Modern Bioimaging Techniques for Elemental Tissue Analysis: Key Parameters, Challenges and Medical Impact
Abstract
1. Introduction
2. Brief Characteristic of Bioimaging Techniques
- Heating of the sample surface by laser;
- Ablation of sample surface components;
- Laser-induced creation of microplasma;
- Dissociation (break down) of ablated materials into ions and atoms at excited state;
- Emission of electromagnetic radiation (in two steps: first, continuously; and second, radiation characteristic of the elements of the ablated sample).
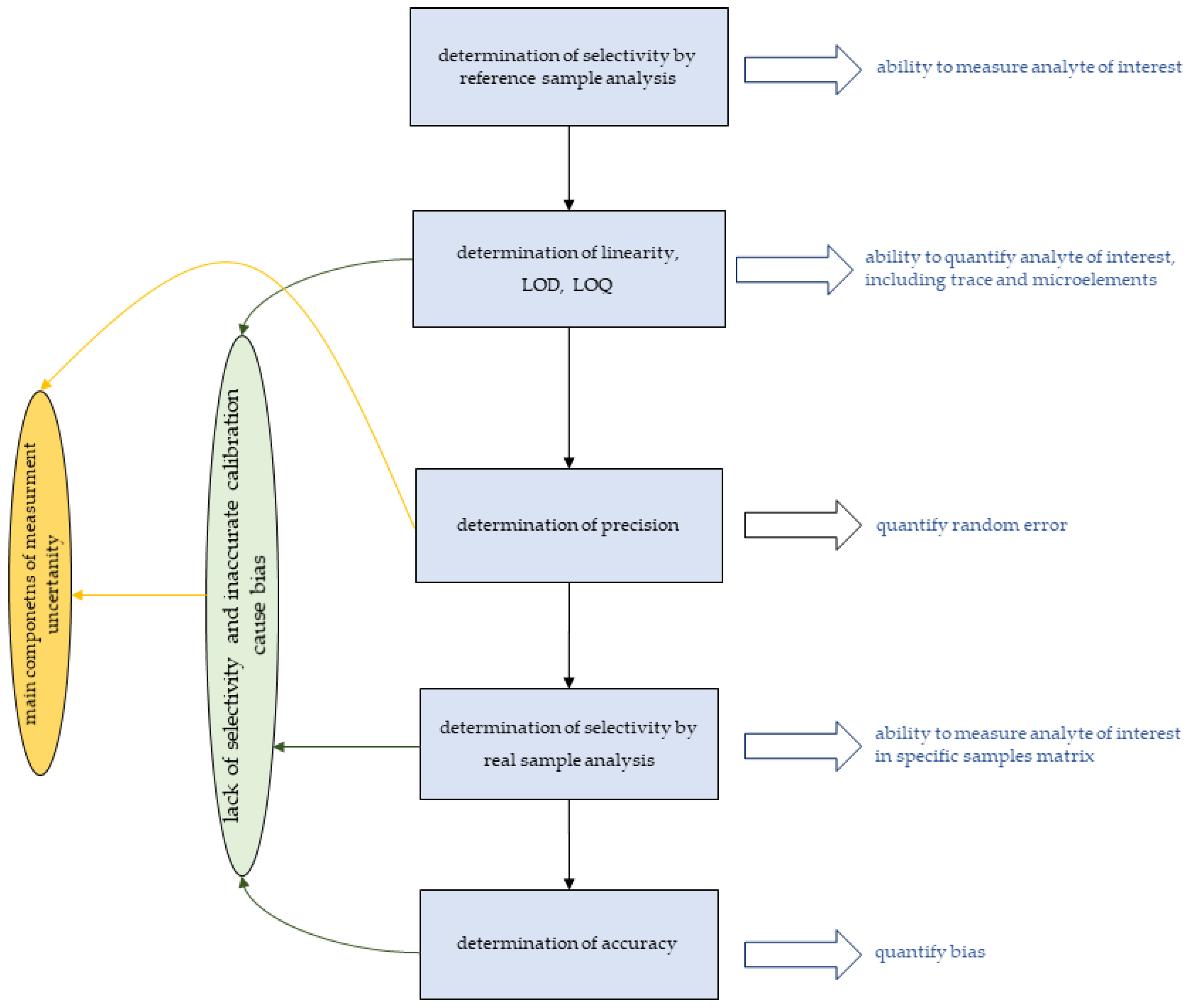
3. Parameters Relevant to the Quality of Measurements
3.1. Selectivity of Measurements
3.2. Linearity and Accuracy
3.3. Limits of Detection and Quantification
3.4. Measurement Uncertainty
- Lack of or limited selectivity hinders the application of bioimaging techniques for diagnostic purposes;
- The lack of selectivity is related mainly to matrix effects;
- Complicated procedures for determining linearity can often lead to unreliable quantitative analysis results;
- Selectivity and the resulting accuracy can be assessed using appropriate reference materials (limited availability) or using enriched samples;
- The quantitative bioimaging will be more challenging as the amount of analyte decreases and the matrix becomes more complex;
- Both bias and random error are main components of measurement uncertainty.
