Rapid Bactericidal Activity of Punica granatum L. Peel Extract: A Natural Alternative for Mastitis Prevention in Dairy Cattle
Abstract
1. Introduction
2. Results
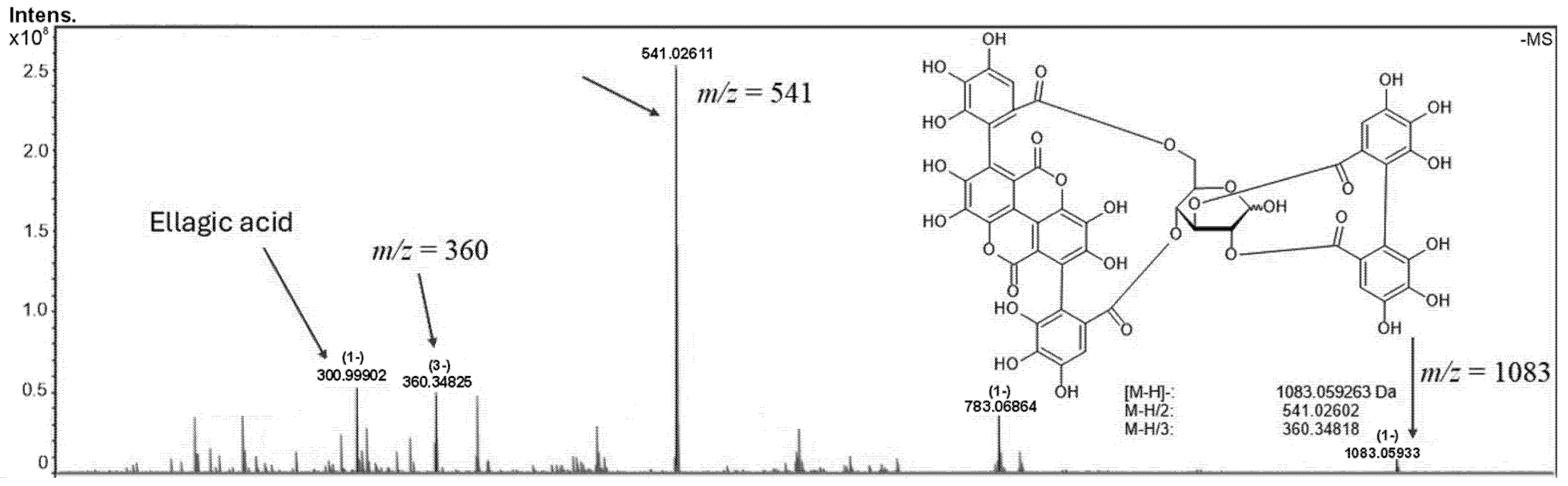
2.1. Phytochemical Analysis of the Hydroalcoholic Extract of Punica granatum
2.2. Analysis of the Integrity of Bacterial Cell Membranes After Contact with Punica granatum Extract
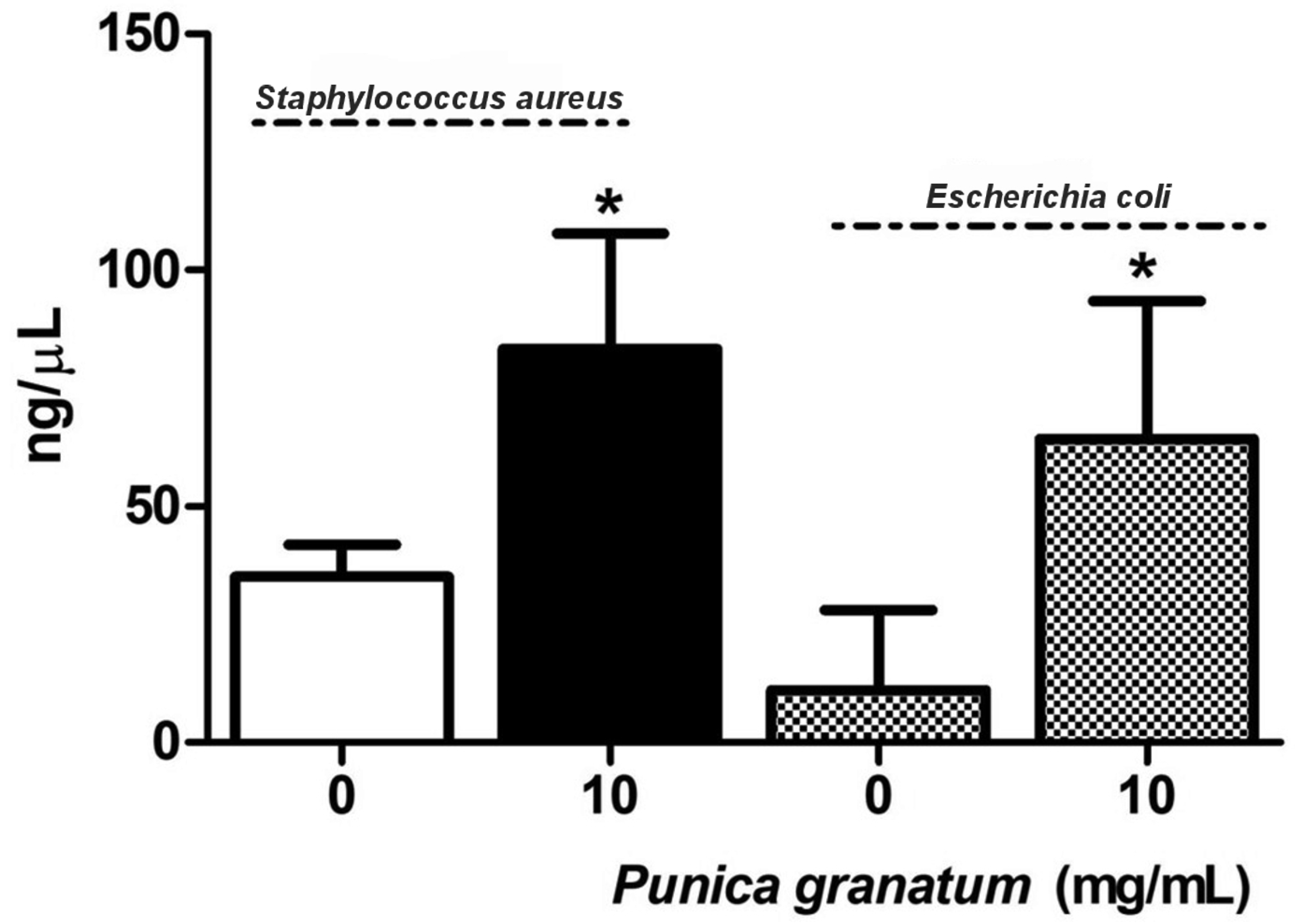
2.3. DNA Concentration in Supernatants of Bacterial Cultures After Treatment with P. granatum Extract
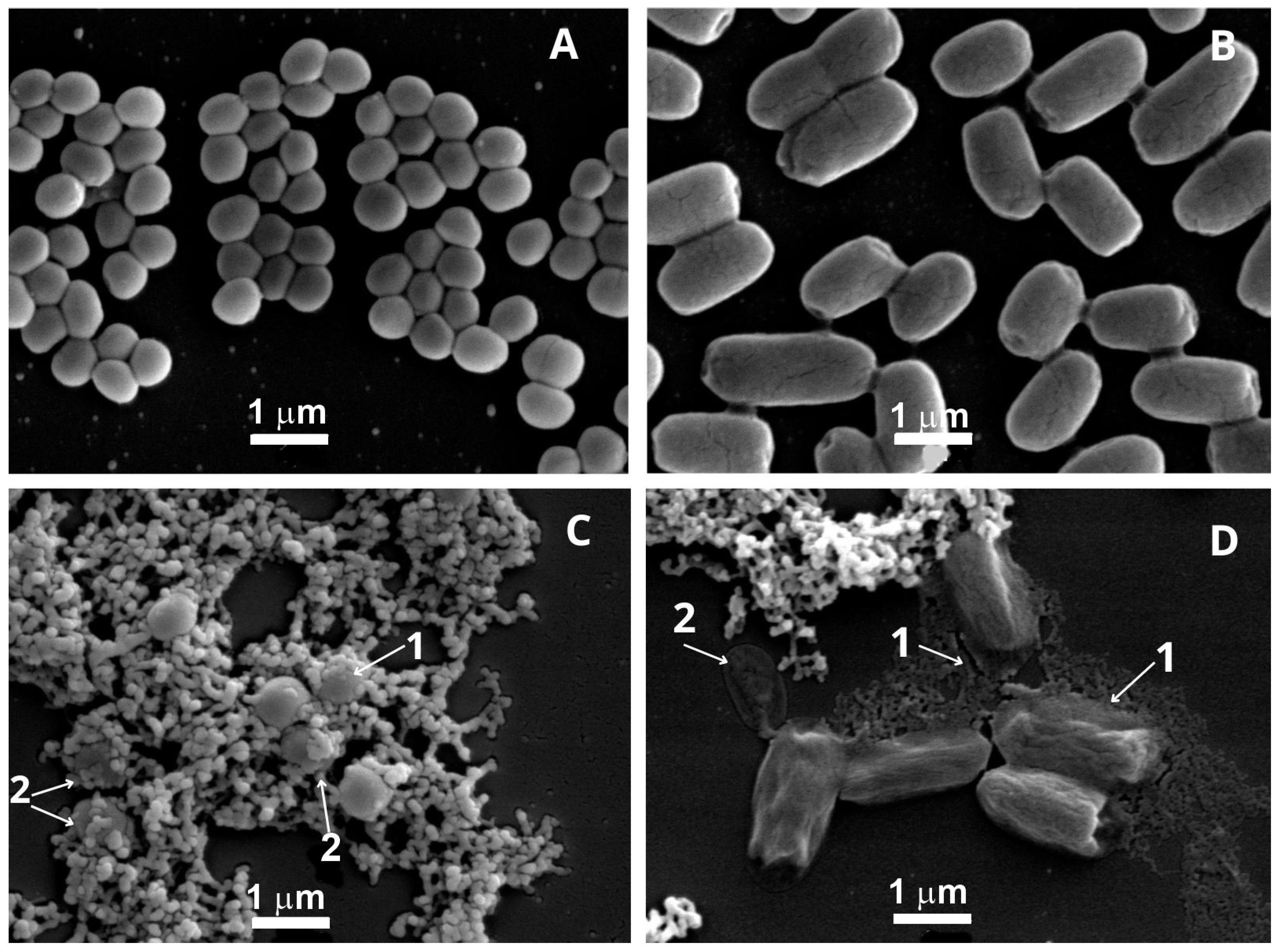
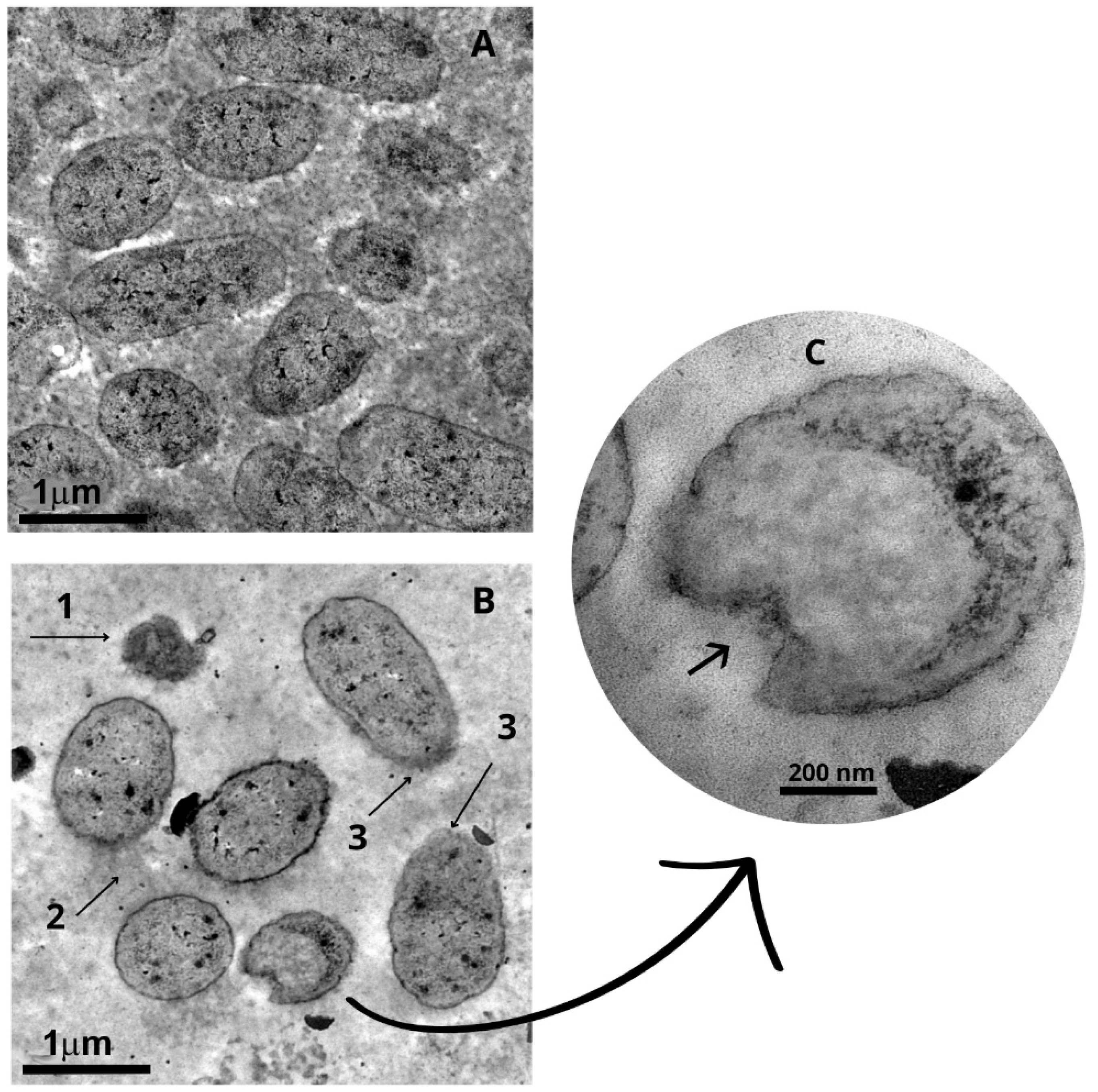
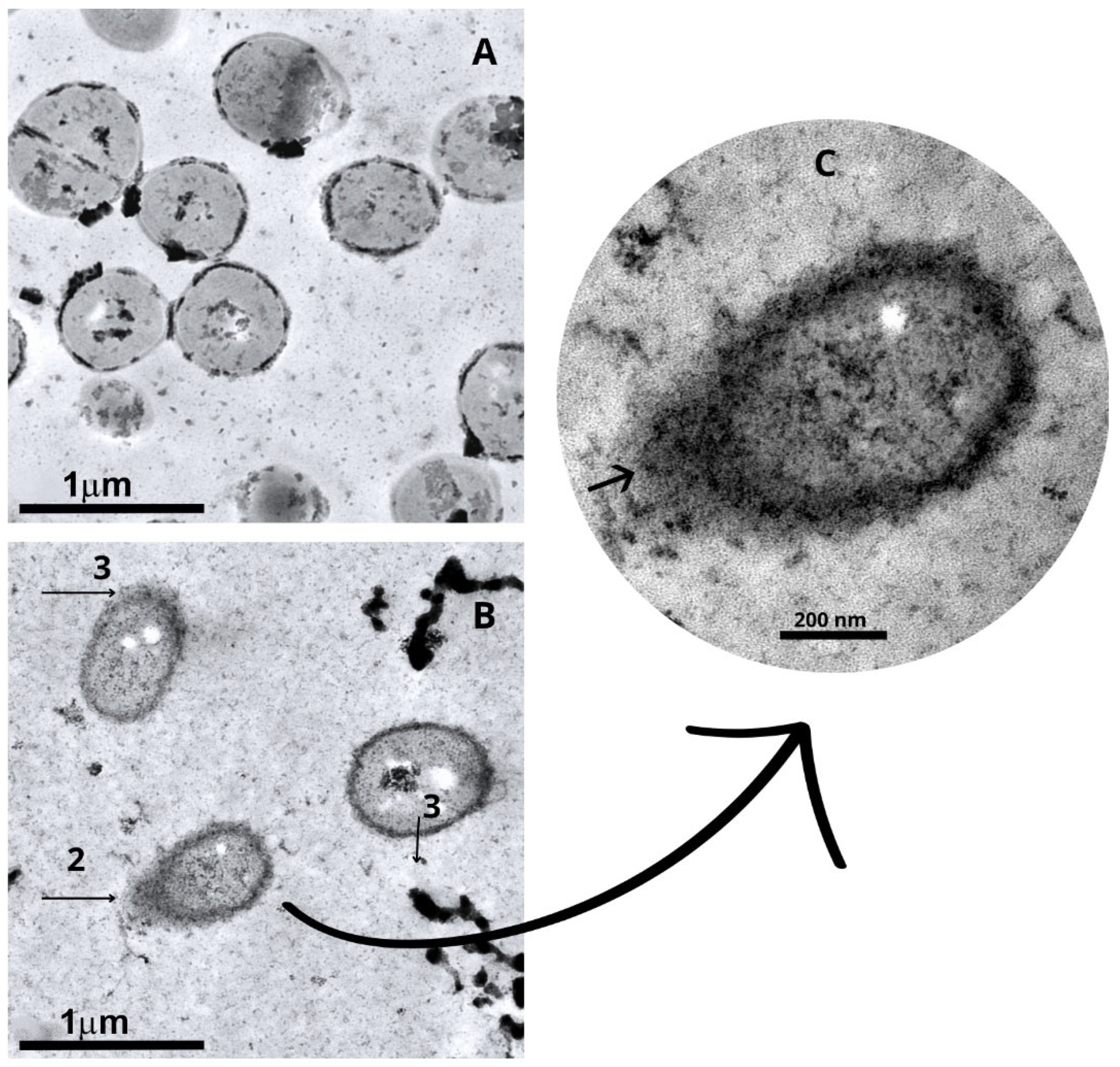
2.4. Ultrastructural Changes of Staphylococcus aureus and Escherichia coli After Treatment with Punica granatum Extract
2.5. Comparison of Bacterial Survival After Treatment with Different Concentrations of Extract and Disinfectants
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extract Preparation
4.2. Phytochemical Analysis of Punica granatum Extract
4.3. Bacterial Strains
4.4. Analysis by Flow Cytometry of Membrane Integrity in Staphylococcus aureus and Escherichia coli Strains After Treatment with Punica granatum Extract
4.5. Quantification of DNA in the Supernatant of Bacterial Strain Cultures After Treatment with Punica granatum Extract
4.6. Evaluation of Morphological and Ultrastructural Characteristics of Bacterial Strains After Treatment with Punica granatum Extract
4.7. Determination of Bacterial Survival After Treatment with Different Concentrations of Extract and Disinfectants
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| AMR | Antimicrobial resistance |
| WHO | World Health Organization |
