Phytochemicals against Osteoarthritis by Inhibiting Apoptosis
Abstract
1. Introduction
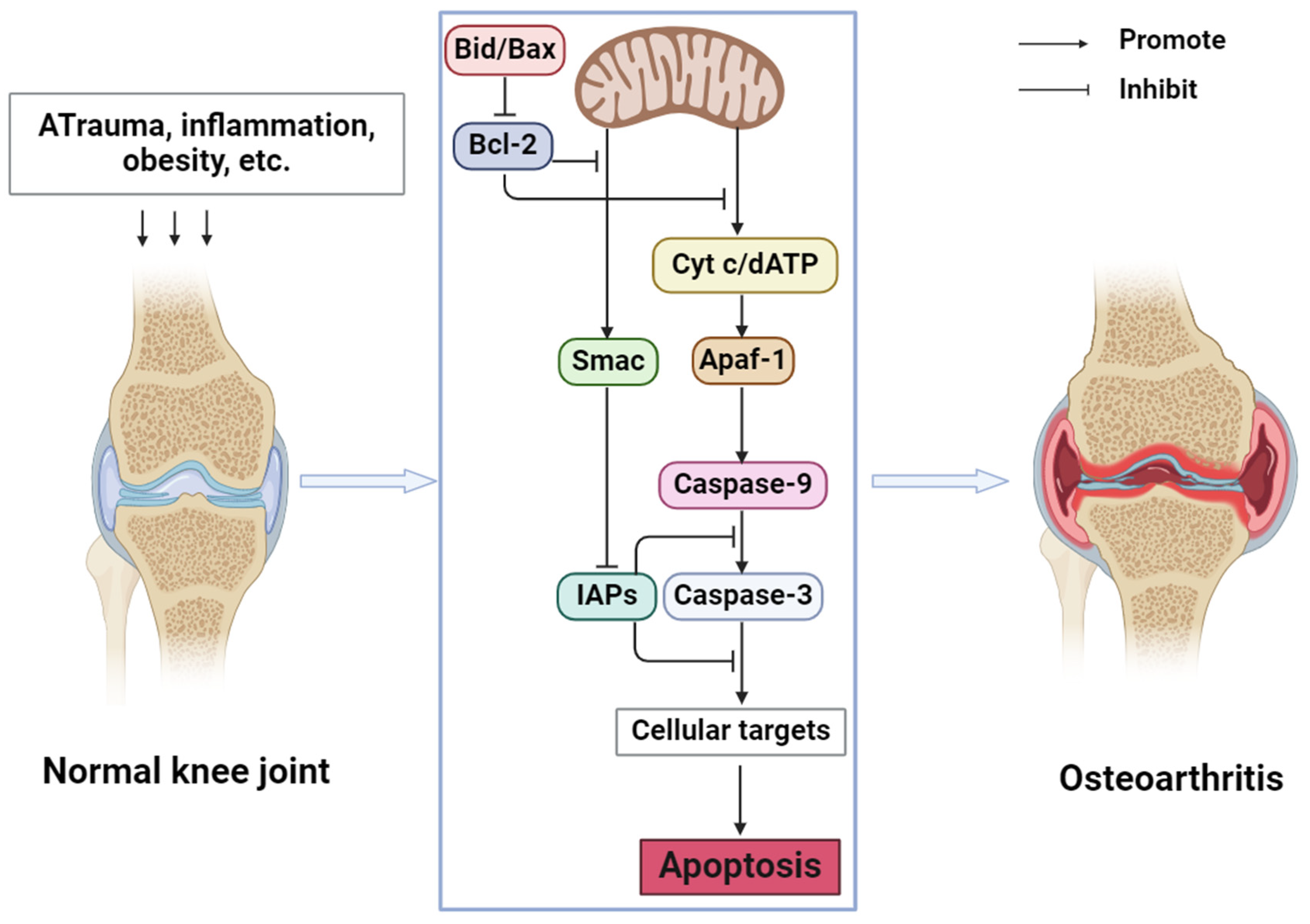
2. Apoptosis and OA
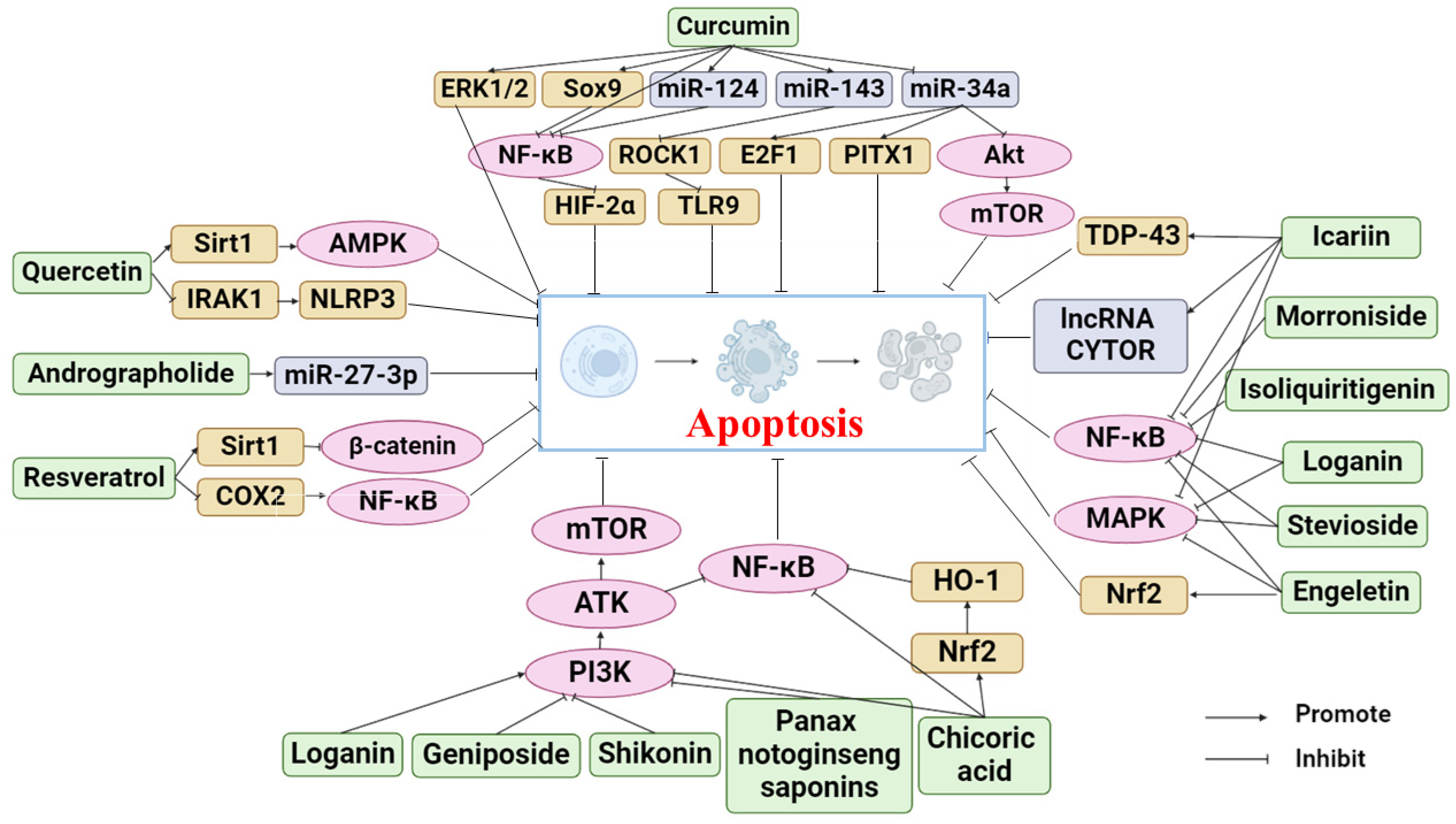
3. Phytochemicals for the Treatment of OA by Inhibiting Apoptosis
3.1. Curcumin
3.2. Resveratrol
3.3. Chicoric Acid
3.4. Icariin
3.5. Quercetin
3.6. Isoliquiritigenin
3.7. Loganin
3.8. Andrographolide
3.9. Geniposide
3.10. Morroniside
3.11. Leonurine
3.12. Panax Notoginseng Saponins
3.13. Shikonin
3.14. Stevioside
3.15. Achyranthes Bidentata Polysaccharides
3.16. Engeletin
3.17. Baicalein
3.18. Genistein
3.19. Epigallocatechin-3-gallate
4. Clinical Application of Phytochemicals in the Treatment of OA
5. Summary and Prospect
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mandl, L.A. Osteoarthritis year in review 2018: Clinical. Osteoarthr. Cartil. 2019, 27, 359–364. [Google Scholar] [CrossRef]
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef]
- Quicke, J.G.; Conaghan, P.G.; Corp, N.; Peat, G. Osteoarthritis year in review 2021: Epidemiology & therapy. Osteoarthr. Cartil. 2022, 30, 196–206. [Google Scholar] [CrossRef]
- Wallace, I.J.; Worthington, S.; Felson, D.T.; Jurmain, R.D.; Wren, K.T.; Maijanen, H.; Woods, R.J.; Lieberman, D.E. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl. Acad. Sci. USA 2017, 114, 9332–9336. [Google Scholar] [CrossRef]
- Felson, D.T.; Zhang, Y.; Anthony, J.M.; Naimark, A.; Anderson, J.J. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann. Intern. Med. 1992, 116, 535–539. [Google Scholar] [CrossRef]
- Bennell, K.L.; Hunter, D.J.; Hinman, R.S. Management of osteoarthritis of the knee. BMJ 2012, 345, e4934. [Google Scholar] [CrossRef]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef]
- Kong, H.; Wang, X.-Q.; Zhang, X.-A. Exercise for Osteoarthritis: A Literature Review of Pathology and Mechanism. Front. Aging Neurosci. 2022, 14, 854026. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Ketelut-Carneiro, N.; Fitzgerald, K.A. Apoptosis, Pyroptosis, and Necroptosis-Oh My! The Many Ways a Cell Can Die. J. Mol. Biol. 2022, 434, 167378. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Riley, J.S.; Bock, F.J. Voices from beyond the grave: The impact of apoptosis on the microenvironment. Biochim. Biophys. Acta Mol. Cell. Res. 2022, 1869, 119341. [Google Scholar] [CrossRef]
