Optimized Solar-Simulated Photocatalysis of Congo Red Dye Using TiO2: Toward a Sustainable Water Treatment Approach
Abstract
1. Introduction
2. Results and Discussion
2.1. Irradiation Spectrum
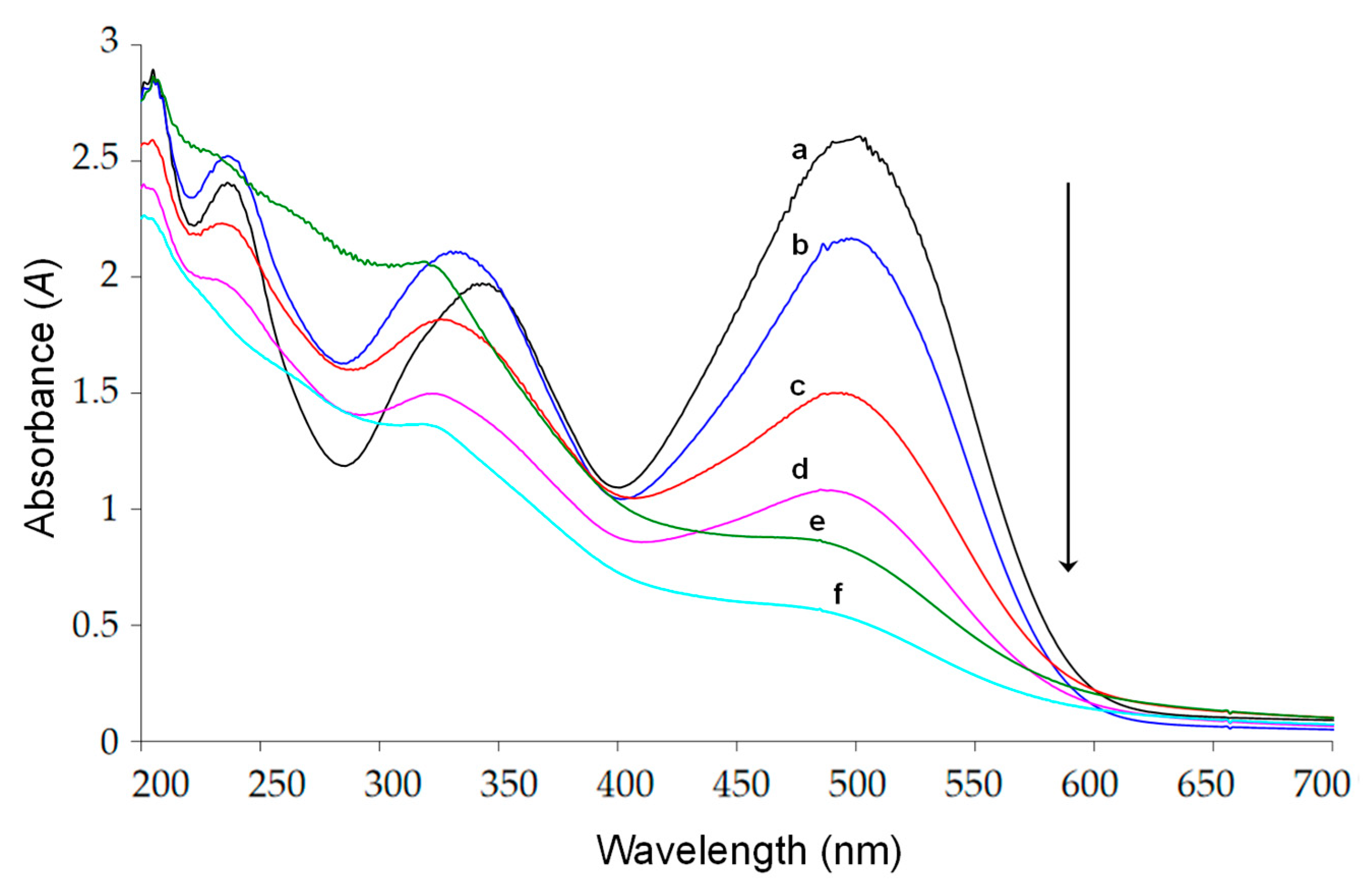
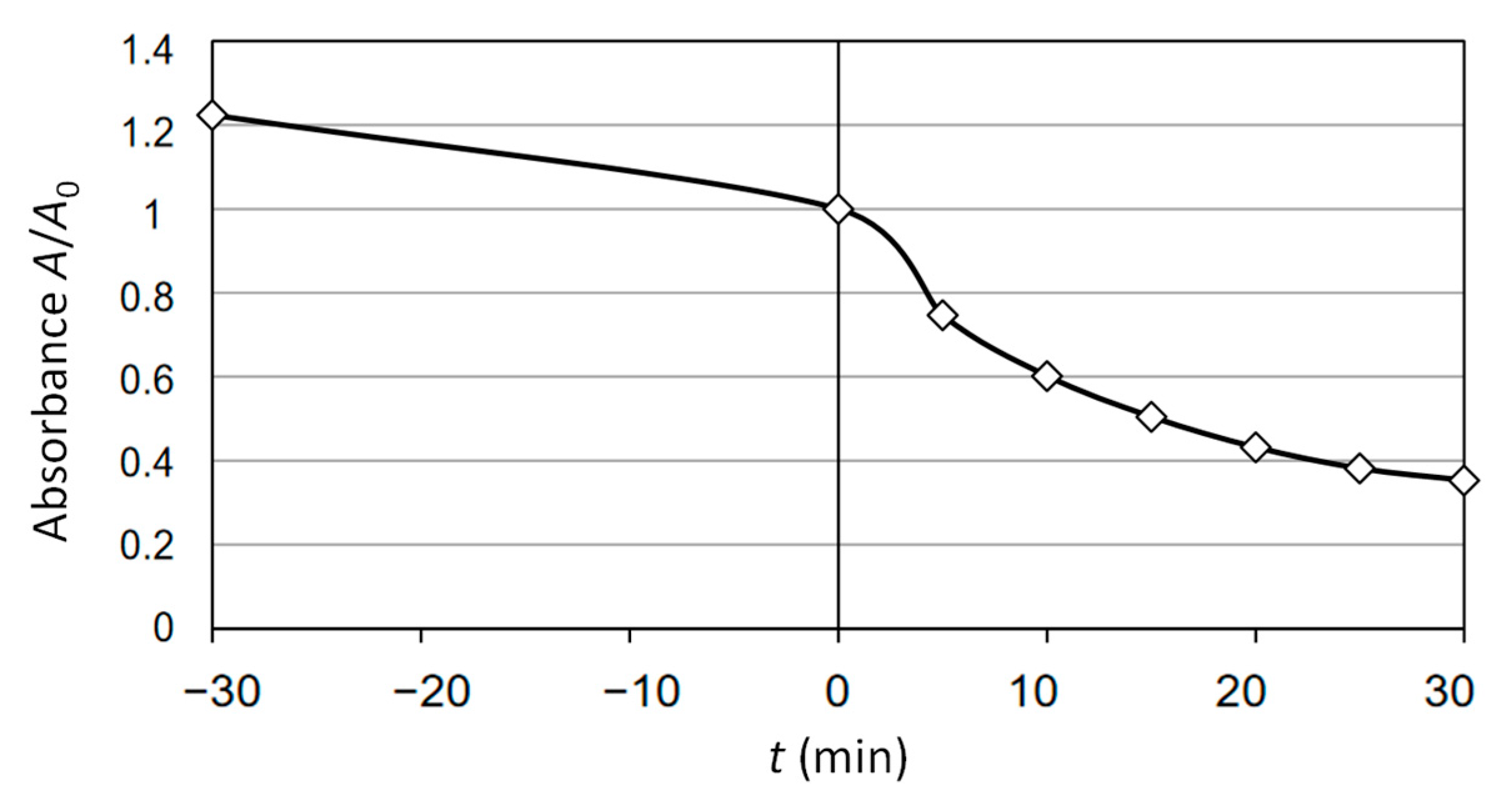
2.2. Absorbance and TOC
2.3. Results of ANOVA 2FI Model
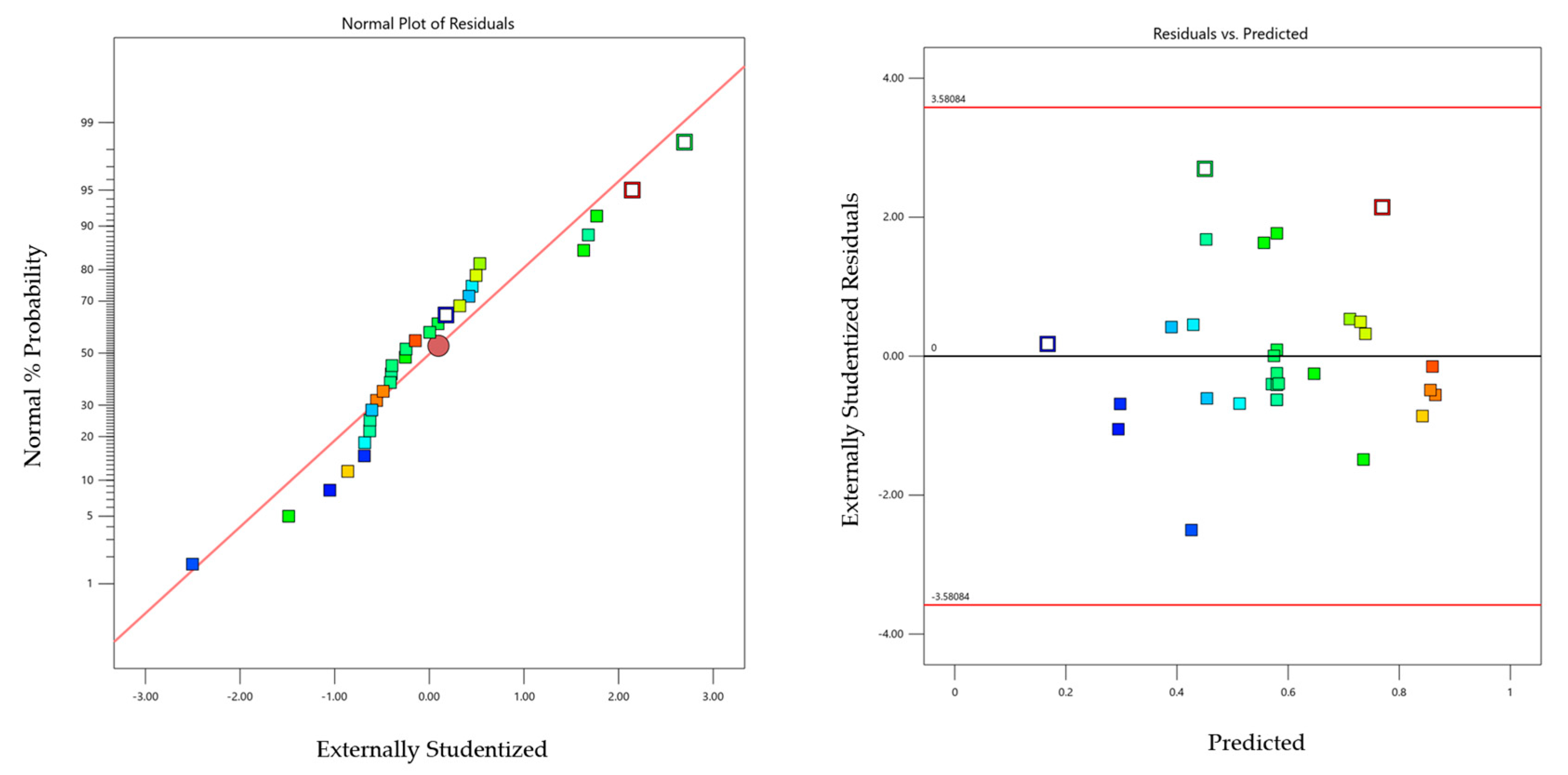
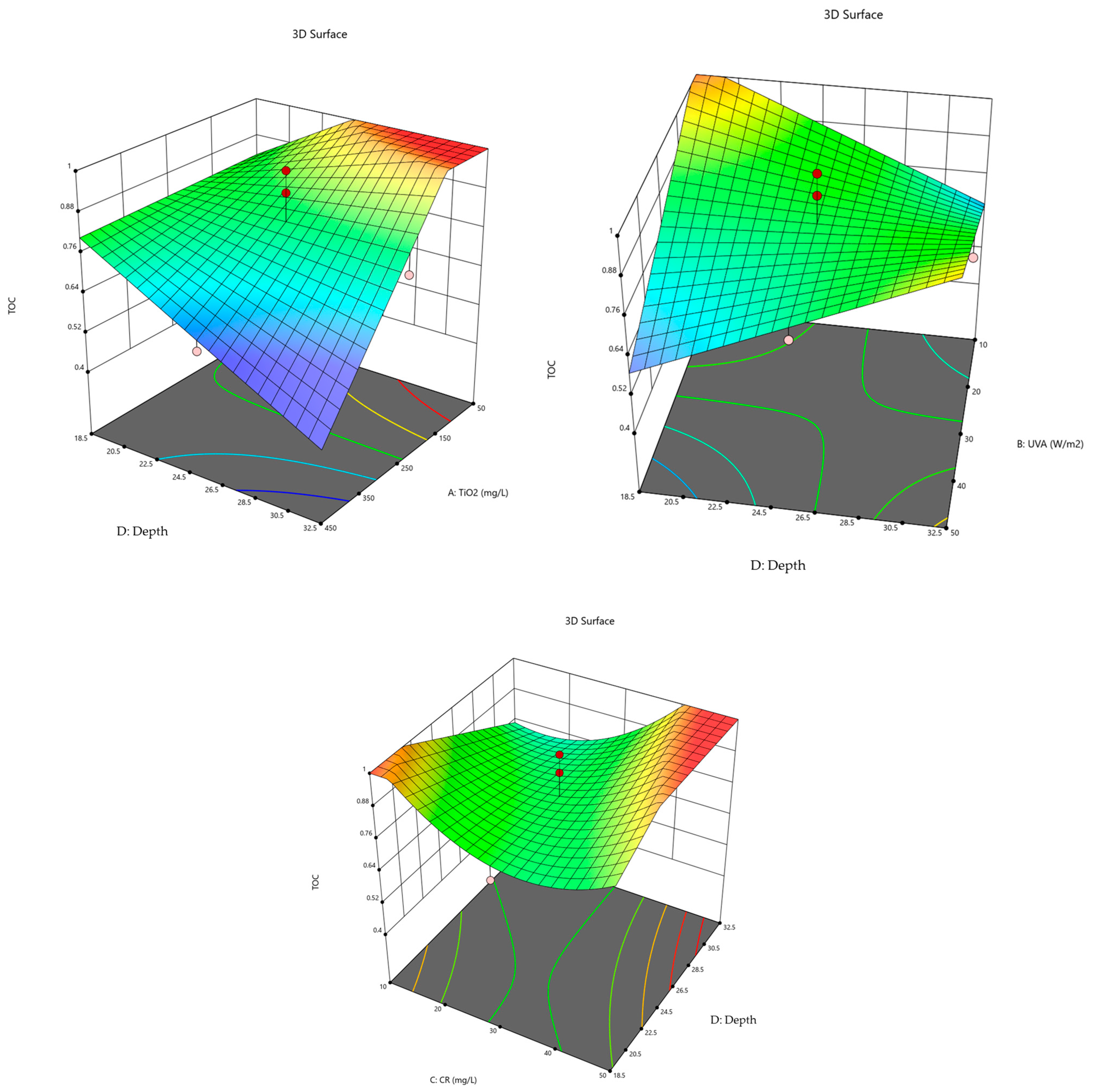
2.4. Model Diagnostics —Graphs
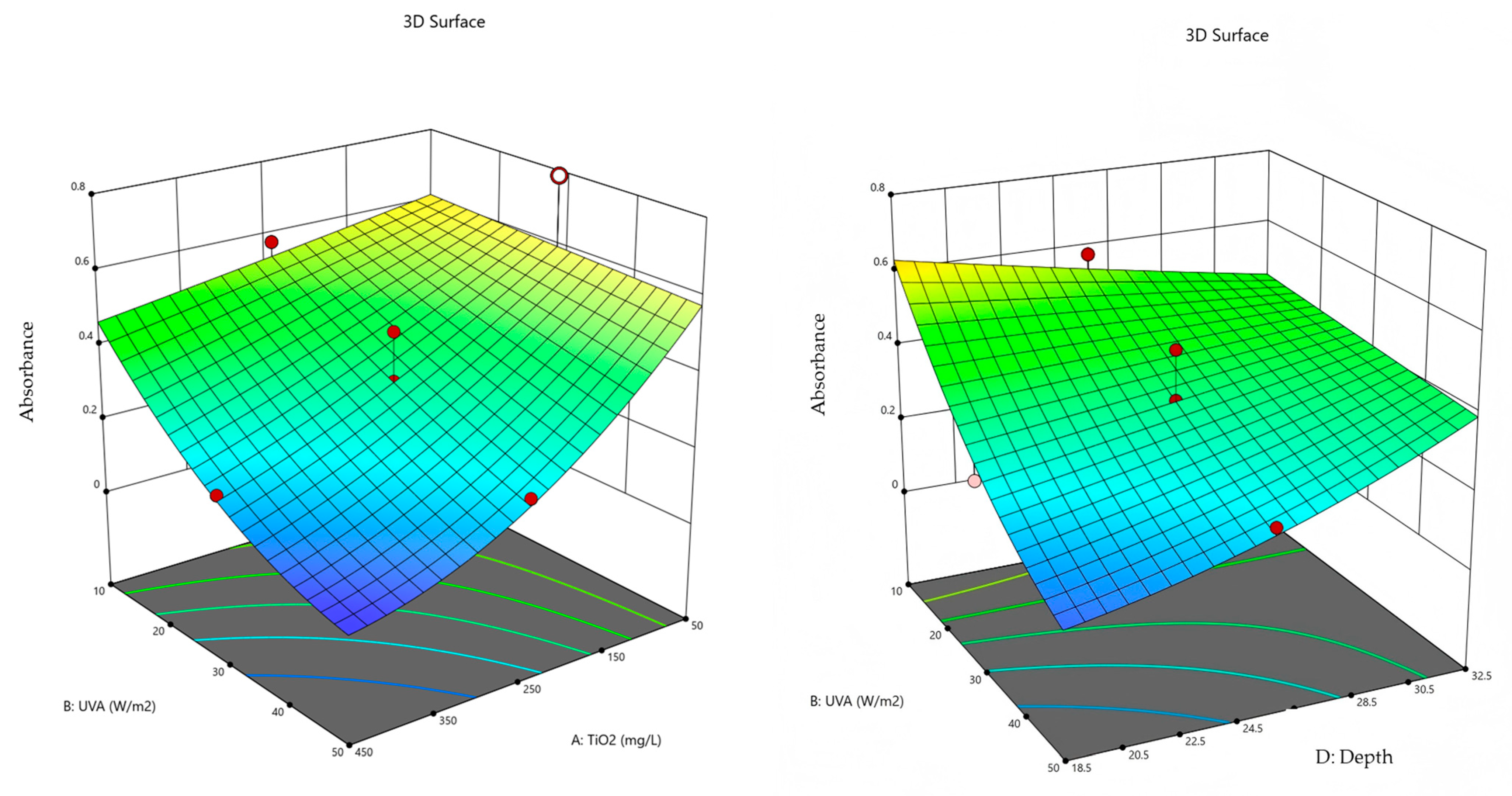
2.5. Conditions for Optimization
2.6. Comparison of Azo Dye Treatment Methods in Wastewaters
3. Materials and Methods
3.1. Methods
3.2. Materials
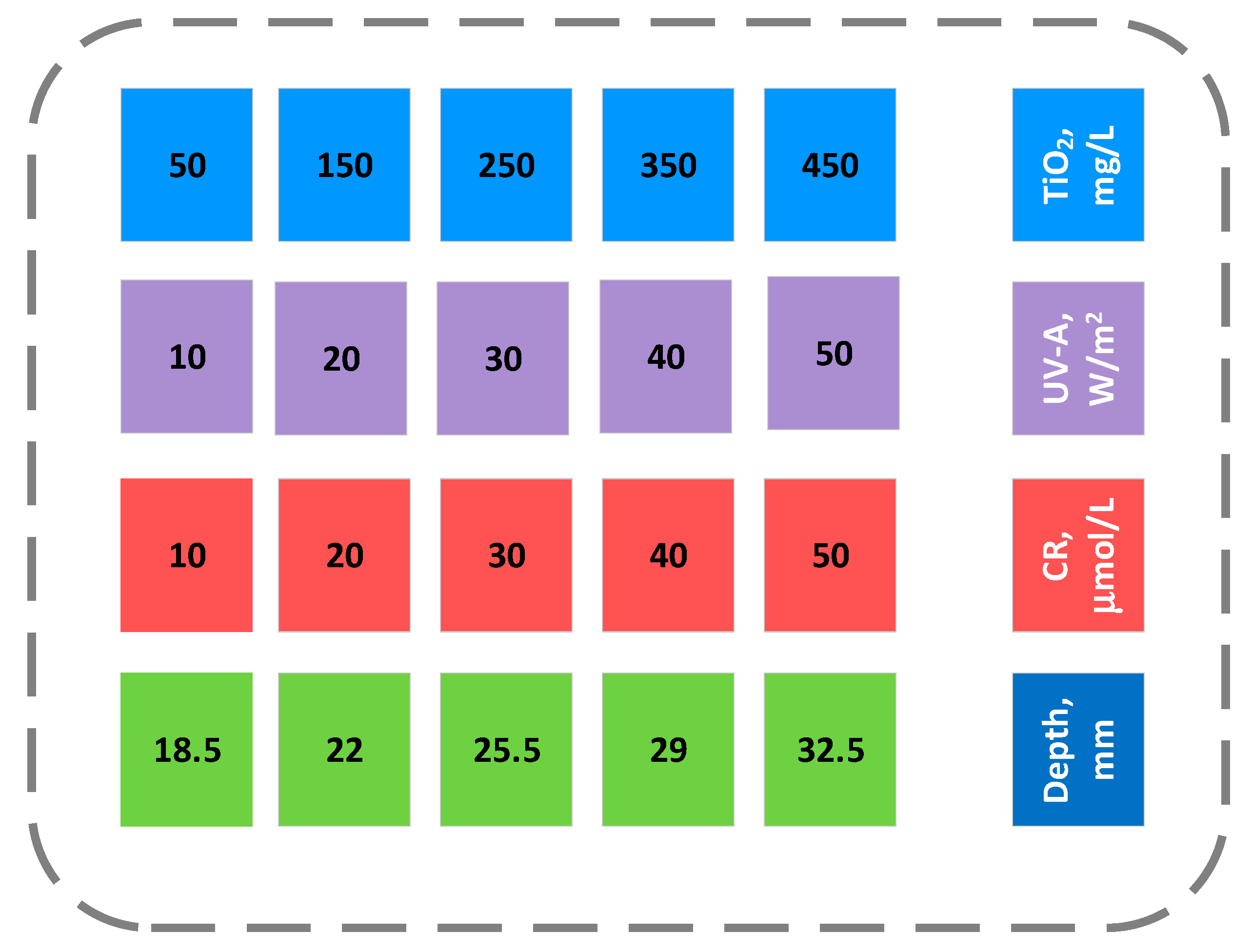
3.3. Experimental Section
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqui, S.I.; Allehyani, E.S.; Al-Harbi, S.A.; Hasan, Z.; Abomuti, M.A.; Rajor, H.K.; Oh, S. Investigation of Congo Red Toxicity towards Different Living Organisms: A Review. Processes 2023, 11, 807. [Google Scholar] [CrossRef]
- Hoang, N.T.T.; Nguyen, D.D. Improving the Degradation Kinetics of Industrial Dyes with Chitosan/TiO2/Glycerol Films for the Sustainable Recovery of Chitosan from Waste Streams. Sustainability 2023, 15, 6979. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Bamigboye, M.O.; Ogunbiyi, O.D.; Akano, M.T. Toxicity and decontamination strategies of Congo red dye. Groundw. Sustain. Dev. 2022, 19, 100844. [Google Scholar] [CrossRef]
- Periyasamy, A.P. Recent Advances in the Remediation of Textile-Dye-Containing Wastewater: Prioritizing Human Health and Sustainable Wastewater Treatment. Sustainability 2024, 16, 495. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Naseem, K.; Farooqi, Z.H.; Begum, R.; Irfan, A. Removal of Congo red dye from aqueous medium by its catalytic reduction using sodium borohydride in the presence of various inorganic nano-catalysts: A review. J. Clean. Prod. 2018, 187, 296–307. [Google Scholar] [CrossRef]
