Magnetically Induced Switching of Circularly Polarized Luminescence Using Electromagnets
Abstract
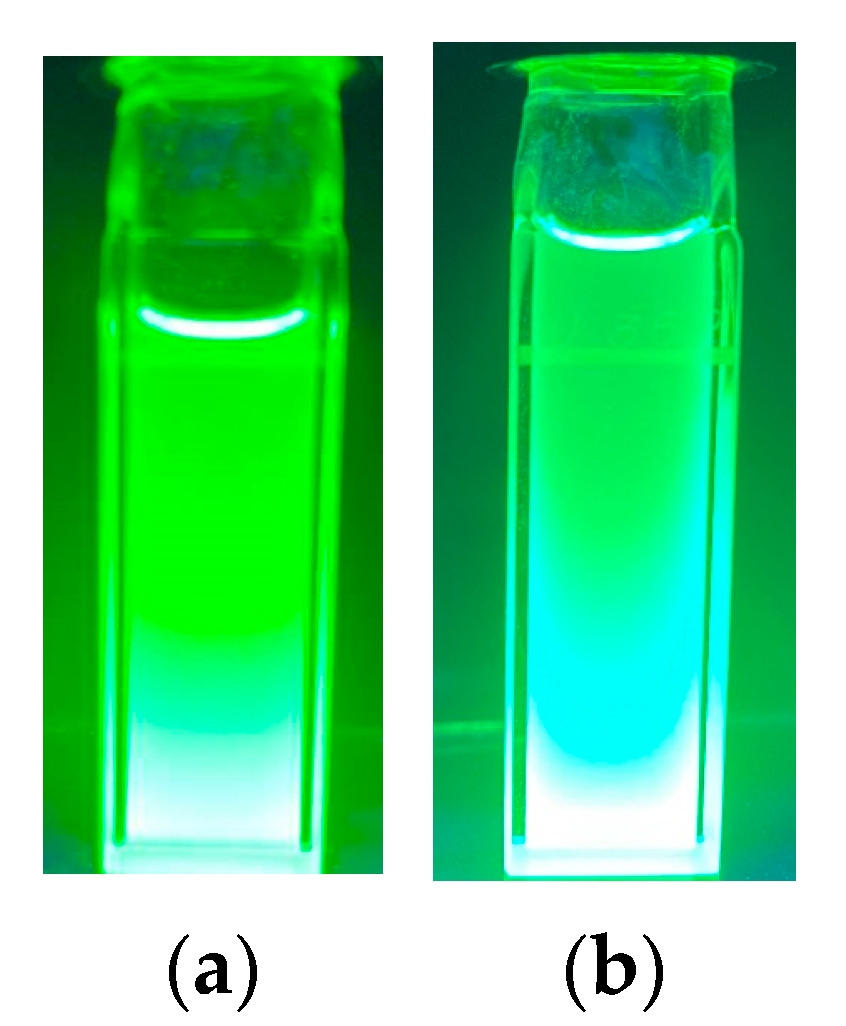
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. MPL and MCPL Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CPL | Circularly polarized luminescence |
| MCPL | Magnetic circularly polarized luminescence |
| MPL | Magnetic photoluminescence |
| PVQD | Perovskite quantum dot |
References
- Field, J.E.; Muller, G.; Riehl, J.P.; Venkataraman, D. Circularly polarized luminescence from bridged triarylamine helicenes. J. Am. Chem. Soc. 2003, 125, 11808–11809. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Bando, Y. Recent progress in research on stimuli-responsive circularly polarized luminescence based on π-conjugated molecules. Pure Appl. Chem. 2013, 85, 1967–1978. [Google Scholar] [CrossRef]
- Sánchez-Carnerero, E.M.; Agarrabeitia, A.R.; Moreno, F.; Maroto, B.L.; Muller, G.; Ortiz, M.J.; de la Moya, S. Circularly polarized luminescence from simple organic molecules. Chemistry 2015, 21, 13488–13500. [Google Scholar] [CrossRef]
- Kumar, J.; Nakashima, T.; Kawai, T. Circularly polarized luminescence in chiral molecules and supramolecular assemblies. J. Phys. Chem. Lett. 2015, 6, 3445–3452. [Google Scholar] [CrossRef]
- Longhi, G.; Castiglioni, E.; Koshoubu, J.; Mazzeo, G.; Abbate, S. Circularly polarized luminescence: A review of experimental and theoretical aspects. Chirality 2016, 28, 696–707. [Google Scholar] [CrossRef]
- Sun, Z.; Suenaga, T.; Sarkar, P.; Sato, S.; Kotani, M.; Isobe, H. Stereoisomerism, crystal structures, and dynamics of belt-shaped cyclonaphthylenes. Proc. Natl. Acad. Sci. USA 2016, 113, 8109–8114. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Inoue, Y.; Mori, T. Circularly polarized luminescence and circular dichroisms in small organic molecules: Correlation between excitation and emission dissymmetry factors. ChemPhotoChem 2018, 2, 386–402. [Google Scholar] [CrossRef]
- Pop, F.; Zigon, N.; Avarvari, N. Main-group-based electro- and photoactive chiral materials. Chem. Rev. 2019, 119, 8435–8478. [Google Scholar] [CrossRef]
- Ma, J.L.; Peng, Q.; Zhao, C.H. Circularly polarized luminescence switching in small organic molecules. Chemistry 2019, 25, 15441–15454. [Google Scholar] [CrossRef]
- Ohishi, Y.; Inouye, M. Circularly polarized luminescence from pyrene excimers. Tetrahedron Lett. 2019, 60, 151232. [Google Scholar] [CrossRef]
- Gao, J.X.; Zhang, W.Y.; Wu, Z.G.; Zheng, Y.X.; Fu, D.W. Enantiomorphic perovskite ferroelectrics with circularly polarized luminescence. J. Am. Chem. Soc. 2020, 142, 4756–4761. [Google Scholar] [CrossRef]
