Abstract
Centaurea calcitrapa is a well-known plant with antioxidant, anti-proliferative, and antimicrobial properties. The plant contains various phenolic compounds, flavonoids, and other bioactive molecules contributing to its medicinal properties. However, little is known about its antidiabetic activity. The study's purpose is the isolation and identification of active compounds of C. calcitrapa aerial parts in diabetic rats induced by streptozotocin. The ethyl acetate extract (E2) was separated into eight subfractions by column chromatography. The subfractions were evaluated for their antidiabetic activity using diabetic-induced rats. The most active subtraction was purified, and the active compounds were identified using UV spectrophotometry, Fourier Transform Infrared Spectroscopy, Mass spectrophotometry, and HPLC. Subfraction E2-VIII showed the most effective reduction in blood glucose levels, comparable to metformin. In HPLC analysis, subfraction E2-VIII showed three main compounds: nepetin, kaempferide, and Luteolin. The nepetin flavonoid was examined using molecular docking, and it showed a high affinity to α-amylase. In conclusion, the aerial parts of C. calcitrapa extract and isolated compounds especially nepetin present promising antidiabetic agents this is probably mediated by its strong antioxidants and α-amylase inhibitory effect.
1. Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder characterized by persistent elevated blood glucose levels and alterations in insulin levels or action. Type 2 diabetes mellitus (T2DM) is the predominant form [1]. T2DM has a considerable burden on the healthcare system; in 2019, the International Diabetes Federation (IDF) reported that diabetes resulted in 4.2 million fatalities, with 463 million people aged 20 to 79 years living with the condition, a figure projected to increase to 700 million by 2045. The incidence and prevalence of T2DM differ by geographical region, with over 80% of patients residing in low- to middle-income nations presenting further obstacles to effective treatment [2].
The pathophysiology of T2DM is attributed to a combination of two principal factors: impaired insulin production by pancreatic β-cells and the inadequate responsiveness of insulin-sensitive tissues to insulin [2], in which a disruption in the feedback mechanisms between insulin activity and secretion leads to elevated blood glucose levels [3,4]. In case of β-cell malfunction, insulin production diminishes, constraining the body’s ability to regulate physiological glucose levels. Meanwhile, insulin resistance (IR) leads to heightened glucose synthesis in the liver and diminished glucose absorption in muscle, liver, and adipose tissue. Although both mechanisms occur early in pathogenesis and contribute to disease progression, β-cell dysfunction is typically more pronounced than IR. Nonetheless, the coexistence of β-cell malfunction and insulin resistance exacerbates hyperglycemia, facilitating the advancement of T2DM [5,6,7].
The persistent and extensive elevation of reactive oxygen species (ROS) plays a crucial role in the pathophysiology of T2DM and IR. A pro-oxidant environment results in mitochondrial malfunction, endoplasmic reticulum stress, NADPH oxidase (NOX) activation, and superoxide formation. The elevation of ROS production stimulates the five principal pathways implicated in the pathogenesis of diabetes complications: augmentation of the polyol pathway, heightened formation of advanced glycation end-products (AGEs), increased expression of AGEs receptors and their activating ligands, activation of protein kinase C (PKC) isoforms, and hyperactivity of the hexosamine pathway [8,9]. Increased intracellular ROS via these pathways leads to impaired angiogenesis in response to ischemia, activates many proinflammatory pathways, and induces constant epigenetic modifications that sustain the expression of proinflammatory genes even when hyperglycemia is resolved [10].
Despite the availability of various therapeutic options to treat T2DM, it still lacks a definitive cure. Consequently, scientists are actively exploring multiple methods to treat and prevent diabetes. One of the methods used is traditional and complementary medicine [11]. The World Health Organization (WHO) has defined traditional medicine, stating that it encompasses the comprehensive understanding of health practices and abilities rooted in indigenous beliefs and experiences. Complementary medicine, on the other hand, refers to a range of health practices that are not considered part of a particular country’s traditional medicine [11].
The Asteraceae family is among the largest flowering plant families, comprising approximately 20,000 species. The Centaurea genus is a prominent group within this family, comprising between 250 and 700 species. These species are herbaceous annuals, biennials, and perennials, and are widely distributed worldwide, especially in the Mediterranean region, Western Asia, and the Americas. The Asteraceae family consists of four main subfamilies, three of which are present in Iraq [12,13]. Centaurea species have been utilized for medicinal use for centuries, including antibacterial, anti-inflammatory, antipyretic, antirheumatic, antidiarrheal, and cytotoxic properties. The leaves and shoots of Stepposa Wagenitz., C. urvillei DC. spp., C. triumfettii All, C. calcitrapa L., and C. pullata L. have traditionally been included in the diet, either in their raw form or after processing. Additionally, certain species are utilized to produce drinks and tonics [14,15,16]. Phytochemical analyses of Centaurea species have revealed the existence of several natural chemicals that display diverse biological functions [14,17,18]. The genus Centaurea contains several active components, such as phenolic acids, sesquiterpene lactones, steroids, and flavonoids. These components contribute to the genus’s wide range of biological activities [19].
Centaurea calcitrapa (known as purple starthistle) is a biennial, herbaceous plant that typically reaches a height of 60 cm. This plant is traditionally used to manage several diseases, including ophthalmic disorders, as well as antipyretic, hepatic, gastrointestinal, and dermatological diseases [20,21]. Prior studies on its extracts demonstrated promising potential for biological action. The aqueous and methanol (MeOH) extracts demonstrated potent antioxidant activity. Additionally, the MeOH extract has shown considerable cytotoxic action against HeLa (human cervical cancer) and Vero (epithelial cells of African green monkey kidney) cell lines [22]. Phytochemical investigations have identified sterols, sesquiterpene lactones, lignans, bisabolenes, triterpenoids, and flavonoids as components of C. calcitrapa extracts [23,24,25]. The MeOH extract showed an antidiabetic effect attributed to its α-glucosidase inhibitory effect [26].
The molecular docking method is a bioinformatics model that examines protein-ligand interactions at the atomic scale. This interaction resembles the lock-and-key approach, utilized to identify target structures for protein active sites and clarify the possible mechanism of action. Conversely, ligands can associate with proteins via several interactions, namely hydrogen bonds, hydrophobic interactions, van der Waals forces, and salt bridges, and are defined by their binding affinity [27,28].
The current study addressed several knowledge gaps regarding C. calcitrapa as an antidiabetic treatment. First, to elucidate if C. calcitrapa works as an antidiabetic agent, the specific bioactive compounds in C. calcitrapa that are responsible for its antidiabetic effects, the mechanism by which the plant’s compounds lower blood glucose levels, and assess the safety of C. calcitrapa. In the current study, we undertook bioassay-guided isolation and active compound identification in diabetic rats induced by streptozotocin. Furthermore, a molecular docking study will be performed for the most active compound isolated.
2. Results
2.1. Centaurea calcitrapa Extracts Ameliorate Renal, Hepatic, and Oxidative Stress Changes Induced by Streptozotocin
At the end of the experimental phase in the first experiment, serum levels of various biomarkers were assessed to examine the effect of C. calcitrapa extracts on liver function, kidney function, and oxidative stress changes induced by streptozocin. All extracts showed a significant reduction in the levels of MDA and CAT activity compared to the induction group and a significant increase in the levels of SOD compared to the induction group (Figure 1A–C). Ethyl acetate extract (E-2) of C. calcitrapa showed the best ameliorative effect on oxidative stress, showing no significant differences compared to the metformin group (Figure 1A–C).
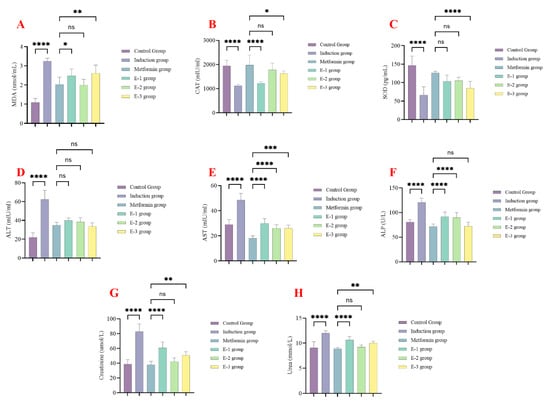
Figure 1.
Centaurea calcitrapa extracts affect oxidative stress, liver enzymes, and kidney function markers. (A) MDA levels, (B) catalase levels, (C) SOD levels, (D) ALT levels, (E) AST levels, (F) ALP levels, (G) creatinine levels, and (H) urea levels. * Indicate p-value < 0.05, ** indicate p-value < 0.01, *** indicate p-value < 0.001, **** indicate p-value < 0.0001, ns indicate p-value > 0.05.
All extracts significantly reduced ALT, AST, and ALP levels compared to the induction group, with comparable levels seen in the metformin group (Figure 1D–F). All extracts significantly reduced serum urea and creatinine compared to the induction group (Figure 1G,H). Extract E-2 demonstrated the most significant reduction in serum urea and creatinine compared to the metformin group. These findings collectively indicate that extract E-2 exhibited the best safety profile among the extracts.
2.2. Antidiabetic Properties of C. calcitrapa Extracts on Diabetic Rats Induced by Streptozotocin
In the first experiment, rats were followed up after 15 and 30 days to examine changes in fasting blood glucose (FBG) and body weight. After 30 days, all rats in the extract groups showed no significant difference in body weight compared to the metformin group (Figure 2A).