4. Application of Bioimaging Techniques in Analysis of Human Tissues
4.1. Laser-Based Technique
4.2. X-Ray-Based Techniques
| Sample | Implant/ Group of Patients | Number of Patients/Samples (Without Control) | Element | Technique | Remark | Reference |
|---|---|---|---|---|---|---|
| lung tissue | healthy and idiopathic pulmonary fibrosis | 2 | Ca, Zn, S, Fe, Al, Cr, Cu, Ti, Mn, P | XRF nano-XRF (100 nm) | mapping | [54] |
| tooth | no determinant | 3 + additional samples (stored for more than a decade) | Ca, P, Ba, Zn, W, Zr, Sr, Fe | μXRF | elemental maps | [5] |
| P, Ca, Ba, Zn, W, Zr | SEM-EDS | |||||
| P, Ca, Ba, Zn, W, Zr | CμXRF | |||||
| synovial sheath tissues | hip | 2 | Co, Cr, Mo | μXRF and μXAFS (3 μm resolution) | mapping | [6] |
| Co, Cr (speciation) | sub-μXRF and sub-μXANES (600 nm resolution) | mapping | ||||
| Co, Cr | nano-XRF and nano-XANES (250 nm resolution) | mass fraction | ||||
| peri-implant cancellous bone | hip, knee | 14 | Co, Cr, Ti (main analytes) Fe, S, P, Ca (matrix structures) | μXRF (10, 3 and 2 µm) | mapping/ mass fraction | [18] |
| Ti (speciation) | μXANES | |||||
| Ti, Zr, Ta | nano-XRF (60 and 30 nm) | mapping | ||||
| tissues | dental | 13 | Ti, Zr (main analytes) P, S (matrix structures) | μXRF (resolution from 1 to 20 μm) | particle density (mass fractions) | [7] |
| Zr, Zn, Fe, Hf, Y, Sr, Cr, Ni, Nb | nano-XRF (60 nm resolution) | particle density (mass fractions) | ||||
| Ti (speciation) | μXANES (between 1 and 10 μm) | |||||
| periprosthetic tissue | hip | 7 | Co, Cr, Ti | μXRF | mapping | [19] |
| Co, Cr, Ti (speciation) | µXANES | |||||
| hair | no determinant | 4 | Hg | nXRF (50 nm) | distribution of mercury | [44] |
| Hg | XANES (high resolution) | speciation | ||||
| liver | hip | 1 | Co, Cr, Ca | μXRF μXAS | mapping | [20] |
| blood | Co, Cr | - | quantitatively | |||
| bone and mucosal tissues | dental | 12 | Ca, Ti, Fe, P | XRF | - | [45] |
| soft tissue, bone marrow, mineralized bone tissue | hip | 13 | Co, Cr | μXRF (80 µm resolution) | mapping and mass fraction | [21] |
| periprosthetic bone marrow | hip, knee | 8 | Co, Cr and Mo | nano-XRF (60 nm resolution) | bioimaging | [43] |
| breast, ovarian tissue | various cancers | 60 (samples) | Zn, Fe, Cu, Ca | μXRF | mapping particle density | [8] |
| soft tissues | dental | 31/36 | C, N, Na, K, O (controls) Ca, P, Ti, Zr, Al, Si, F, Cl, Fe, Zn, Pt, S, Mg, Br, Pb, Ni, Ba, Bi, La | SEM-EDS | mean percentages represent the composition of the elements | [55] |
| periprosthetic tissue | hip | 53 | Cr, Co, Mo, Ti, V, Fe, P, O | SEM-EDS TEM-EDS | mapping | |
| Cr | XRD | crystalline structures | [22] | |||
| blood | Co, Cr | - | quantitatively | |||
| tissue | patients who had tongue and/or lip piercings | 16 | C, K, Ca, O, Na, Mg, Al, Cr, Mn, Fe, Co, Si, S | SEM-EDS | semi-quantitatively | [56] |
| smears of mucosa | Ca, C, O, Na, Mg, Al., Mo, Si | |||||
| capsular tissue, deep hip tissue granuloma tissue | hip | 26 | Ti, Cr, Co, Fe, Ca, Mo, C, Cl, Si, P | SEM-EDS | qualitatively | [39] |
| blood serum | Co, Cr, Ti | - | - | |||
| tissues | Cr, Al | XRD | crystalline structures | |||
| fallopian tube or uterine horn tissue | intrauterine device | 10 | endogenous particles contain Na, P, S, Ca, Cl, K, Fe, Sn, Si, Al, Ca, Fe, Ti, Sb -based Au, Al, Pt | SEM-EDS | qualitatively | [9,57] |
| red bone marrow (postmortem) | hip, knee | 6 | particles of combined Co, Cr, Mo, Fe, Ni, Ti, Al, V | SEM-EDS (resolution from 50 nm to 6 μm) | qualitatively | [58] |
| skin and lymphatic tissues tattooed skin | tattooed skin (postmortem) | 20 (skin) 25 (lymph node) | Br, T, P, Cl, P, S, K, Ca | μXRF (from 0.5 µm to 5 µm) | mapping | [23] |
| Ti | μXANES (from 1 µm to 10 µm) | speciation | ||||
| skin and lymph node tissues | tattooed skin (postmortem) | 5 | Fe, Cr, Ni, Ti, Cu | nano-XRF (50 nm) | elemental maps | [10] |
| Cr, Ni | XANES | speciation | ||||
| periprosthetic tissues | hip | 18 | Cr, Co, Mo, Si, Ca, P, Na | SEM-EDS | elemental composition | [37] |
| synovial fluid | hip | 40 | Co, Cr particles | SEM-EDS | semi quantitatively | [33] |
| periprosthetic tissue | hip | 1 | Cr, Co, Ta, C, O, S, Ti, N, Na | SEM-EDS | area fractions | [51] |
| postmortem neuronal tissues | gadolinium-based contrast agents brain magnetic resonance examinations | 13 | C, Cs, Cu, Gd, O, Os, Pb, Ti, V | TEM-EDS | distribution | [52] |
| cardiac, hepatic splenic postmortem tissues | hip, knee | 5/13 | Co, Cr | LA-ICP-MS | distribution | [24] |
| Co, Cr, Ti | μXRF (5, 3 μm) | mapping | ||||
| μXANES (3 μm) | speciation |
4.3. Alternative Techniques Potentially Applicable to Bioimaging
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaudiuso, R.; Melikechi, N.; Abdel-Salam, Z.A.; Harith, M.A.; Palleschi, V.; Motto-Ros, V.; Busser, B. Laser-induced breakdown spectroscopy for human and animal health: A review. Spectrochim. Acta Part B At. Spectrosc. 2019, 152, 123–148. [Google Scholar] [CrossRef]
- Clases, D.; Gonzalez de Vega, R. Facets of ICP-MS and their potential in the medical sciences—Part 1: Fundamentals, stand-alone and hyphenated techniques. Anal. Bioanal. Chem. 2022, 414, 7337–7361. [Google Scholar] [CrossRef]
- Paunesku, T.; Vogt, S.; Maser, J.; Lai, B.; Woloschak, G. X-ray fluorescence microprobe imaging in biology and medicine. J. Cell. Biochem. 2006, 99, 1489–1502. [Google Scholar] [CrossRef]
- Matusiewicz, H. Sample Preparation for Inorganic Trace Element Analysis. Phys. Sci. Rev. 2017, 2, 20178001. [Google Scholar] [CrossRef]
- Bauer, L.J.; Mustafa, H.A.; Zaslansky, P.; Mantouvalou, I. Chemical mapping of teeth in 2D and 3D: X-ray fluorescence reveals hidden details in dentine surrounding fillings. Acta Biomater. 2020, 109, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Morrell, A.P.; Floyd, H.; Mosselmans, J.F.W.; Grover, L.M.; Castillo-Michel, H.; Davis, E.T.; Parker, J.E.; Martin, R.A.; Addison, O. Improving our understanding of metal implant failures: Multiscale chemical imaging of exogenous metals in ex-vivo biological tissues. Acta Biomater. 2019, 98, 284–293. [Google Scholar] [CrossRef]
