Chiral Salen-Based Organic Salts: Synthesis and Potential Antibacterial Activity
Abstract
1. Introduction
2. Results and Discussion
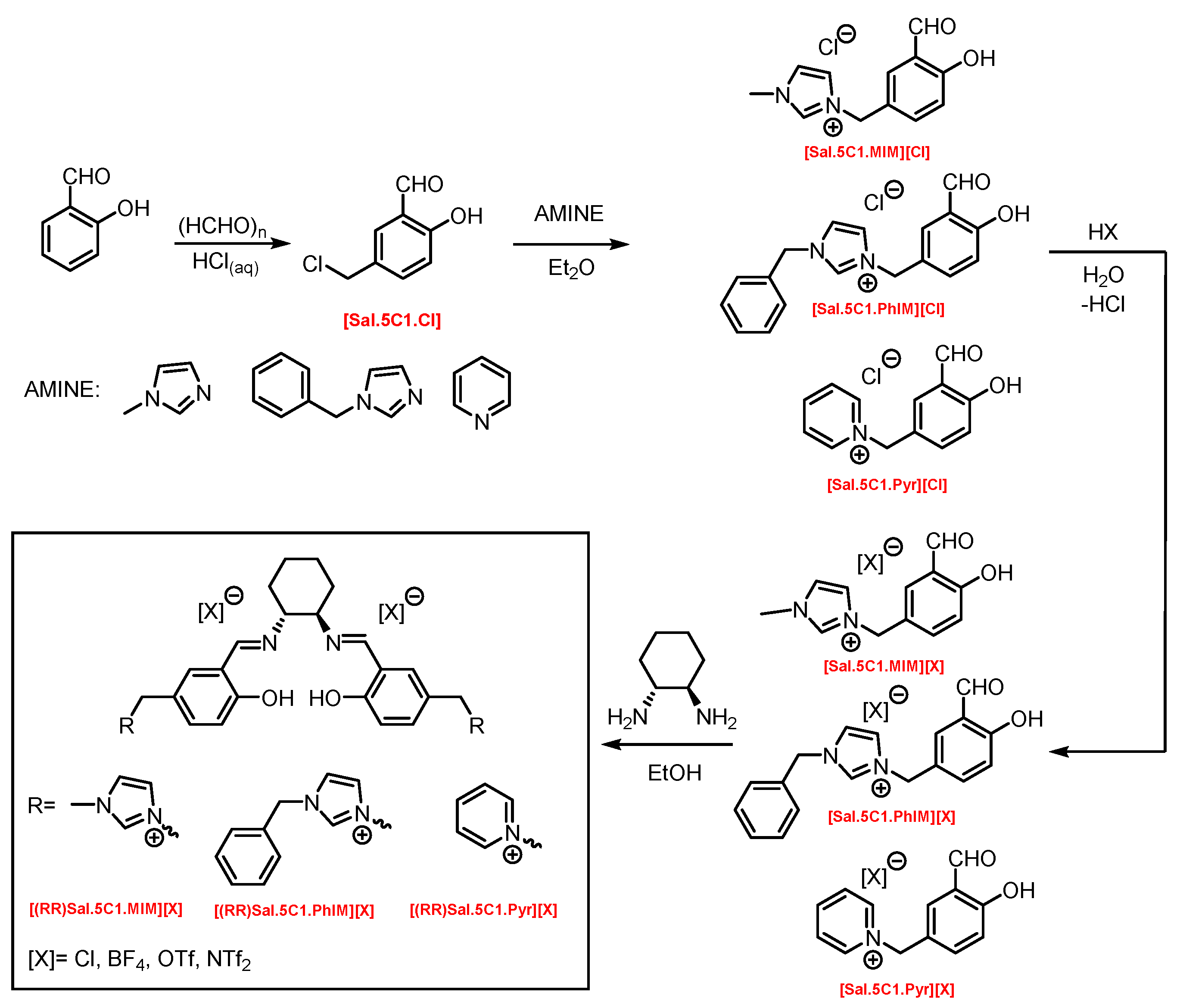
2.1. Synthesis of Chiral Salen Organic Salts
2.2. Identification
2.3. Melting Point, TG, and DSC
2.4. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for the Tested Chiral Organic Salts and Their Substrates
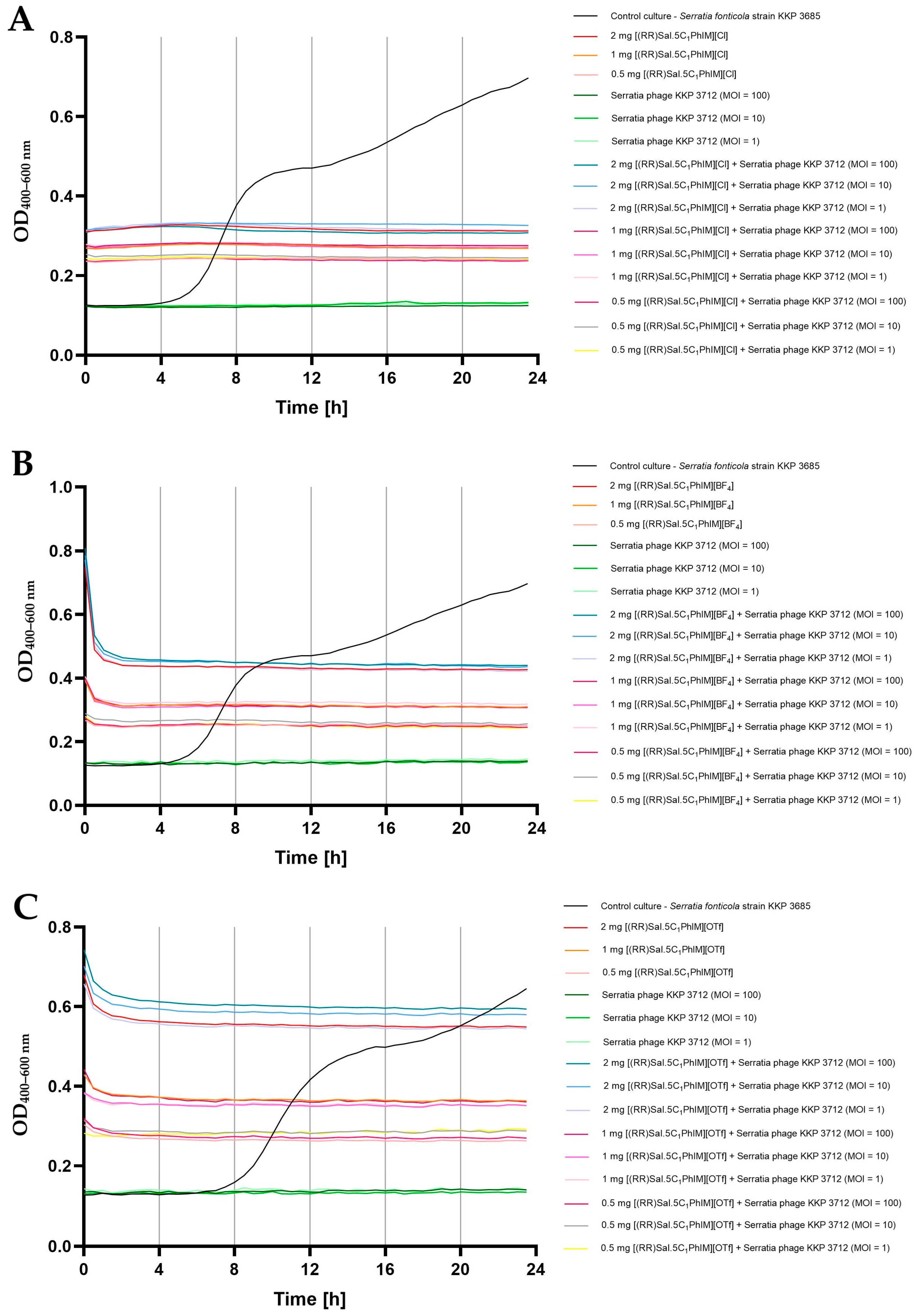
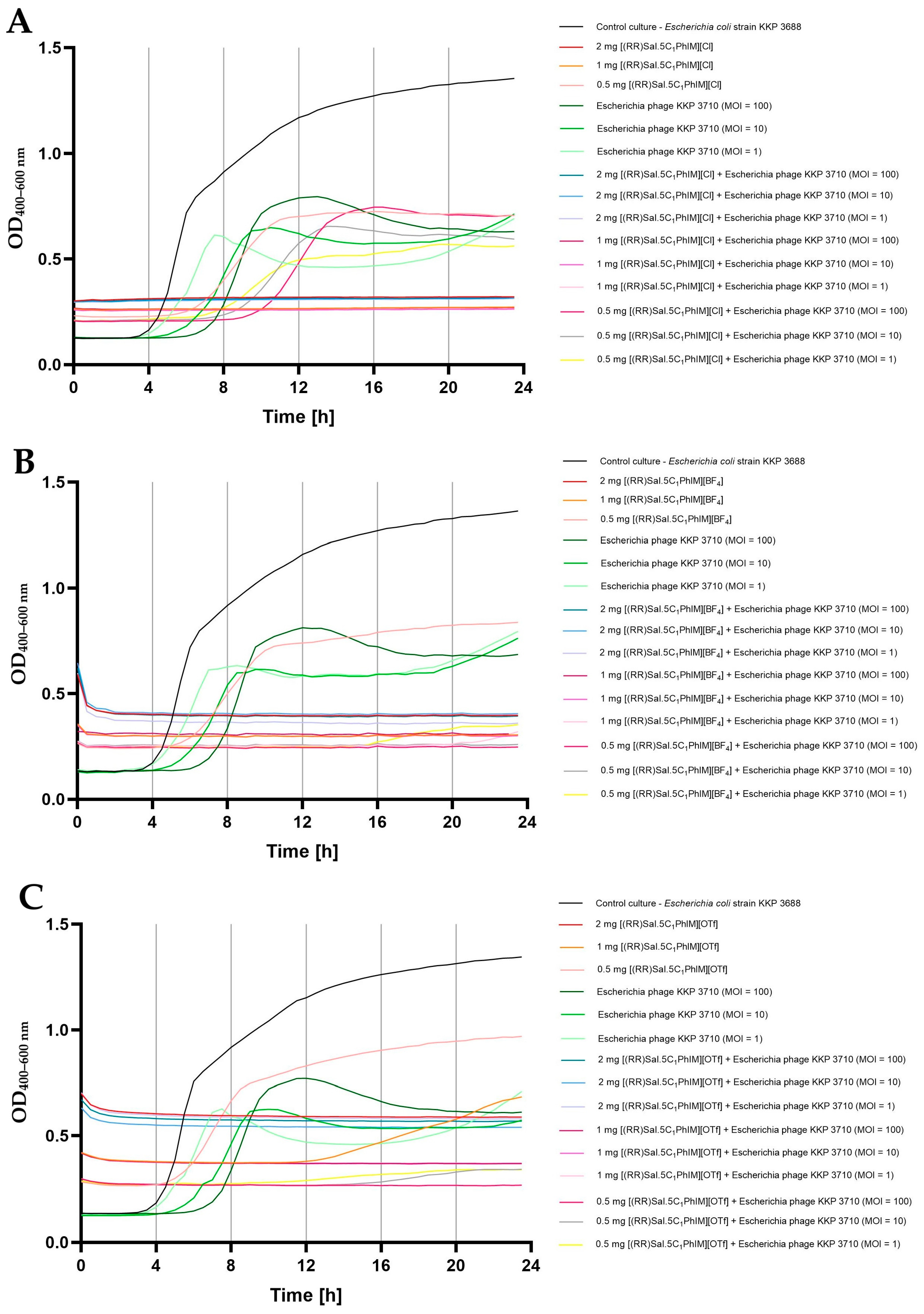
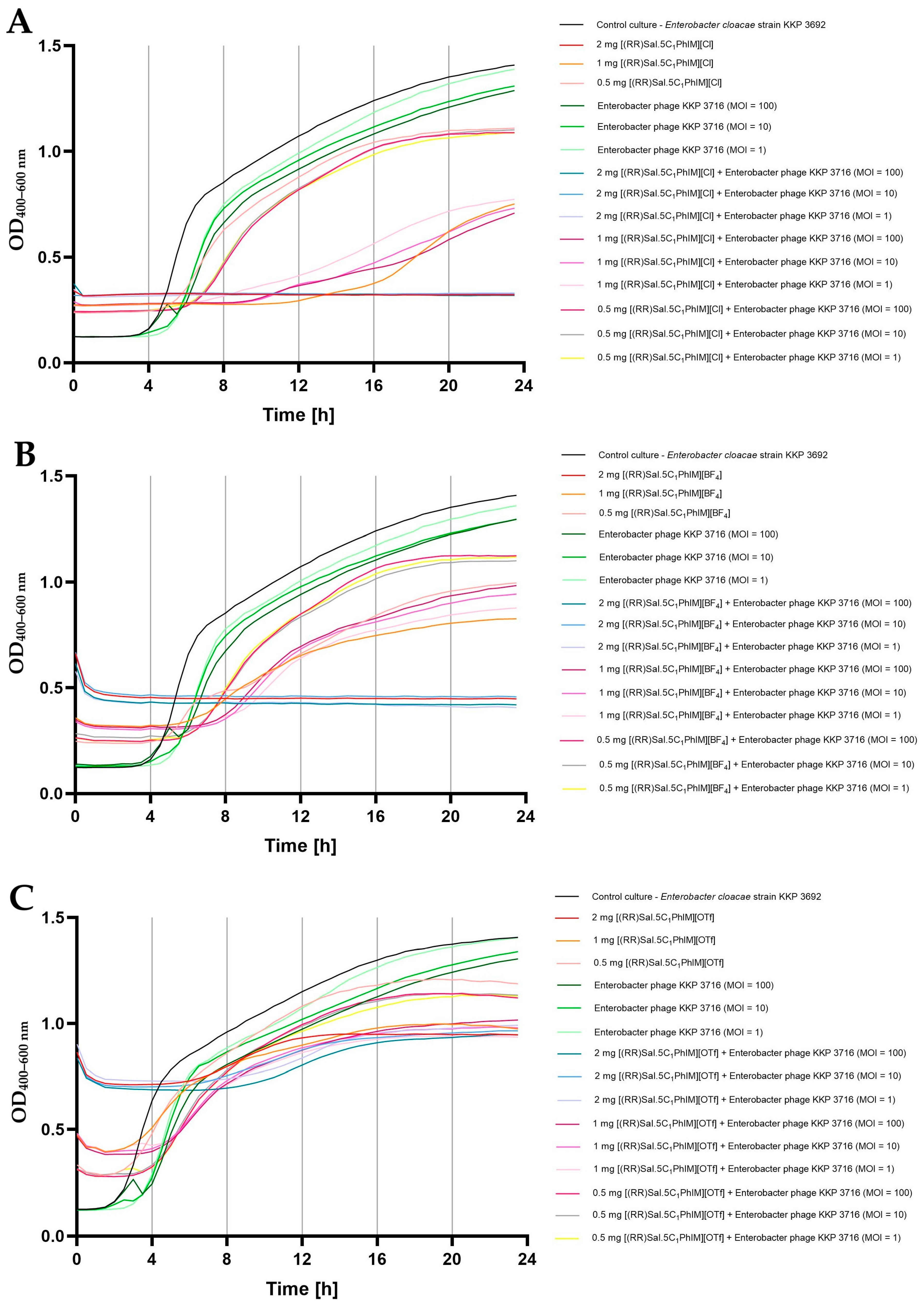
2.5. Influence of Chiral Salen-Based Organic Salts on the Bacteriophage Activity
2.6. Synergistic Effect of Chiral Organic Salts and Lytic Bacteriophages Against Target Bacteria
3. Materials and Methods
3.1. Synthesis of Chiral Salen Organic Salts
3.1.1. Materials Used in the Synthesis of Chiral Salen Organic Salts
3.1.2. Methods Used for the Characteristic of Chiral Salen Organic Salts
3.1.3. Synthesis Protocols
- 5-(2-chloromethyl)-salicylaldehyde, [Sal.5C1.Cl]

The General Procedure of Quaternisation Reaction
- 1-(3-formyl-4-hydroxybenzyl)-3-methylimidazolium chloride, [Sal.5C1.MIM][Cl]

- 1-(3-formyl-4-hydroxybenzyl)-3-benzylimidazolium chloride, [Sal.5C1.PhIM][Cl]