- Xiao, S.-Q.; Cheng, M.; Wang, L.; Cao, J.; Fang, L.; Zhou, X.-P.; He, X.-J.; Hu, Y.-F. The role of apoptosis in the pathogenesis of osteoarthritis. Int. Orthop. 2023, 47, 1895–1919. [Google Scholar] [CrossRef]
- Angwa, L.M.; Jiang, Y.; Pei, J.; Sun, D. Antioxidant Phytochemicals for the Prevention of Fluoride-Induced Oxidative Stress and Apoptosis: A Review. Biol. Trace Elem. Res. 2022, 200, 1418–1441. [Google Scholar] [CrossRef]
- Shen, J.; Shan, J.; Zhong, L.; Liang, B.; Zhang, D.; Li, M.; Tang, H. Dietary Phytochemicals that Can Extend Longevity by Regulation of Metabolism. Plant Foods Hum. Nutr. 2022, 77, 12–19. [Google Scholar] [CrossRef]
- Wang, Z.; Efferth, T.; Hua, X.; Zhang, X.-A. Medicinal plants and their secondary metabolites in alleviating knee osteoarthritis: A systematic review. Phytomedicine 2022, 105, 154347. [Google Scholar] [CrossRef]
- Kumari, S.; Dhapola, R.; Reddy, D.H. Apoptosis in Alzheimer’s disease: Insight into the signaling pathways and therapeutic avenues. Apoptosis 2023, 28, 943–957. [Google Scholar] [CrossRef]
- Li, P.; Dong, X.-R.; Zhang, B.; Zhang, X.-T.; Liu, J.-Z.; Ma, D.-S.; Ma, L. Molecular mechanism and therapeutic targeting of necrosis, apoptosis, pyroptosis, and autophagy in cardiovascular disease. Chin. Med. J. 2021, 134, 2647–2655. [Google Scholar] [CrossRef]
- Tong, X.; Tang, R.; Xiao, M.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Yu, X.; Shi, S. Targeting cell death pathways for cancer therapy: Recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J. Hematol. Oncol. 2022, 15, 174. [Google Scholar] [CrossRef]
- Zhou, R.-P.; Dai, B.-B.; Xie, Y.-Y.; Wu, X.-S.; Wang, Z.-S.; Li, Y.; Wang, Z.-Q.; Zu, S.-Q.; Ge, J.-F.; Chen, F.-H. Interleukin-1β and tumor necrosis factor-α augment acidosis-induced rat articular chondrocyte apoptosis via nuclear factor-kappaB-dependent upregulation of ASIC1a channel. Biochim. Biophys. Acta Mol. Basis. Dis. 2018, 1864, 162–177. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Novak, K.; Haqqi, T.M. ERK1/2-mediated activation of DRP1 regulates mitochondrial dynamics and apoptosis in chondrocytes. Osteoarthr. Cartil. 2022, 30, 315–328. [Google Scholar] [CrossRef]
- He, Y.; Wang, W.; Xu, X.; Yang, B.; Yu, X.; Wu, Y.; Wang, J. Mettl3 inhibits the apoptosis and autophagy of chondrocytes in inflammation through mediating Bcl2 stability via Ythdf1-mediated m6A modification. Bone 2022, 154, 116182. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Sun, G.; Liu, P.; Kong, K.; Chen, X.; Yang, F.; Wang, X. CircFOXO3 protects against osteoarthritis by targeting its parental gene FOXO3 and activating PI3K/AKT-mediated autophagy. Cell Death Dis. 2022, 13, 932. [Google Scholar] [CrossRef]