- Nelson, K.; Hesse, B.; Addison, O.; Morrell, A.P.; Gross, C.; Lagrange, A.; Suárez, V.I.; Kohal, R.; Fretwurst, T. Distribution and Chemical Speciation of Exogenous Micro- And Nanoparticles in Inflamed Soft Tissue Adjacent to Titanium and Ceramic Dental Implants. Anal. Chem. 2020, 92, 14432–14443. [Google Scholar] [CrossRef] [PubMed]
- Al-Ebraheem, A.; Dao, E.; Geraki, K.; Farquharson, M.J. Emerging Patterns in the Distribution of Trace Elements in Ovarian, Invasive and In-Situ Breast Cancer. J. Phys. Conf. Ser. 2014, 499, 012014. [Google Scholar] [CrossRef]
- Catinon, M.; Roux, E.; Auroux, A.; Balter, V.; Fellah, C.; Trunfio-Sfarghiu, A.M.; Sournies, G.; Vincent, M. Identification of inorganic particles resulting from degradation of ESSURE® implants: Study of 10 cases. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 250, 162–170. [Google Scholar] [CrossRef]
- Schreiver, I.; Hesse, B.; Seim, C.; Castillo-Michel, H.; Anklamm, L.; Villanova, J.; Dreiack, N.; Lagrange, A.; Penning, R.; De Cuyper, C.; et al. Distribution of nickel and chromium containing particles from tattoo needle wear in humans and its possible impact on allergic reactions. Part. Fibre Toxicol. 2019, 16, 33. [Google Scholar] [CrossRef]
- Birka, M.; Wentker, K.S.; Lusmöller, E.; Arheilger, B.; Wehe, C.A.; Sperling, M.; Stadler, R.; Karst, U. Diagnosis of Nephrogenic Systemic Fibrosis by means of Elemental Bioimaging and Speciation Analysis. Anal. Chem. 2015, 87, 3321–3328. [Google Scholar] [CrossRef]
- Hare, D.J.; Raven, E.P.; Roberts, B.R.; Bogeski, M.; Portbury, S.D.; McLean, C.A.; Masters, C.L.; Connor, J.R.; Bush, A.I.; Crouch, P.J.; et al. Laser ablation-inductively coupled plasma-mass spectrometry imaging of white and gray matter iron distribution in Alzheimer’s disease frontal cortex. NeuroImage 2016, 137, 124–131. [Google Scholar] [CrossRef]
- Sajnóg, A.; Hanć, A.; Koczorowski, R.; Makuch, K.; Barałkiewicz, D. Usefulness of laser ablation ICP-MS for analysis of metallic particles released to oral mucosa after insertion of dental implants. J. Trace Elem. Med. Biol. 2018, 46, 46–54. [Google Scholar] [CrossRef]
- Bonta, M.; Gonzalez, J.J.; Derrick Quarles, C.; Russo, R.E.; Hegedus, B.; Limbeck, A. Elemental mapping of biological samples by the combined use of LIBS and LA-ICP-MS. J. Anal. At. Spectrom. 2015, 31, 252–258. [Google Scholar] [CrossRef]
- Gondal, M.A.; Aldakheel, R.K.; Almessiere, M.A.; Nasr, M.M.; Almusairii, J.A.; Gondal, B. Determination of heavy metals in cancerous and healthy colon tissues using laser induced breakdown spectroscopy and its cross-validation with ICP-AES method. J. Pharm. Biomed. Anal. 2020, 183, 113153. [Google Scholar] [CrossRef] [PubMed]
- Hachmöller, O.; Aichler, M.; Schwamborn, K.; Lutz, L.; Werner, M.; Sperling, M.; Walch, A.; Karst, U. Element bioimaging of liver needle biopsy specimens from patients with Wilson’s disease by laser ablation-inductively coupled plasma-mass spectrometry. J. Trace Elem. Med. Biol. 2016, 35, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Bonta, M.; Hegedus, B.; Limbeck, A. Application of dried-droplets deposited on pre-cut filter paper disks for quantitative LA-ICP-MS imaging of biologically relevant minor and trace elements in tissue samples. Anal. Chim. Acta 2016, 908, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Schoon, J.; Hesse, B.; Rakow, A.; Ort, M.J.; Lagrange, A.; Jacobi, D.; Winter, A.; Huesker, K.; Reinke, S.; Cotte, M.; et al. Metal-Specific Biomaterial Accumulation in Human Peri-Implant Bone and Bone Marrow. Adv. Sci. 2020, 7, 2000412. [Google Scholar] [CrossRef]
- Di Laura, A.; Quinn, P.D.; Panagiotopoulou, V.C.; Hothi, H.S.; Henckel, J.; Powell, J.J.; Berisha, F.; Amary, F.; Mosselmans, J.F.W.; Skinner, J.A.; et al. The Chemical Form of Metal Species Released from Corroded Taper Junctions of Hip Implants: Synchrotron Analysis of Patient Tissue. Sci. Rep. 2017, 7, 10952. [Google Scholar] [CrossRef]
- Abdel-Gadir, A.; Berber, R.; Porter, J.B.; Quinn, P.D.; Suri, D.; Kellman, P.; Hart, A.J.; Moon, J.C.; Manisty, C.; Skinner, J.A. Detection of metallic cobalt and chromium liver deposition following failed hip replacement using T2∗ and R2 magnetic resonance. J. Cardiovasc. Magn. Reson. 2016, 18, 29. [Google Scholar] [CrossRef]
- Hahn, M.; Busse, B.; Procop, M.; Zustin, J.; Amling, M.; Katzer, A. Cobalt deposition in mineralized bone tissue after metal-on-metal hip resurfacing: Quantitative μ-X-ray-fluorescence analysis of implant material incorporation in periprosthetic tissue. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1855–1862. [Google Scholar] [CrossRef]
- Xia, Z.; Ricciardi, B.F.; Liu, Z.; von Ruhland, C.; Ward, M.; Lord, A.; Hughes, L.; Goldring, S.R.; Purdue, E.; Murray, D.; et al. Nano-analyses of wear particles from metal-on-metal and non-metal-on-metal dual modular neck hip arthroplasty. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1205–1217. [Google Scholar] [CrossRef]
- Schreiver, I.; Hesse, B.; Seim, C.; Castillo-Michel, H.; Villanova, J.; Laux, P.; Dreiack, N.; Penning, R.; Tucoulou, R.; Cotte, M.; et al. Synchrotron-based ν-XRF mapping and μ-FTIR microscopy enable to look into the fate and effects of tattoo pigments in human skin. Sci. Rep. 2017, 7, 11395. [Google Scholar] [CrossRef]
- Swiatkowska, I.; Mosselmans, J.F.W.; Geraki, T.; Wyles, C.C.; Maleszewski, J.J.; Henckel, J.; Sampson, B.; Potter, D.B.; Osman, I.; Trousdale, R.T.; et al. Synchrotron analysis of human organ tissue exposed to implant material. J. Trace Elem. Med. Biol. 2018, 46, 128–137. [Google Scholar] [CrossRef]