| PI | Propidium iodide |
References
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments—A review. Asian-Australas J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Freitas, T.F.; Marques, F.M.; Kuster, R.M.; Salles, J.B.; Victório, C.P.; de Assis, M.C. Punica granatum L. inhibits the growth of microorganisms associated with bovine mastitis. Nat. Prod. J. 2020, 10, 611–620. [Google Scholar] [CrossRef]
- Nader, T.T.; Coppede, J.S.; Taleb-Contini, S.H.; Amaral, L.A.; Pereira, A.M.S. Atividade antibiofilme de substâncias de Croton urucurana em Staphylococcus aureus isoladas de mastite bovina. Pesq. Vet. Bras. 2018, 38, 1713–1719. [Google Scholar] [CrossRef]
- Ashraf, A.; Imran, M. Causes, types, etiological agents, prevalence, diagnosis, treatment, prevention, effects on human health and future aspects of bovine mastitis. Anim. Health Res. Rev. 2020, 21, 36–49. [Google Scholar] [CrossRef]
- Maurice, N.M.; Bedi, B.; Sadikot, R.T. Pseudomonas aeruginosa biofilms: Host response and clinical implications in lung infections. Am. J. Resp. Cell Mol. Biol. 2018, 51, 415–420. [Google Scholar] [CrossRef]
- Pascu, C.; Herman, V.; Iancu, I.; Costinar, L. Etiology of Mastitis and Antimicrobial Resistance in dairy cattle farms in the Western part of Romania. Antibiotics 2022, 11, 57. [Google Scholar] [CrossRef]
- Vidal, C.S.; Carvalho, G.H.S.; Assis, M.C.; Angeli, R.; Victorio, C.P. Patents of plant products applied to bovine mastitis in the INPI. Rev. Fitos. 2024, 19, e1663. [Google Scholar] [CrossRef]
- Vidal, C.S.; de Carvalho, G.H.S.; de Assis, M.C.; Angeli, R.; Victório, C.P. Technological mapping of plant-based patents for treating bovine mastitis. Trop. Anim. Health Prod. 2025, 57, 111. [Google Scholar] [CrossRef]
