Abstract
The precise construction and programmable assembly of structures with specific topologies remain persistent challenges in crystal engineering, primarily constrained by the limited availability of building blocks. Utilizing a synergistic approach that combines an in situ-formed concave polyoxovanadate (POV) cluster {VV4} with specifically designed 120° ditopic carboxylic acid bridging ligands, we successfully synthesized a series of wine-rack-type supramolecular macrocycles characterized by the general formula [(V5O9Cl)4(L)8]8−. The experimental results demonstrate that the introduction of sulfonic acid groups enables controlled structural extension into 1D chain and 2D layer architectures, manifesting the unique advantages of POV-based wine-rack units in constructing framework-based porous materials. This work significantly contributes to the structural diversity of wine-rack-type supramolecular architectures while simultaneously highlighting the great potential of polyoxometalate-driven supramolecular assemblies in materials science.
1. Introduction
The construction of supramolecular macrocyclic complexes represents a pivotal research area in supramolecular chemistry and coordination chemistry [1,2,3,4,5,6], attracting considerable attention in recent years due to these structures’ remarkable application potential across diverse fields such as catalysis, sensing, drug delivery, and materials science [7,8,9,10]. Among these, supramolecular macrocycles featuring “wine-rack” architectures have emerged as a prominent focus of investigation owing to their unique geometric configurations and distinctive mechanical properties. Current research efforts predominantly concentrate on elucidating the structural characteristics and functional behaviors of metal–organic frameworks and covalent organic frameworks incorporating wine-rack motifs [11,12]. However, the systematic design of supramolecular macrocycles with intrinsic wine-rack topologies remains underexplored.
Polyoxometalates (POMs) represent a prominent class of discrete anionic metal–oxo clusters, primarily constructed through corner-, edge-, or face-sharing configurations of early transition metal polyhedra (M = Mo, W, V, Nb, Ta, etc.) [13]. Among these, POV clusters stand out as exceptional building blocks for supramolecular architectures, owing to the pronounced polarization capability of V ions toward terminal oxygen atoms, which facilitates the formation of directional bowl-shaped subunits—a critical feature for assembling closed supramolecular architectures [14,15,16,17]. Pioneering studies by Michael’s group, Wang’s group, and others have systematically demonstrated that {V4} and {VV4} clusters are ideal for constructing capsule-like and octahedral structures [18,19,20], while {V5} and {MV5} (M = W, Mo, V, Nb) motifs exhibit high compatibility with icosahedral architectures [21,22]. Similarly, {V6} and {VV6} units are tailored for tetrahedral and cubic assemblies [23,24,25]. Notably, Navarro’s group and Hartl’s group have exemplified the versatility of {V3} clusters in constructing both tetrahedral and cubic superstructures [26,27], underscoring the structural adaptability of V-oxo clusters in supramolecular design.
In 2015, Michael’s group laid the foundation for wine-rack-type supramolecular macrocycles by constructing three pioneering structures based on {V4} clusters [28]. Herein, we demonstrate that wine-rack-type supramolecular macrocycles with the general formula [(V5O9Cl)4(L)8]8−, derived from {VV4} units, can be functionally controlled through the use of different vanadium sources and rigid angular ligands. A comparison of these two studies highlights the remarkable precision of atomic-level structural modulation achievable in supramolecular chemistry. By employing functionalized 120° dicarboxylic acid linkers for in situ assembly with {VV4} clusters, we successfully synthesized a series of wine-rack-type supramolecular macrocycles (WR-VMOP-1–4). The sulfonic acid-functionalized one-dimensional (1D) and two-dimensional (2D) structures reveal the potential of POV-based wine-rack macrocycles as porous framework materials. POM crystalline materials can be used as solid adsorbents for adsorbing organic dyes [29,30,31]. Two main factors affect the adsorption process: one is the pore size that can accommodate the dye molecules, and the other is the role of ion exchange. Therefore, the dye adsorption properties for WR-VMOP-3 were investigated.