- Moncayo, S.; Trichard, F.; Busser, B.; Sabatier-Vincent, M.; Pelascini, F.; Pinel, N.; Templier, I.; Charles, J.; Sancey, L.; Motto-Ros, V. Multi-elemental imaging of paraffin-embedded human samples by laser-induced breakdown spectroscopy. Spectrochim. Acta Part B At. Spectrosc. 2017, 133, 40–44. [Google Scholar] [CrossRef]
- Boaru, S.G.; Merle, U.; Uerlings, R.; Zimmermann, A.; Flechtenmacher, C.; Willheim, C.; Eder, E.; Ferenci, P.; Stremmel, W.; Weiskirchen, R. Laser ablation inductively coupled plasma mass spectrometry imaging of metals in experimental and clinical Wilson’s disease. J. Cell. Mol. Med. 2015, 19, 806–814. [Google Scholar] [CrossRef]
- Swiatkowska, I.; Martin, N.G.; Henckel, J.; Apthorp, H.; Hamshere, J.; Hart, A.J. Blood and plasma titanium levels associated with well-functioning hip implants. J. Trace Elem. Med. Biol. 2020, 57, 9–17. [Google Scholar] [CrossRef]
- Pechancová, R.; Gallo, J.; Baron, D.; Milde, D.; Antal, P.; Slobodová, Z.; Lemr, K.; Pluháček, T. Detailed insight into chromium species released from failed CoCrMo implants: Ex vivo periprosthetic tissues study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 271–283. [Google Scholar] [CrossRef]
- Kondawar, M.; Wankhede, S.; Kuchekar, B. Laser induced breakdown spectroscopy: An advanced method for element analysis. Indian J. Pharm. Educ. Res. 2007, 41, 3–9. [Google Scholar]
- Becker, J.S.; Matusch, A.; Wu, B. Bioimaging mass spectrometry of trace elements—Recent advance and applications of LA-ICP-MS: A review. Anal. Chim. Acta 2014, 835, 1–18. [Google Scholar] [CrossRef]
- Newville, M. Fundamentals of XAFS. Rev. Mineral. Geochem. 2014, 78, 33–74. [Google Scholar] [CrossRef]
- Sajnóg, A.; Hanć, A.; Koczorowski, R.; Barałkiewicz, D. New procedure of quantitative mapping of Ti and Al released from dental implant and Mg, Ca, Fe, Zn, Cu, Mn as physiological elements in oral mucosa by LA-ICP-MS. Talanta 2017, 175, 370–381. [Google Scholar] [CrossRef]
- De Pasquale, D.; Stea, S.; Squarzoni, S.; Bordini, B.; Amabile, M.; Catalani, S.; Apostoli, P.; Toni, A. Metal-on-metal hip prostheses: Correlation between debris in the synovial fluid and levels of cobalt and chromium ions in the bloodstream. Int. Orthop. 2014, 38, 469–475. [Google Scholar] [CrossRef]
- Sajnóg, A.; Hanć, A.; Makuch, K.; Koczorowski, R.; Barałkiewicz, D. Study on quantitative analysis of Ti, Al and V in clinical soft tissues after placing the dental implants by laser ablation inductively coupled plasma mass spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2016, 125, 1–10. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). ICH Q2(R2) Validation of Analytical Procedures—Scientific Guideline. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 17 April 2025).
- Bonta, M.; Lohninger, H.; Laszlo, V.; Hegedus, B.; Limbeck, A. Quantitative LA-ICP-MS imaging of platinum in chemotherapy treated human malignant pleural mesothelioma samples using printed patterns as standard. J. Anal. At. Spectrom. 2014, 29, 2159–2167. [Google Scholar] [CrossRef]
- Scharf, B.; Clement, C.C.; Zolla, V.; Perino, G.; Yan, B.; Elci, S.G.; Purdue, E.; Goldring, S.; MacAluso, F.; Cobelli, N.; et al. Molecular analysis of chromium and cobalt-related toxicity. Sci. Rep. 2014, 4, 5729. [Google Scholar] [CrossRef]
- DeTata, D.A.; Fillingham, R.M.; D’Uva, J. Explosives: Overview. Encycl. Forensic Sci. 2023, 2, 356–390. [Google Scholar] [CrossRef]
- Munir, S.; Oliver, R.A.; Zicat, B.; Walter, W.L.; Walter, W.K.; Walsh, W.R. The histological and elemental characterisation of corrosion particles from taper junctions. Bone Jt. Res. 2016, 5, 370–378. [Google Scholar] [CrossRef]
- Konz, I.; Fernández, B.; Fernández, M.L.; Pereiro, R.; González-Iglesias, H.; Coca-Prados, M.; Sanz-Medel, A. Quantitative bioimaging of trace elements in the human lens by LA-ICP-MS. Anal. Bioanal. Chem. 2014, 406, 2343–2348. [Google Scholar] [CrossRef]
- Walas, S.; Miliszkiewicz, N.; Telk, A. Current approaches to calibration in LA-ICP-MS analysis. J. Anal. At. Spectrom. 2015, 30, 327–338. [Google Scholar] [CrossRef]
- Konz, I.; Fernández, B.; Fernández, M.L.; Pereiro, R.; González, H.; Álvarez, L.; Coca-Prados, M.; Sanz-Medel, A. Gold internal standard correction for elemental imaging of soft tissue sections by LA-ICP-MS: Element distribution in eye microstructures. Anal. Bioanal. Chem. 2013, 405, 3091–3096. [Google Scholar] [CrossRef]
- Schoon, J.; Hesse, B.; Tucoulou, R.; Geissler, S.; Ort, M.; Duda, G.N.; Perka, C.; Wassilew, G.I.; Perino, G.; Rakow, A. Synchrotron-based characterization of arthroprosthetic CoCrMo particles in human bone marrow. J. Mater. Sci. Mater. Med. 2022, 33, 54. [Google Scholar] [CrossRef]
- Manceau, A.; Enescu, M.; Simionovici, A.; Lanson, M.; Gonzalez-Rey, M.; Rovezzi, M.; Tucoulou, R.; Glatzel, P.; Nagy, K.L.; Bourdineaud, J.P. Chemical Forms of Mercury in Human Hair Reveal Sources of Exposure. Environ. Sci. Technol. 2016, 50, 10721–10729. [Google Scholar] [CrossRef]
- Fretwurst, T.; Buzanich, G.; Nahles, S.; Woelber, J.P.; Riesemeier, H.; Nelson, K. Metal elements in tissue with dental peri-implantitis: A pilot study. Clin. Oral Implant. Res. 2016, 27, 1178–1186. [Google Scholar] [CrossRef]
- Haschke, M.; Boehm, S. Micro-XRF in Scanning Electron Microscopes. Adv. Imaging Electron. Phys. 2017, 199, 1–60. [Google Scholar] [CrossRef]
- Ayers, R.; Miller, M.; Schowinsky, J.; Burger, E.; Patel, V.; Kleck, C. Three cases of metallosis associated with spine instrumentation. J. Mater. Sci. Mater. Med. 2018, 29, 3. [Google Scholar] [CrossRef]
- ABLATOM Launches the First Biomedical LIBS Microscope in a Hospital Setting. Available online: https://www.selectscience.net/article/ablatom-launches-the-first-biomedical-libs-microscope-in-a-hospital-setting (accessed on 24 June 2025).