- Silva, C.R.e.A.; Vidal, C.S.; Victorio, C.P.; Assis, M.C. Natural sanitizers pre- and post-milking in the prevention of bovine mastites. Concilium 2024, 24, 526. [Google Scholar] [CrossRef]
- Sandle, T. Chapter 17—Assessing, Controlling, and Removing Contamination Risks from the Process. In Biocontamination Control for Pharmaceuticals and Healthcare; Academic Press: Cambridge, MA, USA, 2019; pp. 287–314. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Protective effects of pomegranate (Punica granatum) and its main components against natural and chemical toxic agents: A comprehensive review. Phytomedicine 2023, 109, 154581. [Google Scholar] [CrossRef]
- Mohammadi, M.; Boghrati, Z.; Emami, S.A.; Akaberi, M. Pomegranate: A review of the heavenly healer’s past, present, and future. Iran. J. Basic Med. Sci. 2023, 26, 1245. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.W.P.; Casali, A.K. Atividade antimicrobiana in vitro de extratos etanólicos de romã (Punica granatum L.) sobre as bactérias Escherichia coli e Staphylococcus aureus. Braz. J. Develop. 2019, 5, 22970–22980. [Google Scholar] [CrossRef]
- Campos, E.; Silva, L.; Garcia, S.; de Oliveira, P.G.; de Oliveira, M.A.P.; da Silva, C.A.; de Paula, J.R. Atividade antimicrobiana do extrato, frações e punicalagina da casca do fruto de Punica granatum frente a isolados clínicos de vacas com mastite. Res. Soc. Dev. 2021, 16, 1–13. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, C.; Wu, Q.; Zheng, Z.; Liu, P.; Li, G.; Peng, X.; Xia, X. Antimicrobial activity of punicalagin against Staphylococcus aureus and its effect on biofilm formation. Foodborne Pathog Dis. 2017, 14, 282–287. [Google Scholar] [CrossRef]
