Antibacterial and Antifungal Activity of Extracts from Five Portuguese Cowpea (Vigna unguiculata) Accessions
Abstract
1. Introduction
2. Results
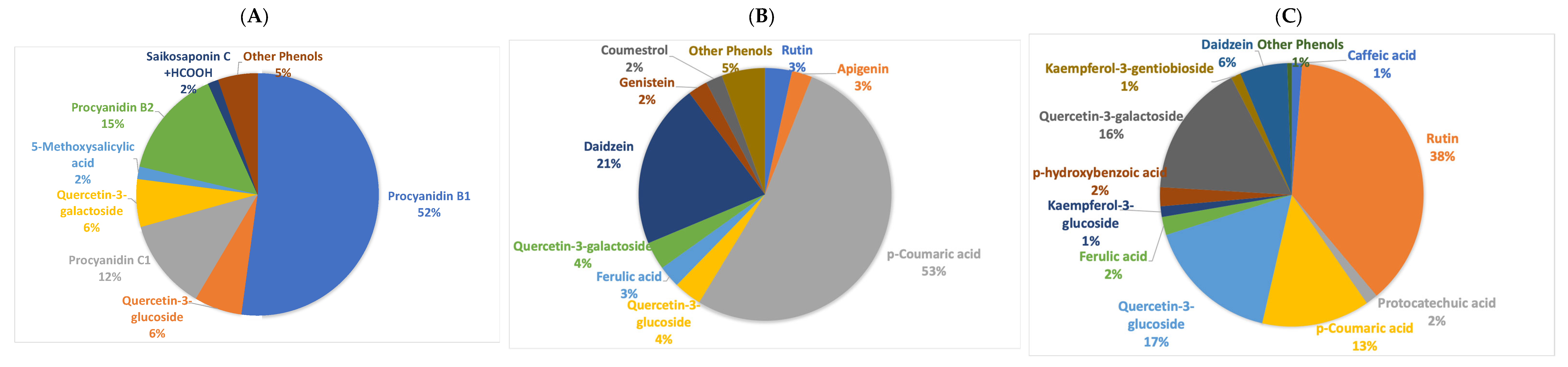
2.1. Phenolic Content of the Extracts
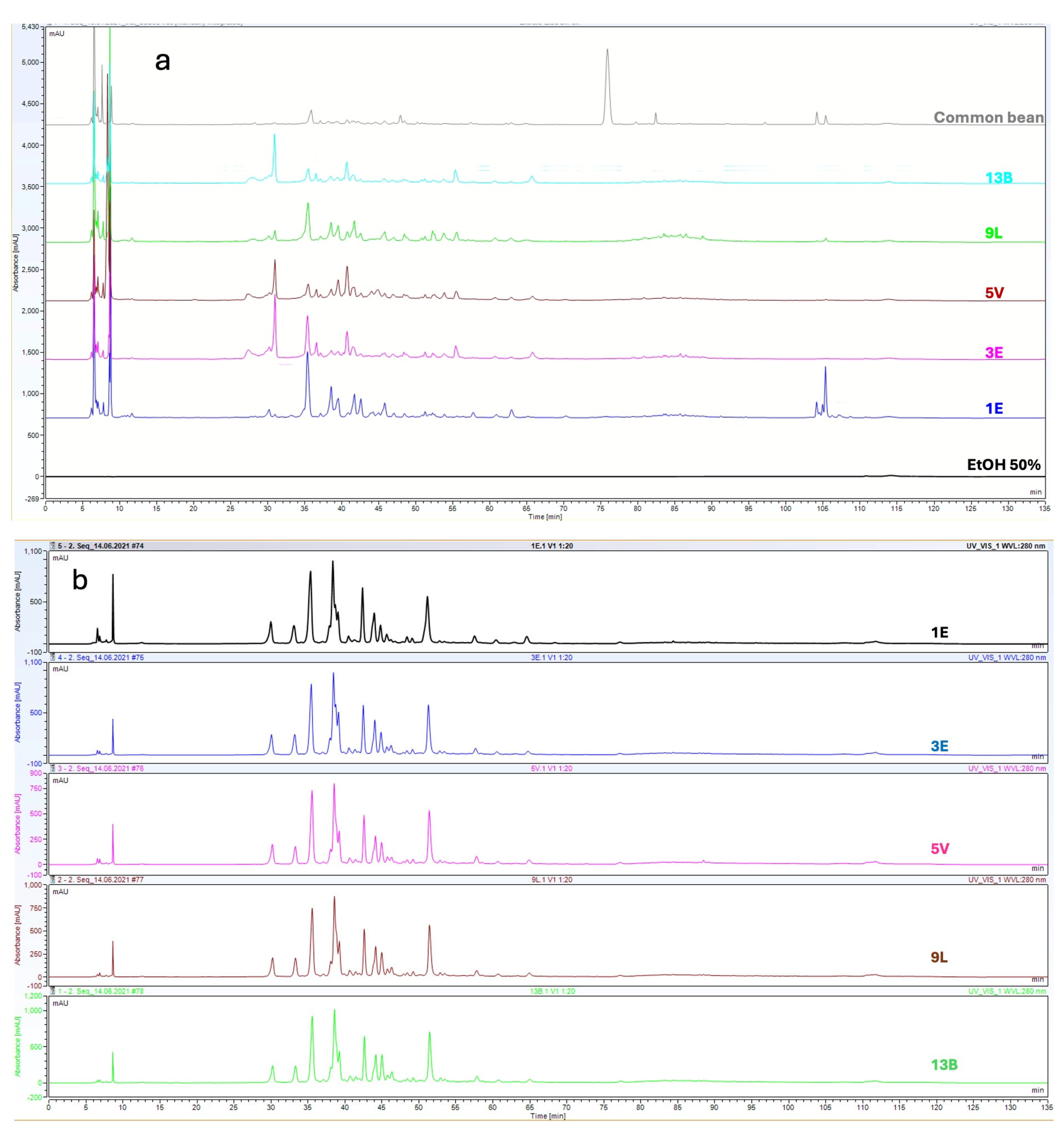
2.2. Metabolic Diversity of Cowpea Extracts
2.3. Antifungal Activity of Cowpea Extracts from Variety 9L (Guarda Do Douro)
2.4. Antibacterial Activity
2.4.1. MIC and MBC of Extracts of Pods and Leaves
2.4.2. Mean Logarithmic Reduction in Bacterial Cell Viability
3. Discussion
4. Methods
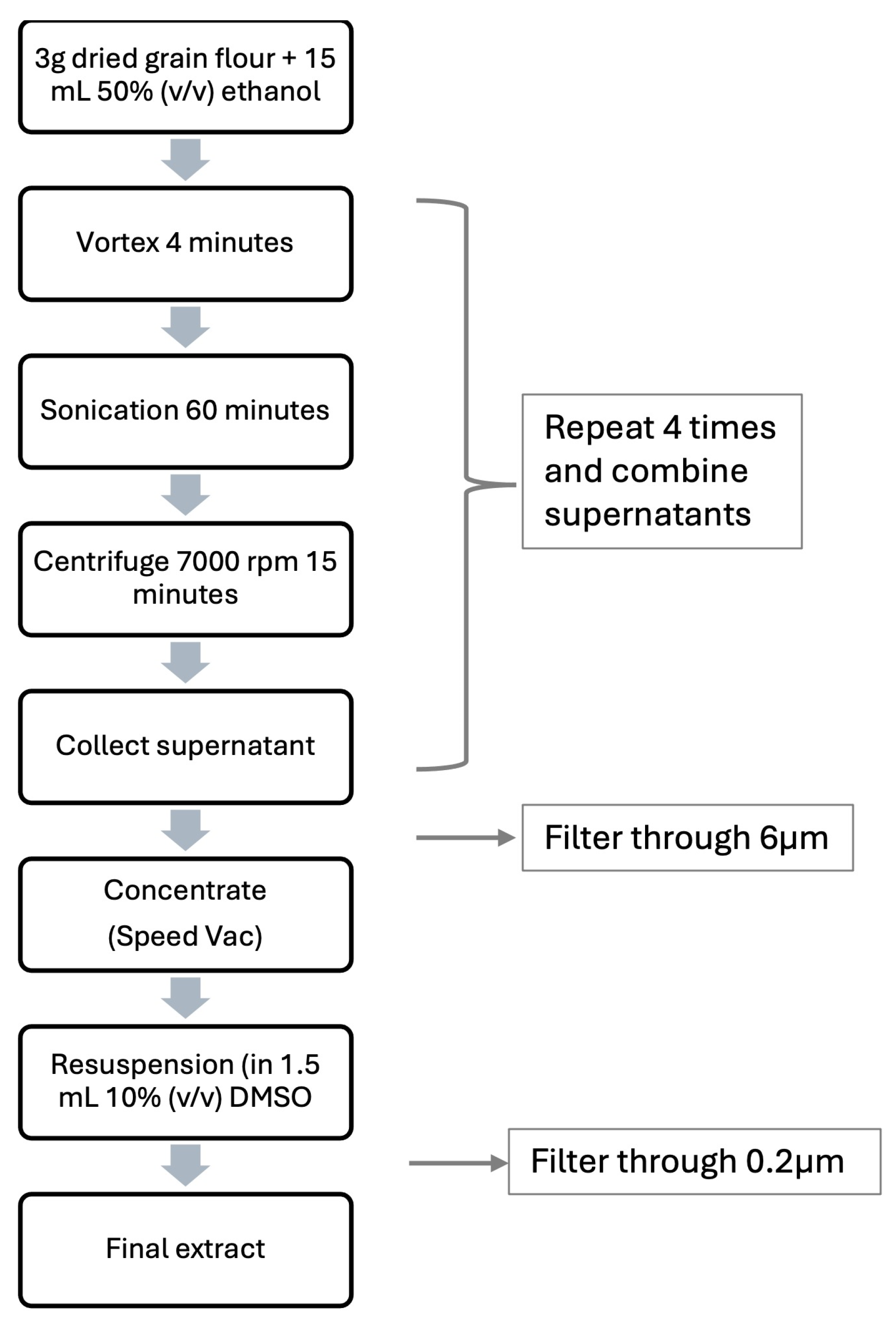
4.1. Plant Extracts
4.2. Total Phenolic Content (TPC)
4.3. In Vitro Antioxidant Activity
4.4. Characterization of Phenolic Compounds Through Quadrupole Time-of-Flight (QTOF) Mass Analyzers
4.5. Data Processing, Identification and Relative Quantification of Compounds
4.6. Bacteria and Fungi Used
4.7. Evaluation of the Antimicrobial Activity of the Extracts
4.7.1. Antifungal Activity
Preparation of the Conidial Suspensions
Determination of the MIC of the Extracts from Landrace 9L Against Fungi
4.7.2. Antibacterial Activity
Preparation of the Inocula
Determination of the MIC and MBC of the Extracts Against Bacteria
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.-K.; Iwar, K.; Ochar, K.; Park, S.-Y.; Go, E.-B.; Lee, K.-D.; Kim, S.-H. Cowpea (Vigna unguiculata) Cultivation and Breeding in the Republic of Korea: Advances and Future Perspectives. Agronomy 2024, 14, 2679. [Google Scholar] [CrossRef]
- Moreira, R.; Nunes, C.; Pais, I.P.; Nobre Semedo, J.; Moreira, J.; Sofia Bagulho, A.; Pereira, G.; Manuela Veloso, M.; Scotti-Campos, P. Are Portuguese Cowpea Genotypes Adapted to Drought? Phenological Development and Grain Quality Evaluation. Biology 2023, 12, 507. [Google Scholar] [CrossRef] [PubMed]
- Lazaridi, E.; Bebeli, P.J. Cowpea Constraints and Breeding in Europe. Plants 2023, 12, 1339. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Moreira, R.; Pais, I.; Semedo, J.; Simões, F.; Veloso, M.M.; Scotti-Campos, P. Cowpea Physiological Responses to Terminal Drought—Comparison between Four Landraces and a Commercial Variety. Plants 2022, 11, 593. [Google Scholar] [CrossRef]
- Ali, A.; Al Saady, N.A.; Waly, M.I.; Bhatt, N.; Al Subhi, A.M.; Khan, A.J. Evaluation of Indigenous Omani Legumes for Their Nutritional Quality, Phytochemical Composition and Antioxidant Properties. Int. J. Postharvest Technol. Innov. 2013, 3, 333. [Google Scholar] [CrossRef]
- Bell, L.W.; Ryan, M.H.; Bennett, R.G.; Collins, M.T.; Clarke, H.J. Growth, Yield and Seed Composition of Native Australian Legumes with Potential as Grain Crops. J. Sci. Food Agric. 2012, 92, 1354–1361. [Google Scholar] [CrossRef]