- Guo, Q.; Chen, X.; Chen, J.; Zheng, G.; Xie, C.; Wu, H.; Miao, Z.; Lin, Y.; Wang, X.; Gao, W.; et al. STING promotes senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the NF-κB signaling pathway. Cell Death Dis. 2021, 12, 13. [Google Scholar] [CrossRef]
- Xi, F.-X.; Wei, C.-S.; Xu, Y.-T.; Ma, L.; He, Y.-L.; Shi, X.-E.; Yang, G.-S.; Yu, T.-Y. MicroRNA-214-3p Targeting Ctnnb1 Promotes 3T3-L1 Preadipocyte Differentiation by Interfering with the Wnt/β-Catenin Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 1816. [Google Scholar] [CrossRef]
- Cao, Y.; Tang, S.a.; Nie, X.; Zhou, Z.; Ruan, G.; Han, W.; Zhu, Z.; Ding, C. Decreased miR-214-3p activates NF-κB pathway and aggravates osteoarthritis progression. EBioMedicine 2021, 65, 103283. [Google Scholar] [CrossRef]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Panda, K.C.; Das, S.; Jena, M.; Bhutia, S.K. Apoptosis and autophagy modulating dietary phytochemicals in cancer therapeutics: Current evidences and future perspectives. Phytother. Res. 2021, 35, 4194–4214. [Google Scholar] [CrossRef]
- Zeng, Y.; Xiong, Y.; Yang, T.; Wang, Y.; Zeng, J.; Zhou, S.; Luo, Y.; Li, L. Icariin and its metabolites as potential protective phytochemicals against cardiovascular disease: From effects to molecular mechanisms. Biomed. Pharmacother. 2022, 147, 112642. [Google Scholar] [CrossRef]
- Lei, S.S.; Su, J.; Zhang, Y.; Huang, X.W.; Wang, X.P.; Huang, M.C.; Li, B.; Shou, D. Benefits and mechanisms of polysaccharides from Chinese medicinal herbs for anti-osteoporosis therapy: A review. Int. J. Biol. Macromol. 2021, 193, 1996–2005. [Google Scholar] [CrossRef]
- Buhrmann, C.; Brockmueller, A.; Mueller, A.-L.; Shayan, P.; Shakibaei, M. Curcumin Attenuates Environment-Derived Osteoarthritis by Sox9/NF-kB Signaling Axis. Int. J. Mol. Sci. 2021, 22, 7645. [Google Scholar] [CrossRef]
- Zhou, Y.; Ming, J.; Deng, M.; Li, Y.; Li, B.; Li, J.; Ma, Y.; Chen, Z.; Wang, G.; Liu, S. Chemically modified curcumin (CMC2.24) alleviates osteoarthritis progression by restoring cartilage homeostasis and inhibiting chondrocyte apoptosis via the NF-κB/HIF-2α axis. J. Mol. Med. 2020, 98, 1479–1491. [Google Scholar] [CrossRef]
- Qiu, B.; Xu, X.; Yi, P.; Hao, Y. Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signalling pathways. J. Cell. Mol. Med. 2020, 24, 10855–10865. [Google Scholar] [CrossRef]