- Imran, M.; Shaharoona, B.; Crowley, D.; Khalid, A.; Hussain, S.; Arshad, M. The stability of textile azo dyes in soil and their impact on microbial phospholipid fatty acid profiles. Ecotoxicol. Environ. Saf. 2015, 120, 163–168. [Google Scholar] [CrossRef]
- Muruganandham, M.; Swaminathan, M. Solar photocatalytic degradation of a reactive azo dye in TiO2-suspension. Sol. Energy Mater. Sol. Cells 2004, 8, 439–457. [Google Scholar] [CrossRef]
- Bouberka, Z.; Kacha, S.; Kameche, M.; Elmaleh, S.; Derriche, Z. Sorption study of an acid dye from aqueous solutions using modified clays. J. Hazard. Mater. 2005, 119, 117–124. [Google Scholar] [CrossRef]
- Scott, J.P.; Ollis, D.F. Integration of chemical and biological oxidation process for water treatment: Review and recommendation. Environ. Prog. 1995, 14, 88–103. [Google Scholar] [CrossRef]
- Amagan, B.; Turan, M.; Celike, M. Equilibrium studies on the adsorption of reactive azo dyes into zeolite. Desalination 2004, 170, 33–39. [Google Scholar] [CrossRef]
- Hashemian, D. Study of adsorption of acid dye from aqueous solutions using bentonite. Main Group Chem. 2007, 6, 97–107. [Google Scholar] [CrossRef]
- Purkait, M.K.; Maiti, A.; DasGupta, S.; De, S. Removal of congo red using activated carbon and its regeneration. J. Hazard. Mater. 2007, 145, 287–295. [Google Scholar] [CrossRef]
- Dai, S.; Zhuang, Y.; Chen, Y.; Chen, L. Study on the relationship between structure of synthetic organic chemicals and their biodegradability. Environ. Chem. 1995, 14, 354–367. [Google Scholar]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical Processes for Water Treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- Goslich, R.; Dillert, R.; Bahnemann, D. Solar water treatment: Principles and reactors. Water Sci. Technol. 1997, 35, 137–148. [Google Scholar] [CrossRef]
