facilitators and barriers to the regulatory frameworks of dietary and herbal supplements: A scoping review. J. Pharm. Policy Pract. 2022, 15, 55. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wang, L.; Liu, Q.; Yang, S.; Wang, C. Advancing herbal medicine: Enhancing product quality and safety through robust quality control practices. Front. Pharmacol. 2023, 14, 1265178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cheng, Y.; Li, H.; Li, M.; Yang, Q.; Hua, K.; Wen, X.; Han, Y.; Liu, G.; Chu, C. Metallic nano-warriors: Innovations in nanoparticle-based ocular antimicrobials. Mater. Today Bio 2024, 28, 101242. [Google Scholar] [CrossRef]
- Subiza, J.; Subiza, J.L.; Alonso, M.; Hinojosa, M.; Garcia, R.; Jerez, M.; Subiza, E. Allergic conjunctivitis to chamomile tea. Ann. Allergy 1990, 65, 127–132. [Google Scholar]
- Ovuru, K.F.; Izah, S.C.; Yasmin, H.; Enerijiofi, K.E.; Das, M.; Ogwu, M.C. Microbial contaminants of herbal remedies: Health risks and sustainable quality control strategies. In Herbal Medicine Phytochemistry; Izah, S.C., Ogwu, M.C., Akram, M., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2024; pp. 1571–1600. [Google Scholar]
- Ibrahim, M.M.; Maria, D.N.; Wang, X.; Simpson, R.N.; Hollingsworth, T.J.; Jablonski, M.M. Enhanced corneal penetration of a poorly permeable drug using bioadhesive multiple microemulsion technology. Pharmaceutics 2020, 12, 704. [Google Scholar] [CrossRef] [PubMed]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin delivery mediated by bio-based nanoparticles: A review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef]
- Mostafa, M.; Al Fatease, A.; Alany, R.G.; Abdelkader, H. Recent advances of ocular drug delivery systems: Prominence of ocular implants for chronic eye diseases. Pharmaceutics 2023, 15, 1746. [Google Scholar] [CrossRef]
- Salazar-Gómez, A.; Velo-Silvestre, A.A.; Alonso-Castro, A.J.; Hernández-Zimbrón, L.F. Medicinal plants used for eye conditions in Mexico—A review. Pharmaceuticals 2023, 16, 1432. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.M.; Gonçalves, M.C.; Marques, M.S.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. In vitro models for anti-aging efficacy assessment: A critical update in dermocosmetic research. Cosmetics 2023, 10, 66. [Google Scholar] [CrossRef]
- Parveen, A.; Parveen, B.; Parveen, R.; Ahmad, S. Challenges and guidelines for clinical trial of herbal drugs. J. Pharm. Bioallied Sci. 2015, 7, 329–333. [Google Scholar] [CrossRef]
- Thakkar, S.; Anklam, E.; Xu, A.; Ulberth, F.; Li, J.; Li, B.; Hugas, M.; Sarma, N.; Crerar, S.; Swift, S.; et al. Regulatory landscape of dietary supplements and herbal medicines from a global perspective. Regul. Toxicol. Pharmacol. 2020, 114, 104647. [Google Scholar] [CrossRef]
- DIRECTIVE 2004/24/EC OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 31 March 2004 Amending, as Regards Traditional Herbal Medicinal Products, Directive 2001/83/EC on the Community Code Relating to Medicinal Products for Human Use. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:136:0085:0090:EN:PDF (accessed on 15 April 2025).
- Guide to Traditional Herbal Medicinal Products Registration Scheme. Available online: https://assets.hpra.ie/data/docs/default-source/external-guidance-document-(tracked)/aut-g0029-guide-to-thmp-registration-scheme-v5-changes-tracked.pdf (accessed on 15 April 2025).
- Dubale, S.; Usure, R.E.; Mekasha, Y.T.; Hasen, G.; Hafiz, F.; Kebebe, D.; Suleman, S. Traditional herbal medicine legislative and regulatory framework: A cross-sectional quantitative study and archival review perspectives. Front. Pharmacol. 2025, 16, 1475297. [Google Scholar] [CrossRef]
- Huang, X.; Li, L.; Chen, Z.; Yu, H.; You, X.; Kong, N.; Tao, W.; Zhou, X.; Huang, J. Nanomedicine for the detection and treatment of ocular bacterial infections. Adv. Mater. 2023, 35, 2302431. [Google Scholar] [CrossRef]

| Group of Antibiotics | Mechanism of Action | Spectrum of Activity |
|---|---|---|
| Aminoglycosides |
| Strong antibacterial activity: effective against Gram-negative bacteria (Haemophilus, Pseudomonas, Enterobacteriaceae) and Staphylococcus aureus (except some MRSA strains). Limited activity against Streptococci. |
| Fluoroquinolones | Inhibition of DNA synthesis: Blocks type II topoisomerases, enzymes responsible for cutting both DNA strands:
| Highly effective against Gram-negative bacteria (Haemophilus, Salmonella, Neisseria, Pseudomonas, Enterobacteriaceae). Fourth-generation fluoroquinolones (e.g., gatifloxacin, moxifloxacin, besifloxacin) are also effective against Gram-positive bacteria. |
| Group of Antibiotics | Exemplary Drugs | Spectrum of Activity |