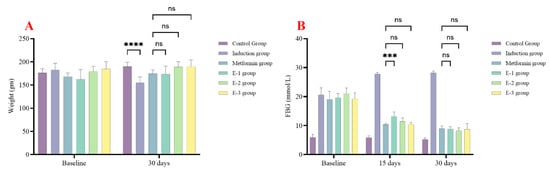
Figure 2.
Centaurea calcitrapa extracts affect rat weight and glycemic control. (A) rat weight, (B) FBG at baseline, after 15 days, and 30 days. *** indicate p-value < 0.001, **** indicate p-value < 0.0001, ns indicate p-value > 0.05.
Regarding glycemic control, after 15 days, only extracts E-2 and E-3 showed no significant difference compared to the metformin group. However, after 30 days, all extracts demonstrated no significant difference compared to the metformin group. At all times (15 and 30 days), all extracts showed significantly lower FBG levels compared to the induction group, as seen in Figure 2B.
As a result, since E-2 extracts showed the best safety profile and glycemic control, E-2 extract was selected for further bioassay.
2.3. Antidiabetic Properties of E-2 Subfractions on Diabetic Rats Induced by Streptozotocin
After selecting the E-2 extract, it was further isolated by column chromatography, yielding eight subfractions. These subfractions were subjected to animal experimentation to determine the best antidiabetic properties. After 8, 15, 22, and 30 days, the E-2VIII subfraction showed no significant difference in FBG levels compared to the metformin group, which indicates that this subfraction contains most of the antidiabetic activity of C. calcitrapa, as seen in Figure 3. Subsequently, subfraction E-2VIII underwent purification and identification of its active phytochemical components.
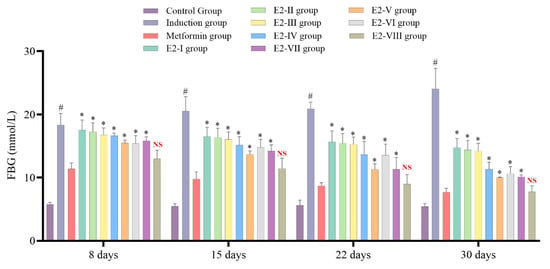
Figure 3.
Centaurea calcitrapa subfraction effect on glycemic control after 8, 15, 22, and 30 days. * Indicates significant difference (p-value < 0.05) compared to the Metformin group, # Indicates significant difference (p-value < 0.05) compared to the Control group, and NS indicates p-value > 0.05.
2.4. Purification of E2-VIII Subfraction by HPLC
The HPLC purification of E2-VIII identified three compounds: compound P-1 showed a peak at 3.0 min, compound P-2 showed a peak at 4.95 min, and compound P-3 showed a peak at 5.95 min, as shown by the three curves in Figure 4. Furthermore, we utilized HPLC to separate these three compounds for further analysis to elucidate their chemical structures.
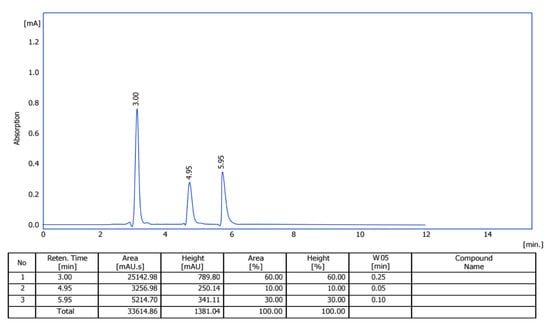
Figure 4.
HPLC diagram of subfraction E2-VIII.
2.5. Calibration of Nepetin, Kaempferide, and Luteolin Compounds by HPLC
2.6. Structural Analysis of E2-VIII Subfractions
The HPLC chromatography’s separation technique separated E2-VIII into three portions; these portions undergo UV spectroscopy, Fourier-transform infrared spectroscopy (FT-IR), and mass spectrometry (MS), elucidating their chemical structure, then confirm it by comparing each one with HPLC with 10 standard compounds (Scutellarin, Luteolin, Nepetin, Apigenin, Kaempferol, Chryseriol, Jaceidin, Kaempferide, Eupatolin, and Centaureidin), as seen in Table 1.

Table 1.
Structural analysis of E2-VIII subfractions.
Compound I (P-1): UV-Vis Spectroscopy (273 nm and 351 nm): These absorbance wavelengths suggest the presence of a benzoyl peak 220–280 nm and a cinnamoyl peak 300–400 nm reference (Figure 5A).
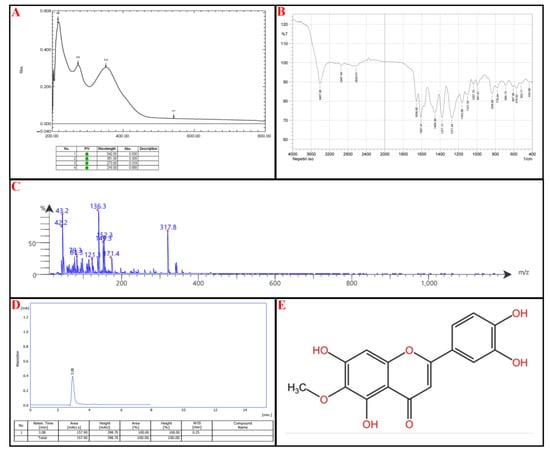
Figure 5.
Identification of subfraction E2-VIII portion P-1, (A) UV spectrophotometry, (B) FT-IR, (C) MS-spectrometry, (D) HPLC, (E) chemical structure.
FTIR Analysis: 3407 cm−1: Indicates the presence of an O-H stretch, from hydroxyl groups. Notably, 2947 cm−1: Suggests aromatic C-H stretching. Notably, 1657 cm−1 and 1607 cm−1: Strong evidence of C=O (carbonyl) and C=C (aromatic) stretches. Notably, 1456 cm−1 and 1377 cm−1: Points to C-H bending vibrations, characteristic of methyl (-CH₃) or methylene (-CH₂-) groups. Notably, 1271–1037 cm−1: Suggests the presence of C-O stretching (Figure 5B).
Mass Spectrometry (MS) Analysis: Parent Peak: 317.8 m/z: Indicates the compound’s molecular weight (M + H + 1), suggesting a relatively large structure. Notably, 316.8 (M + 1) and the molecular fragments 171.4; [M + H-C9H6O2] 149.3; M + H-C7H4O5, 136.3 (Figure 5C).
Based on the spectral data and the listed flavonoids’ structural characteristics, the most likely candidate is Nepetin or Jaceidin. The UV-Vis absorption suggests a flavonoid backbone with extended conjugation, likely a methoxylated flavone; Nepetin and Jaceidin exhibit similar UV profiles due to their conjugated systems. Based on FTIR analysis: 3407 cm−1 (O-H stretch): Present in flavonoids. Notably, 1657 cm−1 and 1607 cm−1 (C=O and C=C stretches): Strong indicators of flavone structures. Notably, 1271–1037 cm−1 (C-O stretches): Suggests ether or ester functionalities common in methoxylated flavones. Based on MS analysis, this molecular weight aligns closely with Nepetin (MW: 316.3 g/mol) and Jaceidin (MW: 330.3 g/mol). Fragmentation patterns (171.4, 152.3, 152.3, 149.3, 136.3, etc.) suggest a breakdown of a flavone core with methoxy substitutions.
The final step relied on HPLC; the isolated compound peaks at a retention time (Rt) of 3.08 min, comparable to the standard nepetin with an Rt of 3.08 min, as seen in Figure 5D. The chemical structure of nepetin, according to the International Union of Pure and Applied Chemistry (IUPAC), is 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-6-methoxy-4H-1-benzopyran-4-one, which is illustrated in Figure 5E (for reference standard of each analytical procedure, see Supplementary Material Figure S2).
Compound II: UV-Vis Spectroscopy (269 nm and 364 nm): These absorbance wavelengths suggest the presence of a benzoyl peak 220–280 nm and a cinnamoyl peak 300–400 nm reference (Figure 6A).
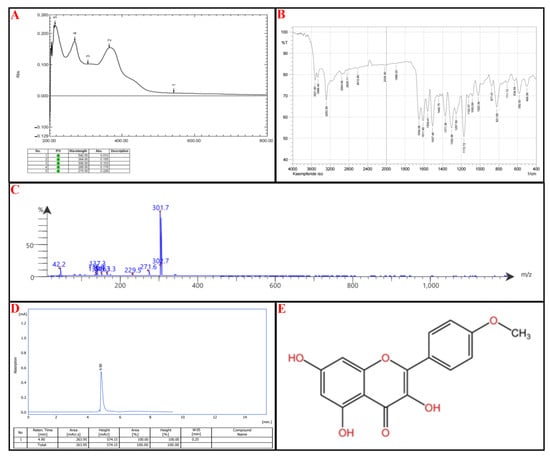
Figure 6.
Identification of subfraction E2-VIII portion P-2, (A) UV spectrophotometry, (B) FT-IR, (C) MS-spectrometry, (D) HPLC, (E) chemical structure.