- Vidal, A. Developments in solar photocatalysis for water purification. Chemosphere 1998, 36, 2593–2606. [Google Scholar] [CrossRef]
- Ljubas, D.; Franzreb, M.; Hansen, H.C.B.; Weidler, P.G. Magnetizing of nano-materials on example of Degussa’s P-25 TiO2 photocatalyst: Synthesis of magnetic aggregates, characterization and possible use. Sep. Purif. Technol. 2014, 136, 274–285. [Google Scholar] [CrossRef]
- Ljubas, D. Solar photocatalysis-a possible step in drinking water treatment. Energy 2005, 30, 1699–1710. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: Principles, Mechanisms and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Serpone, N.; Sauvé, G.; Koch, R.; Tahiri, H.; Pichat, P.; Piccinini, P.; Pelizzeti, E.; Hidaka, H. Standardization protocol of process efficiencies and activation parameters in heterogeneous photocatalysis: Relative photonic efficiencies ζr. J. Photochem. Photobiol. A 1996, 94, 191–203. [Google Scholar] [CrossRef]
- Ponnusamy, S.K.; Subramarian, R. Process optimization studies of Congo red dye adsorption onto cashew nut shell using response surface methodology. Int. J. Ind. Chem. 2013, 4, 17. [Google Scholar] [CrossRef]
- Cho, H.; Zoh, K.D. Photocatalytic degradation of azo dye (Reactive Red 120) in TiO2/UV system: Optimization and modeling using a response surface methodology (RSM) based on the central composite desig. Dyes Pigment. 2007, 75, 533–543. [Google Scholar] [CrossRef]
- Fathinia, M.; Khataee, A.R.; Zarei, M.; Aber, S. Comparative Photocatalytic Degradation of Two Dyes on Immobilized TiO2 Nanoparticles: Effect of Dye Molecular Structure and Response Surface Approach. J. Mol. Catal. A 2010, 333, 73–84. [Google Scholar] [CrossRef]
- Ngulube, K.F.; Abdelhaleem, A.; Fujii, M.; Nasr, M. Synergism of Artificial Intelligence and Techno-Economic for Sustainable Treatment of Methylene Blue Dye-Containing Wastewater by Photocatalysis. Sustainability 2024, 16, 529. [Google Scholar] [CrossRef]