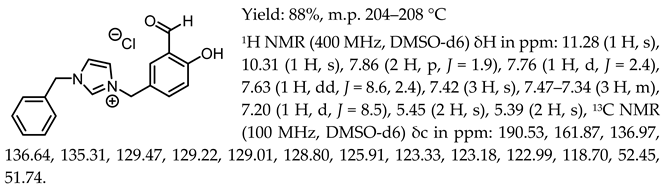
- 1-(3-formyl-4-hydroxybenzyl)-pyridinium chloride, [Sal.5C1.Pyr][Cl]

The General Procedure of Chloride Anion Exchange
- 1-(3-formyl-4-hydroxybenzyl)-3-methylimidazolium tetrafluoroborate, [Sal.5C1.MIM][BF4]

- 1-(3-formyl-4-hydroxybenzyl)-3-methylimidazolium trifluoromethanosulfonate, [Sal.5C1.MIM][OTf]

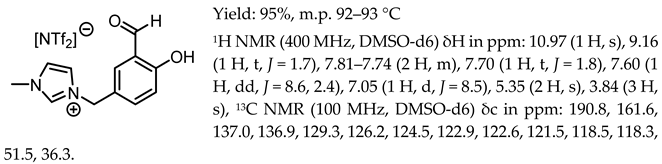
- 1-(3-formyl-4-hydroxybenzyl)-3-methylimidazolium bis(trifluoromethanesulfonyl)imide, [Sal.5C1.MIM][NTf2]
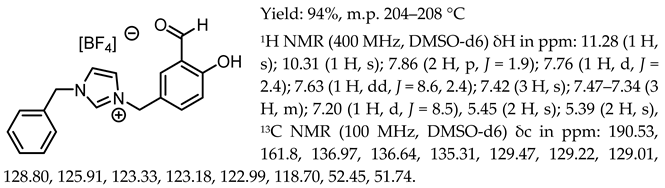
- 1-(3-formyl-4-hydroxybenzyl)-3-benzylimidazolium tetrafluoroborate, [Sal.5C1.PhIM][BF4]
- 1-(3-formyl-4-hydroxybenzyl)-3-benzylimidazolium trifluoromethanesulfonate, [Sal.5C1.PhIM][OTf]

- 1-(3-formyl-4-hydroxybenzyl)-3-benzylimidazolium bis(trifluoromethylosulfonyl)imide, [Sal.5C1.PhIM][NTf2]
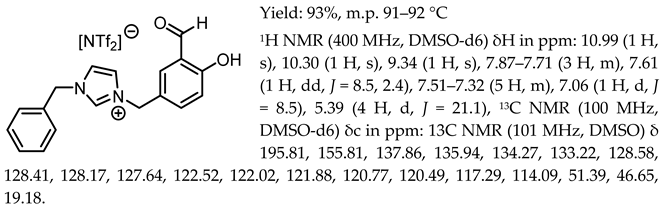
- 1-(3-formyl-4-hydroxybenzyl)-pyridinium tetrafluoroborate, [Sal.5C1.Pyr][BF4]
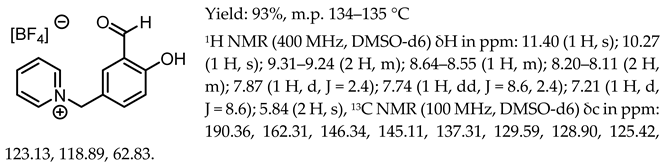
- 1-(3-formyl-4-hydroxybenzyl)-pyridinium trifluoromethanesulfonate, [Sal.5C1.Pyr][OTf]
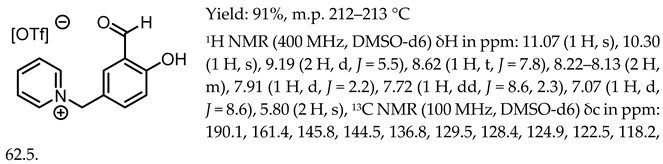
