A Non-Vacuum Coating Process That Fully Achieves Technical Goals of Bipolar Plates via Synergistic Control of Multiple Layer-by-Layer Strategy
Abstract
1. Introduction
2. Results and Discussion
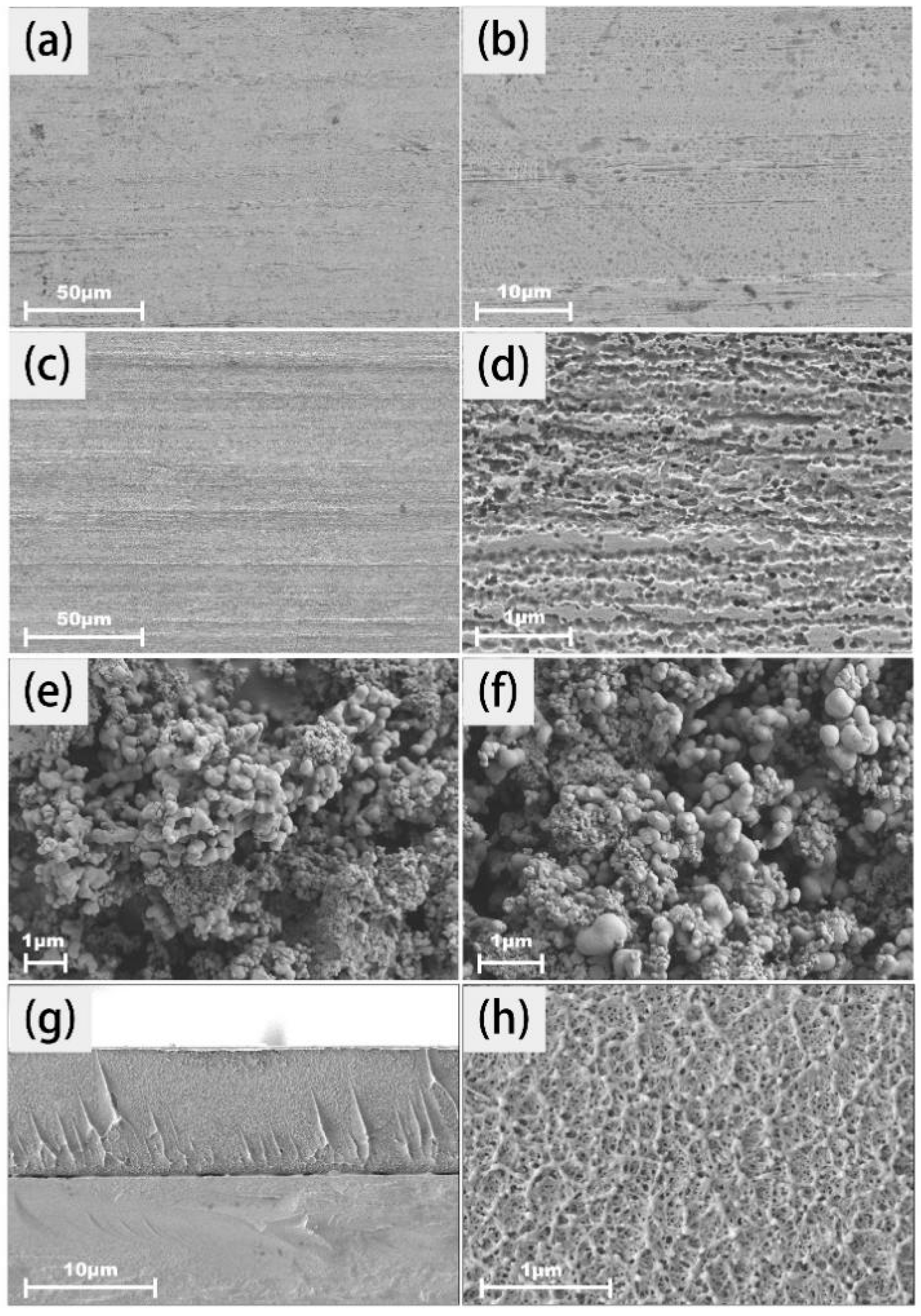
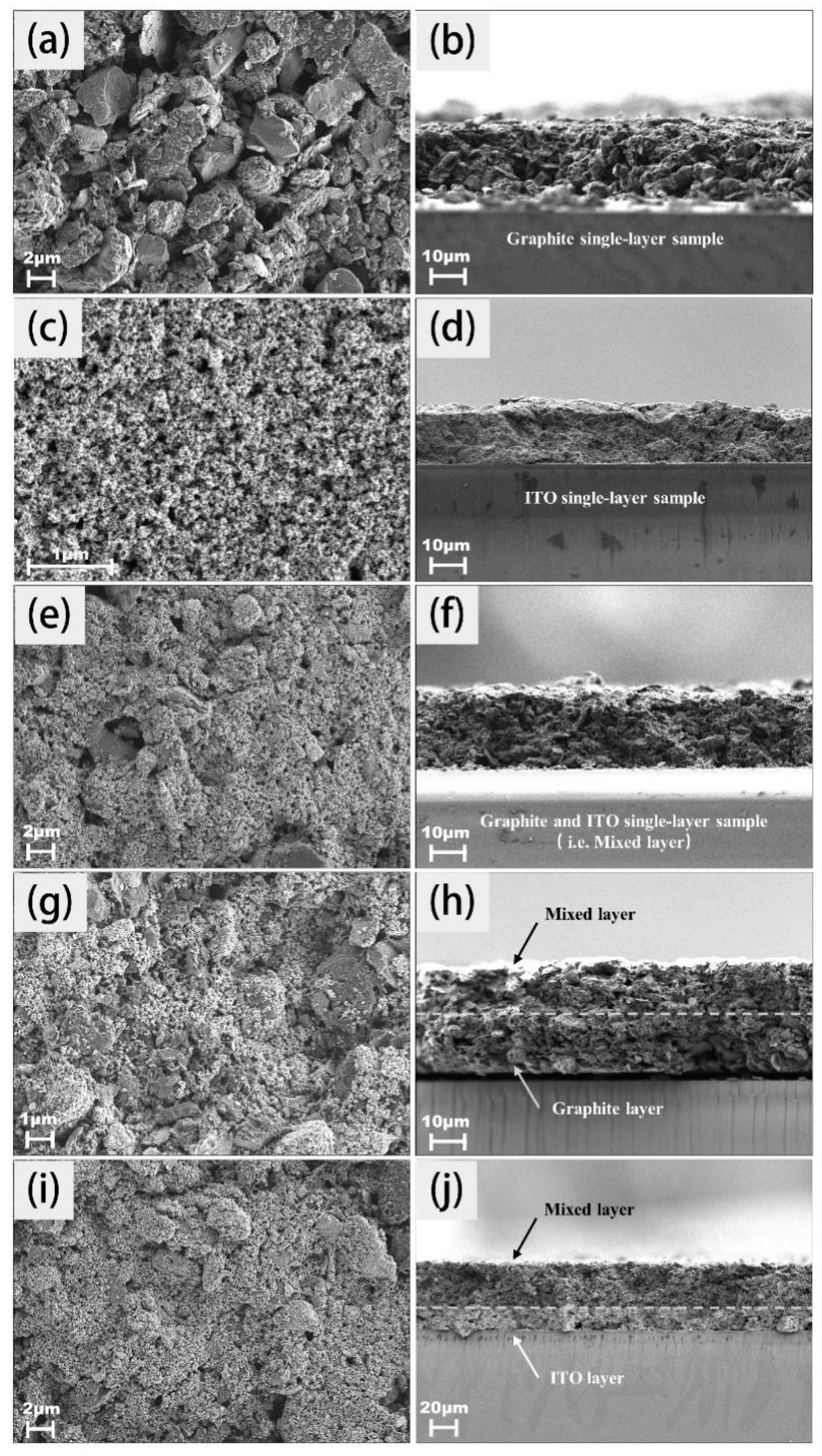
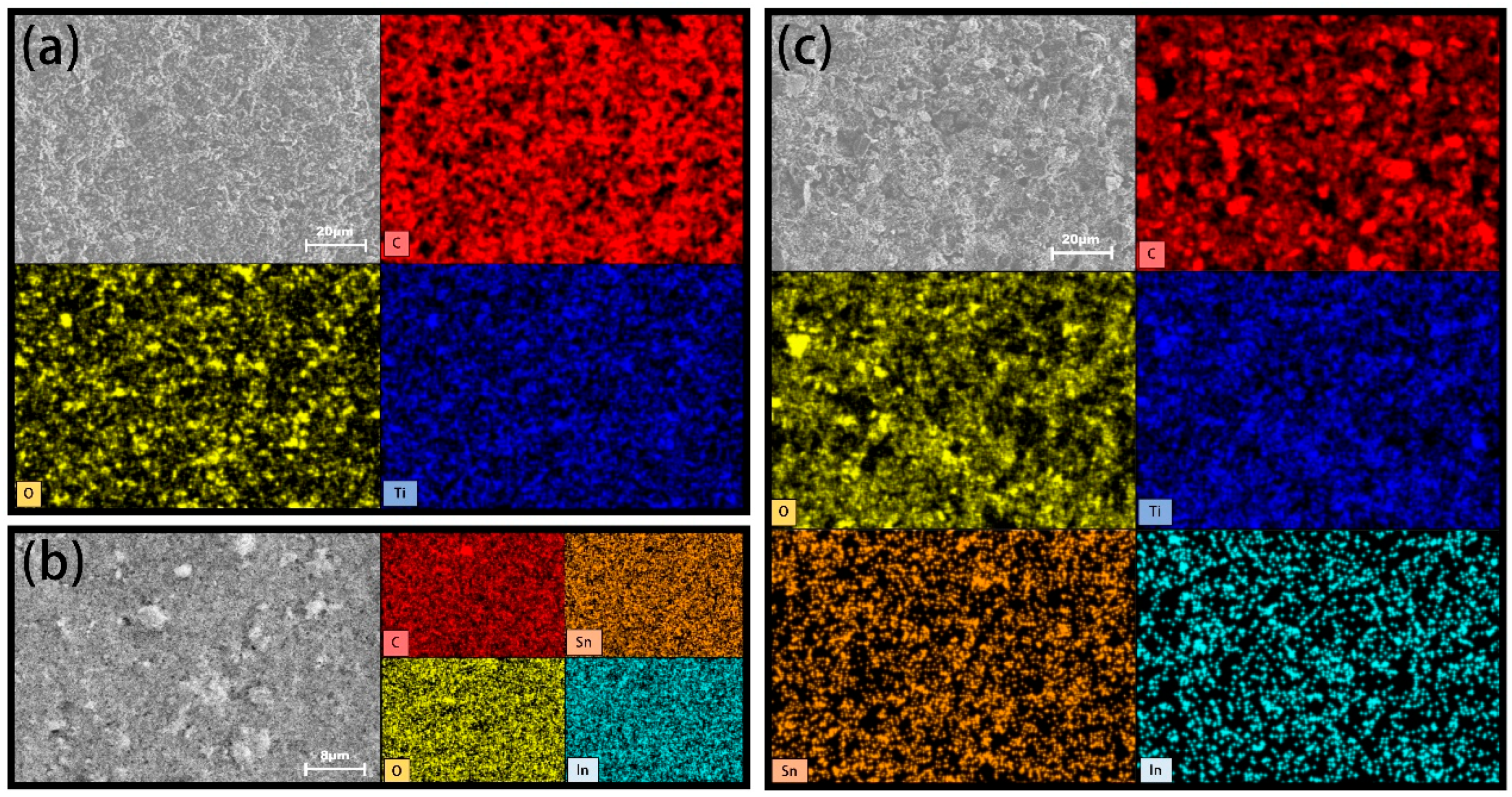
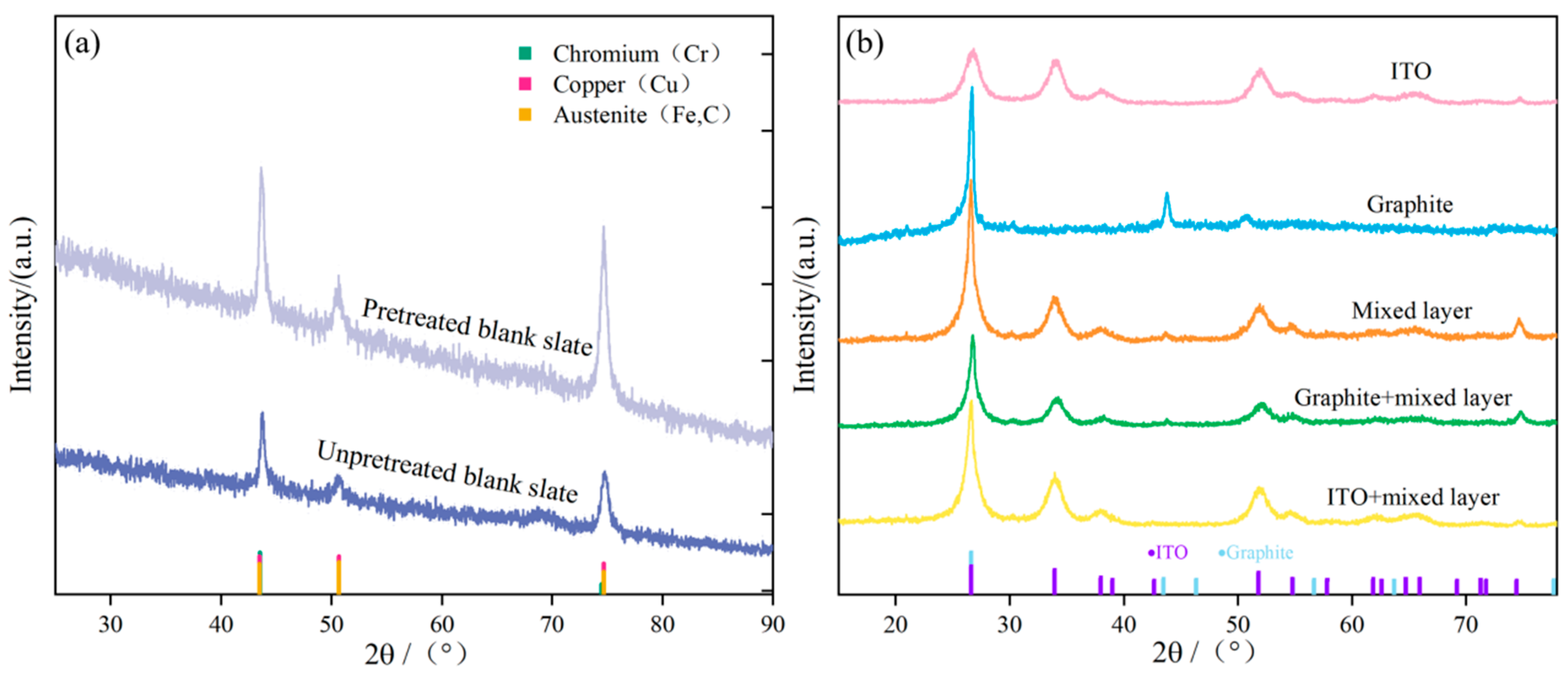
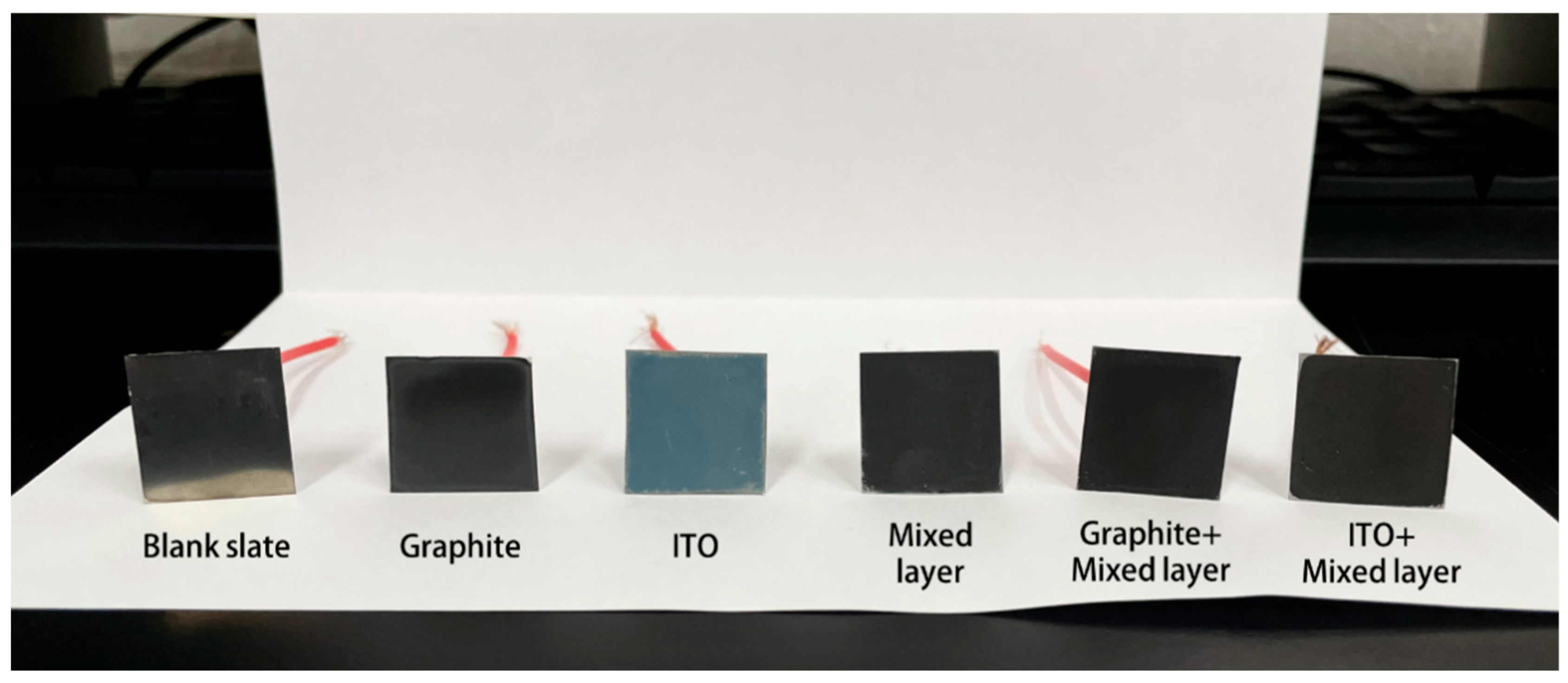
2.1. Characterization of Coating
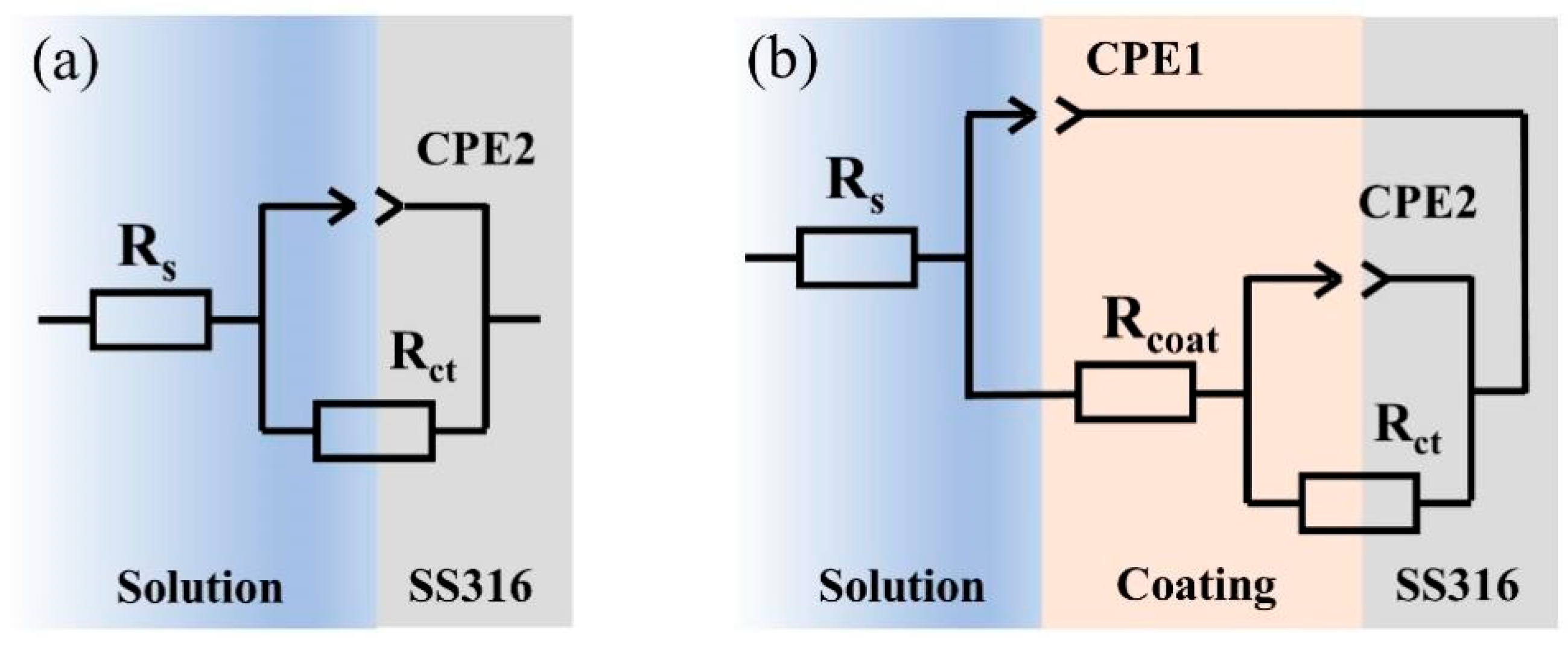
2.2. Characterization of Electrochemical Properties of Coatings
2.3. Characterization of Coating Surface Contact Resistance
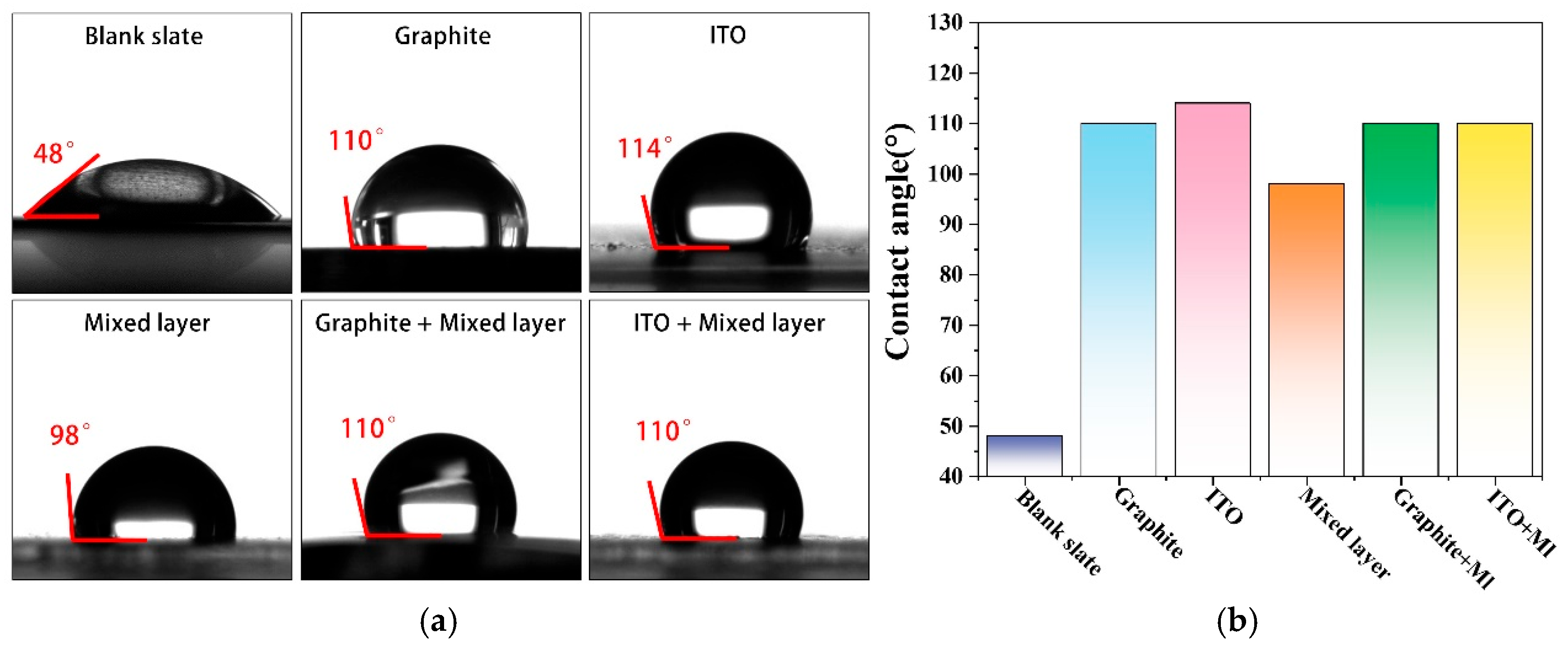
2.4. Characterization of Coating Contact Angle
3. Experimental Section
3.1. Materials
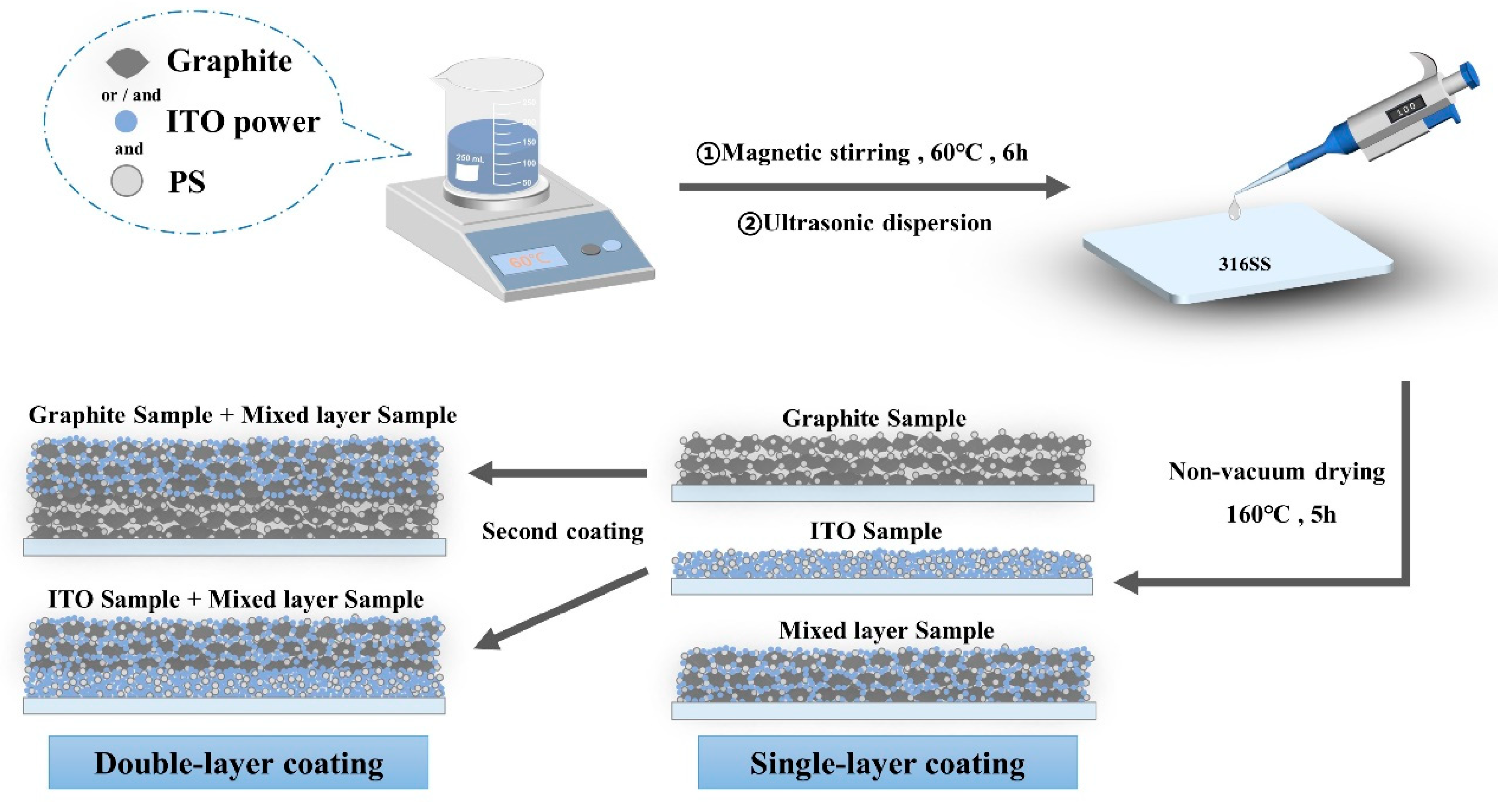
3.2. Preparation of Stainless Steel Substrate
3.3. Preparation of Coating Formulations
3.4. Sample Encapsulation
3.5. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, K.; Shen, Y.; Sun, H.; Liu, D.; An, Q.; Cai, X.; Chu, P.K. Conductive amorphous carbon-coated 316Lstainless steel as bipolar plates in polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2009, 34, 6771–6777. [Google Scholar] [CrossRef]
- Chung, C.Y.; Chen, S.K.; Chiu, P.J.; Chang, M.H.; Hung, T.T.; Ko, T.H. Carbon film-coated 304 stainless steel as PEMFC bipolar plate. J. Power Sources 2008, 176, 276–281. [Google Scholar] [CrossRef]
- Jeon, U.S.; Ahn, S.Y.; Choi, Y.M.; Kim, K.K.; Cho, E.A.; Ha, H.Y.; Hong, S.A.; Oh, I.H. Study on the carbon composite bipolar plates for polymer electrolyte membrane fuel cells. Solid State Ion 2004, 186, 307–314. [Google Scholar]
- Wang, S.H.; Peng, J.; Lui, W.B.; Zhang, J.S. Performance of the gold-plated titanium bipolar plates for the light weight PEM fuel cells. J. Power Sources 2006, 162, 486–491. [Google Scholar] [CrossRef]