- Korbahti, K.; Rauf, M.A. Application of Response Surface Analysis to the Photolytic Degradation of Basic Red 2 Dye. Chem. Eng. J. 2008, 138, 166–171. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.C.; Zakaria, R.; Ying, J.Y. Role of Particle Size in Nanocrystalline TiO2-Based Photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Sanchez Tobon, C.; Panžić, I.; Bafti, A.; Matijašić, G.; Ljubas, D.; Ćurković, L. Rapid Microwave-Assisted Synthesis of N/TiO2/rGO Nanoparticles for the Photocatalytic Degradation of Pharmaceuticals. Nanomaterials 2022, 12, 3975. [Google Scholar] [CrossRef]
- Guimarães, R.R.; Parussulo, A.L.A.; Toma, H.E.; Araki, K. Enlightening the synergic effect of anatase/rutile mixtures in solar cells. Electrochim. Acta 2016, 188, 523–528. [Google Scholar] [CrossRef]
- Kulišić, P. New Energy Sources: Solar Energy and Wind Energy (Novi Izvori Energije: Sunčana Energija i Energija Vjetra); Školska knjiga: Zagreb, Croatia, 1991. (In Croatian) [Google Scholar]
- Guo, J.; Zhou, T.; Guo, H.; Ge, C.; Lu, J. Application of nano-TiO2@adsorbent composites in the treatment of dye wastewater: A review. J. Eng. Fibers Fabr. 2025, 20, 1558–9250. [Google Scholar]
- Sanchez Tobon, C.; Ljubas, D.; Mandić, V.; Panžić, I.; Matijašić, G.; Ćurković, L. Microwave-Assisted Synthesis of N/TiO2 Nanoparticles for Photocatalysis under Different Irradiation Spectra. Nanomaterials 2022, 12, 1473. [Google Scholar] [CrossRef]
- Patil, S.R.; Akpan, U.G.; Hameed, S.K.; Samdarshi, S.K. A comparative study of the photocatalytic efficiency of Degussa P25, Qualigens, and Hombikat UV-100 in the degradation kinetic of congo red dye. Desalination Water Treat. 2012, 46, 188–195. [Google Scholar] [CrossRef]
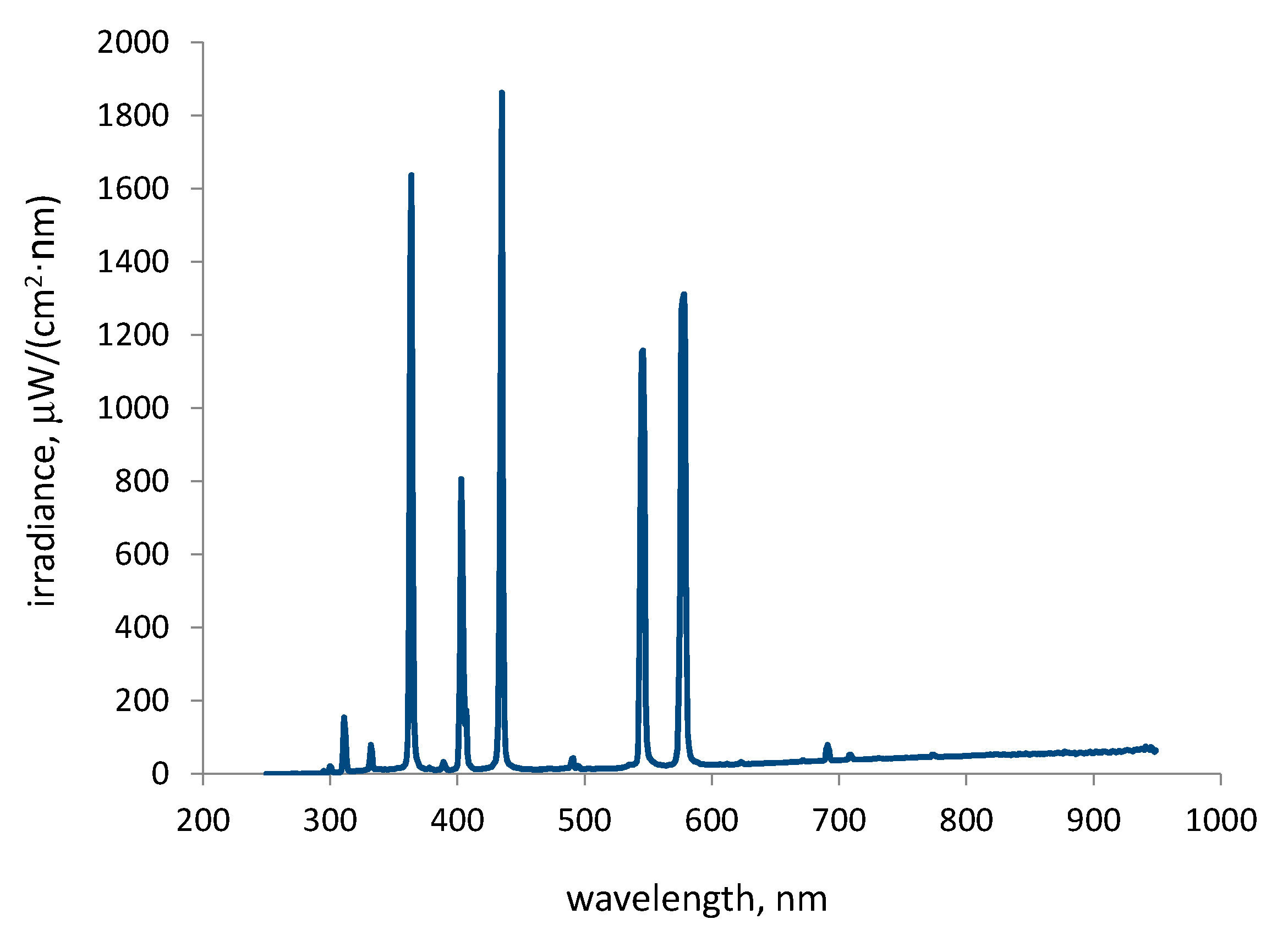