- 1-(3-formyl-4-hydroxybenzyl)-pyridinium bis(trifluoromethanesulfonyl)imide, [Sal.5C1.Pyr][NTf2]
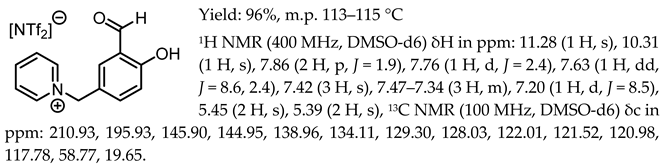
The General Procedure for the Synthesis of Chiral Salen Organic Salts
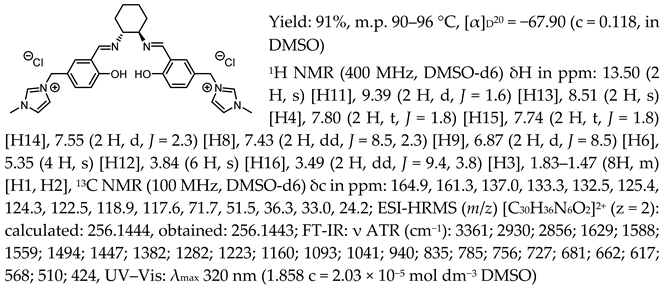
- N,N′-bis-[5-((1-methylimidazol-3-ium)methylene)-salicylidene]-trans-(1R,2R)-cyclohexanediamine dichloride, [(RR)Sal.5C1.MIM][Cl]
- N,N′-bis-[5-((1-methylimidazol-3-ium)methylene)-salicylidene]-trans-(±)-cyclohexanediamine dichloride, [(RR)Sal.5C1.MIM][Cl]
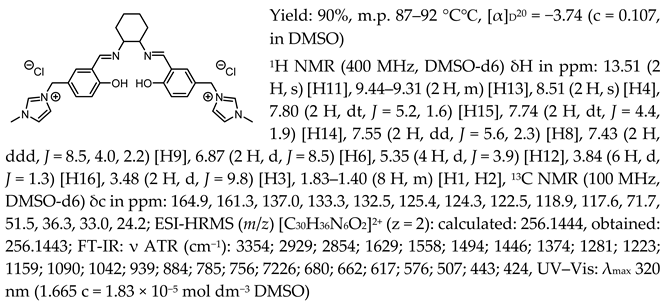
- N,N′-bis-[5-((1-methylimidazol-3-ium)methylene)-salicylidene]-trans-(1R,2R)-cyclohexanediamine ditetrafluoroborate, [(RR)Sal.5C1.MIM][BF4]

- N,N′-bis-[5-((1-methylimidazol-3-ium)methylene)-salicylidene]-trans-(1R,2R)-cyclohexanediamine ditrifluoromethanesulfonate, [(RR)Sal.5C1.MIM][OTf]
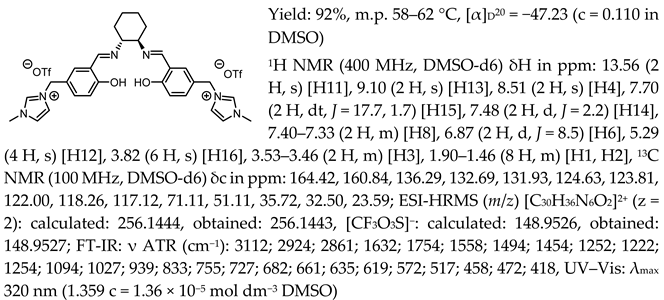
- N,N′-bis-[5-((1-methylimidazol-3-ium)methylene)-salicylidene]-trans-(1R,2R)-cyclohexanediamine di[bis(trifluoromethanesulfonyl)imide], [(RR)Sal.5C1.MIM][NTf2]
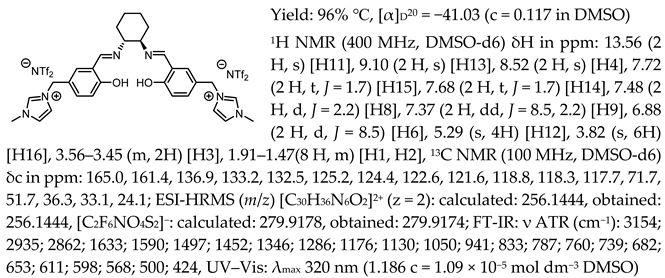
- N,N′-bis-[5-((pyridinium)methylene)-salicylidene]-trans-(1R,2R)-cyclohexanediamine dichloride, [(RR)Sal.5C1.Pyr][Cl]