- Zhao, P.; Cheng, J.; Geng, J.; Yang, M.; Zhang, Y.; Zhang, Q.; Wang, Y.; Lu, B. Curcumin protects rabbit articular chondrocytes against sodium nitroprusside-induced apoptosis in vitro. Eur. J. Pharmacol. 2018, 828, 146–153. [Google Scholar] [CrossRef]
- Zeng, J.-J.; Wang, H.-D.; Shen, Z.-W.; Yao, X.-D.; Wu, C.-J.; Pan, T. Curcumin Inhibits Proliferation of Synovial Cells by Downregulating Expression of Matrix Metalloproteinase-3 in Osteoarthritis. Orthop. Surg. 2019, 11, 117–125. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Song, X.; Bai, H.; Ma, T.; Zhang, Z.; Li, X.; Jiang, R.; Wang, G.; Fan, X.; et al. Effects of baicalein on IL-1β-induced inflammation and apoptosis in rat articular chondrocytes. Oncotarget 2017, 8, 90781–90795. [Google Scholar] [CrossRef]
- Yao, J.; Liu, X.; Sun, Y.; Dong, X.; Liu, L.; Gu, H. Curcumin-Alleviated Osteoarthritic Progression in Rats Fed a High-Fat Diet by Inhibiting Apoptosis and Activating Autophagy via Modulation of MicroRNA-34a. J. Inflamm. Res. 2021, 14, 2317–2331. [Google Scholar] [CrossRef]
- Wang, J.; Gao, J.-S.; Chen, J.-W.; Li, F.; Tian, J. Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit. Rheumatol. Int. 2012, 32, 1541–1548. [Google Scholar] [CrossRef]
- Gu, L.-L.; Zhang, X.-Y.; Xing, W.-M.; Xu, J.-D.; Lu, H. Andrographolide-induced apoptosis in human renal tubular epithelial cells: Roles of endoplasmic reticulum stress and inflammatory response. Environ. Toxicol. Pharmacol. 2016, 45, 257–264. [Google Scholar] [CrossRef]
- Li, W.; Hu, S.; Chen, X.; Shi, J. The Antioxidant Resveratrol Protects against Chondrocyte Apoptosis by Regulating the COX-2/NF-B Pathway in Created Temporomandibular Osteoarthritis. BioMed Res. Int. 2021, 2021, 9978651. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H.; Hu, B.; Zhang, M. Sirt1 regulates apoptosis and extracellular matrix degradation in resveratrol-treated osteoarthritis chondrocytes via the Wnt/β-catenin signaling pathways. Exp. Ther. Med. 2017, 14, 5057–5062. [Google Scholar] [CrossRef]
- Qu, Y.; Shen, Y.; Teng, L.; Huang, Y.; Yang, Y.; Jian, X.; Fan, S.; Wu, P.; Fu, Q. Chicoric acid attenuates tumor necrosis factor-α-induced inflammation and apoptosis via the Nrf2/HO-1, PI3K/AKT and NF-κB signaling pathways in C28/I2 cells and ameliorates the progression of osteoarthritis in a rat model. Int. Immunopharmacol. 2022, 111, 109129. [Google Scholar] [CrossRef]