- Boger, V.; Pirkwieser, P.; Orth, N.; Koehler, M.; Somoza, V. AFM-optimized single-cell level LA-ICP-MS imaging for quantitative mapping of intracellular zinc concentration in immobilized human parietal cells using gelatin droplet-based calibration. Anal. Chim. Acta 2025, 1355, 343999. [Google Scholar] [CrossRef]
- Tareq, M.S.; Hamad, T.K. Heavy metal migration from dental filling materials to calcified oral tissues: An in vitro analysis using LIBS and ICP-MS techniques. Odontology 2025. [Google Scholar] [CrossRef]
- Babis, G.C.; Stavropoulos, N.A.; Sasalos, G.; Ochsenkuehn-Petropoulou, M.; Megas, P. Metallosis and elevated serum levels of tantalum following failed revision hip arthroplasty—A case report. Acta Orthop. 2014, 85, 677–680. [Google Scholar] [CrossRef]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Murray, D.L.; Thielen, K.R.; Williamson, E.E.; Eckel, L.J. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 2015, 275, 772–782. [Google Scholar] [CrossRef]
- Wang, Q.; Eltit, F.; Garbuz, D.; Duncan, C.; Masri, B.; Greidanus, N.; Wang, R. CoCrMo metal release in metal-on-highly crosslinked polyethylene hip implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1213–1228. [Google Scholar] [CrossRef]
- Falcones, B.; Kahnt, M.; Johansson, U.; Svobodová, B.; von Wachenfelt, K.A.; Brunmark, C.; Dellgren, G.; Elowsson, L.; Thånell, K.; Westergren-Thorsson, G. Nano-XRF of lung fibrotic tissue reveals unexplored Ca, Zn, S and Fe metabolism: A novel approach to chronic lung diseases. Cell Commun. Signal. 2025, 23, 67. [Google Scholar] [CrossRef]
- Wilson, T.G.; Valderrama, P.; Burbano, M.; Blansett, J.; Levine, R.; Kessler, H.; Rodrigues, D.C. Foreign Bodies Associated With Peri-Implantitis Human Biopsies. J. Periodontol. 2015, 86, 9–15. [Google Scholar] [CrossRef]
- Domingo, M.G.; Ferrari, L.; Aguas, S.; Alejandro, F.S.; Steimetz, T.; Sebelli, P.; Olmedo, D.G. Oral exfoliative cytology and corrosion of metal piercings. Tissue implications. Clin. Oral Investig. 2019, 23, 1895–1904. [Google Scholar] [CrossRef]
- Catinon, M.; Roux, E.; Auroux, A.; Trunfio-Sfarghiu, A.M.; Lauro-Colleaux, C.; Watkin, E.; Sournies, G.; Vincent, M. Confirmation of the systematic presence of tin particles in fallopian tubes or uterine horns of Essure implant explanted patients: A study of 18 cases with the same pathological process. J. Trace Elem. Med. Biol. 2022, 69, 126891. [Google Scholar] [CrossRef]
- Hall, D.J.; Pourzal, R.; Jacobs, J.J.; Urban, R.M. Metal wear particles in hematopoietic marrow of the axial skeleton in patients with prior revision for mechanical failure of a hip or knee arthroplasty. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 1930–1936. [Google Scholar] [CrossRef]
- Yue, Z.; Sun, C.; Chen, F.; Zhang, Y.; Xu, W.; Shabbir, S.; Zou, L.; Lu, W.; Wang, W.; Xie, Z.; et al. Machine learning-based LIBS spectrum analysis of human blood plasma allows ovarian cancer diagnosis. Biomed. Opt. Express 2021, 12, 2559–2574. [Google Scholar] [CrossRef]
- Skalny, A.V.; Korobeinikova, T.V.; Aschner, M.; Baranova, O.V.; Barbounis, E.G.; Tsatsakis, A.; Tinkov, A.A. Medical application of laser-induced breakdown spectroscopy (LIBS) for assessment of trace element and mineral in biosamples: Laboratory and clinical validity of the method. J. Trace Elem. Med. Biol. 2023, 79, 127241. [Google Scholar] [CrossRef]
- Jablan, J.; Besalú, E.; Žarak, M.; Dumić, J.; Marguí, E. Analytical potential of total reflection X-ray fluorescence spectrometry for simultaneous determination of iron, copper and zinc in human blood serum and plasma. Talanta 2021, 233, 122553. [Google Scholar] [CrossRef]
- Witt, B.; Schaumlöffel, D.; Schwerdtle, T. Subcellular Localization of Copper—Cellular Bioimaging with Focus on Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 2341. [Google Scholar] [CrossRef]
- Risseeuw, S.; Pilgrim, M.G.; Bertazzo, S.; Brown, C.N.; Csincsik, L.; Fearn, S.; Thompson, R.B.; Bergen, A.A.; ten Brink, J.B.; Kortvely, E.; et al. Bruch’s Membrane Calcification in Pseudoxanthoma Elasticum: Comparing Histopathology and Clinical Imaging. Ophthalmol. Sci. 2024, 4, 100416. [Google Scholar] [CrossRef]
- Thompson, R.B.; Reffatto, V.; Bundy, J.G.; Kortvely, E.; Flinn, J.M.; Lanzirotti, A.; Jones, E.A.; McPhail, D.S.; Fearn, S.; Boldt, K.; et al. Identification of hydroxyapatite spherules provides new insight into subretinal pigment epithelial deposit formation in the aging eye. Proc. Natl. Acad. Sci. 2015, 112, 1565–1570. [Google Scholar] [CrossRef]
- Pilgrim, M.G.; Lengyel, I.; Lanzirotti, A.; Newville, M.; Fearn, S.; Emri, E.; Knowles, J.C.; Messinger, J.D.; Read, R.W.; Guidry, C.; et al. Subretinal Pigment Epithelial Deposition of Drusen Components Including Hydroxyapatite in a Primary Cell Culture Model. Investig. Ophthalmol. Vis. Sci. 2017, 58, 708–719. [Google Scholar] [CrossRef]