- Yan, X.Q.; Hou, M.; Zhang, H.F.; Jing, F.N.; Ming, P.W.; Yi, B.L. Performance of PEMFC stack using expanded graphite bipolar plates. J. Power Sources 2006, 160, 252–257. [Google Scholar] [CrossRef]
- Gu, J.P.; Li, X.Q.; Zhou, Z.; Liao, R.S.; Gao, J.W.; Tang, Y.P.; Wang, Q.M. Synergistic regulation of effective detection for hypochlorite based on a dual-mode probe by employing aggregation induced emission (AIE) and intramolecular charge transfer (ICT) effects. Chem. Eng. J. 2019, 368, 157–164. [Google Scholar] [CrossRef]
- Kim, M.; Yu, H.N.; Lim, J.W.; Lee, D.G. Bipolar plates made of plain weave carbon/epoxy composite for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2012, 37, 4300–4308. [Google Scholar] [CrossRef]
- Ji, S.; Hwang, Y.S.; Park, T.; Lee, Y.H.; Paek, J.Y.; Chang, I.; Lee, M.H.; Cha, S.W. Graphite foil based assembled bipolar plates for polymer electrolyte fuel cells. Int. J. Precis. Eng. Manuf. 2012, 13, 2183–2186. [Google Scholar] [CrossRef]
- Wu, S.D.; Yan, H.; Zuo, X.H.; Cao, Z.B.; Li, H.Y.; Yang, W.M.; Zhan, J. Low-cost graphite coated copper as bipolar plates of proton exchange membrane fuel cells for corrosion protection. Fuel Cells 2021, 21, 502–511. [Google Scholar] [CrossRef]
- Liu, M.; Xu, H.F.; Fu, J.; Tian, Y. Conductive and corrosion behaviors of silver-doped carbon-coated stainless steel as PEMFC bipolar plates. Int. J. Miner. Metall. Mater. 2016, 23, 844–849. [Google Scholar] [CrossRef]
- Masood, M.T.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Palazon, F.; Athanassiou, A.; Bayer, I.S. Superhydrophobic high impact polystyrene (HIPS) nanocomposites with wear abrasion resistance. Chem. Eng. J. 2017, 322, 10–21. [Google Scholar] [CrossRef]