- Yang, D.; Cao, G.; Ba, X.; Jiang, H. Epigallocatechin-3-O-gallate promotes extracellular matrix and inhibits inflammation in IL-1β stimulated chondrocytes by the PTEN/miRNA-29b pathway. Pharm. Biol. 2022, 60, 589–599. [Google Scholar] [CrossRef]
- Huang, H.-T.; Cheng, T.-L.; Ho, C.-J.; Huang, H.H.; Lu, C.-C.; Chuang, S.-C.; Li, J.-Y.; Lee, T.-C.; Chen, S.-T.; Lin, Y.-S.; et al. Intra-Articular Injection of (-)-Epigallocatechin 3-Gallate to Attenuate Articular Cartilage Degeneration by Enhancing Autophagy in a Post-Traumatic Osteoarthritis Rat Model. Antioxidants 2020, 10, 8. [Google Scholar] [CrossRef]
- Mi, B.; Wang, J.; Liu, Y.; Liu, J.; Hu, L.; Panayi, A.C.; Liu, G.; Zhou, W. Icariin Activates Autophagy via Down-Regulation of the NF-κB Signaling-Mediated Apoptosis in Chondrocytes. Front. Pharmacol. 2018, 9, 605. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, Z.-F.; Qin, F.-W.; Tang, W.; Liu, D.-H.; Wu, P.-Y.; Jiao, F. Icariin inhibits chondrocyte apoptosis and angiogenesis by regulating the TDP-43 signaling pathway. Mol. Genet. Genom. Med. 2019, 7, e00586. [Google Scholar] [CrossRef]
- Liu, D.; Tang, W.; Zhang, H.; Huang, H.; Zhang, Z.; Tang, D.; Jiao, F. Icariin protects rabbit BMSCs against OGD-induced apoptosis by inhibiting ERs-mediated autophagy via MAPK signaling pathway. Life Sci. 2020, 253, 117730. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Shen, H.; Hao, Q.; Fu, S.; Liu, X. Up-regulation of long non-coding RNA CYTOR induced by icariin promotes the viability and inhibits the apoptosis of chondrocytes. BMC Complement. Med. Ther. 2021, 21, 152. [Google Scholar] [CrossRef]
- Feng, K.; Chen, Z.; Pengcheng, L.; Zhang, S.; Wang, X. Quercetin attenuates oxidative stress-induced apoptosis via SIRT1/AMPK-mediated inhibition of ER stress in rat chondrocytes and prevents the progression of osteoarthritis in a rat model. J. Cell. Physiol. 2019, 234, 18192–18205. [Google Scholar] [CrossRef]
- Hu, Y.; Gui, Z.; Zhou, Y.; Xia, L.; Lin, K.; Xu, Y. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic. Biol. Med. 2019, 145, 146–160. [Google Scholar] [CrossRef]
- Wang, X.-P.; Xie, W.-P.; Bi, Y.-F.; Wang, B.-A.; Song, H.-B.; Wang, S.-L.; Bi, R.-X. Quercetin suppresses apoptosis of chondrocytes induced by IL-1β via inactivation of p38 MAPK signaling pathway. Exp. Ther. Med. 2021, 21, 468. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Tang, Y.; Lu, H.; Qi, Y.; Li, G.; He, H.; Lu, F.; Yang, Y.; Sun, H. Quercetin Alleviates Osteoarthritis Progression in Rats by Suppressing Inflammation and Apoptosis via Inhibition of IRAK1/NLRP3 Signaling. J. Inflamm. Res. 2021, 14, 3393–3403. [Google Scholar] [CrossRef]