2. Results and Discussion
2.1. Synthesis and Structure of Crystals
WR-VMOP-1, formulated as (NH2Me2)12(SO4)4[(V5O9Cl)4(1,3-bdc)8]·[solvents], was synthesized by reacting 3,5-pyridinedicarboxylic acid (Figure 1b) with VCl4 in a mixed solvent system of DMF, methanol, and acetonitrile at 130 °C for 2 days. Single-crystal X-ray diffraction (SCXRD) analysis reveals that WR-VMOP-1 (Figure 1c) crystallizes in the monoclinic space group C 2/c. WR-VMOP-1 comprises four pentanuclear [V5O9Cl(COO)4]2− clusters (Figure 1a) interconnected by eight ligands, forming a wine-rack architecture (Figure 1d). The Vᴠ centers are positioned above four basal Vɪᴠ ions. The oxidation states of the vanadium cations are consistent with previous reports and further verified by bond valence sum calculations [32]. The apical Vᴠ atom adopts a square-pyramidal geometry, coordinated by four μ2-O2− anions and one terminal O2− ligand. Each Vɪᴠ center exhibits an octahedral coordination environment coordinated by two carboxylate groups, two μ2-O2− ligands, one terminal O2− ligand, and one μ4-Cl− ligand. The V=O bond distances range from 1.573 to 1.604 Å, while V–O bond lengths vary between 1.876 and 2.027 Å (d(V–Cl): 2.030–2.900 Å). The cavity of the structure features a Cl···Cl distance of 12.3 Å across the architecture (Figure S1). The dihedral angles between the phenyl rings of the ligands and the plane of the macrocycle range from 34° to 69°.
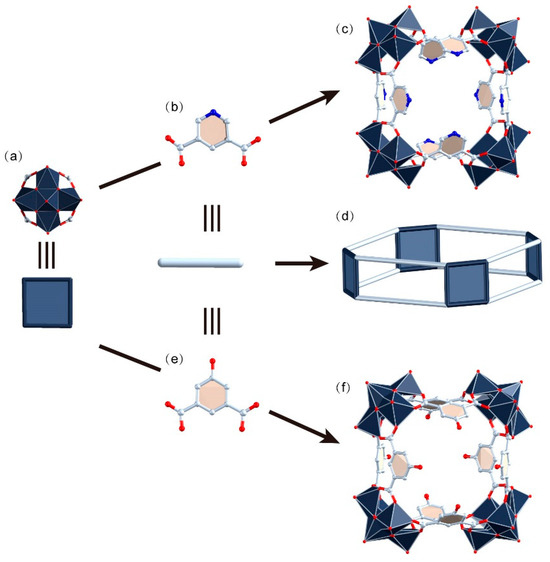
Figure 1.
(a) Polyhedral representation of {VV4} units. (b) Ball-and-stick representation of 3,5-pyridinedicarboxylic acid. (c) Polyhedral representation of WR-VMOP-1. (d) Diagram of the wine-rack-type supramolecular macrocycles. (e) Ball-and-stick representation of 5-hydroxyisophthalic acid. (f) Polyhedral representation of WR-VMOP-2. Dark slate gray V, red O, pale blue C, blue N.
WR-VMOP-2 was synthesized under conditions analogous to those for WR-VMOP-1, with the addition of VOSO4 and substitution of the 5-hydroxyisophthalic acid (Figure 1e) linker for 3,5-pyridinedicarboxylic acid. SCXRD analysis reveals that WR-VMOP-2 (Figure 1f) crystallizes in the cubic space group Pm-3m. The architecture of WR-VMOP-2 resembles that of WR-VMOP-1, except for a reduced window pore in WR-VMOP-2 due to the steric influence of the hydroxyl group at the 5-position of the ligand. Notably, the crystal packing of WR-VMOP-2 diverges significantly from that of WR-VMOP-1. In the c-axis direction of WR-VMOP-1, adjacent layers are separated by a wavy interlayer composed of sulfate anions and dimethylamine cations (Figure S2), resulting in non-overlapping molecular arrangements that preclude the formation of continuous channels. In contrast, for WR-VMOP-2, molecules along the c-axis are fully overlapped, creating unidirectional pore channels mirroring the intrinsic channel of individual macrocycles (Figure S3).