| Irradiation Range | Total Irradiance in the Range, W/m2 |
|---|---|
| UV C: 250–289 nm | 0.173 |
| UV B: 290–319 nm | 4.92 |
| UV A: 320–399 nm | 56.63 |
| VIS: 400–750 nm | 268.20 |
| Run | A(498)0 | TOC0, mg/L | A30/A0 | TOC30/TOC0 |
|---|---|---|---|---|
| 1 | 0.51833 | 5.007 | 0.09241 | 0.922 |
| 2 | 0.72360 | 6.198 | 0.20830 | 0.747 |
| 3 | 0.76529 | 9.047 | 0.39899 | 0.761 |
| 4 | 0.78161 | 8.353 | 0.33588 | 0.986 |
| 5 | 0.83306 | 8.689 | 0.34327 | 0.903 |
| 6 | 1.2368 | 12.875 | 0.63190 | 1.000 |
| 7 | 1.2215 | 8.792 | 0.54837 | 0.845 |
| 8 | 0.40222 | 3.024 | 0.03135 | 0.686 |
| 9 | 1.0387 | 6.873 | 0.78627 | 1.000 |
| 10 | 0.52965 | 4.842 | 0.41176 | 1.000 |
| 11 | 0.79584 | 6.371 | 0.22289 | 0.763 |
| 12 | 0.49248 | 3.535 | 0.30121 | 0.853 |
| 13 | 1.4572 | 9.955 | 0.69174 | 1.000 |
| 14 | 0.70445 | 5.220 | 0.30540 | 0.783 |
| 15 | 0.70198 | 4.989 | 0.17247 | 0.581 |
| 16 | 0.78837 | 5.689 | 0.31761 | 0.791 |
| 17 | 0.94556 | 7.634 | 0.57289 | 0.819 |
| 18 | 0.80515 | 5.957 | 0.29005 | 0.781 |
| 19 | 0.07364 | 0.975 | 0.05423 | 1.000 |
| 20 | 1.0348 | 7.361 | 0.31514 | 0.867 |
| 21 | 0.5229 | 4.478 | 0.33033 | 0.883 |
| 22 | 0.73002 | 6.207 | 0.29018 | 0.968 |
| 23 | 1.0598 | 8.107 | 0.43202 | 0.856 |
| 24 | 0.3419 | 3.813 | 0.06777 | 0.632 |
| 25 | 0.34969 | 4.371 | 0.17695 | 0.581 |
| 26 | 1.4261 | 10.4515 | 0.72512 | 1.000 |
| 27 | 0.82627 | 9.160 | 0.47506 | 0.806 |
| 28 | 0.86167 | 8.704 | 0.57740 | 0.816 |
| 29 | 1.2639 | 9.635 | 0.68785 | 1.000 |
| 30 | 0.95448 | 8.572 | 0.291583 | 0.739 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 0.9019 | 6 | 0.1503 | 34.74 | <0.0001 | significant |
| A-TiO2 | 0.2157 | 1 | 0.2157 | 49.85 | <0.0001 | |
| B-UVA | 0.1359 | 1 | 0.1359 | 31.40 | <0.0001 | |
| C-CR | 0.4881 | 1 | 0.4881 | 112.80 | <0.0001 | |
| D-Depth | 0.0270 | 1 | 0.0270 | 6.23 | 0.0201 | |