FTIR Analysis: 3522–3284 cm−1: Broad O-H stretches, characteristic of phenolic groups. Notably, 2955–2829 cm−1: C-H stretching, likely from aliphatic or aromatic systems. Notably, 2613 cm−1 and 2036 cm−1: Uncommon absorption bands—could suggest weak hydrogen bonding or interactions. Notably, 1654 cm−1, 1611 cm−1, 1559 cm−1: Strong C=O and C=C stretches, confirming flavonoid backbone with carbonyl functional groups. Notably, 1258–1020 cm−1: C-O stretching bands suggest the presence of ether or ester functionalities (Figure 6B).
Mass Spectrometry (MS) Analysis: Parent Ion: 302.7 m/z: Suggests a molecular weight near 302 g/mol, which fits certain flavonoids. Fragmentation Patterns: 301.7, 271.6: Consistent with sequential losses of hydroxyl groups. Notably, 229.5, 163.3, 149, 137.3: Breakdown of the flavone core. Notably, 42.2 m/z: Typical of small alkyl fragment losses (Figure 6C).
Kaempferide has characteristic UV absorptions near 260–370 nm, corresponding to π→π transitions* in its conjugated flavone structure. The presence of a methoxy (-OCH₃) group at position 4′ can shift absorption toward higher wavelengths, which matches 364 nm. FTIR Analysis: Key bands of Kaempferide include a broad O-H stretching (~3500 cm−1) → Present in Compound II (3522, 3468 cm−1). C=O stretching (1650–1620 cm−1) → Found in Compound II (1654, 1611 cm−1). C=C stretching (aromatic core, ~1600–1550 cm−1) → Present (1559, 1507 cm−1). C-O stretching (~1260–1030 cm−1) → Matches Compound II (1258–1020 cm−1).
The final step relied on HPLC; the isolated compound peaks at an Rt of 4.90 min, comparable to the standard kaempferide, which has an Rt of 4.98 min, as seen in Figure 6D. According to IUPAC, the chemical structure of kaempferide is 3,5,7-Trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one, illustrated in Figure 6E (for reference standard of each analytical procedure, see Supplementary Material Figure S3).
Compound III: UV-Vis Spectroscopy (268 nm and 352 nm): These absorbance wavelengths suggest the presence of a benzoyl peak 220–280 nm and a cinnamoyl peak 300–400 nm reference (Figure 7A).
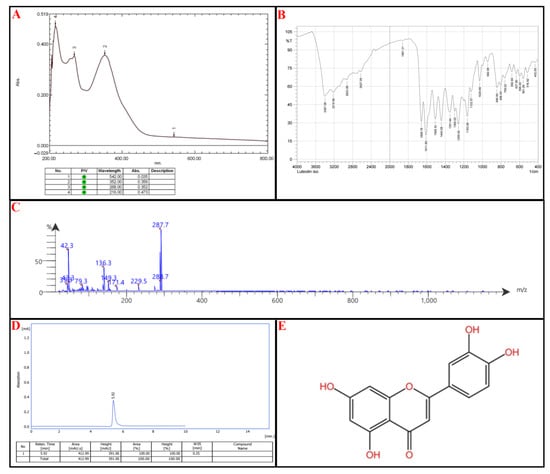
Figure 7.
Identification of subfraction E2-VIII portion P-3, (A) UV spectrophotometry, (B) FT-IR, (C) MS-spectrometry, (D) HPLC, (E) chemical structure.
FTIR Analysis: 3397 cm−1 and 3219 cm−1: Broad O-H stretching, confirming phenolic functional groups. Notably, 2923 cm−1: C-H stretching, typical of aromatic/aliphatic compounds. Notably, 1659 cm−1 and 1612 cm−1: C=O (carbonyl) and C=C (aromatic) stretching, consistent with flavone structures. Notably, 1260–1030 cm−1: C-O stretching, suggesting the presence of ether or ester functionalities (Figure 7B).
Mass Spectrometry (MS) Analysis: Parent Ion: 288.7 m/z → Suggests a molecular weight close to 288 g/mol, which aligns with flavonoids lacking methoxy groups. Fragmentation Patterns: 287.7, 229.5: Loss of hydroxyl groups. Notably, 171.4, 149.3, 136.3: Breakdown of the flavone core. Notably, 43.3, 42.3, 39.3: Alkyl fragment losses, confirming side-group modifications.
Among the listed flavonoids (Scutellarin, Luteolin, Nepetin, Apigenin, Kaempferol, Chrysoeriol, Jaceidin, Kaempferide, Eupatolin, Centaureidin), the best match is Luteolin (MW: 286 g/mol) → Its UV absorption, FTIR bands, and MS fragmentation pattern closely align. Luteolin has hydroxyl groups at key positions, which match the FTIR O-H stretch and mass fragmentation pattern. Other candidates, such as Kaempferol or Apigenin (MW: 286 g/mol), lack the necessary hydroxyl arrangement, making Luteolin the strongest match (Figure 7C).
HPLC was utilized to confirm the compound’s identity; the isolated compound peaks at an Rt of 5.92 min, comparable to the standard Luteolin with an Rt of 5.90 min, as seen in Figure 7D. According to IUPAC, the chemical structure of Luteolin is 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4-one, as illustrated in Figure 7E (for reference standard of each analytical procedure, see Supplementary Figure S4).
2.7. Molecular Docking
The antidiabetic mode of action targets α-amylase. Suppression of α-amylase enzyme activity diminishes glucose absorption in the intestines. It can efficiently postpone the release of glucose into the bloodstream, hence managing deteriorating health issues such as T2DM [30,31].
The nepetin flavonoid was thought to be the main contributor to the subfraction E2-VIII’s in vivo hypoglycemic effect. Therefore, in silico studies were conducted to predict their binding affinity with antidiabetic receptors (α-amylase). The computational approach we used involved docking the flavonoids to form a complex with α-amylase.
The protein-ligand complex is established via electrostatic forces at the binding interface, encompassing hydrogen bonds (from both side chains and backbones), salt bridges, and π-π stacking. Hydrogen bonding confers stability to protein molecules and specific protein-ligand interactions, making it crucial for the interactions of biological macromolecules.
The variation in binding energies of nepetin (44.66 Kcal/mol) indicates a superior binding affinity compared to the reference compound (31.96 Kcal/mol) for the α-amylase target. Nepetin formed four hydrogen bonds (2.758–3 Å) with three amino acids: ASN459, ILE458, and LYS466 in the α-amylase target. Simultaneously, 1U2Y formed five hydrogen bonds (2.685–3.029 Å) with three amino acids: ASN459, CYS462, and ILE458 in α-amylase, as illustrated in Figure 8 and Figure 9.
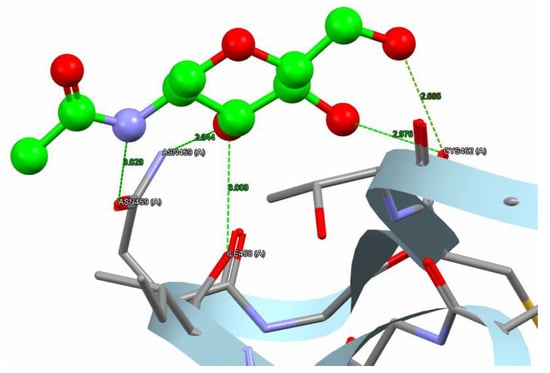
Figure 8.
The reference compound hydrogen bonds and hydrophobic interaction with the α-amylase (PDB code: 1U2Y). The interaction between them via hydrogen bonds [ASN459, CYS462, and ILE458] is green, while brief contact is red. [The reference compound is administered in a ball-and-stick fashion, whereas amino acids are administered as capped sticks].
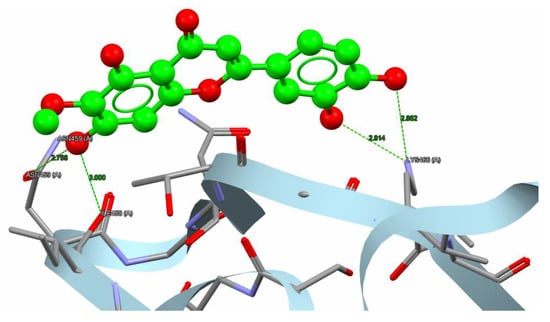
Figure 9.
Nepetin hydrogen bonds besides hydrophobic interaction with α-amylase (PDB code: 1U2Y). The interaction between them via hydrogen bonds [ASN459, ILE458, and LYS 466] is in green, while brief contact is in red. [The nepetin compound is administered in a ball-and-stick fashion, whereas amino acids are administered as capped sticks].
2.8. In Vitro α-Amylase Inhibitory Activity of Nepetin
To confirm the antidiabetic action of nepetin, which was obtained in silico, we conducted an in vitro experiment to confirm the α-amylase inhibitory activity of nepetin utilizing Bernfeld’s technique, with Acarbose serving as the standard. The nepetin exhibited strong α-amylase inhibitory activity, comparable to acarbose, with the IC50 ± SE of 23.73 ± 0.2619 vs. 24.59 ± 0.5151 μg/mL for nepetin and acarbose, respectively, as seen in Figure 10.