- Sajjad, W.; Sohail, M.; Ali, B.; Haq, A.; Din, G.; Hayat, M.; Khan, I.; Ahmad, M.; Khan, S. Antibacterial activity of Punica granatum peel extract. Mycopath 2015, 13, 105–111. [Google Scholar]
- Bach, A.; Vieira, E.; Rosa, P.; Viana, C.; Hoffmann, F.; Fernandes, R.A.A.; Macagnan, R.; da Silva Oliveira, N. Eficácia do uso de desinfetantes no manejo de ordenha de vacas leiteiras no controle da mastite e seus agentes infecciosos—Revisão bibliográfica. Rev. Cient. Rural. 2019, 21, 188–204. [Google Scholar] [CrossRef]
- Silva, D.D.; Medeiros, E.S.; Rolim, M.B.; Marinho, A.V.; Soares, K.D.; Almeida, G.L.; Pereira Neto, L.M.; Silva, T.I. In vitro efficacy of iodine in the pre and post dipping against coagulase negative Staphylococcus isolated in milk of cows with subclinical mastitis. Ciên. Rural. 2021, 51, e20200417. [Google Scholar] [CrossRef]
- Dos Santos, E.P.; Rodrigues, F.S.; De Sousa Santos, I.C.R.; de Sá Silva, K.C.; Coutinho, G.S.L.; Firmo, W.D.C.A.; Pinheiro, A.J.M.C.R. Punica granatum L. (romã) e atividade antimicrobiana contra o biofilme dental: Uma revisão bibliográfica. Ens. Ciência Biológicas Agrárias Saúde 2019, 23, 88–93. [Google Scholar] [CrossRef]
- Guerrero-Solano, J.A.; Jaramillo-Morales, O.A.; Jiménez-Cabrera, T.; Urrutia-Hernández, T.A.; Chehue-Romero, A.; Olvera-Hernández, E.G.; Bautista, M. Punica protopunica Balf., the forgotten sister of the common pomegranate (Punica granatum L.): Features and medicinal properties: A review. Plants 2020, 9, 1214. [Google Scholar] [CrossRef]