- N,N′-bis-[5-((pyridinium)methylene)-salicylidene]-trans-(1R,2R)-cyclohexanediamine ditetrafluoroborate, [(RR)Sal.5C1.Pyr][BF4]
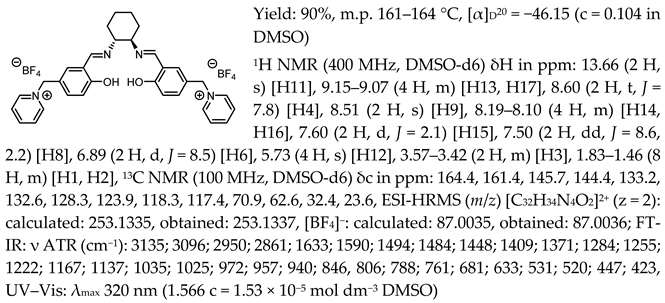
- N,N′-bis-[5-((pyridinium)methylene)-salicylidene]-trans-(1R,2R)-cyclohexanediamine ditrifluoromethanesulfonate, [(RR)Sal.5C1.Pyr][OTf]
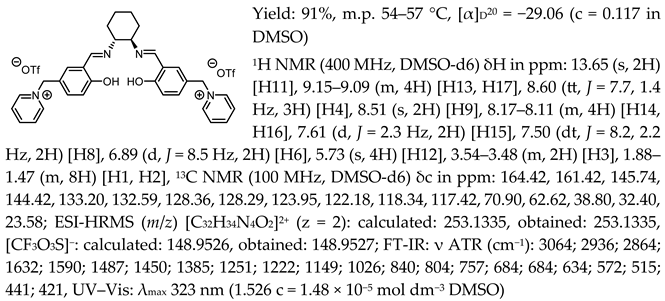
- N,N′-bis-[5-((pyridinium)methylene)-salicylidene]-trans-(1R,2R)-cyclohexanediamine di[bis(trifluoromethanesulfonyl)imide], [(RR)Sal.5C1.Pyr][NTf2]

- N,N′-bis-[5-((1-benzylimidazol-3-ium)methylene)-salicylidene]-trans-(1R,2R)-cyclohexanediamine dichloride, [(RR)Sal.5C1.PhIM][Cl]

- N,N′-bis-[5-((1-benzylimidazol-3-ium)methylene)-salicylidene]-trans-(1R,2R)-cyclohexanediamine ditetrafluoroborate, [(RR)Sal.5C1.PhIM][BF4]
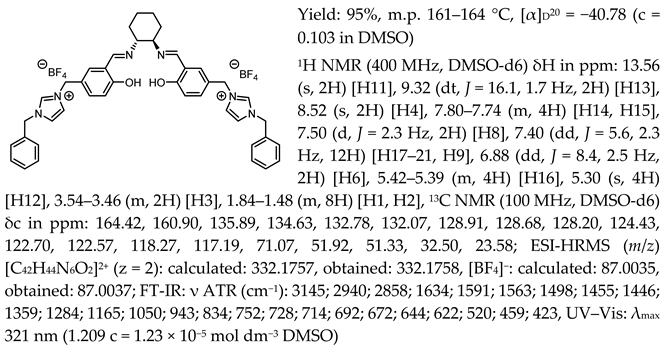
- N,N′-bis-[5-((1-benzylimidazol-3-ium)methylene)-salicylidene]-trans-(1R,2R)-cyclohexanediamine ditrifluoromethanesulfonate, [(RR)Sal.5C1.PhIM][OTf]

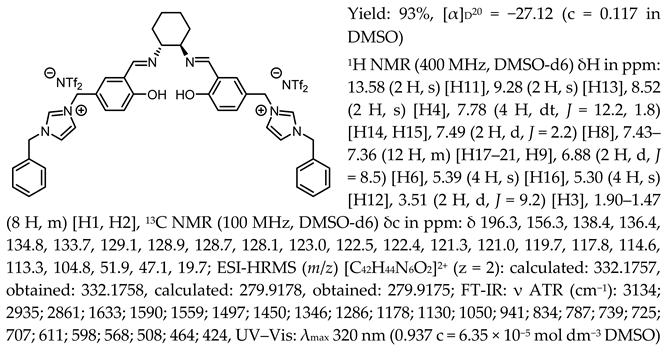
- N,N′-bis-[5-((1-benzylimidazol-3-ium)methylene)-salicylidene]-trans-(1R,2R)-cyclohexanediamine di[bis(trifluoromethanesulfonyl)imide], [(RR)Sal.5C1.PhIM][NTf2]
3.2. Microbiological Resources
3.3. Determination of the Minimum Inhibitory Concentration (MIC)
3.4. Determination of the Minimum Bactericidal Concentration (MBC)
3.5. Synergistic Effect of Chiral Organic Salts and Other Antimicrobial Factors
3.5.1. Influence of Chiral Organic Salts on the Bacteriophage Activity
3.5.2. Synergistic Effect of Chiral Organic Salts and Lytic Bacteriophages Against Target Bacteria
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASM | American Society of Microbiology |
| CLSI | Clinical and Laboratory Standards Institute |
| CFU | Colony-forming unit |
| DMSO | Dimethyl sulfoxide |
| IAFB | Prof. Wacław Dąbrowski Institute of Agricultural and Food Biotechnology—State Re-search Institute |
| MBC | Minimum bactericidal concentration |
| MIC | Minimum inhibitory concentration |
| MOI | Multiplicity of infection |
| NCCLS | National Committee for Clinical Laboratory Standards |
| OD | Optical density |
| PFU | Plaque-forming unit |
| SSO | Salen-based organic salts |
| WHO | World Health Organisation |
References
- Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2023.