| AB | 0.0191 | 1 | 0.0191 | 4.41 | 0.0470 | |
| BD | 0.0161 | 1 | 0.0161 | 3.73 | 0.0660 | |
| Residual | 0.0995 | 23 | 0.0043 | |||
| Lack of Fit | 0.0831 | 18 | 0.0046 | 1.41 | 0.3761 | not significant |
| Pure Error | 0.0164 | 5 | 0.0033 | |||
| Cor Total | 1.00 | 29 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 0.4382 | 8 | 0.0548 | 10.22 | <0.0001 | significant |
| A-TiO2 | 0.2520 | 1 | 0.2520 | 47.00 | <0.0001 | |
| B-UVA | 0.0069 | 1 | 0.0069 | 1.29 | 0.2696 | |
| C-CR | 0.0242 | 1 | 0.0242 | 4.51 | 0.0463 | |
| D-Depth | 0.0001 | 1 | 0.0001 | 0.0232 | 0.8806 | |
| AD | 0.0394 | 1 | 0.0394 | 7.35 | 0.0135 | |
| BD | 0.0348 | 1 | 0.0348 | 6.50 | 0.0191 | |
| CD | 0.0256 | 1 | 0.0256 | 4.78 | 0.0409 | |
| C2 | 0.0275 | 1 | 0.0275 | 5.13 | 0.0348 | |
| Residual | 0.1073 | 20 | 0.0054 | |||
| Lack of Fit | 0.0767 | 15 | 0.0051 | 0.8383 | 0.6412 | not significant |
| Name | Goal | Lower Limit | Upper Limit | Lower Weight | Upper Weight | Importance |
|---|---|---|---|---|---|---|
| A:TiO2 | minimize | 150 | 350 | 1 | 1 | 3 |
| B:UVA | minimize | 20 | 40 | 1 | 1 | 3 |
| C:CR | is target = 25 | 20 | 40 | 1 | 1 | 3 |
| D:Depth | is in range | 22 | 29 | 1 | 1 | 3 |
| Absorbance | minimize | 0.031356 | 0.786271 | 1 | 1 | 3 |
| TOC | minimize | 0.581512 | 1.07353 | 1 | 1 | 3 |
| Number | TiO2 | UV-A | CR | Depth | Absorbance | TOC | Desirability | |
|---|---|---|---|---|---|---|---|---|
| 1 | 222.372 | 20.000 | 25.000 | 29.000 | 0.367 | 0.805 | 0.674 | Selected |
| 2 | 222.075 | 20.000 | 24.997 | 29.000 | 0.367 | 0.806 | 0.674 | |
| 3 | 218.487 | 20.000 | 25.000 | 29.000 | 0.370 | 0.811 | 0.674 | |
| 4 | 226.254 | 20.000 | 25.000 | 29.000 | 0.364 | 0.799 | 0.674 | |
| 5 | 216.159 | 20.000 | 25.000 | 29.000 | 0.371 | 0.815 | 0.674 |
| Water Treatment Method | Advantages and Positive Aspects | Drawbacks and Limitations |
|---|---|---|