- Biesemeier, A.; Eibl, O.; Eswara, S.; Audinot, J.-N.; Wirtz, T.; Schraermeyer, U. Transition metals and trace elements in the retinal pigment epithelium and choroid: Correlative ultrastructural and chemical analysis by analytical electron microscopy and nano-secondary ion mass spectrometry. Metallomics 2018, 10, 296–308. [Google Scholar] [CrossRef]
- Proetto, M.T.; Anderton, C.R.; Hu, D.; Szymanski, C.J.; Zhu, Z.; Patterson, J.P.; Kammeyer, J.K.; Nilewski, L.G.; Rush, A.M.; Bell, N.C.; et al. Cellular Delivery of Nanoparticles Revealed with Combined Optical and Isotopic Nanoscopy. ACS Nano 2016, 10, 4046–4054. [Google Scholar] [CrossRef]
- Lee, R.F.S.; Escrig, S.; Croisier, M.; Clerc-Rosset, S.; Knott, G.W.; Meibom, A.; Davey, C.A.; Johnsson, K.; Dyson, P.J. NanoSIMS analysis of an isotopically labelled organometallic ruthenium(II) drug to probe its distribution and state in vitro. Chem. Commun. 2015, 51, 16486–16489. [Google Scholar] [CrossRef]
- Legin, A.A.; Schintlmeister, A.; Jakupec, M.A.; Galanski, M.S.; Lichtscheidl, I.; Wagner, M.; Keppler, B.K. NanoSIMS combined with fluorescence microscopy as a tool for subcellular imaging of isotopically labeled platinum-based anticancer drugs. Chem. Sci. 2014, 5, 3135–3143. [Google Scholar] [CrossRef]
- Wedlock, L.E.; Kilburn, M.R.; Liu, R.; Shaw, J.A.; Berners-Price, S.J.; Farrell, N.P. NanoSIMS multi-element imaging reveals internalisation and nucleolar targeting for a highly-charged polynuclear platinum compound. Chem. Commun. 2013, 49, 6944–6946. [Google Scholar] [CrossRef]
- Gulin, A.A.; Pavlyukov, M.S.; Gularyan, S.K.; Nadtochenko, V.A. Visualization of the spatial distribution of Pt+ ions in cisplatin-treated glioblastoma cells by time-of-flight secondary ion mass spectrometry. Biochem. Mosc. Suppl. Ser. A 2015, 9, 202–209. [Google Scholar] [CrossRef]
- Aldossari, S.; McMahon, G.; Lockyer, N.P.; Moore, K.L. Microdistribution and quantification of the boron neutron capture therapy drug BPA in primary cell cultures of human glioblastoma tumour by NanoSIMS. Analyst 2019, 144, 6214–6224. [Google Scholar] [CrossRef]
- Wang, F. The Disturbance of Anticancer Drug Cisplatin to Cellular Homeostasis of Trace Elements Revealed by ICP-MS and ToF-SIMS. At. Spectrosc. 2021, 42, 166–174. [Google Scholar] [CrossRef]
- Liu, X.; Hummon, A.B. Chemical Imaging of Platinum-Based Drugs and their Metabolites. Sci. Rep. 2016, 6, 38507. [Google Scholar] [CrossRef]
- Larroque, M.; Mounicou, S.; Sgarbura, O.; Arnaudguilhem, C.; Rebel, L.; Leaha, C.; Faye, P.-A.; Enjalbal, C.; Quénet, F.; Bouyssiere, B.; et al. Study of oxaliplatin penetration into ovaries of patients treated with hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal metastases of colorectal and appendiceal origin using mass spectrometry imaging. Pleura Peritoneum 2021, 6, 67–74. [Google Scholar] [CrossRef]
- Bianga, J.; Bouslimani, A.; Bec, N.; Quenet, F.; Mounicou, S.; Szpunar, J.; Bouyssiere, B.; Lobinski, R.; Larroque, C. Complementarity of MALDI and LA ICP mass spectrometry for platinum anticancer imaging in human tumor. Metallomics 2014, 6, 1382–1386. [Google Scholar] [CrossRef]
- Pavelicova, K.; Do, T.; Vejvodova, M.; Vaculovic, T.; Nowak, K.; Matczuk, M.; Wu, S.; Krężel, A.; Adam, V.; Vaculovicova, M. Joint forces of mass spectrometric techniques (ICP-MS and MALDI-TOF-MS) and fluorescence spectrometry in the study of platinum-based cytostatic drugs interactions with metallothionein MT2 and MT3. Talanta 2024, 274, 125920. [Google Scholar] [CrossRef]
- Jovanović-Stević, S.; Radisavljević, S.; Scheurer, A.; Ćoćić, D.; Šmit, B.; Petković, M.; Živanović, M.N.; Virijević, K.; Petrović, B. Bis(triazinyl)pyridine complexes of Pt(II) and Pd(II): Studies of the nucleophilic substitution reactions, DNA/HSA interactions, molecular docking and biological activity. J. Biol. Inorg. Chem. 2021, 26, 625–637. [Google Scholar] [CrossRef]
- Milutinović, M.M.; Čanović, P.P.; Stevanović, D.; Masnikosa, R.; Vraneš, M.; Tot, A.; Zarić, M.M.; Simović Marković, B.; Misirkić Marjanović, M.; Vučićević, L.; et al. Newly Synthesized Heteronuclear Ruthenium(II)/Ferrocene Complexes Suppress the Growth of Mammary Carcinoma in 4T1-Treated BALB/c Mice by Promoting Activation of Antitumor Immunity. Organometallics 2018, 37, 4250–4266. [Google Scholar] [CrossRef]
- Jovanović, S.; Obrenčević, K.; Bugarčić, Ž.D.; Popović, I.; Žakula, J.; Petrović, B. New bimetallic palladium(II) and platinum(II) complexes: Studies of the nucleophilic substitution reactions, interactions with CT-DNA, bovine serum albumin and cytotoxic activity. Dalton Trans. 2016, 45, 12444–12457. [Google Scholar] [CrossRef]
- Kriegel, F.L.; Krause, B.-C.; Reichardt, P.; Singh, A.V.; Tentschert, J.; Laux, P.; Jungnickel, H.; Luch, A. The Vitamin A and D Exposure of Cells Affects the Intracellular Uptake of Aluminum Nanomaterials and Its Agglomeration Behavior: A Chemo-Analytic Investigation. Int. J. Mol. Sci. 2020, 21, 1278. [Google Scholar] [CrossRef]