The peripheral regions of the POVs clusters in WR-VMOP-1 (Figure 2a) and WR-VMOP-2 (Figure 2b) are enriched with oxygen atoms, rendering them highly suitable for coordinating with metal cations. This observation prompted us to explore the use of 5-sulfoisophthalic acid monosodium salt, a 120°-angled ligand bearing intrinsic sulfonate groups, to introduce additional coordination sites into the system. Employing the same synthetic protocol, we successfully obtained WR-VMOP-3 (Figure 2c), a one-dimensional (1D) chain structure (Figure 3a) in which individual macrocycles retain the targeted wine-rack topology. Dihedral angles between the benzene rings and the macrocyclic plane (57–86°) confirm the structural flexibility of the macrocycle. Sodium ions coordinate with sulfonate groups to interconnect the macrocycles into 1D chains, while dimethylamine cations and sulfate anions balance the charge in the crystalline lattice. Crystallographic analysis reveals three distinct Na+ ions (Figure S4): one coordinates exclusively with three sulfonate groups within a single macrocycle, while the remaining two bridge adjacent macrocycles vertically to propagate the 1D chain. The Na–O bond distances range from 2.24 to 2.66 Å, and Na–Cl interactions are observed at approximately 2.67 Å. Substituting VCl4 with VOCl3 yielded WR-VMOP-4, a two-dimensional (2D) layered structure (Figure 3b), further demonstrating the structural extensibility of this wine-rack macrocycle series. In WR-VMOP-4, two Na+ ions link sulfate groups between vertically adjacent macrocycles to sustain the 1D chain, while vanadium ions bridge neighboring V5 clusters laterally to form a 2D network, creating parallelogram-shaped cavities bounded by four macrocycles. The bridging V center adopts a six-coordinate geometry (Figure S5) with V–O single bonds (1.95–2.15 Å), distinct from the V centers in the SBUs, which feature a V=O double bond. Comparative analysis highlights that WR-VMOP-3 (1D) exhibits abundant Cl− coordination sites, whereas WR-VMOP-4 (2D) is enriched with sulfonate moieties. Both frameworks are classified as polyanionic architectures, underscoring their versatility in hosting diverse functional groups for targeted applications.
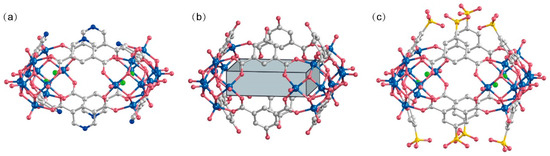
Figure 2.
Molecule structure of (a) WR-VMOP-1, (b) WR-VMOP-2, (c) WR-VMOP-3. Royal blue V, Indian red O, grey C, blue N, bright green Cl.
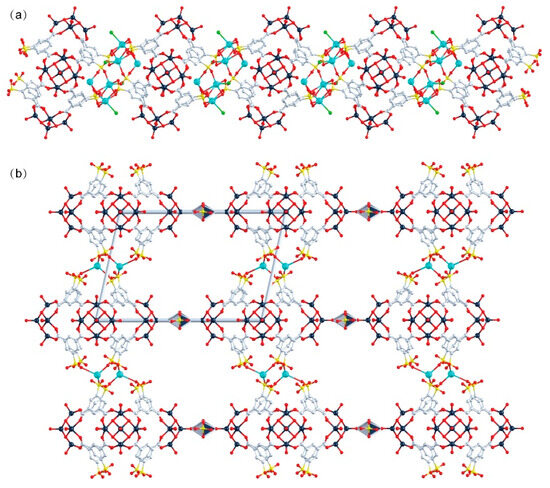
Figure 3.
(a) One-dimensional structure of WR-VMOP-3, (b) Two-dimensional structure of WR-VMOP-4. Dark slate gray V, red O, pale blue C, yellow S, bright green Cl, turquoise Na.
2.2. Dye Adsorption Study
Given the anionic nature of this series of wine-rack-type macrocycles, we selected WR-VMOP-3 (Figures S6 and S7) to evaluate its dye adsorption properties. In the first phase, we compared the adsorption efficiency of WR-VMOP-3 crystals toward dyes with similar molecular sizes but varying charges: methylene blue (MB+, cationic), methyl yellow (MY0, neutral), and methyl orange (MO−, anionic). Remarkably, the crystals exhibited strong adsorption affinity for the cationic dye MB+, achieving nearly complete adsorption within 2 h, as evidenced by the transition of the solution from intense blue to nearly colorless (Figure 4a). In contrast, negligible adsorption was observed for neutral MY0 and anionic MO−, with no significant changes in solution color (Figure S8), UV-vis absorption peak positions, or intensities after 2 h. These results demonstrate the inherent negative charge of WR-VMOP-3, which selectively adsorbs cationic dyes via electrostatic interactions.