- El-Hamamsy, S.M.A.; El-Khamissi, H.A.Z. Phytochemicals, antioxidant activity and identification of phenolic compounds by HPLC of pomegranate (Punica granatum L.) 84 Peel extracts. J. Agr. Chem. Biotechnol. 2020, 11, 79–84. [Google Scholar] [CrossRef]
- Shahkoomahally, S.; Shin, D.; Habibi, F.; Kim, J.; Sarkhosh, A. Profiling phenolic compounds in juice and peel of fourteen pome granate (Punica granatum L.) varieties grown in Florida, USA. Food Chem. Adv. 2023, 2, 2. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Stability of total phenolic concentration and antioxidant capacity of extracts from pomegranate co-products subjected to in vitro digestion. BMC Complement Altern. Med. 2016, 16, 358. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, A.; Magri, A.; Petriccione, M.; Vaio, D. Effects of cold storage on quality parameters and nutraceutical compounds of pomegranate fruits (cv. Acco). Adv. Hortic. Sci. 2023, 37, 15. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Almajano, M.P.; Delgado, M.E.; Gordon, M.H. Changes in the antioxidant properties of protein solutions in the presence of epigallocatechin gallate. Food Chem. 2007, 101, 126–130. [Google Scholar] [CrossRef]
- Oulahal, N.; Degraeve, P. Phenolic-Rich Plant Extracts with Antimicrobial Activity: An Alternative to Food Preservatives and Biocides? Front. Microbiol. 2022, 12, 753518. [Google Scholar] [CrossRef] [PubMed]
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart, C.N., Jr. The potential of systems biology to discover antibacterial mechanisms of plant phenolics. Front. Microbiol. 2017, 8, 422. [Google Scholar] [CrossRef]