| Adsorption (on granulated or powdered activated carbon) | Provides effective dye removal from wastewater; the treatment is easy to implement and represents a well-established and widely adopted approach. | Efficiency depends on the properties of the adsorbent and environmental conditions; there is a risk of secondary pollution generation. |
| Membrane Separation (ultrafiltration, nanofiltration, reverse osmosis) | Capable of removing various dye types, allows selective separation, and occupies a relatively limited spatial footprint. | Installation and operational costs are high; it requires specific conditions such as pressurization; it is susceptible to clogging and has a limited operational lifespan; and there is a problem with concentrated flow with pollutants. |
| Coagulation and flocculation | A straightforward process requiring low-cost equipment; especially effective for hydrophobic dyes. | Generates significant amounts of sludge that are difficult to manage; less suitable for hydrophilic dye removal, equipment material can easily corrode. |
| Photocatalysis | Efficient in degrading persistent dye molecules, enabling complete breakdown; characterized by high energy efficiency if natural solar radiation is used | Separation of photocatalyst; if the process uses suspended particles, intermediate products can be more toxic after treatment than the parent product |
| Biological treatment (biodegradation by microorganisms) | Enables selective biodegradation via microorganisms, with minimal ecological toxicity. | Ineffective for high-concentration dye wastewaters or those with poor light penetration; slow process dependent on specific environmental conditions. |
| Run | TiO2, mg/L | UV-A, W/m2 | CR, μmol/L | Depth, mm |
|---|---|---|---|---|
| 1 | 150 | 40 | 20 | 22 |
| 2 | 250 | 50 | 30 | 25.5 |
| 3 | 250 | 30 | 30 | 32.5 |
| 4 | 350 | 20 | 20 | 22 |