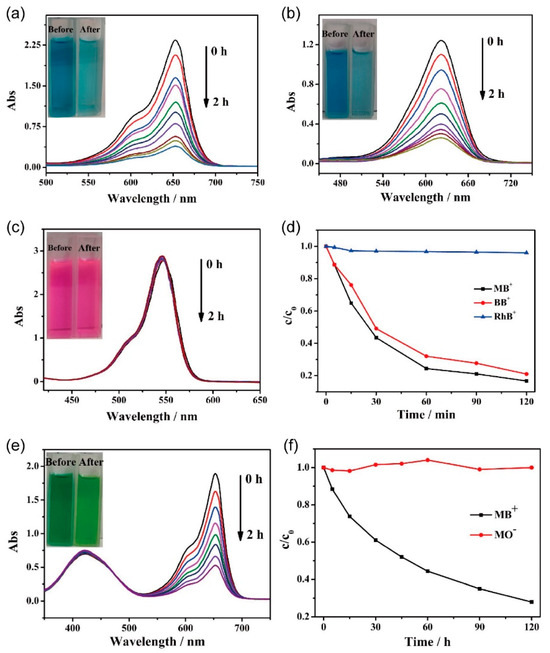
Figure 4.
UV-vis adsorption spectra of methanol solution of (a) MB+, (b) BB+ and (c) RhB+ in WR-VMOP-3. (d) The change curve of the concentration of the dyes in the solution with time during the dyes adsorption process. (e) UV-vis adsorption spectra of the selective adsorption of WR-VMOP-3 on mixed dye MB+/MO−. (f) The change curve of the concentration of dyes in the solution with time during the selective adsorption process.
In the subsequent phase, we investigated the size-dependent adsorption behavior of WR-VMOP-3 toward cationic dyes by selecting three model dyes with identical single positive charge but varied molecular dimensions: rhodamine B (RhB+), brilliant blue (BB+), and methylene blue (MB+) (molecular size: RhB+ > BB+ > MB+). Remarkably, the crystals exhibited strong adsorption affinity for the smallest dye, MB+, achieving near-complete adsorption within 2 h, as evidenced by the transition of the solution from intense blue to nearly colorless (Figure 4a). For the medium-sized dye BB+, a moderate adsorption efficiency was observed, with a slight attenuation (Figure 4b) of the blue after 2 h. In contrast, negligible adsorption occurred for the largest dye, RhB+, as the solution retained its characteristic pink color (Figure 4c) throughout the experiment, with no discernible changes in UV-vis absorption spectra (Figure 4d). In general, as the size of the cationic dye is increased, the ion-exchange process becomes slower and slower. And steric constraints imposed by the macrocyclic framework also limit the size of the dyes that can be adsorbed. These results highlight the size-selective adsorption capability of WR-VMOP-3.
To further investigate the selective adsorption capability of WR-VMOP-3 for mixed dyes with similar molecular dimensions, the crystals were immersed in a mixed solution containing MB+ and MO−. As shown in Figure 4e, the characteristic absorption peaks of MB+ exhibited rapid attenuation, while both the position and intensity of characteristic peak for MO− remained essentially unchanged. This observation demonstrates that WR-VMOP-3 can selectively capture the cationic dye MB+ from the mixed dyes system. Further verification was achieved by plotting the concentration ratio (C/C0) versus adsorption time (Figure 4f), where C0 represents the initial concentration. The relative concentration of MB+ showed a dramatic decline, whereas that of MO− remained nearly constant at around 1 throughout the adsorption process. Minor fluctuations in MO− concentration could be attributed to measurement errors during time-dependent sampling. These complementary analytical approaches collectively confirm the preferential adsorption behavior of WR-VMOP-3 toward cationic dyes. After dye adsorption, WR-VMOP-3 retained its crystalline integrity, as evidenced by the PXRD pattern (Figure S9).
3. Materials and Methods
All reagents were commercially sourced and utilized directly without additional purification. Note: VCl4 must be used in a fume hood.
3.1. Materials and Physical Techniques
All the reagents were obtained from commercial sources and used without further purification. Powder X-ray diffraction (PXRD) patterns were recorded ranging from 5 to 50° at room temperature on a Siemens D5005 diffractometer (Siemens AG, Munich, Germany) with Cu Kα (λ = 1.5418 Å). Thermogravimetric analysis (TGA) of the samples were performed using a Perkin–Elmer TG–7 analyzer (PerkinElmer Inc., Waltham, MA, USA) heated from room temperature to 800 °C under nitrogen at the heating rate of 10 °C·min−1. IR spectrum was performed in the range 4000–400 cm−1 using KBr pellets on an Alpha Centaurt FT-IR spectrophotometer (Bruker, Billerica, MA, USA).