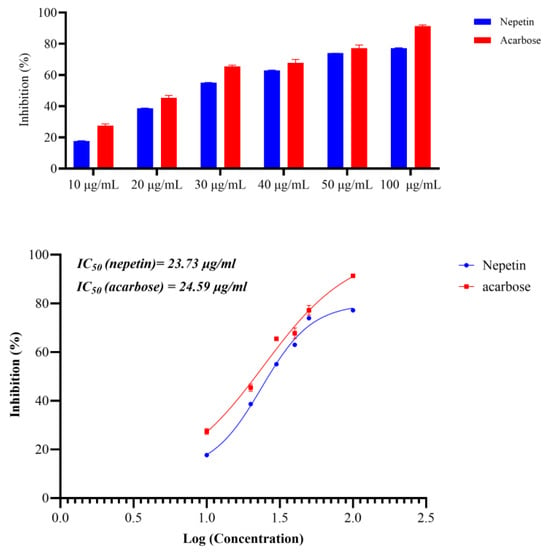
Figure 10.
Inhibitory activity of nepetin and acarbose against α-amylase showing the percentage inhibition and IC50 (R2 of nepetin is 0.9933 and for acarbose is 0.9779, using log(inhibitor) vs. response-Variable slope (four parameters).
3. Discussion
The present study examines the potential of C. calcitrapa extract and its subfractions as an antidiabetic agent; one subfraction showed potent antidiabetic activity and high safety (E2-extract). The E2 extract demonstrated no toxicity, as evidenced by the ALT, AST, and ALP levels, which are crucial indicators for liver toxicity assessment. Similarly, the E2 extract showed no renal toxicity, as confirmed by the urea and creatinine levels in rats’ serum. This safety profile of the E2 extract provides reassurance for its potential use as an antidiabetic agent. The E2 extract demonstrates significant antioxidant activity, as evidenced by the levels of MDA, catalase, and SOD. Since oxidative stress is considered an important factor in the pathogenesis of T2DM, diabetes is associated with a reduction in antioxidant levels due to protein glycation. In contrast, elevated MDA levels suggest increased lipid peroxidation and free radicals. Following treatment with the plant extract, the antioxidant enzyme levels were compared to those in rats treated with metformin, indicating the antioxidant activity of the C. calcitrapa extract.
Our findings agreed with a previous study examining the renoprotective effects of Centaurea choulettiana leaves in mice treated with cisplatin, in which the plant n-butanol extract reduced urea and creatinine levels. In addition, the n-butanol extract showed potent antioxidant activity, as evidenced by improvement in renal tissue levels of MDA, reduced glutathione (GSH), CAT, SOD, and myeloperoxidase (MPO) [32]. Another study that examined the n-butanol extract of the aerial part of Centaurea tougourensis showed hepatorenal protection in mice treated with streptozotocin, as evidenced by a reduction in the levels of ALT, AST, creatinine, and urea [33]. The aqueous extract of Ephedra foeminea showed hepatorenal protective effects and potent antioxidant activity in diabetic rats treated with streptozotocin, with a significant reduction in ALT, AST, ALP, bilirubin, urea, and creatinine. Additionally, it showed a reduction in the interleukin (IL)-1 and GSH levels, which is in agreement with the current study [34]. These findings indicate that the species of the genus Centaurea, including C. calcitrapa, protect against streptozotocin-induced diabetic changes.
Streptozocin-induced diabetes can be attributed to pancreatic beta-cell death, leading to insulin insufficiency [35]. Additionally, streptozotocin-induced diabetes leads to heightened proinflammatory status, a hyperlipidemic state, and a redox imbalance, all of which are hallmarks of diabetes mellitus [10,36]. In the current study, metformin showed successful antidiabetic activity in rats; metformin is recognized for its efficacy in reducing blood glucose levels in individuals with type 2 diabetes; this is mainly achieved by suppressing hepatic gluconeogenesis and enhancing peripheral insulin sensitivity [37,38].
The diabetes condition is linked to a widespread elevation in tissue oxidative stress, potentially demonstrated by alterations in the tissue antioxidant system [39]. Oxidative stress may lead to excessive generation of oxygen-free radical precursors and/or diminished efficacy of the antioxidant system. The emergence of oxygen-free radicals is linked to the auto-oxidation of glucose, disrupted glutathione metabolism, modifications in antioxidant enzymes, and the production of lipid peroxides [40]. Numerous research studies have established the correlation between oxidative stress and the pathophysiology of insulin resistance through the suppression of insulin signaling and dysregulation of adipocytokines [41]. Elevated ROS synthesis among T2DM patients is believed to initiate numerous unfavorable processes, including hexosamine pathways, the development of advanced glycation end-products (AGEs), and PKCβ1/2 activation [42]. Hyperglycemia may produce oxidative stress through multiple pathways, including glucose autoxidation, the polyol pathway, advanced glycation end-product (AGE) production, and PKCβ1/2 kinase activation. In T2DM patients, increased levels of free fatty acids, leptin, and other circulating variables may contribute to the overproduction of ROS [40].
In the present study, subfraction E2-VIII after 8, 15, 22, and 30 days showed a sustained and consistent glucose-lowering effect compared to metformin. The E2-VIII subfractions were purified to determine the active components, and three components were identified: nepetin, kaempferide, and Luteolin. All these flavonoids were previously reported in C. calcitrapa, including nepetin, kaempferide, and Luteolin [12,14]. Furthermore, Nepetin was examined using molecular docking, showing that nepetin has strong α-amylase inhibitory activity. Based on these findings, we performed an in vitro study to show nepetin α-amylase inhibitory activity; nepetin had strong α-amylase inhibitory activity comparable to the active standard (acarbose) (IC50 ± SE of 23.73 ± 0.2619 μg/mL). These findings indicate that nepetin is the most important constituent and shows antidiabetic activity mediated through inhibiting the α-amylase enzyme.
Nepetin has been documented to exhibit several biological functions, notably anti-inflammatory responses [43] and antidiabetic activity [44]. Recently, nepetin was reported to inhibit the catalytic activity of protein tyrosine phosphatases (PTPN)-1, PTPN2, and PTPN11 in vitro, indicating that nepetin acts as a multi-targeting inhibitor; furthermore, treatment of mature 3T3-L1 adipocytes with 20 μM nepetin stimulates glucose uptake through AMPK activation, which indicates nepetin activity as an antidiabetic agent through ameliorating IR [44]. Nepetin can hinder the degranulation and the production of leukotriene C4 and prostaglandin D2 in IgE/antigen (Ag) stimulated bone marrow-derived mast cells in mice. The IgE/Ag-mediated signaling pathway indicated that nepetin inhibited intracellular Ca2+ levels and activated PLCγ1 and cPLA2. Moreover, nepetin administration diminished prostaglandin D2 synthesis and inhibited cyclooxygenase-2 protein expression by obstructing the Akt and nuclear factor-κB signaling pathways [43]. Nepetin also inhibits the activity of IL-1β-induced IL-6, IL-8, and MCP-1 secretion and mRNA expression by repressing the activation of NF-κB and MAPKs [45]; other studies showed that nepetin possesses anti-inflammatory activity by attenuating NF-κB [46].
Kaempferide showed multiple biological activities, like antioxidant [47], anti-inflammation [48], anticancer [49], antihypertension [50], and improving glycolipid metabolism disorder [51]. As an antioxidant and anti-inflammatory agent, Kaempferide exerts its biological activity by inhibiting the TLR4/IκBα/NFκB signaling pathways [48]. Kaempferide improves glycolipid metabolism disorder by activating the PPARγ and its downstream signaling pathway [51]. PPARγ modulates glucose metabolism primarily by enhancing the sensitivity of peripheral tissues to insulin, promoting glucose consumption in muscle, and suppressing hepatic glycogen production [52,53]. PPARγ may facilitate insulin sensitivity through many mechanisms. PI3K is a crucial enzyme facilitating glucose entry into cells. Activated PPARγ may enhance the PI3K/AKT signaling pathway to improve insulin sensitivity [54]; Activated PPARγ can elevate glucose transporter-4 expression, augment glucose uptake, and ameliorate insulin resistance [55]; PI3K activation may expedite triglyceride decomposition in peripheral tissues, elevate its synthesis in adipose tissue, and suppress glucagon production [56]. These findings suggest that kaempferide may exert its antidiabetic activity via activation of PPARγ, leading to insulin sensitization.
Luteolin is utilized to address multiple medical conditions by modulating oxidative stress, inflammation, and dyslipidemia, and decelerating carbohydrate digestion and absorption through interaction with α-glucosidase [57,58]. Recent research demonstrated that Luteolin had strong antidiabetic benefits in streptozotocin-induced diabetic rats. Luteolin supplementation over three weeks markedly reduced hyperglycemia, HbA1c levels, hyperlipidemia, and inflammation and enhanced antioxidant enzyme activity [59]. Luteolin can regenerate pancreatic β-cells and secrete insulin due to its ability to stimulate the release of bound insulin from β-cells by inhibiting ATP-sensitive K+ channels [60].
Thus, the heightened antidiabetic activity shown by subfraction E2-VIII could be attributed to its individual component to enhance insulin levels, insulin sensitivity, and anti-inflammatory activity.
4. Materials and Methods
4.1. Chemicals, Reagents
The solvents (methanol, ethyl acetate, chloroform, n-hexane, and petroleum ether) were purchased from Central Drug House (P) Ltd., Delhi, India. HPLC analytical grade solvents (Water and methanol) were purchased from Loba Chemie; the lab is in Mumbai, India. Soxhlet apparatus (Adarsh Scientific Industry, Ambala Cantt, India), rotavapor R-100 (Buchi Labortechnik AG, Flawil, Switzerland), fertigfolien/pre-coated thin-layer chromatography sheet allugram Xtra sil G/uv 254, Layer: 0.20 mm with fluorescent indicator UV 254 (Düren, Germany), column chromatography comax column, Czech Republic, packed with silica gel (60 μm, Merck Co., Darmstadt, Germany).