- Wang, T.L.; Domoney, C.; Hedley, C.L.; Casey, R.; Grusak, M.A. Can We Improve the Nutritional Quality of Legume Seeds? Plant Physiol. 2003, 131, 886–891. [Google Scholar] [CrossRef]
- Lonardi, S.; Muñoz-Amatriaín, M.; Liang, Q.; Shu, S.; Wanamaker, S.I.; Lo, S.; Tanskanen, J.; Schulman, A.H.; Zhu, T.; Luo, M.; et al. The Genome of Cowpea (Vigna unguiculata [L.] Walp.). Plant J. 2019, 98, 767–782. [Google Scholar] [CrossRef]
- Boukar, O.; Belko, N.; Chamarthi, S.; Togola, A.; Batieno, J.; Owusu, E.; Haruna, M.; Diallo, S.; Umar, M.L.; Olufajo, O.; et al. Cowpea (Vigna unguiculata): Genetics, Genomics and Breeding. Plant Breed. 2019, 138, 415–424. [Google Scholar] [CrossRef]
- Lenny, S.; Rizky, D.W. Potential Antibacterial and Antioxidant Activiy of Methanolic Extract of Vigna unguiculata (L.) Walp Leaves. In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation, Medan, Indonesia, 21–22 July 2019; SCITEPRESS—Science and Technology Publications: Setúbal, Portugal, 2019; pp. 215–217. [Google Scholar]
- Kritzinger, Q.; Lall, N.; Aveling, T.A.S.; van Wyk, B.-E. Antimicrobial Activity of Cowpea (Vigna unguiculata) Leaf Extracts. S. Afr. J. Bot. 2005, 71, 45–48. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Haney, E.F.; Gill, E.E. The Immunology of Host Defence Peptides: Beyond Antimicrobial Activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Potential Role of Bioactive Peptides in Prevention and Treatment of Chronic Diseases: A Narrative Review. Br. J. Pharmacol. 2017, 174, 1378–1394. [Google Scholar] [CrossRef] [PubMed]
- Ashraduzzaman, M.; Alam, M.; Khatun, S.; Absar, N. Antimicrobial Activity of Vigna unguiculata L. Walp Seed Oil. Int. J. Biotechnol. Wellness Ind. 2016, 5, 70–75. [Google Scholar] [CrossRef]
- Sardar, H.; Hadi, F.; Alam, W.; Halawani, I.F.; Alzahrani, F.M.; Saleem, R.A.; Cerqua, I.; Khan, H.; Capasso, R. Unveiling the Therapeutic and Nutritious Potential of Vigna unguiculata in Line with Its Phytochemistry. Heliyon 2024, 10, e37911. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural Products as Antimicrobial Agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef]
- Thery, T.; Arendt, E.K. Antifungal Activity of Synthetic Cowpea Defensin Cp-Thionin II and Its Application in Dough. Food Microbiol. 2018, 73, 111–121. [Google Scholar] [CrossRef]
- Shashirekha, M.N.; Mallikarjuna, S.E.; Rajarathnam, S. Status of Bioactive Compounds in Foods, with Focus on Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2015, 55, 1324–1339. [Google Scholar] [CrossRef]
- Sharma, M.; Kaushik, P. Vegetable Phytochemicals: An Update on Extraction and Analysis Techniques. Biocatal Agric. Biotechnol. 2021, 36, 102149. [Google Scholar] [CrossRef]
- Ahmad, I.; Huang, P.-J.; Malak, N.; Khan, A.; Asad, F.; Chen, C.-C. Antioxidant Potential of Alkaloids and Polyphenols of Viola Canescens Wall Using in Vitro and in Silico Approaches. Front. Chem. 2024, 12, 1379463. [Google Scholar] [CrossRef]