- Yang, R.; Guan, Y.; Zhou, J.; Sun, B.; Wang, Z.; Chen, H.; He, Z.; Jia, A. Phytochemicals from Camellia nitidissima chi flowers reduce the pyocyanin production and motility of Pseudomonas aeruginosa PAO1. Front. Microbiol. 2018, 8, 2640. [Google Scholar] [CrossRef]
- Kamiya, M.; Mori, T.; Nomura, M.; Inagaki, T.; Nonogaki, T.; Nagatsu, A.; Yamagishi, Y.; Mikamo, H.; Ikeda, Y. Tradescantia pallida extract inhibits biofilm formation in Pseudomonas aeruginosa. Nagoya J. Med. Sci. 2019, 81, 439–452. [Google Scholar] [CrossRef]
- Saraiva, A.; Coutinho, F.; Silva, R.; Randau, K.P.; Xavier, H.S.; Pisciottano, M.N.C. Atividade antimicrobiana de polifenóis isolados das folhas de Schinopsis brasiliensis (Engl.) guiado por bioautografia. Rev. Fitos. 2020, 14, 10–25. [Google Scholar] [CrossRef]
- Estrela, C.; Ribeiro, R.G.; Estrela, C.R.; Pécora, J.D.; Sousa-Neto, M.D. Antimicrobial effect of 2% sodium hypochlorite and 2% chlorhexidine tested by different methods. Braz. Dent. J. 2003, 14, 58–62. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Ver. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed]
- Dinu, S.; Matichescu, A.; Buzatu, R.; Marcovici, I.; Geamantan-Sirbu, A.; Semenescu, A.D.; Bratu, R.C.; Bratu, D.C. Insights into the cytotoxicity and irritant potential of chlorhexidine digluconate: An in vitro and in ovo safety screening. Dent. J. 2024, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Hayouni, E.A.; Miled, K.; Boubaker, S.; Bellasfar, Z.; Abedrabba, M.; Iwaski, H.; Oku, H.; Matsui, T.; Limam, F.; Hamdi, M. Hydroalcoholic extract based-ointment from Punica granatum L. peels with enhanced in vivo healing potential on dermal wounds. Phytomedicine 2011, 18, 976–984. [Google Scholar] [CrossRef]