Catalase (Cusabio, Wuhan, China), Alkaline Phosphatase (ALP) (Cusabio, China), Rat aspartate aminotransferase (AST) (Cusabio, China), alanine aminotransferase (ALT) (Cusabio, China), superoxide dismutase (SOD) (Cusabio, China), Malondialdehyde (MDA) (MyBioSource, UDA, San Diego, CA, USA), urea (Linear, Barcelona, Spain), creatinine (Linear, Spain)).
4.2. Plant Materials
Assistant professor Dr Abd Al-Moein authenticated the plant in the University of Kirkuk, College of Medicinal and Industrial Plants, Iraq, a collection of C. calcitrapa flowers, leaves, and stem (the whole aerial part at blooming season).
A voucher specimen was deposited in the scientific affairs of the University of Kirkuk, College of Medicinal and Industrial Plants, under the accession number (2597/40/7).
The aerial parts of the plant are dried in a well-ventilated place in the shade, away from sunlight [61], and collected from Al-Taji town in Baghdad (33.494606, 44.170728). The whole aerial plant part is ground by an industrial grinder to reduce the plant particle size [62].
4.3. Extraction
The ground plant materials (4.0 kg) were defatted using petroleum ether [63], followed by the continuous extraction method (Soxhlet) using three different solvents with increasing polarity: chloroform, ethyl acetate, and methanol for 4.5 h for each solvent [64]. The resulting extract solution was filtered and concentrated using a rotary evaporator (Rotavapor® R-100, BÜCHI Labortechnik AG, Flawil, Switzerland).
These extracts were evaluated for their potential antioxidant, antidiabetic, kidney function, and hepatic function activities: chloroform extract (E1), ethyl acetate extract (E2), and methanol extract (E3) in the diabetic-induced model in rats. (see Supplementary Figure S5).
4.4. Column Chromatography
Column chromatography technology was used to separate the components of E2, the normal phase (silica gel 60 μm), and the mobile phase gradient chloroform-methanol (100:0 to 0:100 v/v). The eluent is collected (each 10 mL) and examined by thin-layer chromatography (TLC) on silica gel with fluorescent indicator 254 nm on aluminum cards (layer thickness 0.2 mm) using n-hexane: ethyl acetate (3:1) as eluent (v/v), combining similar parts [65].
Eight fractions were isolated: E2-I, E2-II, E2-III, E2-IV, E2-V, E2-VI, E2-VII, and E2-VIII. All were evaluated for their antidiabetic activity, and the most active fraction was subjected to identification and structure elucidation. Details are illustrated in Supplementary Figure S4.
4.5. Purification of E2-VIII Subfraction by High-Performance Liquid Chromatography (HPLC)
The chromatographic separation and purification of E2-VIII individual components were conducted using a Hypersil ODS-C18 column (250 mm × 4.6 mm, 5 μm) reverse-phase HPLC system (SYKAM, Eresing, Germany) coupled with a UV diode array detector, L-2200 autosampler, and L-2130 pump. Chromatographic data were processed by Clarity Chromatography data station software v6.0. The isocratic mobile phase consisted of Methanol: Water (50:50, v/v), eluted at a 1 mL/min flow rate. The detector’s wavelength was 260 nm, and the sample injection volume was 100 μL.
4.6. Identification of the Isolated Compounds
4.6.1. UV Spectrometry
The UV spectrometric experiments were conducted on a Shimadzu-1600, Japan. The scan range was 200 to 800 nm, the spectral band was 2.0 nm, and the spectral resolution was 0.1 nm. The light source was 340.8 nm. The samples analyzed were diluted in methanol into quartz cuvettes.
4.6.2. Fourier-Transform Infrared Spectroscopy (FTIR)
The FTIR spectrometric experiments were carried out on a Shimadzu FTIR Prestige 21, Tokyo, Japan, with a scan range of 4000 to 400 cm−1, on KBr disks, spectral resolution 2 cm−1, He-Ne laser.
4.6.3. Mass Spectrophotometry (MS)
The analysis was performed using Advion expression, New York, NY, USA. Ion Source: APCI, Polarity: Positive ion, m/z Range 10 to 1200, Acquisition Speed 10,000 m/z units/s, stability ± 0.1 m/z units over 12 h period (65–75 °F (18–24 °C) operating temperature), Polarity Switching Speed 50 Ms, Dynamic Range 4–5 orders of magnitude, Gas Supply 60 psi, >98% pure Nitrogen, Gas Consumption < 10 L/min, Pirani pressure 7.21 × 10−3 mbar, Turbo speed 99.6%, Capillary temperature 255 °C, Source gas temperature 354 °C, Transfer line temperature 0 °C, Capillary voltage 192.4 volts, Source voltage 76.5 volts, Extraction electrode 9.44 volts, Esi voltage 3.69 KV, Apci current 5.34 μamps, Hexapole bias 8.42 volts, Pole bias −5.11 volts, Hexapole RF 200.2 volts, Rectified RF 4.12 volts, Dc1 −97.29 volts, Dc2 104.52 volts, Detector −1.15 KV, and Dynode −10.36 KV.
4.6.4. Calibration Curve
Nepetin, kaempferide, and luteolin standard compounds were dissolved in MeOH (1 mg/mL). All samples were filtered through a syringe filter before analysis. Standard solutions were serially diluted to prepare different concentrations (3, 5, 7, and 9 μg/mL). A calibration curve for each standard was constructed by plotting concentration (x, ppm) versus peak area (y). Linearity was assessed from the correlation coefficient value (r2) [66]. Details are illustrated in Supplementary Figure S5.
4.7. In Vitro α-Amylase Inhibitory Assay
The α-amylase inhibitory activity of nepetin was assessed using Bernfeld’s technique, with Acarbose serving as the standard [67]. A series of nepetin and acarbose with concentrations of 10, 20, 30, 40, 50, and 100 μg/mL was made and permitted to react with α-amylase and 2 mM phosphate buffer (pH 6.9). Following a 20-minute incubation, 0.1 mL of 1% starch solution was included in the reaction mixture. A similar method was executed for control samples devoid of the enzyme. Subsequently, 0.5 mL of dinitro salicylic acid reagent was introduced to both the control and test samples and maintained in a boiling water bath for 5 min. The absorbance was subsequently measured at 405 nm utilizing a spectrophotometer, and the percentage inhibition was computed using the formula [26,68]:
AC indicates the absorbance of the control (containing all reagents except the test solution), and AT indicates the absorbance of the tested solution.
The half-maximal inhibitory concentration (IC50) values were identified by applying nonlinear regression to fit inhibition parameters with standard log inhibitors against 4P-response models [69].
4.8. Experimental Design and Settings
Sprague-Dawley albino male rats aged 12 to 16 weeks, weighing 140–220 grams, were obtained from a biotechnology research center at Tikrit University. All care for animals and scientific experiments were conducted in strict compliance with regulations established by the animal ethics committee at the Al-Mustafa University College animal facility in Baghdad, Iraq (following AVMA guideline 2020 [70]).
Animals were adjusted for seven days in laboratory settings prior to the commencement of the assessments. Water and standard food pellets (Elazig Food Company, Elazig, Turkey) were provided ad libitum in the ventilated room. Before and during the experimental period, all rats were assessed for their health status, including food and water intake. A 12 h light-dark period, ambient temperature of 18–22 °C, and 40% humidity. The room was adequately aired with entirely fresh air. The authors adhered to the ARRIVE 2.0 criteria [71].
The study was conducted at Al-Mustafa University College’s animal house between 5 January 2022, and 23 April 2024.
The experimental phase includes two animal studies. The initial study aimed to determine the antidiabetic activity of plant extracts, and then the extract with the highest hepatorenal safety and best antidiabetic activity would be further investigated; the second experimental study aimed to identify the active compound with the highest antidiabetic activity.
4.8.1. Diabetic Induction
The Sprague-Dawley albino male rats were acclimated and subjected to an overnight fast. Subsequently, they were administered a single intraperitoneal injection of a freshly prepared solution of streptozotocin (STZ) (60 mg/kg body weight) in a 0.1 M cold citrate buffer (pH 4.5). During the induction process, the rats were given a 5% glucose solution overnight to prevent severe low blood sugar caused by the excessive release of insulin caused by the administration of STZ. The rats were categorized as diabetic if their blood glucose levels surpassed 13.9 mmol/L 72 h following the treatment with STZ. The administration of investigational treatment commenced on the fourth day following the STZ injection. Rats in the control group that did not have diabetes induced were exposed to the identical procedure; instead of receiving STZ, they were given intraperitoneal injections of a solution containing 0.9% saline [38,72].
4.8.2. Special Considerations to Minimize the Suffering and Distress of Animals
Every attempt was made to reduce suffering and the number of animals participating in the tests. The animal was observed immediately following the STZ injection, approximately 10 min later, and the subsequent day. In the event of bleeding, gauze was applied, and pressure was exerted. After the hemorrhage ceased, the area was sanitized with gauze and water. In instances of peritonitis, internal organ laceration, and/or infection, a veterinarian was consulted to evaluate the animal’s suitability for further participation in the experiment [73,74].