- Zhou, Z.R.; Vincent, L. Lubrication by thin polystyrene coating in fretting. Wear 1999, 231, 179–184. [Google Scholar] [CrossRef]
- Qiao, G.X.; Liu, L.; Hao, X.X.; Zheng, J.K.; Liu, W.Q.; Gao, J.W.; Zhang, C.C.; Wang, Q.M. Signal transduction from small particles: Sulfur nanodots featuring mercury sensing cell entry mechanism and in vitro tracking performance. Chem. Eng. J. 2020, 382, 122907. [Google Scholar] [CrossRef]
- Zhang, X.; Geng, T.; Guo, Y.G.; Zhang, Z.J.; Zhang, P.Y. Facile fabrication of stable superhydrophobic SiO2/polystyrene coating and separation of liquids with different surface tension. Chem. Eng. J. 2013, 231, 414–419. [Google Scholar] [CrossRef]
- Radwan, A.B.; Mannah, C.A.; Sliem, M.H.; Al Qahtani, N.H.S.; Okonkwo, P.C.; Berdimurodov, E.; Mohamed, A.M.; Abdullah, A.M. Electrospun highly corrosion-resistant polystyrene-nickel oxide superhydrophobic nanocomposite coating. J. Appl. Electrochem. 2021, 51, 1605–1618. [Google Scholar] [CrossRef]
- Li, X.Q.; Gu, J.P.; Zhou, Z.; Ma, L.F.; Tang, Y.P.; Gao, J.W.; Wang, Q.M. New lanthanide ternary complex system in electrospun nanofibers: Assembly, physico-chemical property and sensor application. Chem. Eng. J. 2019, 358, 67–73. [Google Scholar] [CrossRef]
- Yu, F.; Wang, K.; Cui, L.Z.; Wang, S.L.; Hou, M.; Xiong, F.; Zou, R.Q.; Gao, P.; Peng, H.L.; Liu, Z.F. Vertical-Graphene-Reinforced Titanium Alloy Bipolar Plates in Fuel Cells. Adv. Mater. 2022, 34, 2110565. [Google Scholar] [CrossRef]
- Ahmad, S.; Nawaz, T.; Ali, A.; Orhan, M.F.; Samreen, A.; Kannan, A.M. An overview of proton exchange membranes for fuel cells: Materials and manufacturing. Int. J. Hydrogen Energy 2022, 47, 19086–19131. [Google Scholar] [CrossRef]