- Parvez, A.K.; Jubyda, F.T.; Ayaz, M.; Sarker, A.; Haque, N.; Khan, M.S.; Mou, T.J.; Rahman, M.A.; Huq, M.A. Microbial- and Plant-Derived Bioactive Peptides and Their Applications against Foodborne Pathogens: Current Status and Future Prospects. Int. J. Microbiol. 2024, 2024, 9978033. [Google Scholar] [CrossRef] [PubMed]
- Avanza, M.V.; Álvarez-Rivera, G.; Cifuentes, A.; Mendiola, J.A.; Ibáñez, E. Phytochemical and Functional Characterization of Phenolic Compounds from Cowpea (Vigna unguiculata (L.) Walp.) Obtained by Green Extraction Technologies. Agronomy 2021, 11, 162. [Google Scholar] [CrossRef]
- Cetin-Karaca, H.; Newman, M.C. Antimicrobial Efficacy of Plant Phenolic Compounds against Salmonella and Escherichia Coli. Food Biosci. 2015, 11, 8–16. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic Acid with Antiviral and Antibacterial Activity as A Promising Component of Biomaterials—A Minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef] [PubMed]
- Cipriano-Salazar, M.; Rojas-Hernández, S.; Olivares-Pérez, J.; Jiménez-Guillén, R.; Cruz-Lagunas, B.; Camacho-Díaz, L.M.; Ugbogu, A.E. Antibacterial Activities of Tannic Acid against Isolated Ruminal Bacteria from Sheep. Microb. Pathog. 2018, 117, 255–258. [Google Scholar] [CrossRef]
- Hochma, E.; Yarmolinsky, L.; Khalfin, B.; Nisnevitch, M.; Ben-Shabat, S.; Nakonechny, F. Antimicrobial Effect of Phytochemicals from Edible Plants. Processes 2021, 9, 2089. [Google Scholar] [CrossRef]
- Pina-Pérez, M.C.; Ferrús Pérez, M.A. Antimicrobial Potential of Legume Extracts against Foodborne Pathogens: A Review. Trends Food Sci. Technol. 2018, 72, 114–124. [Google Scholar] [CrossRef]
- Uuh Narvaez, J.J.; Castellanos Ruelas, A.F.; Olivera Castillo, L.; Puerto Castillo, C.; Segura Campos, M.R. Saponins from Vigna unguiculata Husks Obtained by Microwave-Assisted Extraction: Identification and Mechanism of Inhibition on Urease Activity. S. Afr. J. Bot. 2023, 154, 265–272. [Google Scholar] [CrossRef]
- Oulahal, N.; Degraeve, P. Phenolic-Rich Plant Extracts With Antimicrobial Activity: An Alternative to Food Preservatives and Biocides? Front. Microbiol. 2022, 12, 753518. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Al-Mohammadi, A.-R.; Osman, A.; Enan, G.; Abdel-Hameid, S.; Sitohy, M. Characterization and Antibacterial Activity of 7S and 11S Globulins Isolated from Cowpea Seed Protein. Molecules 2019, 24, 1082. [Google Scholar] [CrossRef]