- Mori, T. Circularly Polarized Luminescence of Isolated Small Organic Molecules; Mori, T., Ed.; Springer: Singapore, 2020. [Google Scholar]
- Imai, Y. Non-classical circularly polarized luminescence of organic and organometallic luminophores. Chem. Lett. 2021, 50, 1131–1141. [Google Scholar] [CrossRef]
- Lin, Y.C.; Karlsson, M.; Bettinelli, M. Photoluminescent Materials and Electroluminescent Devices. In Topics in Current Chemistry Collections; Armaroli, N., Bolink, H., Eds.; Springer: Cham, Switzerland, 2016; pp. 1–47. [Google Scholar]
- Xia, Z.; Liu, Q. Progress in discovery and structural design of color conversion phosphors for LEDs. Prog. Mater. Sci. 2016, 84, 59–117. [Google Scholar] [CrossRef]
- Diaconu, C.V.; Batista, E.R.; Martin, R.L.; Smith, D.L.; Crone, B.K.; Crooker, S.A.; Smith, D.L. Circularly polarized photoluminescence from platinum porphyrins in organic hosts: Magnetic field and temperature dependence. J. Appl. Phys. 2011, 109, 073513. [Google Scholar] [CrossRef]
- Liew, J.Y.; Lo, S.C.; Burn, P.L.; Krausz, E.R.; Hall, J.D.; Moore, E.G.; Riley, M.J. Analysis of the emitting states of an Ir(III) complex with strong blue emission. Chem. Phys. Lett. 2015, 641, 62–67. [Google Scholar] [CrossRef]
- Wu, T.; Kapitán, J.; Andrushchenko, V.; Bouř, P. Identification of lanthanide (III) luminophores in magnetic circularly polarized luminescence using Raman optical activity instrumentation. Anal. Chem. 2017, 89, 5043–5049. [Google Scholar] [CrossRef]
- Ivchenko, E.L. Magnetic circular polarization of exciton photoluminescence. Phys. Solid State 2018, 60, 1514–1526. [Google Scholar] [CrossRef]
- Imai, Y. Circularly Polarized Luminescence (CPL) Induced by an External Magnetic Field: Magnetic CPL (MCPL). ChemPhotoChem 2021, 5, 969–973. [Google Scholar] [CrossRef]
- Imai, Y. Magnetic circularly polarized luminescence (MCPL) by applying an external magnetic field. Photochemistry 2021, 8, 75–78. [Google Scholar]
- Foxley, J.; Knappenberger, K. Magneto-Optical Properties of Noble Metal Nanostructures. Annu. Rev. Phys. Chem. 2023, 74, 53–72. [Google Scholar] [CrossRef]
- Zinna, F.; Pescitelli, G. Magnetic Circularly Polarized Luminescence of Organic Compounds. Eur. J. Org. Chem. 2023, 26, e202300509. [Google Scholar] [CrossRef]
- Fuse, M.; Mazzeo, G.; Chidinelli, S.; Evidente, A.; Abbate, S.; Longhi, G. Experimental and theoretical aspects of magnetic circular dichroism and magnetic circularly polarized luminescence in the UV, visible and IR ranges: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 319, 124583. [Google Scholar] [CrossRef] [PubMed]