|---|---|---|
| Aminoglycosides | Streptomycin, gentamicin, kanamycin, tobramycin, neomycin, amikacin, sisomicin, netilmicin | -Enterobacterales: Escherichia coli, Klebsiella, Enterobacter, Proteus -Pseudomonas (including Pseudomonas aeruginosa) -Haemophilus spp. -Brucella spp. -Pasteurella spp. -Mycobacterium tuberculosis -Staphylococci (including S. aureus) -Resistance in some strains of S. aureus, especially methicillin-resistant strains (MRSA). |
| Fluoroquinolones | Norfloxacin, enoxacin (Generation I). Ciprofloxacin, ofloxacin, lomefloxacin (Generation II). Levofloxacin (Generation III). | Generation I: Gram-negative bacteria: Haemophilus influenzae, Moraxella, Neisseria, Chlamydia spp. Generation II: Gram-negative bacteria: Haemophilus, Pseudomonas, Salmonella, Neisseria, Moraxella Generation III: Gram-negative bacteria: Pseudomonas, Neisseria, Haemophilus; Gram-positive bacteria: S. aureus, Streptococcus pneumoniae, S. pyogenes -Poor sensitivity to anaerobic bacteria. -Inherited resistance in some strains of P. aeruginosa. |
| Type of Compound | Plant Extracts (Phytoconstituents) | Mechanism and Activity | Refs. |
|---|---|---|---|
| Polyphenolics (curcuminoids, stilbenes) | Turmeric (curcumin) Grapes (resveratrol) | Curcumin disrupts the bacterial cell membrane and inhibits quorum sensing, a key mechanism for biofilm formation in resistant bacteria. Resveratrol has been tested and showed efficacy against Staphylococcus aureus biofilms. | [67,68,69,70,71,72] |
| Essential oils | Eyebright (thymol) Chamomile (α-bisabolol and its oxides A and B, β-farnesene) Rosemary (1,8-cyneole, α-pinene) Oregano (carvacrol) Thyme (thymol) Cinnamon (cinnamaldehyde) | Disrupt bacterial membranes and metabolic routes. Oregano has efficacy against multidrug-resistant Escherichia coli and Pseudomonas aeruginosa. | [74,75,76,77] |
| Alkaloids | Berberis (berberine) | Effective against Gram-positive bacteria, including MRSA. Interferes with bacterial DNA and cell wall synthesis; distinct mode of action compared to conventional antibiotics. | [75,78] |
| Flavonoids | Green tea (catechins) Onions (quercetin) | Inhibit bacterial enzymes and cell walls. Catechins show synergy with conventional antibiotics, enhancing their action against resistant strains. | [79,80,81] |
| Tannins and Terpenoids | Neem (Azadirachta indica), Eucalyptus (Eucalyptus globulus) Abietic acid (derived from pine tree resin) | Exhibit antimicrobial, antifungal, and antiviral activities. Neem extracts are effective against resistant strains of Helicobacter pylori. | [71,82,83,84] |
| Type of Compound | Compounds/Plant Extracts | Mechanism of Action | Refs. |
|---|---|---|---|
| Polyphenolics (curcuminoids, stilbenes) | Curcumin (turmeric) Resveratrol (grapes) | Curcumin disrupts the bacterial cell membrane and inhibits quorum sensing, a key mechanism for biofilm formation in resistant bacteria. Resveratrol has been tested and showed efficacy against Staphylococcus aureus biofilms | [67,68,69,70,71,72] |
| Essential oils | α-Bisabolol and its oxides, β-farnesene (chamomile) 1,8-Cyneole, α-Pinene (rosemary) Carvacrol (oregano) Thymol (eyebright, thyme) Cinnamaldehyde (cinnamon) | Disrupt bacterial membranes and metabolic routes. Oregano has efficacy against multidrug-resistant Escherichia coli and Pseudomonas aeruginosa | [74,75,76,77] |
| Terpenoids | Abietic acid Conifer resins, mainly from Pinus species | Disrupts microbial cell membranes, increases permeability, and causes leakage of intracellular components. Effective against among others Pseudomonas aeruginosa, Staphylococcus aureus, Candida albicans | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzięgielewska, M.; Tomczyk, M.; Wiater, A.; Woytoń, A.; Junka, A. Targeting Ocular Biofilms with Plant-Derived Antimicrobials in the Era of Antibiotic Resistance. Molecules 2025, 30, 2863. https://doi.org/10.3390/molecules30132863
Dzięgielewska M, Tomczyk M, Wiater A, Woytoń A, Junka A. Targeting Ocular Biofilms with Plant-Derived Antimicrobials in the Era of Antibiotic Resistance. Molecules. 2025; 30(13):2863. https://doi.org/10.3390/molecules30132863
Chicago/Turabian StyleDzięgielewska, Monika, Michał Tomczyk, Adrian Wiater, Aleksandra Woytoń, and Adam Junka. 2025. "Targeting Ocular Biofilms with Plant-Derived Antimicrobials in the Era of Antibiotic Resistance" Molecules 30, no. 13: 2863. https://doi.org/10.3390/molecules30132863
APA StyleDzięgielewska, M., Tomczyk, M., Wiater, A., Woytoń, A., & Junka, A. (2025). Targeting Ocular Biofilms with Plant-Derived Antimicrobials in the Era of Antibiotic Resistance. Molecules, 30(13), 2863. https://doi.org/10.3390/molecules30132863