- Sombié, P.; Compaoré, M.; Coulibaly, A.; Ouédraogo, J.; Tignégré, J.-B.; Kiendrébéogo, M. Antioxidant and Phytochemical Studies of 31 Cowpeas (Vigna unguiculata (L. Walp.)) Genotypes from Burkina Faso. Foods 2018, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Marathe, S.A.; Rajalakshmi, V.; Jamdar, S.N.; Sharma, A. Comparative Study on Antioxidant Activity of Different Varieties of Commonly Consumed Legumes in India. Food Chem. Toxicol. 2011, 49, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.; Lino-Neto, T.; Rosa, E.; Carnide, V. Cowpea: A Legume Crop for a Challenging Environment. J. Sci. Food Agric. 2017, 97, 4273–4284. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M.; Duodu, K.G. Bioactive Polyphenols and Peptides in Cowpea (Vigna unguiculata) and Their Health Promoting Properties: A Review. J. Funct. Foods 2017, 38, 686–697. [Google Scholar] [CrossRef]
- Wolff, S.M.; da Silveira, A.C.; Lazzarotto, M. Metodologia Para Extração de Fenólicos Totais e Antioxidantes Da Erva-Mate. Iniciação Científica Cesumar 2019, 21, 45. [Google Scholar] [CrossRef]
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic Interactions between Phenolic Compounds Identified in Grape Pomace Extract with Antibiotics of Different Classes against Staphylococcus Aureus and Escherichia Coli. PLoS ONE 2017, 12, e0172273. [Google Scholar] [CrossRef]
- Mattos, M.M.G.; Filho, S.A.; Martins, G.R.; Venturi, L.S.; Canetti, V.B.; Ferreira, F.A.; Foguel, D.; da Silva, A.S. Antimicrobial and Antibiofilm Properties of Procyanidins: Potential for Clinical and Biotechnological Applications. Crit. Rev. Microbiol. 2024, 1–24. [Google Scholar] [CrossRef]
- De Rossi, L.; Rocchetti, G.; Lucini, L.; Rebecchi, A. Antimicrobial Potential of Polyphenols: Mechanisms of Action and Microbial Responses—A Narrative Review. Antioxidants 2025, 14, 200. [Google Scholar] [CrossRef]
- Dinev, T.; Tzanova, M.; Velichkova, K.; Dermendzhieva, D.; Beev, G. Antifungal and Antioxidant Potential of Methanolic Extracts from Acorus Calamus L., Chlorella Vulgaris Beijerinck, Lemna Minuta Kunth and Scenedesmus Dimorphus (Turpin) Kützing. Appl. Sci. 2021, 11, 4745. [Google Scholar] [CrossRef]
- Medeiros, J.G.F.; Araujo Neto, A.C.; Silva, E.C.; Rodrigues, R.d.M.; Demartelaere, A.C.F.; da Silva, J.V.B. Phytochemical Profile and Antifungal Action of Anadenanthera Colubrina Extract on the Quality of Maize Seeds. Arq. Inst. Biol. 2021, 88, e0762019. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R. Antifungal Efficacy of Some Natural Phenolic Compounds against Significant Pathogenic and Toxinogenic Filamentous Fungi. Chemosphere 2013, 93, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Šimović, M.; Delaš, F.; Gradvol, V.; Kocevski, D.; Pavlović, H. Antifungal Effect of Eugenol and Carvacrol against Foodborne Pathogens Aspergillus Carbonarius and Penicillium Roqueforti in Improving Safety of Fresh-Cut Watermelon. J. Intercult. Ethnopharmacol. 2014, 3, 91. [Google Scholar] [CrossRef] [PubMed]