- Chandra, P.; Unnikrishnan, M.K.; Vandana, K.E.; Mukhopadhyay, C.; Dinesh Acharya, U.; Surulivel Rajan, M.; Rajesh, V. Antimicrobial Resistance and the Post Antibiotic Era: Better Late than Never Effort. Expert Opin. Drug Saf. 2021, 20, 1375–1390. [Google Scholar] [CrossRef]
- Wójcicki, M.; Shymialevich, D.; Średnicka, P.; Emanowicz, P.; Ostrowska, A.; Cieślak, H.; Sokołowska, B. Phenotypic Characterization and Genome Analysis of New Broad-Spectrum Virulent Salmophage, Salmonella Phage KKP_3822, for Biocontrol of Multidrug-Resistant Salmonella Enterica Strains. Int. J. Mol. Sci. 2024, 25, 12930. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef]
- Kose, A.; Colak, C. Knowledge and Awareness of Physicians About Rational Antibiotic Use and Antimicrobial Resistance Before and After Graduation: A Cross-Sectional Study Conducted in Malatya Province in Turkey. IDR 2021, 14, 2557–2568. [Google Scholar] [CrossRef]
- Wójcicki, M.; Świder, O.; Gientka, I.; Błażejak, S.; Średnicka, P.; Shymialevich, D.; Cieślak, H.; Wardaszka, A.; Emanowicz, P.; Sokołowska, B.; et al. Effectiveness of a Phage Cocktail as a Potential Biocontrol Agent against Saprophytic Bacteria in Ready-To-Eat Plant-Based Food. Viruses 2023, 15, 172. [Google Scholar] [CrossRef]
- Liu, S.; Quek, S.-Y.; Huang, K. Advanced Strategies to Overcome the Challenges of Bacteriophage-Based Antimicrobial Treatments in Food and Agricultural Systems. Crit. Rev. Food Sci. Nutr. 2024, 64, 12574–12598. [Google Scholar] [CrossRef]
- Shymialevich, D.; Wójcicki, M.; Sokołowska, B. The Novel Concept of Synergically Combining: High Hydrostatic Pressure and Lytic Bacteriophages to Eliminate Vegetative and Spore-Forming Bacteria in Food Products. Foods 2024, 13, 2519. [Google Scholar] [CrossRef]
- Wójcicki, M.; Świder, O.; Średnicka, P.; Shymialevich, D.; Ilczuk, T.; Koperski, Ł.; Cieślak, H.; Sokołowska, B.; Juszczuk-Kubiak, E. Newly Isolated Virulent Salmophages for Biocontrol of Multidrug-Resistant Salmonella in Ready-to-Eat Plant-Based Food. Int. J. Mol. Sci. 2023, 24, 10134. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Zhang, X.; Liu, Y.; Hu, J.; Yang, C.; Zhao, X. The Effects of Ultrasound on the Growth, Nutritional Quality and Microbiological Quality of Sprouts. Trends Food Sci. Technol. 2021, 111, 292–300. [Google Scholar] [CrossRef]
- Shymialevich, D.; Wójcicki, M.; Wardaszka, A.; Świder, O.; Sokołowska, B.; Błażejak, S. Application of Lytic Bacteriophages and Their Enzymes to Reduce Saprophytic Bacteria Isolated from Minimally Processed Plant-Based Food Products—In Vitro Studies. Viruses 2023, 15, 9. [Google Scholar] [CrossRef]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human Health Risk Assessment of Antibiotic Resistance Associated with Antibiotic Residues in the Environment: A Review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Godziszewska, J.; Guzek, D.; Głąbski, K.; Wierzbicka, A. Mobile Antibiotic Resistance—The Spread of Genes Determining the Resistance of Bacteria through Food Products. Postępy Hig. Med. Doświadczalnej (Online) 2016, 70, 803–810. [Google Scholar] [CrossRef]
- Salminen, J.; Papaiconomou, N.; Kumar, R.A.; Lee, J.-M.; Kerr, J.; Newman, J.; Prausnitz, J.M. Physicochemical Properties and Toxicities of Hydrophobic Piperidinium and Pyrrolidinium Ionic Liquids. Fluid Phase Equilibria 2007, 261, 421–426. [Google Scholar] [CrossRef]
- Croitoru, C.; Roata, I.C. Ionic Liquids as Antifungal Agents for Wood Preservation. Molecules 2020, 25, 4289. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; e Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Coleman, D.; Špulák, M.; Garcia, M.T.; Gathergood, N. Antimicrobial Toxicity Studies of Ionic Liquids Leading to a ‘Hit’ MRSA Selective Antibacterial Imidazolium Salt. Green Chem. 2012, 14, 1350–1356. [Google Scholar] [CrossRef]
- Křen, V.; Řezanka, T. Sweet Antibiotics—The Role of Glycosidic Residues in Antibiotic and Antitumor Activity and Their Randomization. FEMS Microbiol. Rev. 2008, 32, 858–889. [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gilmore, B.F. The Antimicrobial Potential of Ionic Liquids: A Source of Chemical Diversity for Infection and Biofilm Control. Int. J. Antimicrob. Agents 2015, 46, 131–139. [Google Scholar] [CrossRef]
- Hossain, M.I.; El-Harbawi, M.; Alitheen, N.B.M.; Noaman, Y.A.; Lévêque, J.-M.; Yin, C.-Y. Synthesis and Anti-Microbial Potencies of 1-(2-Hydroxyethyl)-3-Alkylimidazolium Chloride Ionic Liquids: Microbial Viabilities at Different Ionic Liquids Concentrations. Ecotoxicol. Environ. Saf. 2013, 87, 65–69. [Google Scholar] [CrossRef]