4.8.3. Plant Extracts Experimental Design
The initial study included 60 rats divided into six groups (n = 10 for each group). The experiment continued for 30 days with daily administration of metformin and the investigated extracts; at the end of the experiment, all animals survived, as illustrated in Table 2 and Figure 11.

Table 2.
Experimental design of plant extracts animal study.
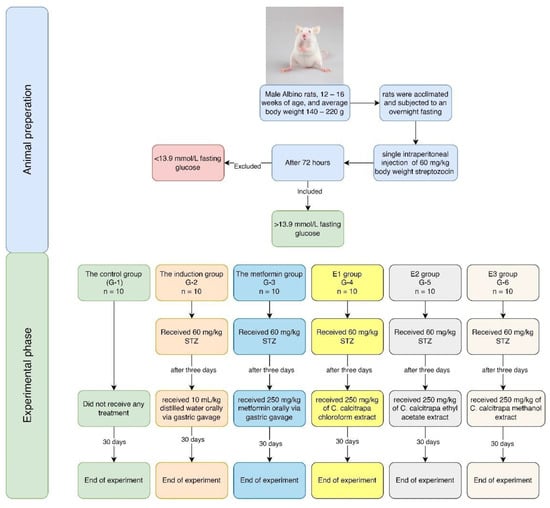
Figure 11.
Flow chart of the experiment.
4.8.4. C. calcitrapa Ethyl Acetate Extract (E2) Fractions Experimental Design
The second experiment involved 88 rats divided into 11 groups (n = 8 for each group). It continued for 30 days with daily administration of metformin and the investigated subfractions. As illustrated in Table 3, all animals survived at the end of the experiment.

Table 3.
Experimental design of the plant extract fractions animal study.
4.9. Clinical and Laboratory Assessment
4.9.1. Body Weight Monitoring
Rats’ body weights were observed at the experiment’s start and end.
4.9.2. Monitoring the Blood Glucose Level
The blood glucose level was measured by scratching the tail vein using the Accu-Chek Performa blood glucometer (Roche, Mannheim, Germany) [38]. The fasting blood glucose (FBG) level measurement was taken in the first experiment on the 1st, 15th, and 30th day of the experimental work. In the second experiment, the blood glucose level measurement was taken on the 1st, 8th, 15th, 22nd, and 30th day of the experimental work.
4.9.3. Biochemical Analysis (Conducted on Stage 1)
Blood was collected in a clot activator tube, and the serum was separated by a centrifuge at 3000 rpm for 10 min. The serum was placed at −20 °C until analyses were performed.
Biomarkers of serum samples were assessed using the enzyme-linked immunosorbent assay (ELISA) technique. Frozen specimens were allowed to thaw at ambient temperature, and the biomarkers necessitating evaluation for the study comprised ALP (Cat# CSB E11865r), catalase (Cat# CSB E13439r), AST (Cat# CSB E13023r), ALT (Cat# CSB E13024r), SOD (CSB EL022397RA), MDA (Cat# MBS263626).
Creatinine (Ref# 1123005) was assessed using the kinetic colorimetric method based on picrate reaction (Jaffe) [78], and urea (Ref# 1158005) was assessed using an enzymatic method in which urea is hydrolyzed by urease to ammonia and carbon dioxide. The ammonia is converted to glutamate by glutamate dehydrogenase in the presence of NADH and oxoglutarate [79,80].
4.10. In Silico Molecular Docking Studies
The structure of nepetin had been drawn in ChemDraw Professional software (v. 16.0). The energy of each molecule was minimized using licensed CCDC genetic optimization for ligand docking (GOLD) Hermes 2021.2.0 (Build 327809) was used to achieve the molecular docking studies for the compounds and to envisage: the protein, ligands, interactions of hydrogen bonding, short contacts, and length of bonds calculation, to carry out the docking simulation. Protein molecules of α-amylase (PDB: 1U2Y) were retrieved from the protein data bank. The receptors were removed, and only polar hydrogen charges were added to the water molecules. GOLD was compiled and run under Windows 10.0 Professional operating system.
4.11. Ethical Considerations
The study was approved by the research ethical committee of Al-Mustafa University College (ID: AP023, date: 20 November 2021).
4.12. Statistical Analysis and Sample Size Calculation
Program G.Power3.1 was utilized for sample size calculation; post hoc sample size was conducted with an effect size of 0.5, power 80%, and an alpha level of 0.05, F-family tests with a total sample size of 60 for each group of 10 animals for the first experiment, while for the second experiment, an effect size of 0.47, power 80%, and an alpha level of 0.05, F-family tests with a total sample size of 88 for each group of 8 animals [81,82]. Random numbers were utilized to create groups in an Excel spreadsheet. The rats were placed in labeled cages and assigned tail tags to reduce confusion [83].
The Anderson-Darlin normality test was performed, and all variables followed a normal distribution. Ordinary One-way ANOVA with post hoc Tukey test was used to assess the effect of renal, hepatic, and oxidative stress markers. In contrast, two-way repeated measures ANOVA with post hoc Šídák’s multiple comparisons test was used to assess the difference in FBG and body weight [84]. The significance level was defined by p-value ≤ 0.05 (alpha level). All analyses used GraphPad Prism version 10.2.0 for Windows, GraphPad Software, and Boston, MA, USA.
5. Conclusions
This study underscores the potential of C. calcitrapa extract, particularly the E2 subfraction, as a promising antidiabetic agent with a strong safety profile. The absence of hepatotoxicity and renal toxicity, combined with its potent antioxidant activity, supports its viability for therapeutic applications. The extract not only demonstrated significant glucose-lowering effects but also provided antioxidant protection against oxidative stress—a major contributor to T2DM pathogenesis. The identified bioactive compounds, including nepetin, kaempferide, and luteolin, exhibit multifaceted biological activities that enhance insulin sensitivity, reduce inflammation, and promote antioxidant defenses. The in silico and in vitro studies indicate that the main compound shows activity against α-amylase.
Future research should focus on elucidating the precise molecular mechanisms through which these flavonoids exert their antidiabetic effects. Investigating their potential for synergistic interactions or formulation into optimized drug delivery systems may enhance their efficacy and stability. Moreover, expanding preclinical studies to include long-term toxicity assessments and evaluating their impact on insulin signaling pathways will be crucial for translating these findings into clinical applications. Given the promising results observed, the C. calcitrapa extract could pave the way for novel phytotherapeutic interventions in diabetes management, offering a natural and effective alternative to conventional therapies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30112394/s1, Figures S1–S5, which contain data about the Calibration curve (Figure S1), the standards of nepetin (Figure S2), the standards of kaempferide (Figure S3), the standards of Luteolin (Figure S4), and the plant material extraction procedure (Figure S5).
Author Contributions
Conceptualization, H.M.K. and K.G.; Data curation, H.A.F.; Formal analysis, H.A.F.; Investigation, H.M.K., Z.M.A.K., A.M.J. and K.G.; Methodology, H.M.K., H.A.F. and K.G.; Resources, Y.M.K. and K.G.; Software, H.A.F.; Supervision, K.G.; Validation, Y.M.K., H.A.F., Z.M.A.K. and A.M.J.; Visualization, H.M.K., H.A.F. and K.G.; Writing—original draft, H.M.K., Y.M.K., H.A.F., Z.M.A.K., A.M.J. and K.G.; Writing—review and editing, H.M.K., Y.M.K., H.A.F., Z.M.A.K., A.M.J. and K.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Research Ethics Committee of Al-Mustafa University College (ID: AP023, approval date: 20 November 2021), following the American Veterinary Association Guidelines (AVMA) [70]. The authors complied with the ARRIVE 2.0 guidelines [71].
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in [Zenodo] at [https://doi.org/10.5281/zenodo.14983246] (date: 1 February 2025), reference number [14983246].
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript: Diabetes mellitus (DM), Type 2 diabetes mellitus (T2DM), methanol (MeOH), Alkaline Phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), superoxide dismutase (SOD), Malondialdehyde (MDA), thin-layer chromatography (TLC), Fourier Transform Infrared Spectroscopy (FTIR), high-performance liquid chromatography (HPLC), Mass spectrophotometry (MS), streptozotocin (STZ), enzyme-linked immunosorbent assay (ELISA), fasting blood glucose (FBG), genetic optimization for ligand docking (GOLD).