- Amasaki, R.; Kitahara, M.; Kimoto, T.; Fujiki, M.; Imai, Y. Mirror-image magnetic circularly polarized luminescence from perovskite (M+Pb2+Br3, M+ = Cs+ and amidinium) quantum dots. Eur. J. Inorgan. Chem. 2022, 2022, e202101066. [Google Scholar] [CrossRef]
- Amasaki, R.; Kitahara, M.; Tanaka, S.; Imai, Y. Multi-color magnetic circularly polarized luminescence from achiral perovskite (Cs+Pb2+X3) quantum dots. Eur. J. Inorgan. Chem. 2024, 27, e202300621. [Google Scholar] [CrossRef]
- Zhang, J.; Dai, L.; Webster, A.M.; Chan, W.T.K.; Mackenzie, L.E.; Pal, R.; Cobb, S.L.; Law, G.-L. Unusual Magnetic Field Responsive Circularly Polarized Luminescence Probes with Highly Emissive Chiral Europium (III) Complexes. Angew. Chem. Int. Ed. 2021, 60, 1004–1010. [Google Scholar] [CrossRef]




| PVQDs | Faraday Configuration | λex (nm) | λMCPL (nm) | gMCPL /10−3 (T−1) |
|---|---|---|---|---|
| 1 | N-up | 398 | 535 | −9.2 |
| S-up | 537 | +9.3 | ||
| 2 | N-up | 360 | 512 | −5.0 |
| S-up | 516 | +7.9 |
| PVQDs | Magnetic Field Strength (T) | λMCPL (nm) | |gMCPL| /10−3 |
|---|---|---|---|
| 1 | 1.7 [20] | 538 | 9.43 |
| 0.198 | 537 | 1.53 | |
| 0.158 | 536 | 1.46 | |
| 2 | 1.7 [20] | 514 | 9.37 |
| 0.198 | 513 | 1.13 | |
| 0.158 | 514 | 1.02 |
| PVQDs | Faraday Configuration | 15 min | 45 min | 75 min | 105 min | |
|---|---|---|---|---|---|---|
| 1 | ΔI | SNSN | +83 | −35 | +62 | −39 |
| gMCPL /10−3 (T−1) | +8.4 | −3.6 | +6.3 | −3.9 | ||
| ΔI | NSNS | −39 | +83 | −39 | +45 | |
| gMCPL /10−3 (T−1) | −3.9 | +8.5 | −4.1 | +4.7 | ||
| 2 | ΔI | SNSN | +51 | −56 | +93 | −66 |
| gMCPL /10−3 (T−1) | +5.1 | −5.6 | +9.4 | −6.6 | ||
| ΔI | NSNS | −81 | +72 | −62 | +71 | |
| gMCPL /10−3 (T−1) | −8.3 | +7.3 | −6.3 | +7.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imai, Y.; Fukuchi, K.; Yanagihashi, Y.; Suzuki, S. Magnetically Induced Switching of Circularly Polarized Luminescence Using Electromagnets. Molecules 2025, 30, 2426. https://doi.org/10.3390/molecules30112426
Imai Y, Fukuchi K, Yanagihashi Y, Suzuki S. Magnetically Induced Switching of Circularly Polarized Luminescence Using Electromagnets. Molecules. 2025; 30(11):2426. https://doi.org/10.3390/molecules30112426
Chicago/Turabian StyleImai, Yoshitane, Kota Fukuchi, Yoshihiko Yanagihashi, and Satoko Suzuki. 2025. "Magnetically Induced Switching of Circularly Polarized Luminescence Using Electromagnets" Molecules 30, no. 11: 2426. https://doi.org/10.3390/molecules30112426
APA StyleImai, Y., Fukuchi, K., Yanagihashi, Y., & Suzuki, S. (2025). Magnetically Induced Switching of Circularly Polarized Luminescence Using Electromagnets. Molecules, 30(11), 2426. https://doi.org/10.3390/molecules30112426