- Qin, J.; Guo, J.; Xu, Q.; Zheng, Z.; Mao, H.; Yan, F. Synthesis of Pyrrolidinium-Type Poly(Ionic Liquid) Membranes for Antibacterial Applications. ACS Appl. Mater. Interfaces 2017, 9, 10504–10511. [Google Scholar] [CrossRef]
- Saraswat, J.; Aldahmash, B.; AlOmar, S.Y.; Imtiyaz, K.; Rizvi, M.M.A.; Patel, R. Synergistic Antimicrobial Activity of N-Methyl Substituted Pyrrolidinium–Based Ionic Liquids and Melittin against Gram-Positive and Gram-Negative Bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 10465–10479. [Google Scholar] [CrossRef]
- Cole, M.R.; Li, M.; El-Zahab, B.; Janes, M.E.; Hayes, D.; Warner, I.M. Design, Synthesis, and Biological Evaluation of β-Lactam Antibiotic-Based Imidazolium- and Pyridinium-Type Ionic Liquids. Chem. Biol. Drug Des. 2011, 78, 33–41. [Google Scholar] [CrossRef]
- Fernandes, M.M.; Carvalho, E.O.; Correia, D.M.; Esperança, J.M.S.S.; Padrão, J.; Ivanova, K.; Hoyo, J.; Tzanov, T.; Lanceros-Mendez, S. Ionic Liquids as Biocompatible Antibacterial Agents: A Case Study on Structure-Related Bioactivity on Escherichia coli. ACS Appl. Bio Mater. 2022, 5, 5181–5189. [Google Scholar] [CrossRef]
- Fang, Z.; Zheng, X.; Li, L.; Qi, J.; Wu, W.; Lu, Y. Ionic Liquids: Emerging Antimicrobial Agents. Pharm. Res. 2022, 39, 2391–2404. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Asztemborska, M.; Caille, J.-C.; Jurczak, J. Enantioselective [4+2] Cycloaddition of Buta-1,3-Dienes to Alkyl Glyoxylates Catalyzed by the Chiral (Salen)Chromium(III) Complex. Adv. Synth. Catal. 2003, 345, 506–509. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Asztemborska, M.; Jurczak, J. The Enantioselective Diels–Alder Reaction of 1-Methoxybuta-1,3-Diene with n-Butyl Glyoxylate Catalyzed by the (Salen)Cr(III)Cl and Co(II) Complexes. Tetrahedron Asymmetry 2004, 15, 3189–3194. [Google Scholar] [CrossRef]
- Katsuki, T. Chiral Metallosalen Complexes: Structures and Catalyst Tuning for Asymmetric Epoxidation and Cyclopropanation. Adv. Synth. Catal. 2002, 344, 131–147. [Google Scholar] [CrossRef]
- Bonaccorso, C.; Brancatelli, G.; Ballistreri, F.P.; Geremia, S.; Pappalardo, A.; Tomaselli, G.A.; Toscano, R.M.; Sciotto, D. Novel Chiral (Salen)Mn(III) Complexes Containing a Calix [4]Arene Unit in 1,3-Alternate Conformation as Catalysts for Enantioselective Epoxidation Reactions of (Z)-Aryl Alkenes. Dalton Trans. 2013, 43, 2183–2193. [Google Scholar] [CrossRef]
- Vigato, P.A.; Tamburini, S. The Challenge of Cyclic and Acyclic Schiff Bases and Related Derivatives. Coord. Chem. Rev. 2004, 248, 1717–2128. [Google Scholar] [CrossRef]
- Titze, M.; Heitkämper, J.; Junge, T.; Kästner, J.; Peters, R. Highly Active Cooperative Lewis Acid—Ammonium Salt Catalyst for the Enantioselective Hydroboration of Ketones. Angew. Chem. Int. Ed. 2021, 60, 5544–5553. [Google Scholar] [CrossRef]
- Latendorf, K.; Mechler, M.; Schamne, I.; Mack, D.; Frey, W.; Peters, R. Titanium Salen Complexes with Appended Silver NHC Groups as Nucleophilic Carbene Reservoir for Cooperative Asymmetric Lewis Acid/NHC Catalysis. Eur. J. Org. Chem. 2017, 2017, 4140–4167. [Google Scholar] [CrossRef]
- Kull, T.; Peters, R. Contact Ion Pair Directed Lewis Acid Catalysis: Asymmetric Synthesis of Trans-Configured β-Lactones. Angew. Chem. Int. Ed. 2008, 47, 5461–5464. [Google Scholar] [CrossRef]
- Elshaarawy, R.F.M.; Janiak, C. Toward New Classes of Potent Antibiotics: Synthesis and Antimicrobial Activity of Novel Metallosaldach–Imidazolium Salts. Eur. J. Med. Chem. 2014, 75, 31–42. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Hiller, K.-A.; Wenzl, V.; Forster, E.-M.; Cieplik, F.; Maisch, T. The Optimal Effective Concentration Combination (OPECC) as a Novel Method for Evaluating the Effects of Binary Application of Antibacterial Compounds. Microorganisms 2023, 11, 830. [Google Scholar] [CrossRef]
- Platania, V.; Kaldeli-Kerou, A.; Karamanidou, T.; Kouki, M.; Tsouknidas, A.; Chatzinikolaidou, M. Antibacterial Effect of Colloidal Suspensions Varying in Silver Nanoparticles and Ions Concentrations. Nanomaterials 2022, 12, 31. [Google Scholar] [CrossRef]
- CLSI. Performance Standards Antimicrobial Susceptibility Testing, 33rd ed.; CLSI Supplement M100; CLSI: Wayne, PA, USA, 2023. [Google Scholar]

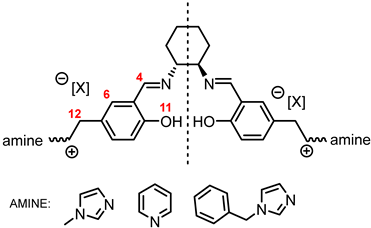 | ||||
|---|---|---|---|---|
| H4 | H6 | H11 | H12 | |
| [(RR)Sal.5C1.MIM][Cl] | 8.51 | 6.87 | 13.50 | 5.35 |
| [(rac)Sal.5C1.MIM][Cl] | 8.51 | 6.87 | 13.51 | 5.35 |