- García-Alegría, A.M.; Anduro-Corona, I.; Pérez-Martínez, C.J.; Guadalupe Corella-Madueño, M.A.; Rascón-Durán, M.L.; Astiazaran-Garcia, H. Quantification of DNA through the NanoDrop Spectrophotometer: Methodological Validation Using Standard Reference Material and Sprague Dawley Rat and Human DNA. Int. J. Anal. Chem. 2020, 2020, 8896738. [Google Scholar] [CrossRef]
- Pau, G.; Fuchs, F.; Sklyar, O.; Boutros, M.; Huber, W. EBImage-an R package for image processing with applications to cellular phenotypes. Bioinformatics 2010, 26, 979–981. [Google Scholar] [CrossRef]
| Time (s) | Control | 50 mg/mL | 25 mg/mL | 10 mg/mL |
|---|---|---|---|---|
| 30 | 13.1 (12.4/20.9) | 214.8 (167.0/235.0) | 201.7 (118.8/239.3) | 91.4 (87.8/179.4) |
| 60 | 18.6 (13.3/22.3) | 197.7 (126.4/269.0) | 189.4 (86.0/239.0) | 111.4 (61.0/118.6) |
| 120 | 18.5 (13.0/22.5) | 218.7 (100.9/250.3) | 181.1 (67.9/245.8) | 118.6 (82.0/129.8) |
| Time (s) | Control | 50 mg/mL | 25 mg/mL | 10 mg/mL |
|---|---|---|---|---|
| 30 | 12.6 (11.6/24.9) | 179.4 (164.0/198.1) | 138.9 (129.8/283.9) | 129.8 (63.8/189.4) |
| 60 | 12.5 (12.1/14.5) | 191.1 (120.8/212.9) | 115.5 (79.8/177.4) | 121.9 (107.5/220.7) |
| 120 | 13.5 (11.6/41.4) | 196.3 (148.6/199.9) | 64.36 (50.5/160.9) | 82.79 (77.0/259.5) |
| Bacterial Species | Control | Punica granatum Extract (mg/mL) | Chlorhexidine (%) | Sodium Hypochlorite (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 50 | 25 | 10 | 2 | 0.5 | 2.5 | 0.5 | |
| Staphylococcus aureus | 1.0 × 102 (1.0/1.7) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.3 × 102 (1.0/1.6) |