- Stefanović, O.D.; Tešić, J.D.; Čomić, L.R. Melilotus Albus and Dorycnium Herbaceum Extracts as Source of Phenolic Compounds and Their Antimicrobial, Antibiofilm, and Antioxidant Potentials. J. Food Drug Anal. 2015, 23, 417–424. [Google Scholar] [CrossRef]
- Korukluoglu, M.; Sahan, Y.; Yigit, A. Antifungal Properties of Olive Leaf Extracts and Their Phenolic Compounds. J. Food Saf. 2008, 28, 76–87. [Google Scholar] [CrossRef]
- Cui, E.-J.; Song, N.-Y.; Shrestha, S.; Chung, I.-S.; Kim, J.-Y.; Jeong, T.-S.; Baek, N.-I. Flavonoid Glycosides from Cowpea Seeds (Vigna Sinensis K.) Inhibit LDL Oxidation. Food Sci. Biotechnol. 2012, 21, 619–624. [Google Scholar] [CrossRef]
- Brown, L.; Wolf, J.M.; Prados-Rosales, R.; Casadevall, A. Through the Wall: Extracellular Vesicles in Gram-Positive Bacteria, Mycobacteria and Fungi. Nat. Rev. Microbiol. 2015, 13, 620–630. [Google Scholar] [CrossRef]
- Nohynek, L.J.; Alakomi, H.-L.; Kähkönen, M.P.; Heinonen, M.; Helander, I.M.; Oksman-Caldentey, K.-M.; Puupponen-Pimiä, R.H. Berry Phenolics: Antimicrobial Properties and Mechanisms of Action Against Severe Human Pathogens. Nutr. Cancer 2006, 54, 18–32. [Google Scholar] [CrossRef]
- Candeias, M.R. Avaliação Das Propriedades Antimicrobianas de Extratos de Feijão-Frade Dissertação Para a Obtenção Do Grau de Mestre Em Engenharia Alimentar. Master’s Thesis, Universidade de Lisboa, Lisboa, Portugal, 2021. [Google Scholar]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial Herb and Spice Compounds in Food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Erny, G.L.; Acunha, T.; Simó, C.; Cifuentes, A.; Alves, A. Finnee—A Matlab Toolbox for Separation Techniques Hyphenated High Resolution Mass Spectrometry Dataset. Chemom. Intell. Lab. Syst. 2016, 155, 138–144. [Google Scholar] [CrossRef]
- Chong, S.; Dugast-Darzacq, C.; Liu, Z.; Dong, P.; Dailey, G.M.; Cattoglio, C.; Heckert, A.; Banala, S.; Lavis, L.; Darzacq, X.; et al. Imaging Dynamic and Selective Low-Complexity Domain Interactions That Control Gene Transcription. Science 1979, 2018, 361. [Google Scholar] [CrossRef]
- Singh, U.; Akhtar, S.; Mishra, A.; Sarkar, D. A Novel Screening Method Based on Menadione Mediated Rapid Reduction of Tetrazolium Salt for Testing of Anti-Mycobacterial Agents. J. Microbiol. Methods 2011, 84, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.T.; Gholami, H.; Kavoosi, G.; Rowshan, V.; Tafsiry, A. Chemical Composition, Antioxidant, Antimicrobial and Cytotoxic Activities of Tagetes Minuta and Ocimum Basilicum Essential Oils Essential Oils. Food Sci. Nutr. 2014, 2, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- CLSI M26-A; Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline This Document Provides Procedures for Determining the Lethal Activity of Antimicrobial Agents. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 1999; Volume 19.