- Ji, B.; Guo, W.; Ma, H.; Xu, B.; Mu, W.; Zhang, Z.; Amat, A.; Cao, L. Isoliquiritigenin suppresses IL-1β induced apoptosis and inflammation in chondrocyte-like ATDC5 cells by inhibiting NF-κB and exerts chondroprotective effects on a mouse model of anterior cruciate ligament transection. Int. J. Mol. Med. 2017, 40, 1709–1718. [Google Scholar] [CrossRef]
- Yi, N.; Mi, Y.; Xu, X.; Li, N.; Zeng, F.; Yan, K.; Tan, K.; Kuang, G.; Lu, M. Baicalein Alleviates Osteoarthritis Progression in Mice by Protecting Subchondral Bone and Suppressing Chondrocyte Apoptosis Based on Network Pharmacology. Front. Pharmacol. 2021, 12, 788392. [Google Scholar] [CrossRef]
- Li, B.; Chen, K.; Qian, N.; Huang, P.; Hu, F.; Ding, T.; Xu, X.; Zhou, Q.; Chen, B.; Deng, L.; et al. Baicalein alleviates osteoarthritis by protecting subchondral bone, inhibiting angiogenesis and synovial proliferation. J. Cell. Mol. Med. 2021, 25, 5283–5294. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Chen, X.; Zhang, Y.; Zhang, Y.; Jia, Y.; Wang, H.; Liu, Y.; Xiao, L. Baicalein ameliorates inflammatory-related apoptotic and catabolic phenotypes in human chondrocytes. Int. Immunopharmacol. 2014, 21, 301–308. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Q.; Guo, P.; Huang, Y.; Ye, Z.; Hu, J. Anti-chondrocyte apoptosis effect of genistein in treating inflammation-induced osteoarthritis. Mol. Med. Rep. 2020, 22, 2032–2042. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, Y.; Zhao, H.; Zhang, S. Loganin Attenuates Osteoarthritis in Rats by Inhibiting IL-1β-Induced Catabolism and Apoptosis in Chondrocytes Via Regulation of Phosphatidylinositol 3-Kinases (PI3K)/Akt. Med. Sci. Monit. 2019, 25, 4159–4168. [Google Scholar] [CrossRef]
- Chen, S.; Luo, Z.; Chen, X. Andrographolide mitigates cartilage damage via miR-27-3p-modulated matrix metalloproteinase13 repression. J. Gene Med. 2020, 22, e3187. [Google Scholar] [CrossRef]
- Pan, T.; Shi, X.; Chen, H.; Chen, R.; Wu, D.; Lin, Z.; Zhang, J.; Pan, J. Geniposide Suppresses Interleukin-1β-Induced Inflammation and Apoptosis in Rat Chondrocytes via the PI3K/Akt/NF-κB Signaling Pathway. Inflammation 2018, 41, 390–399. [Google Scholar] [CrossRef]