- Hartnell, D.; Andrews, W.; Smith, N.; Jiang, H.; McAllum, E.; Rajan, R.; Colbourne, F.; Fitzgerald, M.; Lam, V.; Takechi, R.; et al. A Review of ex vivo Elemental Mapping Methods to Directly Image Changes in the Homeostasis of Diffusible Ions (Na+, K+, Mg2+, Ca2+, Cl−) Within Brain Tissue. Front. Neurosci. 2020, 13, 1415. [Google Scholar] [CrossRef]
- Pain, C.D.; Egan, G.F.; Chen, Z. Deep learning-based image reconstruction and post-processing methods in positron emission tomography for low-dose imaging and resolution enhancement. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3098–3118. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Gao, J.; Sun, H.; Chen, Z.; Qiu, X. Surface-enhanced Raman scattering (SERS) technology: Emerging applications in cancer imaging and precision medicine. Methods 2025, 241, 67–93. [Google Scholar] [CrossRef] [PubMed]
- de Vega, R.G.; Sanchez, M.L.F.; Eiro, N.; Vizoso, F.J.; Sperling, M.; Karst, U.; Medel, A.S. Multimodal laser ablation/desorption imaging analysis of Zn and MMP-11 in breast tissues. Anal. Bioanal. Chem. 2018, 410, 913–922. [Google Scholar] [CrossRef]
- Bhartiya, A.; Batey, D.; Cipiccia, S.; Shi, X.; Rau, C.; Botchway, S.; Yusuf, M.; Robinson, I.K. X-ray Ptychography Imaging of Human Chromosomes After Low-dose Irradiation. Chromosome Res. 2021, 29, 107–126. [Google Scholar] [CrossRef]
- Najafinobar, N.; Venkatesan, S.; von Sydow, L.; Klarqvist, M.; Olsson, H.; Zhou, X.-H.; Cloonan, S.M.; Malmberg, P. ToF-SIMS mediated analysis of human lung tissue reveals increased iron deposition in COPD (GOLD IV) patients. Sci. Rep. 2019, 9, 10060. [Google Scholar] [CrossRef] [PubMed]
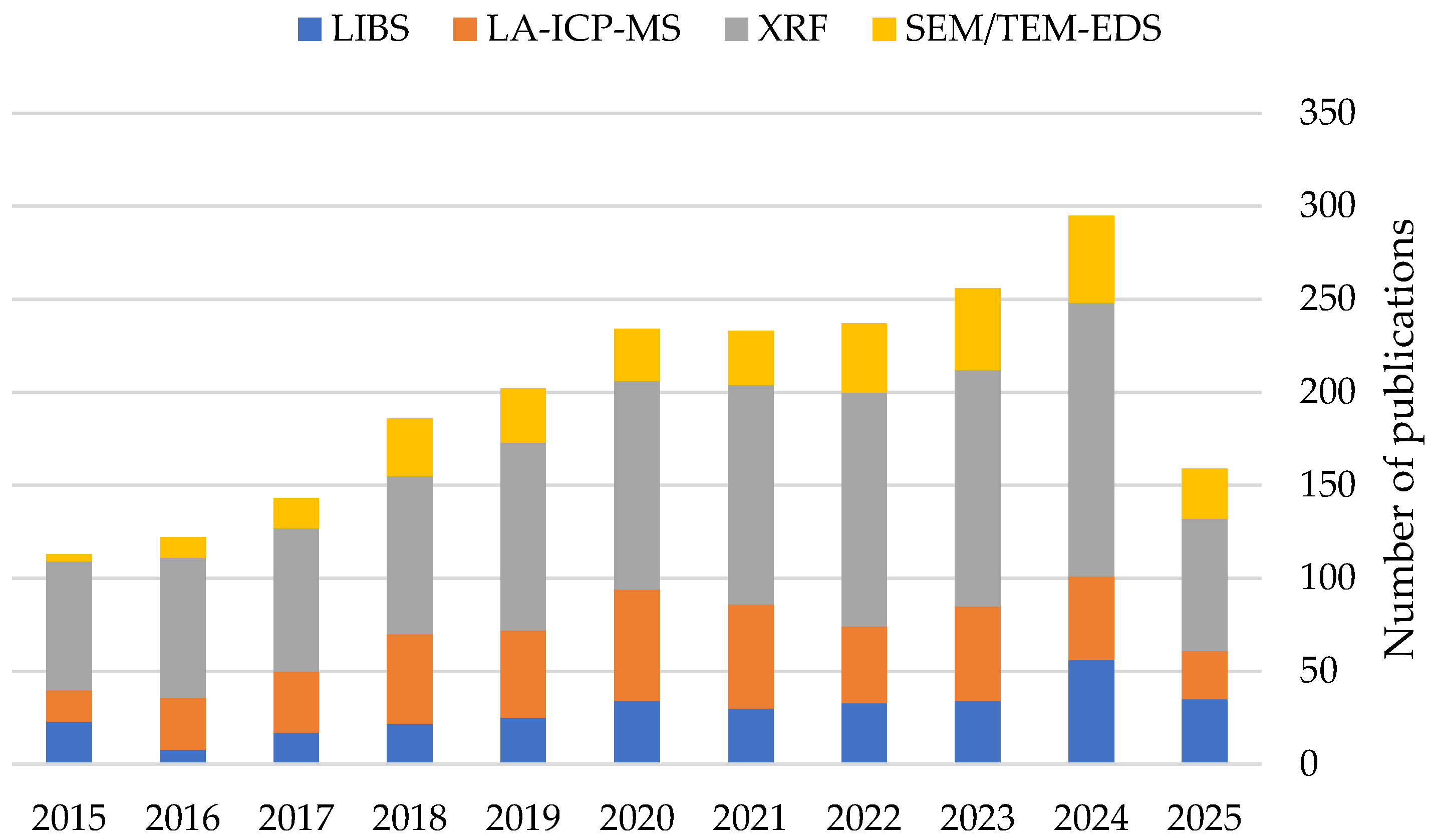
| Feature | LA-ICP-MS | LIBS | XRF | SEM-EDS | TEM-EDS |
|---|---|---|---|---|---|
| Full Name | Laser Ablation Inductively Coupled Plasma Mass Spectrometry | Laser-Induced Breakdown Spectroscopy | X-ray Fluorescence | Scanning Electron Microscopy with Energy-Dispersive X-ray | Transmission Electron Microscopy with Energy-Dispersive X-ray |
| Spatial Resolution | 5–100 µm | ~10–100 µm | ~0.05–100 µm | ~1 µm | <20 nm |
| Detection Limit (LOD) | µg/kg | mg/kg | mg/kg | Tenths of weight % | Tenths of weight % |
| Quantification | Yes | Yes | Yes | Semi-quantitative | Semi-quantitative |
| Sample Destruction * | Semi-non-destructive | Non-/minimally destructive | No | No | No |
| Suitable Sample State | Solid (flat and polished) | Solid (minimal preparation) | Solid (minimal preparation) | Solid (degreased and dried) | Ultrathin slices (~100–150 nm) |
| Light Element Detection | Limited | Yes (H, C, N detectable) | Limited | Limited | Limited |
| Sample Preparation Complexity | Medium (polishing, standards) | Low (clean surface) | Low (minimal preparation) | Medium (mounting, coating with Au/C) | High (FIB, ultramicrotomy, thinning to electron transparency) |
| Analysis Time | Minutes to hours (mapping) | Seconds to minutes | Seconds to minutes | Minutes per point or map | Long (due to preparation and imaging) |
| LA-ICP-MS | LIBS | XRF | SEM-EDS |
|---|---|---|---|
| laser energy ablation area isobaric interferences polyatomic interferences doubly charged ions plasma robustness dwell time instrumental drift gas flows | laser energy ablation area readout time delay surface roughness gate width measurement atmosphere repetition rate | absorption effects power of the excitation source type of radiation source signal intensity for speciation topography of the layer measurement atmosphere | power of the excitation source measurement atmosphere surface topography sample conductivity |
| Analyte | Internal Standard | Reference |
|---|---|---|
| 157Gd, 158Gd, 160Gd, 31P, 44Ca | 103Rh, 115In | [11] |
| 54Fe, 56Fe, 63Cu, 65Cu, 64Zn, 68Zn | 197Au (pseudo-internal standard) | [40] |
| 194Pt,195Pt,196Pt, 13C, 31P, 34S | 97Au (pseudo-internal standard) | [36] |
| 23Na, 24Mg, 25Mg, 39K, 42Ca, 44Ca, 55Mn, 56Fe, 57Fe, 58Ni, 60Ni, 63Cu, 64Zn, 65Cu, 66Zn | 115In, 197Au (pseudo-internal standard) | [17] |
| 27Al, 49Ti, 51V | 34S | [13] |
| 54Fe, 56Fe, 63Cu, 65Cu | 69Ga, Rh | [16] |
| Na, Mg, P, K, Ca, Ti, Cr, Ni, Cu, Zn, Pb | C, S | [26] |
| Isotope | IDL [µg/g] | IQL [µg/g] | MDL [µg/g] | MQL [µg/g] |
|---|---|---|---|---|
| 23Na | 5.7 [17] | |||
| 26Mg | 14 [32] | 10.6 [17] 419 [32] | ||
| 24Mg | 2.3 [17] | |||
| 27Al | 0.83/0.24 1 [34] | 2.5/0.72 1 [34] | 4.8/1.8 1 [34] | 14/5.3 1 [34] |
| 2.2 [13] | 6.9 [13] | |||
| 4.1 [32] | 14 [32] | |||
| 39K | 13.2 [17] | |||
| 43Ca | 450 [32] | 1174 [32] | ||
| 49Ti | 0.78/0.55 1 [34] | 2.4/1.7 1 [34] | 0.84/1.5 1 [34] | 2.5/4.4 1 [34] |
| 1.1 [13] | 8.1 [13] | |||
| 14 [32] | 21 [32] | |||
| 51V | 0.24/0.1 1 [34] | 0.73/0.30 1 [34] | 0.58/0.82 1 [34] | 1.8/2.5 1 [34] |
| 0.80 [13] | 4.6 [13] | |||
| 55Mn | 1.8 [32] | 0.1 [17] 4.7 [32] | ||
| 56Fe | 5 2 [16] | 18 2 [16] | 0.8 [17] | |
| 57Fe | 43 [32] | 3.4 [17] 98 [32] | ||
| 58Ni | 0.1 [17] | |||