| Phenolic Content (mg Gallic Acid Equivalents/100 g Dry Mass) | Antioxidant Activity (μmole of Trolox Equivalents Antioxidant Capacity per g of Seed’s Dry Weight) | |||
|---|---|---|---|---|
| Cowpea Reference | Grains | Leaves | Pods | Grains |
| 1E—commercial Fradel | 70.5 ± 15.5 | 117 ± 9.1 | 516.7 ± 12.3 | 38.6 ± 11.7 |
| 3E—Sátão/Viseu | 263.7 ± 12.1 | 279.4 ± 37.8 | 569.2 ± 16.0 | 163.8 ± 4.6 |
| 5V—Vale Pedro, Vila Maior/Viseu | 219.9 ± 4.7 | nd | 405.9 ± 17.0 | 130.5 ± 8.6 |
| 9L—Lardosa/Castelo Branco | 64.0 ± 5.2 | 95.4 ± 16.1 | 877.2 ± 29.7 | 29.7 ± 7.6 |
| 13B—Guarda do Douro | 257.5 ± 28.2 | 158.1 ± 32.3 | 868.7 ± 47.4 | 125.7 ± 10.6 |
| Compound | Family; Class | Formula | Found at Mass | Grains | Pods | Leaves |
|---|---|---|---|---|---|---|
| Catechin | Flavonoids; Flavanol | C15H14O6 | 291.088 | x | x | |
| Caffeic acid | Phenolic acids; Hydroxycinnamic acid | C9H8O4 | 181.0513 | x | x | x |
| Rutin | Flavonoids; Flavonol glycoside | C27H30O16 | 611.1643 | x | x | x |
| Quercetin | Flavonoids; Flavonol | C15H10O7 | 303.0508 | x | x | x |
| Kaempferol | Flavonoids; Flavonol | C15H10O6 | 287.057 | x | x | x |
| Apigenin | Flavonoids; Flavonol | C15H10O5 | 271.0617 | x | x | x |
| Protocatechuic acid | Phenolic acids; Hydroxybenzoic acid | C7H6O4 | 155.0353 | x | x | x |
| Procyanidin B1 | Flavonoids; Proanthocyanidin (dimer) | C30H26O12 | 579.1503 | x | ||
| p-Coumaric acid | Phenolic acids; Hydroxycinnamic acid | C9H8O3 | 165.0555 | x | x | x |
| Quercetin-3-glucoside | Flavonoids; Flavonol glycoside | C21H20O12 | 465.1038 | x | x | x |
| Epicatechin | Flavonoids; Flavanol | C15H14O6 | 289.0723 | x | x | |
| Procyanidin C1 | Flavonoids; Proanthocyanidin (trimer) | C45H38O18 | 867.2143 | x | x | |
| Ferulic acid | Phenolic acids; Hydroxycinnamic acid | C10H10O4 | 195.0663 | x | x | x |
| Kaempferol-3-glucoside | Flavonoids; Flavonol glycoside | C21H20O11 | 449.1102 | x | x | x |
| Quercetin-3-arabinoside | Flavonoids; Flavonol glycoside | C20H18O11 | 435.0918 | x | ||
| Vanillic acid | Phenolic acids; Hydroxybenzoic acid | C8H8O4 | 169.0508 | x | x | |
| p-hydroxybenzoic acid | Phenolic acids; Hydroxybenzoic acid | C7H6O3 | 139.0399 | x | x | |
| Syringic acid | Phenolic acids; Hydroxybenzoic acid | C9H10O5 | 199.0609 | x | ||
| Quercetin-3-galactoside | Flavonoids; Flavonol glycoside | C21H20O12 | 465.1038 | x | x | x |
| Phloretin | Dihydrochalcones; Flavonoid-like polyphenol | C15H14O5 | 275.0967 | x | ||
| 5-Methoxysalicylic acid | Phenolic acids; Methoxylated hydroxybenzoic acid | C8H8O4 | 167.0355 | x | ||
| Procyanidin B2 | Flavonoids; Proanthocyanidin (dimer) | C30H26O12 | 579.1503 | x | ||
| Quercetagetin-7-O-glucoside | Flavonoids; Flavonol glycoside | C21H19O13− | 479.0854 | x | ||