3.2. X-Ray Crystallography
All data collections were performed on a Bruker D8–Venture diffractometer (Bruker) with a Turbo X-ray source (Cu Kα radiation, λ = 1.5418 Å and Mo Kα radiation, λ = 0.71069 Å) adopting the direct drive rotating anode technique and a CMOS detector at 296 K. The data frames were collected using the program APEX 3 and processed using the program SAINT routine in APEX 3. The structures were solved by direct methods and refined by the full matrix least-squares on F2 using the SHELXL–2014 program. The diffused electron densities resulting from these residual solvent molecules were removed from the data set using the SQUEEZE routine of PLATON and refined further using the data generated. The restrained DFIX, SIMU, and ISOR instructions were used to make the structures more reasonable. We assigned a CCDC number of 2431104 for WR-VMOP-1, 2431105 for WR-VMOP-2, 2431106 for WR-VMOP-3, and 2431107 for WR-VMOP-4.
3.3. Dyes Adsorption Study
To evaluate the dye adsorption properties of WR-VMOP-3, five representative dyes were selected and tested in two phases. The dyes included methyl orange (MO−), which carries a single negative charge; methyl yellow (MY0), which is uncharged; and three dyes with a single positive charge but varying molecular sizes: rhodamine B (RhB+), basic blue 12 (BB+), and methylene blue (MB+). The molecular sizes of the cationic dyes follow the order RhB+ > BB+ > MB+, and the molecular structures of the dyes are illustrated in Figure S10. For the adsorption experiments, 20 mg of thoroughly washed and dried WR-VMOP-3 was immersed in 5 mL of a dye/methanol solution with an initial concentration of 2.5 × 10−5 mol/L. To prevent any potential photochemical effects, the sample vials containing the mixtures were wrapped in aluminum foil and kept in the dark except during measurements. The changes in dye concentration were monitored by periodically measuring the absorbance of the dye solutions using a quartz cuvette with 3 cm length for ultraviolet–visible (UV-Vis) absorption spectroscopy.
3.4. Synthesis of WR-VMOP-1
3,5-Pyridinedicarboxylic acid (15 mg), DMF (2 mL), CH3OH (0.3 mL), CH3CN (0.2 mL), and VCl4 (4 drops) were sequentially added to a Parr Teflon-lined autoclave and kept at 130 °C for 48 h. After cooling down to room temperature, dark-green crystals were obtained and washed with CH3OH (yield: 30%, based on ligand).
3.5. Synthesis of WR-VMOP-2
5-Hydroxyisophthalic acid (15 mg), VOSO4 (20 mg), DMF (2 mL), CH3OH (0.3 mL), CH3CN (0.2 mL), and VCl4 (2 drops) were sequentially added to a Parr Teflon-lined autoclave and kept at 130 °C for 48 h. After cooling down to room temperature, dark-green crystals were obtained and washed with CH3OH (yield: 10%, based on ligand).
3.6. Synthesis of WR-VMOP-3
5-Sulfoisophthalic acid monosodium salt (25 mg), VOSO4 (20 mg), DMF (2 mL), CH3OH (0.3 mL), CH3CN (0.2 mL), and VCl4 (2 drops) were sequentially added to a Parr Teflon-lined autoclave and kept at 130 °C for 48 h. After cooling down to room temperature, dark-green crystals were obtained and washed with CH3OH (yield: 45%, based on ligand).
3.7. Synthesis of WR-VMOP-4
5-Sulfoisophthalic acid monosodium salt (20 mg), VOSO4 (20 mg), DMF (2 mL), CH3OH (0.3 mL), CH3CN (0.2 mL), and VOCl3 (2 drops) were sequentially added to a Parr Teflon-lined autoclave and kept at 130 °C for 48 h. After cooling down to room temperature, dark-green crystals were obtained and washed with CH3OH (yield: 5%, based on ligand).