| 60Ni | 0.4 [17] | |||
| 63Cu | 1 2 [16] | 4 2 [16] | 0.1 [17] 6.5 [32] | |
| 65Cu | 0.2 [17] | |||
| 64Zn | 0.1 [17] | |||
| 66Zn | 18 [32] | 0.2 [17] 61 [32] | ||
| 157Gd,158Gd,160Gd | 3.0 [11] | 9.0 [11] | ||
| 195Pt | 1.6 [36] |
| Sample | Implant/ Group of Patients | Number of Patients/Samples (Without Control) | Element | Technique | Remark | Reference |
|---|---|---|---|---|---|---|
| gastric cancer cells | human cell line | - | Zn | LA-ICP-MS | 35 μm spot size | [49] |
| skin biopsy samples | nephrogenic systemic fibrosis | 1 | Gd, Ca, P | LA-ICP-MS | KED 1 50 μm spot size | [11] |
| oral mucosa tissues | dental | 30 | Ti, Al, V, S | LA-ICP-MS (quantitatively) | 50 μm spot size | [13] |
| oral mucosa tissues | dental | no information | Ti, Al, Ca, Mg, Zn, Cu, Fe, Mn, S, C | LA-ICP-MS (quantitatively) | 25 μm spot size | [32] |
| oral mucosa tissues | dental | 12 | Ti, Al, V S, C, Mg, Ca | LA-ICP-MS (quantitatively) | 100 μm spot size | [34] |
| white and grey matter and frontal cortex tissues (postmortem) | Alzheimer’s disease | 4 | P, Fe, C | LA-ICP-MS (quantitative imaging) | CRC 2 80 × 80 μm laser beam | [12] |
| liver | Wilson’s disease | 3 | Fe, Cu, Ga | LA–ICP–MS (elemental bioimaging) | KED 1 100 μm spot size | [16] |
| tissue | human malignant mesothelioma | 1 | Na, Mg, K, Ca, Mn, Fe, Ni, Cu, Zn, In, Au | LA-ICP-MS (elemental bioimaging) | 40 μm laser diameter | [17] |
| liver | Wilson’s disease | 6 | C, Na, Mg, P, S, K, Ca, Ti, Cr, Fe, Mn, Ni, Cu, Zn, Pb | LA-ICP-MS | - | [26] |
| tissue | human malignant mesothelioma | 1 | Pt, C, P, S, Au | LA-ICP-MS | 50 μm laser diameter | [36] |
| human lens | eye | 5 | Fe, Cu, Zn, Au | LA-ICP-MS (quantitative bioimaging) | 200 μm | [40] |
| healthy teeth, deciduous teeth, teeth filled with amalgam and composite restorative materials | - | - | Ca, K, Mg, P, Na, Sr, Cu, Cr, Fe, Ba, Pb, Zn, Hg, Al | LIBS qualitative | - | [50] |
| tissue | human tumour | 1 | P, Fe, Cu, Zn, Pt | LA-ICP-MS | mapping 40 μm per pixel | [14] |
| C, H, O, Na, K, Ca, Mg | LIBS | - | ||||
| skin tissues | various cancers | 3 | P, Al, Mg, Na, Zn, Si, Fe, Cu, Ca | LIBS (multi-elemental imaging) | - | [25] |
| colon tissues | colon cancer | 15 | Pb, Cr, Ce, Hg | LIBS | - | [15] |
| Feature | LA-ICP-MS | LIBS | XRF | SEM-EDS | TEM-EDS |
|---|---|---|---|---|---|
| Trace element detection | most suitable | suitable | suitable | limited | limited |
| Macro-elements | limited | suitable | limited | limited | limited |
| Sample re-analysis | limited | limited | possible | possible | possible |
| Depth profile analysis | tens of micrometres | <500 μm | several tens of micrometres | few micrometres | limited by sample thickness |
| Speciation | not possible | not possible | possible | not possible | not possible |
| Throughput | moderate | high | high | slow | slow |
| Operating costs/availability | expensive | moderate | moderate | expensive | very expensive |
| Solid samples | suitable | suitable (solid surfaces) | tissue slices | excellent resolution | excellent resolution |
| Liquid samples (blood, plasma) | requires dry blood spot or cryogenic ablation | requires drying | plasma, serum with or without dilution; blood need preparation step | difficult due to high vacuum environment | difficult due to high vacuum environment |
| Relative sensitivity to matrix effects | high (due to fluctuations in composition, structure and moisture content) | very high (due to laser energy absorption, plasma formation) | moderate to high (due to varying density, thickness and water content) | low to moderate (depend on atomic number and competing absorption-fluorescence effects) | low (due to sample thickness) |
| Linear range (in mg/kg) | ~0.01–10,000 | ~10–10,000 | ~1–100,000 | ~100–100,000 | ~100–50,000 |
| Maximum mapping area | >cm2 | ~cm2–several cm2 | ~cm2–several cm2 | ~mm2 | <<mm2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawicki, J.; Feldo, M.; Skalska-Kamińska, A.; Sowa, I. Modern Bioimaging Techniques for Elemental Tissue Analysis: Key Parameters, Challenges and Medical Impact. Molecules 2025, 30, 2864. https://doi.org/10.3390/molecules30132864
Sawicki J, Feldo M, Skalska-Kamińska A, Sowa I. Modern Bioimaging Techniques for Elemental Tissue Analysis: Key Parameters, Challenges and Medical Impact. Molecules. 2025; 30(13):2864. https://doi.org/10.3390/molecules30132864
Chicago/Turabian StyleSawicki, Jan, Marcin Feldo, Agnieszka Skalska-Kamińska, and Ireneusz Sowa. 2025. "Modern Bioimaging Techniques for Elemental Tissue Analysis: Key Parameters, Challenges and Medical Impact" Molecules 30, no. 13: 2864. https://doi.org/10.3390/molecules30132864
APA StyleSawicki, J., Feldo, M., Skalska-Kamińska, A., & Sowa, I. (2025). Modern Bioimaging Techniques for Elemental Tissue Analysis: Key Parameters, Challenges and Medical Impact. Molecules, 30(13), 2864. https://doi.org/10.3390/molecules30132864