| Kaempferol-3-gentiobioside | Flavonoids; Flavonol diglycoside | C27H30O16 | 609.1478 | x | x | |
| Quercetin 3-O-β-D-glucose-6′-acetate | Flavonoids; Acylated flavonol glycoside | C23H22O13 | 505.1008 | x | x | |
| 5,7,3′,4′,5′-Pentahydroxyflavone | Flavonoids; Flavonol | C15H12O7 | 301.0364 | x | ||
| Daidzein | Isoflavonoids; Isoflavone | C15H10O4 | 253.0514 | x | x | x |
| (+)-Abscisic acid | Terpenoids; Sesquiterpenoid | C15H20O4 | 263.1294 | x | x | |
| Genistein | Isoflavonoids; Isoflavone | C15H10O5 | 269.0463 | x | x | |
| Coumestrol | Coumestans; Phytoestrogen | C15H8O5 | 267.0308 | x | x | |
| 2-Hydroxymyristic acid | Fatty acids; Hydroxy fatty acid (not a phenoplic compound) | C14H28O3 | 243.1972 | x | ||
| Apigeninidin cation | Anthocyanidins; Flavylium cation | C15H11O4+ | 255.2331 | x | x | |
| 5,7-Dimethoxyapigeninidin cation | Anthocyanidins; Methoxylated flavylium cation | C17H15O4 | 283.2651 | x |
| Bacteria | Pod Extracts | Leaf Extracts | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Accessions | |||||||||
| 1E | 3E | 5V | 9L | 13B | 1E | 3E | 9L | 13B | |
| MIC (mg/mL) | |||||||||
| Listeria innocua (Gram-positive and non-pathogenic) | 12.9 | 14.2 | 5.1 | 21.9 | − | 1.1 | 1.1 | 2.3 | 1.1 |
| Listeria monocytogenes (Gram-positive and pathogenic) | 36.9 | 20.4 | 29.0 | 31.4 | 62.1 | 1.1 | 1.1 | 2.3 | 2.3 |
| Escherichia coli (Gram-negative and non-pathogenic) | 51.7 | 56.9 | 10.1 | 87.7 | 46.5 | 9.1 | 9.1 | 9.1 | 9.1 |
| Salmonella enterica Thyphimurium (Gram-negative and pathogenic) | 73.9 | 20.4 | 29.0 | 62.7 | 62.1 | 4.5 | 9.1 | 9.1 | 9.1 |
| Bacteria | Pod Extracts | Leaf Extracts | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Accessions | |||||||||
| 1E | 3E | 5V | 9L | 13B | 1E | 3E | 9L | 13B | |
| MIC (mg/mL) | |||||||||
| Listeria innocua (Gram-positive and non-pathogenic) | 25.8 | 28.5 | 20.3 | 43.9 | − | 2.3 | 2.3 | 2.3 | 1.1 |
| Listeria monocytogenes (Gram-positive and pathogenic) | 39.6 | 40.7 | 29.0 | 62.7 | 62.1 | 2.3 | 1.1 | 4.5 | 2.3 |
| Escherichia coli (Gram-negative and non-pathogenic) | 51.7 | 56.9 | 40.6 | 87.7 | 46.5 | 9.1 | 9.1 | 9.1 | 9.1 |
| Salmonella Thyphimurium (Gram-negative and pathogenic) | − | 40.7 | 29.0 | 62.7 | 62.1 | 4.5 | 9.1 | 9.1 | 9.1 |
| Bacteria | MBC/MIC Ratio for the Extracts of the Five Cowpea Accessions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1E | 3E | 5V | 9L | 13B | |||||
| Pod | Leaf | Pod | Leaf | Pod | Pod | Leaf | Pod | Leaf | |
| Listeria innocua (Gram-positive and non-pathogenic) | 2 | 2 | 2 | 2 | 4 | 2 | 1 | b) | 1 |
| Listeria monocytogenes (Gram-positive and pathogenic) | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 |
| Escherichia coli (Gram-negative and non-pathogenic) | 1 | 1 | 1 | 1 | 4 | 1 | 1 | 1 | 1 |