4. Conclusions
In this study, we successfully constructed a series of wine-rack-type supramolecular macrocycles based on polyoxovanadates through the self-assembly of in situ-generated VV4 units and 120°-angled dicarboxylate ligands. Structural analyses reveal that the dihedral angles between the phenyl rings of the ligands and the macrocyclic plane vary from 34° to 86°, demonstrating significant conformational flexibility in these wine-rack-type macrocycles. The structural features of WR-VMOP-4 particularly highlight the potential of extending the wine-rack motif into a framework structure. Furthermore, dye adsorption experiments were conducted to validate the anionic nature of this macrocycle series, providing experimental evidence for their potential applications in selective molecular recognition and separation processes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30092075/s1, Table S1. The Crystallographic data for WR-VMOP-1 and WR-VMOP-2. Table S2. The Crystallographic data for WR-VMOP-3 and WR-VMOP-4. Figure S1. Molecule structure of WR-VMOP-1. Figure S2. Packing structure of WR-VMOP-1. Figure S3. Packing structure of WR-VMOP-2. Figure S4. Coordination mode of Na⁺ ions in WR-VMOP-3. Figure S5. Coordination mode of bridging V center in WR-VMOP-4. Figure S6. Packing structure of supramolecular macrocycles in WR-VMOP-3. Figure S7. Packing diagram of wine-rack macrocycles in WR-VMOP-3. Figure S8. UV-vis adsorption spectra of methanol solutions of (a) MY0 and (b) MO− in WR-VMOP-3. Figure S9. PXRD pattern of WR-VMOP-3 and dye@WR-VMOP-3. Figure S10. Structure formula of organic dyes. Figure S11. PXRD pattern of (a) WR-VMOP-1, (b) WR-VMOP-3, (c) WR-VMOP-2. Figure S12. IR curves of (a) WR-VMOP-1, (b) WR-VMOP-3, (c) WR-VMOP-2. Figure S13. TG curves of (a) WR-VMOP-1, (b) WR-VMOP-3, (c) WR-VMOP-2. Figure S14. IR (a) and TG (b) curves of WR-VMOP-4.
Author Contributions
The synthesis of crystals was conducted by N.B. and H.G.; measurements were conducted by B.H. and H.G.; data processing and visualization were conducted by N.B., B.H. and H.G.; the writing of the original draft was conducted by B.H.; writing—review and editing was conducted by all authors; supervision and funding acquisition were conducted by N.B. and H.G. The manuscript was written through the contributions of all the authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Doctoral Startup Fund Program of Yili Normal University (No. 2022YSB004).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhao, Y.B.; Lu, Y.F.; Liu, A.; Zhang, Z.-Y.; Li, C.J.; Sue, A.C.-H. Macrocycle with Equatorial Coordination Sites Provides New Opportunity for Structure-Diverse Metallacages. Molecules 2023, 28, 2537. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-W.; Huang, Z.-J.; Li, Y.-X.; Wu, X.M.; Shi, W.; Zhang, Y.-B.; Ma, X.M.; Ouyang, G.F.; Ye, B.-H.; Liu, G.-F.; et al. An Ultrastable, Easily Scalable and Regenerable Macrocycle-Based Hydrogen-Bonded Organic Framework. CCS Chem. 2025, 7, 293–306. [Google Scholar] [CrossRef]
- Yang, M.; Qiu, F.; El-Sayed, M.E.-S.; Wang, W.; Du, S.; Su, K.; Yuan, D. Water-stable hydrazone-linked porous organic cages. Chem. Sci. 2021, 12, 13307–13315. [Google Scholar] [CrossRef] [PubMed]
- Ilic, S.; May, A.M.; Usov, P.M.; Cornell, H.D.; Gibbons, B.; Celis-Salazar, P.; Cairnie, D.R.; Alatis, J.; Slebodnick, C.; Morris, A.J. An Aluminum-Based Metal–Organic Cage for Cesium Capture. Inorg. Chem. 2022, 61, 6604–6611. [Google Scholar] [CrossRef]