References
- Fattaheian-Dehkordi, S.; Hojjatifard, R.; Saeedi, M.; Khanavi, M. A Review on Antidiabetic Activity of Centaurea spp.: A New Approach for Developing Herbal Remedies. Evid.-Based Complement. Altern. Med. 2021, 2021, 5587938. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Numan, A.T.; Jawad, N.K.; Fawzi, H.A. Biochemical study of the effect of lead exposure in nonobese gasoline station workers and risk of hyperglycemia: A retrospective case-control study. Medicine 2024, 103, e39152. [Google Scholar] [CrossRef] [PubMed]
- Numan, A.T.; Jawad, N.K.; Fawzi, H.A. Biochemical study of the risk of diabetes, prediabetic and insulin resistance in car painters and its association with mercury exposure: A retrospective case-control study. Toxicol. Res. 2024, 13, tfae221. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Cerf, M.E. Beta cell dysfunction and insulin resistance. Front. Endocrinol. 2013, 4, 37. [Google Scholar] [CrossRef]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Belén Crujeiras, A.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef]
- Dali-Youcef, N.; Mecili, M.; Ricci, R.; Andrès, E. Metabolic inflammation: Connecting obesity and insulin resistance. Ann. Med. 2013, 45, 242–253. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Jansen, C.; Baker, J.D.; Kodaira, E.; Ang, L.; Bacani, A.J.; Aldan, J.T.; Shimoda, L.M.N.; Salameh, M.; Small-Howard, A.L.; Stokes, A.J.; et al. Medicine in motion: Opportunities, challenges and data analytics-based solutions for traditional medicine integration into western medical practice. J. Ethnopharmacol. 2021, 267, 113477. [Google Scholar] [CrossRef]
- Dimkić, I.; Petrović, M.; Gavrilović, M.; Gašić, U.; Ristivojević, P.; Stanković, S.; Janaćković, P. New perspectives of purple starthistle (Centaurea calcitrapa) leaf extracts: Phytochemical analysis, cytotoxicity and antimicrobial activity. AMB Express 2020, 10, 183. [Google Scholar] [CrossRef]
- Al-Joboury, K.R.; Aliwy, S.A. Survey with revised checklist of compositae in the herbarium of iraq natural history research center and museum. Bull. Iraq Nat. Hist. Mus. 2023, 17, 375–407. [Google Scholar] [CrossRef]
- Khammar, A.; Djeddi, S. Pharmacological and biological properties of some Centaurea species. Eur. J. Sci. Res. 2012, 84, 398–416. [Google Scholar]
- Pieroni, A.; Janiak, V.; Dürr, C.M.; Lüdeke, S.; Trachsel, E.; Heinrich, M. In vitro antioxidant activity of non-cultivated vegetables of ethnic Albanians in southern Italy. Phytother. Res. 2002, 16, 467–473. [Google Scholar] [CrossRef]
- Lentini, F. The role of ethnobotanics in scientific research. State of ethnobotanical knowledge in Sicily. Fitoterapia 2000, 71 (Suppl. 1), S83–S88. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Dumlu, M.U.; Gürkan, E. A new active compound from Centaurea species. Z. Naturforsch C 2006, 61, 44–46. [Google Scholar] [CrossRef]
- Csupor, D.; Blazsó, G.; Balogh, A.; Hohmann, J. The traditional Hungarian medicinal plant Centaurea sadleriana Janka accelerates wound healing in rats. J. Ethnopharmacol. 2010, 127, 193–195. [Google Scholar] [CrossRef]
- Sarker, S.D.; Kumarasamy, Y.; Shoeb, M.; Celik, S.; Eucel, E.; Middleton, M.; Nahar, L. Antibacterial and antioxidant activities of three Turkish species of the genus Centaurea. Adv. Tradit. Med. 2005, 5, 246–250. [Google Scholar]
- Erol-Dayi, Ö.; Pekmez, M.; Bona, M.; Aras-Perk, A.; Arda, N. Total phenolic contents, antioxidant activities cytotoxicity of three Centaurea species: C. calcitrapa subsp. calcitrapa, C. ptosimopappa C. spicata. Free Radic. Antioxid. 2011, 1, 31–36. [Google Scholar] [CrossRef]
- Kitouni, R.; Benayache, F.; Benayache, S. Flavonoids of the Exudate of Centaurea calcitrapa. Chem. Nat. Compd. 2015, 51, 762–763. [Google Scholar] [CrossRef]
- Bruno, M.; Bancheva, S.; Rosselli, S.; Maggio, A. Sesquiterpenoids in subtribe Centaureinae (Cass.) Dumort (tribe Cardueae, Asteraceae): Distribution, (13)C NMR spectral data and biological properties. Phytochemistry 2013, 95, 19–93. [Google Scholar] [CrossRef] [PubMed]
- Formisano, C.; Rigano, D.; Senatore, F.; Bancheva, S.; Maggio, A.; Rosselli, S.; Bruno, M. Flavonoids in subtribe Centaureinae (Cass.) Dumort. (tribe Cardueae, Asteraceae): Distribution and (13)C-NMR spectral data. Chem. Biodivers. 2012, 9, 2096–2158. [Google Scholar] [CrossRef] [PubMed]
- Kaskoos, R.A. In-vitro α-glucosidase inhibition and antioxidant activity of methanolic extract of Centaurea calcitrapa from Iraq. Am. J. Essent. Oils Nat. Prod. 2013, 1, 122–125. [Google Scholar]
- Pham, E.C.; Truong, T.N.; Dong, N.H.; Vo, D.D.; Hong Do, T.T. Synthesis of a Series of Novel 2-Amino-5-substituted 1,3,4-oxadiazole and 1,3,4-thiadiazole Derivatives as Potential Anticancer, Antifungal and Antibacterial Agents. Med. Chem. 2022, 18, 558–573. [Google Scholar] [CrossRef]
- Ibraheem, H.H.; Queen, B.K.; Al-Sabti, M.D.; Issa, A.A.; Al-Majedy, Y.K.; Jabir, M.S.; Sulaiman, G.M.; Hasoon, B.A.; Eshaq, M.M.; Jawad, K.H.; et al. Insights into the pharmaceutical properties and in silico study of novel hydrazone derivatives. Sci. Rep. 2024, 14, 29912. [Google Scholar] [CrossRef]
- Feng, W.; Hao, Z.; Li, M. Isolation and Structure Identification of Flavonoids. In Flavonoids—From Biosynthesis to Human Health; Justino, G., Ed.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Date, K.; Satoh, A.; Iida, K.; Ogawa, H. Pancreatic α-Amylase Controls Glucose Assimilation by Duodenal Retrieval through N-Glycan-specific Binding, Endocytosis, and Degradation. J. Biol. Chem. 2015, 290, 17439–17450. [Google Scholar] [CrossRef]
- de Souza, P.M.; de Oliveira Magalhães, P. Application of microbial α-amylase in industry—A review. Braz. J. Microbiol. 2010, 41, 850–861. [Google Scholar] [CrossRef]
- Kenza, B.; Djihane, A.; Mouad, B.; Ratiba, M.; Samir, B.; Fadila, B.; Souad, A. Renoprotective Effect of Centaurea choulettiana Pomel (Asteraceae) Leaves on Cisplatin-induced Oxidative Stress and Renal dysfunction in Mice. J. Appl. Pharm. Sci. 2017, 7, 147–154. [Google Scholar]
- Bensaad, M.; Dassamiour, S.; Hambaba, L.; Saidi, A.; Melakhsou, M.; Nouicer, F.; Baghiani, A.; Khennouf, S.; Kahoul, M.; Kadrine, N. In vivo investigation of antidiabetic, hepatoprotective, anti-inflammatory and antipyretic activities of Centaurea tougourensis Boiss. & Reut. J. Physiol. Pharmacol. 2021, 72, 439–449. [Google Scholar]
- Hajleh, M.N.A.; Khleifat, K.M.; Alqaraleh, M.; Al-Hraishat, E.a.; Al-Limoun, M.O.; Qaralleh, H.; Al-Dujaili, E.A. Antioxidant and antihyperglycemic effects of ephedra foeminea aqueous extract in streptozotocin-induced diabetic rats. Nutrients 2022, 14, 2338. [Google Scholar] [CrossRef] [PubMed]
- Erendor, F.; Eksi, Y.E.; Sahin, E.O.; Balci, M.K.; Griffith, T.S.; Sanlioglu, S. Lentivirus Mediated Pancreatic Beta-Cell-Specific Insulin Gene Therapy for STZ-Induced Diabetes. Mol. Ther. 2021, 29, 149–161. [Google Scholar] [CrossRef]
- Maiti, R.; Das, U.K.; Ghosh, D. Attenuation of hyperglycemia and hyperlipidemia in streptozotocin-induced diabetic rats by aqueous extract of seed of Tamarindus indica. Biol. Pharm. Bull. 2005, 28, 1172–1176. [Google Scholar] [CrossRef]
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef]
- Abd-Alhussain, G.K.; Alatrakji, M.; Ahmed, S.J.; Fawzi, H.A. Efficacy of oral insulin nanoparticles for the management of hyperglycemia in a rat model of diabetes induced with streptozotocin. J. Med. Life 2024, 17, 217–225. [Google Scholar] [CrossRef]
- Gomathi, D.; Kalaiselvi, M.; Ravikumar, G.; Devaki, K.; Uma, C. Evaluation of antioxidants in the kidney of streptozotocin induced diabetic rats. Indian J. Clin. Biochem. IJCB 2014, 29, 221–226. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Ji, N.; Kim, S.-G.; Park, H.-H.; Lee, E.; Lee, Y.J.; Jin, M.; Lee, E. Nepetin, a natural compound from Inulae flos, suppresses degranulation and eicosanoid generation through PLCγ1 and Akt signaling pathways in mast cells. Arch. Pharmacal Res. 2020, 43, 224–232. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Ahn, D.; Kim, J.K.; Seo, S.O.; Chung, S.J. Nepetin Acts as a Multi-Targeting Inhibitor of Protein Tyrosine Phosphatases Relevant to Insulin Resistance. Chem. Biodivers. 2022, 19, e202100600. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Han, R.; Hao, P.; Wang, L.; Liu, M.; Jin, M.; Kong, D.; Li, X. Nepetin inhibits IL-1β induced inflammation via NF-κB and MAPKs signaling pathways in ARPE-19 cells. Biomed. Pharmacother. 2018, 101, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Clavin, M.; Gorzalczany, S.; Macho, A.; Muñoz, E.; Ferraro, G.; Acevedo, C.; Martino, V. Anti-inflammatory activity of flavonoids from Eupatorium arnottianum. J. Ethnopharmacol. 2007, 112, 585–589. [Google Scholar] [CrossRef]