| Salmonella Thyphimurium (Gram-negative and pathogenic) | a) | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Bacteria | Pod Extracts | Leaf Extracts | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accessions | ||||||||||||||||||
| 1E | 3E | 5V | 9L | 13B | 1E | 3E | 9L | 13B | ||||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| ∆ (Log Initial Number of CFU/mL—Log Final Number of CFU/mL) | ||||||||||||||||||
| Listeria innocua (Gram-positive and non-pathogenic) | 2.0 | 6.2 | 2.1 | 4.1 | 1.9 | 3.9 | 1.6 | 4.6 | - | - | 2.6 | 6.5 | 2.7 | 6.5 | 4.5 | 4.5 | 4.3 | 4.3 |
| Listeria monocytogenes (Gram-positive and pathogenic) | 5.3 | 5.3 | 2.4 | 6.8 | 6.8 | 6.8 | 2.4 | 6.8 | 6.8 | 6.8 | 1.8 | 6.5 | 3.3 | 3.3 | 2.8 | 7.2 | 6.5 | 6.5 |
| Escherichia coli (Gram-negative and non-pathogenic) | 5.3 | 4.8 | 6.3 | 6.3 | 2.0 | 6.3 | 6.3 | 6.3 | 3.7 | 3.7 | 7.2 | 7.2 | 7.2 | 7.2 | 5.2 | 5.2 | 7.4 | 7.4 |
| Salmonella Thyphimurium (Gram-negative and pathogenic) | 1.9 | - | 2.0 | 6.7 | 6.7 | 6.7 | 5.7 | 5.7 | 5.1 | 5.1 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 | 7.4 |
| Type | Reference | Species |
|---|---|---|
| Bacteria | CBISA3008 =NCTC11288 =ATCC33090 | Listeria innocua |
| CBISA3001 =NCTC11994 = CECT4032 | Listeria monocytogenes serovar 4b | |
| CBISA3965 | Escherichia coli B | |
| CBISA3969 =ATCC14028 | Salmonella enterica serovar Typhimurium | |
| Filamentous fungi | Unnamed internal collection | Penicillium expansum |
| Aspergillus flavus | ||
| Aspergillus niger |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamandane, A.; Candeias, M.; Lourenço, S.; Vieira, E.J.F.; Mecha, E.; Gomes, R.; Bronze, R.; Nunes, C.; Brito, L. Antibacterial and Antifungal Activity of Extracts from Five Portuguese Cowpea (Vigna unguiculata) Accessions. Molecules 2025, 30, 2348. https://doi.org/10.3390/molecules30112348
Salamandane A, Candeias M, Lourenço S, Vieira EJF, Mecha E, Gomes R, Bronze R, Nunes C, Brito L. Antibacterial and Antifungal Activity of Extracts from Five Portuguese Cowpea (Vigna unguiculata) Accessions. Molecules. 2025; 30(11):2348. https://doi.org/10.3390/molecules30112348
Chicago/Turabian StyleSalamandane, Acácio, Mariana Candeias, Susana Lourenço, Emília Joana F. Vieira, Elsa Mecha, Ricardo Gomes, Rosário Bronze, Cátia Nunes, and Luisa Brito. 2025. "Antibacterial and Antifungal Activity of Extracts from Five Portuguese Cowpea (Vigna unguiculata) Accessions" Molecules 30, no. 11: 2348. https://doi.org/10.3390/molecules30112348
APA StyleSalamandane, A., Candeias, M., Lourenço, S., Vieira, E. J. F., Mecha, E., Gomes, R., Bronze, R., Nunes, C., & Brito, L. (2025). Antibacterial and Antifungal Activity of Extracts from Five Portuguese Cowpea (Vigna unguiculata) Accessions. Molecules, 30(11), 2348. https://doi.org/10.3390/molecules30112348