| [(RR)Sal.5C1.MIM][BF4] | 8.51 | 6.87 | 13.56 | 5.29 |
| [(RR)Sal.5C1.MIM][OTf] | 8.51 | 6.87 | 13.56 | 5.29 |
| [(RR)Sal.5C1.MIM][NTf2] | 8.52 | 6.88 | 13.56 | 5.29 |
| [(RR)Sal.5C1.PhIM][Cl] | 8.51 | 6.86 | 13.52 | 5.34 |
| [(RR)Sal.5C1.PhIM][BF4] | 8.52 | 6.88 | 13.56 | 5.30 |
| [(RR)Sal.5C1.PhIM][OTf] | 8.52 | 6.88 | 13.58 | 5.30 |
| [(RR)Sal.5C1.PhIM][NTf2] | 8.52 | 6.88 | 13.58 | 5.30 |
| [(RR)Sal.5C1.Pyr][Cl] | 8.61 | 6.86 | 13.57 | 5.85 |
| [(RR)Sal.5C1.Pyr][BF4] | 8.60 | 6.86 | 13.66 | 5.73 |
| [(RR)Sal.5C1.Pyr][OTf] | 8.60 | 6.89 | 13.65 | 5.73 |
| [(RR)Sal.5C1.Pyr][NTf2] | 8.59 | 6.88 | 13.65 | 5.72 |
| Chiral Salen-Based Organic Salt | State * | Tm [°C] | Tg [℃] | Tonset [°C] |
|---|---|---|---|---|
| [(RR)Sal.5C1.MIM][Cl] | Solid | 90–96 | –nd | 242.9 |
| [(rac)Sal.5C1.MIM][Cl] | Solid | 81–87 | –nd | 236.0 |
| [(RR)Sal.5C1.MIM][BF4] | Solid | 138–140 | –nd | 249.1 |
| [(RR)Sal.5C1.MIM][OTf] | Solid | 58–62 | –nd | 246.3 |
| [(RR)Sal.5C1.MIM][NTf2] | Liquid | –nd | 4.1 | 297.2 |
| [(RR)Sal.5C1.PhIM][Cl] | Solid | 76–80 | –nd | 246.4 |
| [(RR)Sal.5C1.PhIM][BF4] | Solid | 147–150 | –nd | 227.3 |
| [(RR)Sal.5C1.PhIM][OTf] | Solid | 53–57 | –nd | 219.5 |
| [(RR)Sal.5C1.PhIM][NTf2] | Liquid | –nd | 17.3 | 327.7 |
| [(RR)Sal.5C1.Pyr][Cl] | Solid | 91–97 | –nd | 181.9 |
| [(RR)Sal.5C1.Pyr][BF4] | Solid | 161–164 | –nd | 154.4 |
| [(RR)Sal.5C1.Pyr][OTf] | Solid | 54–57 | –nd | 190.5 |
| [(RR)Sal.5C1.Pyr][NTf2] | Liquid | –nd | 16.8 | 280.6 |
| Chiral Salen Organic Salt | Specific Rotation [α]D20 | Absorbance * λmax |
|---|---|---|
| [(RR)Sal.5C1.MIM][Cl] | −67.8 | 320 |
| [(rac)Sal.5C1.MIM][Cl] | −3.7 | 320 |
| [(RR)Sal.5C1.MIM][BF4] | −64.9 | 320 |
| [(RR)Sal.5C1.MIM][OTf] | −47.3 | 320 |
| [(RR)Sal.5C1.MIM][NTf2] | −41.0 | 320 |
| [(RR)Sal.5C1.PhIM][Cl] | −46.4 | 320 |
| [(RR)Sal.5C1.PhIM][BF4] | −40.8 | 320 |
| [(RR)Sal.5C1.PhIM][OTf] | −34.8 | 323 |
| [(RR)Sal.5C1.PhIM][NTf2] | −27.1 | 320 |
| [(RR)Sal.5C1.Pyr][Cl] | −45.1 | 320 |
| [(RR)Sal.5C1.Pyr][BF4] | −40.8 | 321 |
| [(RR)Sal.5C1.Pyr][OTf] | −34.9 | 321 |
| [(RR)Sal.5C1.Pyr][NTf2] | −27.1 | 320 |
| Tested Compounds | Serratia fonticola Strain KKP 3685 | Escherichia coli Strain KKP 3688 | Enterobacter cloacae Strain KKP 3692 | |||
|---|---|---|---|---|---|---|
| MIC [µg mL−1] | MBC [µg mL−1] | MIC [µg mL−1] | MBC [µg mL−1] | MIC [µg mL−1] | MBC [µg mL−1] | |
| [(RR)Sal.5C1.MIM][Cl] | 2000 | 2000 | 4000 | 4000 | 4000 | >4000 |
| [(rac)Sal.5C1.MIM][Cl] | 1000 | 2000 | 4000 | 4000 | 4000 | >4000 |
| [(RR)Sal.5C1.MIM][BF4] | 2000 | 2000 | 4000 | 4000 | 4000 | >4000 |
| [(RR)Sal.5C1.MIM][OTf] | 1000 | 2000 | 4000 | 4000 | 4000 | >4000 |
| [(RR)Sal.5C1.MIM][NTf2] | 2000 | 2000 | 2000 | 4000 | 2000 | >4000 |
| [(RR)Sal.5C1.Pyr][Cl] | 1000 | 2000 | 2000 | 4000 | 4000 | 4000 |
| [(RR)Sal.5C1.Pyr][BF4] | 2000 | 2000 | 2000 | 4000 | 4000 | >4000 |
| [(RR)Sal.5C1.Pyr][OTf] | 1000 | 4000 | 4000 | 4000 | >4000 | >4000 |
| [(RR)Sal.5C1.Pyr][NTf2] | 2000 | 2000 | 2000 | 4000 | 2000 | >4000 |
| [(RR)Sal.5C1.PhIM][Cl] | 500 | 2000 | 1000 | 1000 | 2000 | 2000 |
| [(RR)Sal.5C1.PhIM][BF4] | 500 | 2000 | 1000 | 1000 | 2000 | 2000 |
| [(RR)Sal.5C1.PhIM][OTf] | 500 | 1000 | 2000 | 2000 | 2000 | 2000 |
| [(RR)Sal.5C1.PhIM][NTf2] | 1000 | 2000 | 2000 | 2000 | >4000 | >4000 |
| Substrates | ||||||
| CHDA | 2000 | 2000 | 2000 | 2000 | 4000 | 4000 |
| [Sal.5C1.PhIM][Cl] | 2000 | 2000 | 2000 | 2000 | 4000 | 4000 |
| [Sal.5C1.PhIM][BF4] | 2000 | 2000 | 2000 | 2000 | 4000 | 4000 |
| [Sal.5C1.PhIM][OTf] | 2000 | 2000 | 2000 | 2000 | 4000 | 4000 |
| Control compound | ||||||
| BC | 7.813 | 7.813 | 7.813 | 7.813 | 7.813 | 7.813 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gano, M.; Wójcicki, M.; Janus, E. Chiral Salen-Based Organic Salts: Synthesis and Potential Antibacterial Activity. Molecules 2025, 30, 2173. https://doi.org/10.3390/molecules30102173
Gano M, Wójcicki M, Janus E. Chiral Salen-Based Organic Salts: Synthesis and Potential Antibacterial Activity. Molecules. 2025; 30(10):2173. https://doi.org/10.3390/molecules30102173
Chicago/Turabian StyleGano, Marcin, Michał Wójcicki, and Ewa Janus. 2025. "Chiral Salen-Based Organic Salts: Synthesis and Potential Antibacterial Activity" Molecules 30, no. 10: 2173. https://doi.org/10.3390/molecules30102173
APA StyleGano, M., Wójcicki, M., & Janus, E. (2025). Chiral Salen-Based Organic Salts: Synthesis and Potential Antibacterial Activity. Molecules, 30(10), 2173. https://doi.org/10.3390/molecules30102173