- Sundar, A.; Bhattacharya, S.; Oberstein, J.; Ma, X.; Bassil, B.S.; Nisar, T.; Taffa, D.H.; Wark, M.; Wagner, V.; Kortz, U. Organically Functionalized Mixed-Valent Polyoxo-30-molybdate Wheel and Neutral Tetramolybdenum(V) Oxo Cluster. Inorg. Chem. 2022, 61, 11524–11528. [Google Scholar] [CrossRef]
- Øien-Ødegaard, S.; Bazioti, C.; Redekop, E.A.; Prytz, Ø.; Lillerud, K.P.; Olsbye, U. A toroidal Zr70 oxysulfate cluster and its diverse packing structures. Angew. Chem. Int. Ed. 2020, 59, 21397–21402. [Google Scholar] [CrossRef]
- Tian, Y.-Q.; Dai, L.-F.; Mu, W.-L.; Yu, W.-D.; Yan, J.; Liu, C. Atomically accurate site-specific ligand tailoring of highly acid- and alkali-resistant Ti(IV)-based metallamacrocycle for enhanced CO2 photoreduction. Chem. Sci. 2023, 14, 14280–14289. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Z.Q.; Zhang, Y.N.; Yang, B.; Yang, Y.-W. Synthesis of an Acidochromic and Nitroaromatic Responsive Hydrazone-Linked Pillararene Framework by a Macrocycle-To-Framework Strategy. Angew. Chem. Int. Ed. 2022, 61, e202206144. [Google Scholar] [CrossRef]
- Li, P.F.; Jia, Y.W.; Chen, P.K. Design and Synthesis of New Type of Macrocyclic Architectures Used for Optoelectronic Materials and Supramolecular Chemistry. Chem. Eur. J. 2023, 29, e202300300. [Google Scholar] [CrossRef]
- Abdurakhmanova, E.R.; Mondal, D.; Jędrzejewska, H.; Cmoch, P.; Danylyuk, O.; Chmielewski, M.J.; Szumna, A. Supramolecular umpolung: Converting electron-rich resorcin[4]arenes into potent CH-bonding anion receptors and transporters. Chem 2024, 10, 1910–1924. [Google Scholar] [CrossRef]
- Zeng, Q.X.; Wang, K.; Zou, B. Negative Linear Compressibility Response to Pressure in Multitype Wine-Rack Metal–Organic Frameworks. ACS Mater. Lett. 2020, 2, 291–295. [Google Scholar] [CrossRef]
- Auras, F.; Ascherl, L.; Bon, V.; Vornholt, S.M.; Krause, S.; Döblinger, M.; Bessinger, D.; Reuter, S.; Chapman, K.W.; Kaskel, S.; et al. Dynamic two-dimensional covalent organic frameworks. Nat. Chem. 2024, 16, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-C.; Zhao, J.-W.; Streb, C.; Song, Y.-F. Recent advances on high-nuclear polyoxometalate clusters. Coord. Chem. Rev. 2022, 471, 214734. [Google Scholar] [CrossRef]
- Kondinski, A.; Rasmussen, M.; Mangelsen, S.; Pienack, N.; Simjanoski, V.; Nather, C.; Stares, D.L.; Schalley, C.A.; Bensch, W. Composition-driven archetype dynamics in polyoxovanadates. Chem. Sci. 2022, 13, 6397–6412. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.B.; Zhang, D.; Dong, J.; Feng, Y.Q.; Liu, C.P.; Yao, L.Y.; Chi, Y.N.; Hu, C.W. Open Hollow Polyoxovanadate Cage based on {Nb(V5)} Pentagons with Size-Selective Encapsulation Properties. Chem. Commun. 2025. advance article. [Google Scholar] [CrossRef]
- Zhang, T.; Hou, Y.H.; Hou, B.S.; Zhao, L.; Wang, X.L.; Qin, C.; Su, Z.M. High-nuclear polyoxovanadates assembled from pentagonal building blocks. Chem. Commun. 2022, 58, 11111. [Google Scholar] [CrossRef]
- Xiong, X.L.; Fu, Y.M.; Wu, S.X.; Qin, C.; Wang, X.L.; Su, Z.M. Two High-Nuclear Wheel-Hub-Shaped Transition-Metal-Doped Polyoxovanadates. Inorg. Chem. 2024, 63, 14296–14300. [Google Scholar] [CrossRef]