| Escherichia coli | 6.3 × 102 (1.0/1.7) | 0.0 | 0.0 | 1.3 × 102 (0/1.3) | 0.0 | 0.0 | 0.0 | 0.7 × 102 (0.3/1.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, C.R.e.A.; Vidal, C.S.; Filho, S.M.d.A.; Agatão, I.M.; Berbert, L.C.; Salles, J.B.d.; Cardoso, A.M.; Kuster, R.M.; Victório, C.P.; Assis, M.C.d. Rapid Bactericidal Activity of Punica granatum L. Peel Extract: A Natural Alternative for Mastitis Prevention in Dairy Cattle. Molecules 2025, 30, 2387. https://doi.org/10.3390/molecules30112387
Silva CReA, Vidal CS, Filho SMdA, Agatão IM, Berbert LC, Salles JBd, Cardoso AM, Kuster RM, Victório CP, Assis MCd. Rapid Bactericidal Activity of Punica granatum L. Peel Extract: A Natural Alternative for Mastitis Prevention in Dairy Cattle. Molecules. 2025; 30(11):2387. https://doi.org/10.3390/molecules30112387
Chicago/Turabian StyleSilva, Carenn Rodrigues e Almeida, Camila Silva Vidal, Sergio Martins de Andrade Filho, Izabela Martins Agatão, Lidiane Coelho Berbert, João Bosco de Salles, Alexander Machado Cardoso, Ricardo Machado Kuster, Cristiane Pimentel Victório, and Maria Cristina de Assis. 2025. "Rapid Bactericidal Activity of Punica granatum L. Peel Extract: A Natural Alternative for Mastitis Prevention in Dairy Cattle" Molecules 30, no. 11: 2387. https://doi.org/10.3390/molecules30112387
APA StyleSilva, C. R. e. A., Vidal, C. S., Filho, S. M. d. A., Agatão, I. M., Berbert, L. C., Salles, J. B. d., Cardoso, A. M., Kuster, R. M., Victório, C. P., & Assis, M. C. d. (2025). Rapid Bactericidal Activity of Punica granatum L. Peel Extract: A Natural Alternative for Mastitis Prevention in Dairy Cattle. Molecules, 30(11), 2387. https://doi.org/10.3390/molecules30112387