- Liu, X.Y.; Gao, J.W.; Wang, Q.M. Structural-property correlations of all-inorganic CsPbBr3 perovskites via synergetic controls by PbBr2, 2-mercapto-3-methyl-4-thiazoleacetic acid and water. Chem. Eng. J. 2022, 428, 131117. [Google Scholar] [CrossRef]
- Shen, L.; Guo, A.; Zhu, X.Y. Tween surfactants: Adsorption, self-organization, and protein resistance. Surf. Sci. 2011, 605, 494–499. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.Q.; Tang, Y.P.; Zhang, C.C.; Fu, H.R.; Wu, N.T.; Ma, L.F.; Gao, J.W.; Wang, Q.M. Oxidative deoximation reaction induced recognition of hypochlorite based on a new fluorescent lanthanide-organic framework. Chem. Eng. J. 2018, 351, 364–370. [Google Scholar] [CrossRef]
- Cao, S.Y.; Liu, D.; Ding, H.; Lu, H.; Gui, J.Z. Towards understanding corrosion inhibition of sulfonate/carboxylate functionalized ionic liquids: An experimental and theoretical study. J. Colloid Interface Sci. 2020, 579, 315–329. [Google Scholar] [CrossRef]
- Tai, W.D.; Jia, J.C.; Zhang, W.C.; Dai, Y.Q.; Wang, Q.M.; Zeng, W. A n-Type ionic thermoelectric generator with high thermopower and energy density enabled by modulation of thermodiffusion and thermogalvanic effect. Chem. Eng. J. 2025, 509, 161288. [Google Scholar] [CrossRef]
- Chen, Z.H.; Zhang, G.H.; Yang, W.Z.; Xu, B.; Chen, Y.; Yin, X.S.; Liu, Y. Superior conducting polypyrrole anti-corrosion coating containing functionalized carbon powders for 304 stainless steel bipolar plates in proton exchange membrane fuel cells. Chem. Eng. J. 2020, 393, 124675. [Google Scholar] [CrossRef]
- López León, L.D.; Sánchez Ortiz, W.; Guerrero, A.L.; Ruiz Ochoa, J.A.; Lizárraga Mendiola, L.; Castañeda Robles, I.E. Electrochemical evaluation of mucilage and cochineal pigments as a hybrid film coating on aluminum surfaces. Mater. Res. Express 2022, 9, 065306. [Google Scholar] [CrossRef]