- Mahimaidoss, M.B.; Krasnikov, S.A.; Reck, L.; Onet, C.I.; Breen, J.M.; Zhu, N.Y.; Marzec, B.; Shvets, I.V.; Schmitt, W. Homologous size-extension of hybrid vanadate capsules—Solid state structures, solution stability and surface deposition. Chem. Commun. 2014, 50, 2265–2267. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Wojtas, L.; Zaworotko, M.J. Organic–inorganic hybrid polyhedra that can serve as supermolecular building blocks. Chem. Sci. 2014, 5, 927–931. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Wang, X.-L.; Zhou, E.-L.; Wu, X.-S.; Song, B.-Q.; Shao, K.-Z.; Su, Z.-M. Polyoxovanadate-based organic–inorganic hybrids: From {V5O9Cl} clusters to nanosized octahedral cages. Dalton Trans. 2016, 45, 3698–3701. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Gan, H.M.; Qin, C.; Wang, X.L.; Su, Z.M.; Zaworotko, M.J. Self-Assembly of Goldberg Polyhedra from a Concave [WV5O11(RCO2)5(SO4)]3− Building Block with 5-Fold Symmetry. J. Am. Chem. Soc. 2018, 140, 17365–17368. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.M.; Bao, L.Y.; Xu, N.; Hou, B.S.; Wang, X.L.; Su, Z.M. Self-Assembly Engineering of Fullerene-Like Polyhedra: V60, V66, V72 From {MV5} Pentagonal Second Building Block. Adv. Sci. 2025, 2408863. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Wang, X.-L.; Li, S.-B.; Gong, Y.-R.; Song, B.-Q.; Shao, K.-Z.; Su, Z.-M. Anderson-like alkoxo-polyoxovanadate clusters serving as unprecedented second building units to construct metal–organic polyhedral. Chem. Commun. 2016, 52, 9632–9635. [Google Scholar] [CrossRef]
- Gong, Y.R.; Qin, C.; Zhang, Y.T.; Sun, C.Y.; Pan, Q.H.; Wang, X.L.; Su, Z.M. Face-Directed Assembly of Molecular Cubes by In Situ Substitution of a Predetermined Concave Cluster. Angew. Chem. Int. Ed. 2020, 59, 22034–22038. [Google Scholar] [CrossRef]
- Hou, B.S.; Gu, X.Y.; Gan, H.M.; Zheng, H.Y.; Zhu, Y.; Wang, X.L.; Su, Z.M. Face-Directed Construction of a Metal–Organic Isohedral Tetrahedron for the Highly Efficient Capture of Environmentally Toxic Oxoanions and Iodine. Inorg. Chem. 2022, 61, 7103–7110. [Google Scholar] [CrossRef]
- Augustyniak, A.W.; Fandzloch, M.; Domingo, M.; Łakomskab, I.; Navarro, J.A.R. A vanadium(IV) pyrazolate metal–organic polyhedron with permanent porosity and adsorption selectivity. Chem. Commun. 2015, 51, 14724–14727. [Google Scholar] [CrossRef]
- Spandl, J.; Brüdgam, I.; Hartl, H. Solvothermal Synthesis of a 24-Nuclear, Cube-Shaped Squarato-oxovanadium(IV) Framework: [N(nBu)4]8[V24O24(C4O4)12(OCH3)32]. Angew. Chem. Int. Ed. 2001, 40, 4018–4020. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Gao, W.-Y.; Wojtas, L.; Zhang, Z.J.; Zaworotko, M.J. A new family of anionic organic–inorganic hybrid doughnut-like nanostructures. Chem. Commun. 2015, 51, 9223–9226. [Google Scholar] [CrossRef]
- Wu, S.-X.; Yang, Y.; Qin, C.; Hou, Y.-H.; Wang, X.-L.; Su, Z.-M. Organophosphate functionalized of {Mo240} polyoxomolybdate dodecahedra. Tungsten 2023, 5, 247–253. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Wang, Y.; Li, L.; Xu, N.; Wang, X.L. Anderson-type polyoxometalate-based complexes constructed from a new ‘V’-like bis-pyridine–bisamide ligand for selective adsorption of organic dyes and detection of Cr(VI) and Fe(III) ions. Inorg. Chem. Front. 2021, 8, 4458–4466. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, Y.M.; Wu, S.X.; Zhao, L.; Qin, C.; Wang, X.L.; Su, Z.M. Endohedral Functionalization for Structural Transformation of Polyoxovanadate-Based Metal–Organic Cube. Inorg. Chem. 2023, 62, 648–652. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wang, X.L.; Li, S.B.; Song, B.Q.; Shao, K.Z.; Su, Z.M. Ligand-Directed Assembly of Polyoxovanadate-Based Metal–Organic Polyhedra. Inorg. Chem. 2016, 55, 8770–8775. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).