- Dumon, M.F.; Freneix-Clerc, M.; Carbonneau, M.A.; Thomas, M.J.; Perromat, A.; Clerc, M. In vitro demonstration of the 3′-5,7-trihydroxy-4′-methoxy flavone rutinoside antilipoperoxidant activity. Ann. Biol. Clin. 1994, 52, 265–270. [Google Scholar]
- Tang, H.; Zeng, Q.; Ren, N.; Wei, Y.; He, Q.; Chen, M.; Pu, P. Kaempferide improves oxidative stress and inflammation by inhibiting the TLR4/IκBα/NF-κB pathway in obese mice. Iran. J. Basic Med. Sci. 2021, 24, 493–498. [Google Scholar] [CrossRef]
- Nguyen, V.-S.; Shi, L.; Luan, F.-Q.; Wang, Q.-A. Synthesis of kaempferide Mannich base derivatives and their antiproliferative activity on three human cancer cell lines. Acta Biochim. Pol. 2015, 62, 547–552. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, X.; Li, D.; Hao, W.; Meng, F.; Wang, B.; Han, J.; Zheng, Q. Kaempferide protects against myocardial ischemia/reperfusion injury through activation of the PI3K/Akt/GSK-3β pathway. Mediat. Inflamm. 2017, 2017, 5278218. [Google Scholar] [CrossRef]
- Tang, H.; Zeng, Q.; Tang, T.; Wei, Y.; Pu, P. Kaempferide improves glycolipid metabolism disorder by activating PPARγ in high-fat-diet-fed mice. Life Sci. 2021, 270, 119133. [Google Scholar] [CrossRef]
- He, X.-W.; Yu, D.; Li, W.-L.; Zheng, Z.; Lv, C.-L.; Li, C.; Liu, P.; Xu, C.-Q.; Hu, X.-F.; Jin, X.-P. Anti-atherosclerotic potential of baicalin mediated by promoting cholesterol efflux from macrophages via the PPARγ-LXRα-ABCA1/ABCG1 pathway. Biomed. Pharmacother. 2016, 83, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Bossé, Y.; Weisnagel, S.J.; Bouchard, C.; Després, J.P.; Pérusse, L.; Vohl, M.C. Combined effects of PPARγ2 P12A and PPARα L162V polymorphisms on glucose and insulin homeostasis: The Québec Family Study. J. Hum. Genet. 2003, 48, 614–621. [Google Scholar] [CrossRef]
- Shyni, G.L.; Sasidharan, K.; Francis, S.K.; Das, A.A.; Nair, M.S.; Raghu, K.G. Licarin B from Myristica fragrans improves insulin sensitivity via PPARγ and activation of GLUT4 in the IRS-1/PI3K/AKT pathway in 3T3-L1 adipocytes. RSC Adv. 2016, 6, 79859–79870. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Stalin, A.; Balakrishna, K.; Ignacimuthu, S.; Paulraj, M.G.; Vishal, R. Insulin sensitization via partial agonism of PPARγ and glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway by embelin in type 2 diabetic rats. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef]
- Sangeetha, R. Luteolin in the management of type 2 diabetes mellitus. Curr. Res. Nutr. Food Sci. J. 2019, 7, 393–398. [Google Scholar] [CrossRef]
- Kahksha; Alam, O.; Al-Keridis, L.A.; Khan, J.; Naaz, S.; Alam, A.; Ashraf, S.A.; Alshammari, N.; Adnan, M.; Beg, M.A. Evaluation of Antidiabetic Effect of Luteolin in STZ Induced Diabetic Rats: Molecular Docking, Molecular Dynamics, In Vitro and In Vivo Studies. J. Funct. Biomater. 2023, 14, 126. [Google Scholar] [CrossRef]
- Sheng, Y.; Zheng, S.; Ma, T.; Zhang, C.; Ou, X.; He, X.; Xu, W.; Huang, K. Mulberry leaf alleviates streptozotocin-induced diabetic rats by attenuating NEFA signaling and modulating intestinal microflora. Sci. Rep. 2017, 7, 12041. [Google Scholar] [CrossRef]
- Bogers, R.J.; Craker, L.E.; Lange, D. Medicinal and Aromatic Plants: Agricultural, Commercial, Ecological, Legal, Pharmacological and Social Aspects; Springer: Berlin/Heidelberg, Germany, 2006; Volume 17. [Google Scholar]
- Prasedya, E.S.; Frediansyah, A.; Martyasari, N.W.R.; Ilhami, B.K.; Abidin, A.S.; Padmi, H.; Fahrurrozi; Juanssilfero, A.B.; Widyastuti, S.; Sunarwidhi, A.L. Effect of particle size on phytochemical composition and antioxidant properties of Sargassum cristaefolium ethanol extract. Sci. Rep. 2021, 11, 17876. [Google Scholar] [CrossRef]
- Subramaniam, S.; Vaughn, K.; Carrier, D.J.; Clausen, E.C. Pretreatment of milk thistle seed to increase the silymarin yield: An alternative to petroleum ether defatting. Bioresour. Technol. 2008, 99, 2501–2506. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Jandera, P.; Churacek, J. Gradient Elution in Column Liquid Chromatography: Theory and Practice; Elsevier: Amsterdam, The Netherlands, 1985. [Google Scholar]
- Garayev, E.; Di Giorgio, C.; Herbette, G.; Mabrouki, F.; Chiffolleau, P.; Roux, D.; Sallanon, H.; Ollivier, E.; Elias, R.; Baghdikian, B. Bioassay-guided isolation and UHPLC-DAD-ESI-MS/MS quantification of potential anti-inflammatory phenolic compounds from flowers of Inula montana L. J. Ethnopharmacol. 2018, 226, 176–184. [Google Scholar] [CrossRef]
- Colowick, S.P.; Kaplan, N.O. Amylase, α and β in Methods in Enzymology; Bernfeld, P., Ed.; Academic Press: New York, NY, USA, 1955; pp. 149–158. [Google Scholar]
- Rao, P.S.; Mohan, G.K. In vitro alpha-amylase inhibition and in vivo antioxidant potential of Momordica dioica seeds in streptozotocin-induced oxidative stress in diabetic rats. Saudi J. Biol. Sci. 2017, 24, 1262–1267. [Google Scholar] [CrossRef]
- Buchwald, P. A single unified model for fitting simple to complex receptor response data. Sci. Rep. 2020, 10, 13386. [Google Scholar] [CrossRef]
- .Leary, S.; Underwood, W.; Anthony, R.; Cartner, S.; Grandin, T.; Greenacre, C.; Gwaltney-Brant, S.; McCrackin, M.A.; Meyer, R.; Miller, D.; et al. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2020. [Google Scholar]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Jeddi, S. Streptozotocin as a tool for induction of rat models of diabetes: A practical guide. EXCLI J. 2023, 22, 274–294. [Google Scholar] [CrossRef]
- Ramírez, K.; Quesada-Yamasaki, D.; Jaime, F.-T.C. A Protocol to Perform Systemic Lipopolysacharide (LPS) Challenge in Rats. Odovtos 2019, 21, 53–66. [Google Scholar] [CrossRef]
- Khafaji, A.W.; Al-Zubaidy, A.A.; Farhood, I.G.; Fawzi, H.A. Effects of topical isoxsuprine ointment on imiquimod-induced psoriasiform skin inflammation in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 398, 1545–1556. [Google Scholar] [CrossRef]
- Bouhrim, M.; Bencheikh, N.; Imtara, H.; Daoudi, N.E.; Mechchate, H.; Ouassou, H.; Kharchoufa, L.; Elachouri, M.; Mekhfi, H.; Ziyyat, A.; et al. Protective Effect of Opuntia dillenii (Ker Gawl.) Haw. Seed Oil on Gentamicin-Induced Nephrotoxicity: A Biochemical and Histological Analysis. Sci. World J. 2021, 2021, 2173012. [Google Scholar] [CrossRef]
- Han, X.; Tao, Y.L.; Deng, Y.P.; Yu, J.W.; Cai, J.; Ren, G.F.; Sun, Y.N.; Jiang, G.J. Metformin ameliorates insulitis in STZ-induced diabetic mice. PeerJ 2017, 5, e3155. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Mostofa, M.; Hoque, M.; Das, S.; Sarkar, A. Comparative efficacy of Neem (Azadirachta indica) and Metformin hydrochloride (Comet®) in streptozotocin induced diabetes melitus in rats. Bangl. J. Vet. Med. 2010, 8, 75–80. [Google Scholar] [CrossRef]
- Heinegård, D.; Tiderström, G. Determination of serum creatinine by a direct colorimetric method. Clin. Chim. Acta Int. J. Clin. Chem. 1973, 43, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.-U. Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Talke, H.; Schubert, G.E. Enzymatic urea determination in the blood and serum in the warburg optical test. Klin. Wochenschr. 1965, 43, 174–175. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Festing, M.F.W. Design and Statistical Methods in Studies Using Animal Models of Development. ILAR J. 2006, 47, 5–14. [Google Scholar] [CrossRef]
- García-Pérez, M.A. Use and misuse of corrections for multiple testing. Methods Psychol. 2023, 8, 100120. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).