- Li, J.F.; Zhao, M.; Dai, C.S.; Wang, Z.B.; Pecht, M. A mathematical method for open-circuit potential curve acquisition for lithium-ion batteries. J. Electroanal. Chem. 2021, 895, 115488. [Google Scholar] [CrossRef]
- Gyftou, P.; Pavlatou, E.A.; Spyrellis, N. Effect of pulse electrodeposition parameters on the properties of Ni/nano-SiC composites. Appl. Surf. Sci. 2008, 254, 5910–5916. [Google Scholar] [CrossRef]
- Kumagai, M.; Myung, S.T.; Kuwata, S.; Asaishi, R.; Yashiro, H. Corrosion behavior of austenitic stainless steels as a function of pH for use as bipolar plates in polymer electrolyte membrane fuel cells. Electrochim. Acta 2008, 53, 4205–4212. [Google Scholar] [CrossRef]
- Meng, Q.; Yu, L.; Shang, L.L.; Wang, F.; Liu, X.X.; Zhang, G.A. Corrosive behavior and interfacial conductivity of stampable a-C film on titanium bipolar plate in proton exchange membrane fuel cells. Diam. Relat. Mater. 2023, 135, 109796. [Google Scholar] [CrossRef]
- Yuan, R.X.; Wu, S.Q.; Yu, P.; Wang, B.H.; Mu, L.W.; Zhang, X.G.; Zhu, Y.X.; Wang, B.; Wang, H.Y.; Zhu, J.H. Superamphiphobic and Electroactive Nanocomposite toward Self Cleaning, Antiwear, and Anticorrosion Coatings. ACS Appl. Mater. Interfaces 2016, 8, 12481–12493. [Google Scholar] [CrossRef]
- Liu, R.X.; Jia, Q.; Zhang, B.; Lai, Z.G.; Chen, L. Protective coatings for metal bipolar plates of fuel cells: A review. Int. J. Hydrogen Energy 2022, 47, 22915–22937. [Google Scholar] [CrossRef]
- Zhou, Y.; Lin, G.; Shih, A.J.; Hu, S.J. A micro-scale model for predicting contact resistance between bipolar plate and gas diffusion layer in PEM fuel cells. J. Power Sources 2007, 163, 777–783. [Google Scholar] [CrossRef]