| 5 | 250 | 30 | 30 | 25.5 |
| 6 | 150 | 40 | 40 | 29 |
| 7 | 150 | 40 | 40 | 22 |
| 8 | 350 | 40 | 20 | 22 |
| 9 | 50 | 30 | 30 | 25.5 |
| 10 | 150 | 40 | 20 | 29 |
| 11 | 250 | 30 | 30 | 18.5 |
| 12 | 150 | 20 | 20 | 22 |
| 13 | 250 | 30 | 50 | 25.5 |
| 14 | 250 | 30 | 30 | 25.5 |
| 15 | 450 | 30 | 30 | 25.5 |
| 16 | 250 | 30 | 30 | 25.5 |
| 17 | 350 | 20 | 40 | 29 |
| 18 | 250 | 30 | 30 | 25.5 |
| 19 | 250 | 30 | 10 | 25.5 |
| 20 | 350 | 40 | 40 | 29 |
| 21 | 150 | 20 | 20 | 29 |
| 22 | 250 | 30 | 30 | 25.5 |
| 23 | 350 | 20 | 40 | 22 |
| 24 | 350 | 40 | 20 | 29 |
| 25 | 350 | 20 | 20 | 29 |
| 26 | 150 | 20 | 40 | 29 |
| 27 | 250 | 30 | 30 | 25.5 |
| 28 | 250 | 10 | 30 | 25.5 |
| 29 | 150 | 20 | 40 | 22 |
| 30 | 350 | 40 | 40 | 22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ljubas, D.; Vučemilović, A.; Briševac, D.; Cajner, H.; Juretić, H. Optimized Solar-Simulated Photocatalysis of Congo Red Dye Using TiO2: Toward a Sustainable Water Treatment Approach. Molecules 2025, 30, 2388. https://doi.org/10.3390/molecules30112388
Ljubas D, Vučemilović A, Briševac D, Cajner H, Juretić H. Optimized Solar-Simulated Photocatalysis of Congo Red Dye Using TiO2: Toward a Sustainable Water Treatment Approach. Molecules. 2025; 30(11):2388. https://doi.org/10.3390/molecules30112388
Chicago/Turabian StyleLjubas, Davor, Ante Vučemilović, Debora Briševac, Hrvoje Cajner, and Hrvoje Juretić. 2025. "Optimized Solar-Simulated Photocatalysis of Congo Red Dye Using TiO2: Toward a Sustainable Water Treatment Approach" Molecules 30, no. 11: 2388. https://doi.org/10.3390/molecules30112388
APA StyleLjubas, D., Vučemilović, A., Briševac, D., Cajner, H., & Juretić, H. (2025). Optimized Solar-Simulated Photocatalysis of Congo Red Dye Using TiO2: Toward a Sustainable Water Treatment Approach. Molecules, 30(11), 2388. https://doi.org/10.3390/molecules30112388








