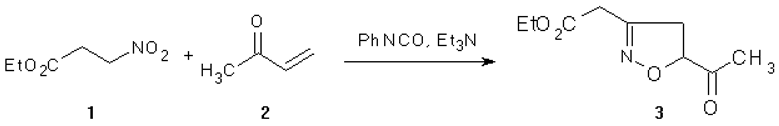
The isoxazoline 3 was prepared by the [3+2] cycloaddition of the nitroderivative 1 [1] with methyl vinyl ketone 2 under Mukaiyama conditions [2].
Phenyl isocyanate (2.5 ml, 23 mmol) was slowly added (4 h) to a mixture of ethyl-nitropropionate 1 (1.46 g, 10 mmol) and methyl vinyl ketone 2 (1.6 ml, 20 mmol) in dry benzene (60 ml) containing a few drops of Et3N. The mixture was stirred at room temperature for two days. The suspension was filtered to eliminate the precipitate of diphenylurea and to the filtrate was added H2O (100 ml). The resulting biphasic system was stirred for two hours at room temperature. After separation, the organic phase was dried and concentrated in vacuo. The crude residue was purified by flash chromatography (ether/light petroleum = 7:3) to give the desired compound 3 as a yellow oil (1 g, 50%).
TLC (ether/light petroleum = 6:4) Rf 0.42
IR (neat, cm-1): 2985, 1737, 1722, 1599, 1555, 1503, 1444, 1400, 1381, 1260, 1203, 1096, 1027.
1HNMR (CDCl3) d: 4.9 (dd, 1H, J = 9.4 Hz, 4.2 Hz, CH2CHO); 4.18 (q, 2H, J = 7.2 Hz, CO2CH2CH3);
3.07 (dd, 1H, J = 17.2 Hz, J = 9.4 Hz, CHHCHO); 2.76 (dd, 1H, J = 17.2 Hz, 4.2 Hz, CHHCHO); 2.54 (d, 2H, J = 7 Hz, CH2CO2); 2.17 (s, 3H, COCH3); 1.25 (t, 3H, J = 7.2 Hz, CO2CH2CH3)
Anal. calc. for C9H13NO4 (199.20): C 54.26, H 6.58, N 7.03; found: C 54.61, H 6.65, N 7.10
Acknowledgment
We gratefully acknowledge the Ministero Pubblica Istruzione (Grant 40% and 60%) for their generous support.
References
- Taylor, M. D.; Badger, E. W.; Steffen, R. P.; Haleen, S. J.; Pugsley, T. A.; Shih, Y. H.; Weishaar, R. E. 2-(2-Aryl-2-oxoethylidene)-1,2,3,4-tetrahydropyridines. Novel isomers of 1,4-dihyropyridine calcium channel blockers. J. Med. Chem. 1988, 31, 1659. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Hoshino, T. The reactions of primary nitro paraffins with isocyanates. J. Am. Chem. Soc. 1960, 82, 5339. [Google Scholar] [CrossRef]
- Baraldi, P. G.; Bigoni, A.; Cacciari, B.; Caldari, C.; Manfredini, S.; Spalluto, G. Nitrile oxide [3+2] cycloaddition: application to the synthesis of 6-substituted 3(2H)- pyridazinones and 6- substituted 4,5-Dihydro-4-hydroxy-3 (2H)-pyridazinones. Synthesis 1994, 11, 1158. [Google Scholar] [CrossRef]
Sample availability: Available from the authors. |
© 1998 by the author. All rights reserved. Molecules website www.mdpi.org/molecules/.