- Lædre, S.; Kongstein, O.E.; Oedegaard, A.; Seland, F.; Karoliussen, H. Measuring In Situ Interfacial Contact Resistance in a Proton Exchange Membrane Fuel Cell. J. Electrochem. Soc. 2019, 166, F853–F859. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, Y.B.; Kim, K.M.; Jeong, M.G.; Lim, D.S. Electrically conductive polymer composite coating on aluminum for PEM fuel cells bipolar plate. Renew. Energy 2013, 54, 46–50. [Google Scholar] [CrossRef]
- Bahremand, F.; Shahrabi, T.; Ramezanzadeh, B. Development of a nanostructured film based on samarium (III)/polydopamine on the steel surface with superior anti-corrosion and water-repellency properties. J. Colloid Interface Sci. 2021, 582, 342–352. [Google Scholar] [CrossRef]
- Sidane, D.; Khireddine, H.; Yala, S.; Ziani, S.; Bir, F.; Chicot, D. Morphological and Mechanical Properties of Hydroxyapatite Bilayer Coatings Deposited on 316L SS by Sol-Gel Method. Metall. Mater. Trans. B 2015, 46, 2340–2347. [Google Scholar] [CrossRef]
- Yu, M.; Xue, B.; Liu, J.H.; Li, S.M.; Zhang, Y. Electrophoretic deposition of hybrid coatings on aluminum alloy by combining 3-aminopropyltrimethoxysilan to silicon-zirconium sol solutions for corrosion protection. Thin Solid Film. 2015, 590, 33–39. [Google Scholar] [CrossRef]
- Chen, M.H.; Wen, Q.; Gu, F.L.; Gao, J.W.; Zhang, C.C.; Wang, Q.M. Mussel chemistry assembly of a novel biosensing nanoplatform based on polydopamine fluorescent dot and its photophysical features. Chem. Eng. J. 2018, 342, 331–338. [Google Scholar] [CrossRef]
- Wang, Y.; Northwood, O. Effects of O2 and H2 on the corrosion of SS316L metallic bipolar plate materials in simulated anode and cathode environments of PEM fuel cells. Electrochim. Acta 2007, 52, 6793–6798. [Google Scholar] [CrossRef]
- Wu, C.Q.; Bo, S.L.; Luo, Z.J.; Zheng, Y.H.; Wang, Q.M. Sulfur quantum dots integration in conductive microrod array enable superior oxygen evolution performance. J. Colloid Interf. Sci. 2025, 694, 137714. [Google Scholar] [CrossRef]
- Xu, S.S.; Wang, Q.; Wang, N.; Zheng, X. Fabrication of superhydrophobic green surfaces with good self-cleaning, chemical stability and anti-corrosion properties. J. Mater. Sci. 2019, 54, 13006–13016. [Google Scholar] [CrossRef]



| Sample | Ecorr (V) | Icorr (μA/cm2) | P.E. (%) |
|---|---|---|---|
| Graphite | 0.42 | 0.59 | 99.867 |
| ITO | 0.16 | 9.52 | 97.862 |
| Mixed layer | 0.64 | 0.76 | 99.829 |
| Graphite + Mixed layer | 0.98 | 0.52 | 99.883 |
| ITO + Mixed layer | 0.69 | 1.89 | 99.575 |
| Blank slate | −0.38 | 445.24 | — |
| Interfacial Contact Resistance (mΩ·cm2) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Force (N/cm2) | Force (Mpa) | Graphite | ITO | Mixed Layer | Graphite+ Mixed Layer | ITO+ Mixed Layer | Unpretreated Blank Slate | Pretreated Blank Slate |
| 15 | 0.15 | 1948 | 1668 | 1188 | 1868 | 1508 | 2748 | 328 |
| 30 | 0.3 | 1450 | 1370 | 1010 | 1430 | 1250 | 2190 | 290 |
| 45 | 0.45 | 1140 | 980 | 980 | 1260 | 1140 | 1740 | 260 |
| 60 | 0.6 | 806.8 | 746.8 | 866.8 | 1146.8 | 946.8 | 1326.8 | 206.8 |
| 75 | 0.75 | 509.4 | 309.4 | 569.4 | 949.4 | 869.4 | 1149.4 | 109.4 |
| 90 | 0.9 | 497.6 | 297.6 | 477.6 | 817.6 | 457.6 | 1017.6 | 97.6 |
| 105 | 1.05 | 486.8 | 286.8 | 426.8 | 386.8 | 406.8 | 746.8 | 66.8 |
| 120 | 1.2 | 474.6 | 214.6 | 374.6 | 294.6 | 314.6 | 674.6 | 54.6 |
| 135 | 1.35 | 361.8 | 161.8 | 221.8 | 241.8 | 201.8 | 561.8 | 41.8 |
| 150 | 1.5 | 136.4 | 16.4 | 56.4 | 16.4 | 16.4 | 536.4 | 36.4 |
| 165 | 1.65 | 85.4 | 5.4 | 45.4 | 3.4 | 1.4 | 505.4 | 5.4 |
| Coating | Graphite Powder (g) | ITO Powder (g) | Polystyrene (in DMF/mL) | Tween 60 (in Deionized Water/mL) |
|---|---|---|---|---|
| Single-layer sample | ||||
| Graphite | 0.5 | — | 3 | 2 |
| ITO | — | 0.5 | 3 | 2 |
| Mixed layer (Ml) | 0.3 | 0.2 | 3 | 2 |
| Double-layer sample | ||||
| Graphite/Ml | 0.5 + 0.3 | — | 3 + 3 | 2 + 2 |
| ITO/Ml | — | 0.5 + 0.2 | 3 + 3 | 2 + 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Chen, X.; Wu, M.; Wang, W.; Lin, Y.; Chen, Z.; Yang, S.; Zheng, Y.; Wang, Q. A Non-Vacuum Coating Process That Fully Achieves Technical Goals of Bipolar Plates via Synergistic Control of Multiple Layer-by-Layer Strategy. Molecules 2025, 30, 2543. https://doi.org/10.3390/molecules30122543
Liu Q, Chen X, Wu M, Wang W, Lin Y, Chen Z, Yang S, Zheng Y, Wang Q. A Non-Vacuum Coating Process That Fully Achieves Technical Goals of Bipolar Plates via Synergistic Control of Multiple Layer-by-Layer Strategy. Molecules. 2025; 30(12):2543. https://doi.org/10.3390/molecules30122543
Chicago/Turabian StyleLiu, Qiaoling, Xiaole Chen, Menghan Wu, Weihao Wang, Yinru Lin, Zilong Chen, Shuhan Yang, Yuhui Zheng, and Qianming Wang. 2025. "A Non-Vacuum Coating Process That Fully Achieves Technical Goals of Bipolar Plates via Synergistic Control of Multiple Layer-by-Layer Strategy" Molecules 30, no. 12: 2543. https://doi.org/10.3390/molecules30122543
APA StyleLiu, Q., Chen, X., Wu, M., Wang, W., Lin, Y., Chen, Z., Yang, S., Zheng, Y., & Wang, Q. (2025). A Non-Vacuum Coating Process That Fully Achieves Technical Goals of Bipolar Plates via Synergistic Control of Multiple Layer-by-Layer Strategy. Molecules, 30(12), 2543. https://doi.org/10.3390/molecules30122543