- Yu, H.; Yao, S.; Zhou, C.; Fu, F.; Luo, H.; Du, W.; Jin, H.; Tong, P.; Chen, D.; Wu, C.; et al. Morroniside attenuates apoptosis and pyroptosis of chondrocytes and ameliorates osteoarthritic development by inhibiting NF-κB signaling. J. Ethnopharmacol. 2021, 266, 113447. [Google Scholar] [CrossRef]
- Hu, P.-F.; Sun, F.-F.; Qian, J. Leonurine Exerts Anti-Catabolic and Anti-Apoptotic Effects via Nuclear Factor kappa B (NF-κB) and Mitogen-Activated Protein Kinase (MAPK) Signaling Pathways in Chondrocytes. Med. Sci. Monit. 2019, 25, 6271–6280. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, W.; Han, G.; Zhou, S.; Li, J.; Chen, M.; Li, H. Panax notoginseng saponins prevent senescence and inhibit apoptosis by regulating the PI3K-AKT-mTOR pathway in osteoarthritic chondrocytes. Int. J. Mol. Med. 2020, 45, 1225–1236. [Google Scholar] [CrossRef]
- Fu, D.; Shang, X.; Ni, Z.; Shi, G. Shikonin inhibits inflammation and chondrocyte apoptosis by regulation of the PI3K/Akt signaling pathway in a rat model of osteoarthritis. Exp. Ther. Med. 2016, 12, 2735–2740. [Google Scholar] [CrossRef]
- Wang, L.; Gai, P.; Xu, R.; Zheng, Y.; Lv, S.; Li, Y.; Liu, S. Shikonin protects chondrocytes from interleukin-1beta-induced apoptosis by regulating PI3K/Akt signaling pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 298–308. [Google Scholar]
- Cai, T.; Ye, H.; Jiang, H.; Lin, C.; Lou, C.; Wang, W.; Yan, Z.; Xue, X.; Pan, X.; Lin, J. Stevioside targets the NF-κB and MAPK pathways for inhibiting inflammation and apoptosis of chondrocytes and ameliorates osteoarthritis in vivo. Int. Immunopharmacol. 2023, 115, 109683. [Google Scholar] [CrossRef]
- Fu, C.; Qiu, Z.; Huang, Y.; Lin, Q.; Jin, L.; Tu, H.; Ye, J.; Zheng, C.; Zhong, W.; Ma, D. Achyranthes bidentata polysaccharides alleviate endoplasmic reticulum stress in osteoarthritis via lncRNA NEAT1/miR-377-3p pathway. Biomed. Pharmacother. 2022, 154, 113551. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Z.; Pang, Z.; Qi, G.; Hua, B.; Yan, Z.; Yuan, H. Engeletin Protects Against TNF-α-Induced Apoptosis and Reactive Oxygen Species Generation in Chondrocytes and Alleviates Osteoarthritis in vivo. J. Inflamm. Res. 2021, 14, 745–760. [Google Scholar] [CrossRef]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzińska, B. Curcumin and Its Potential Impact on Microbiota. Nutrients 2021, 13, 2004. [Google Scholar] [CrossRef]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef]
- Li, X.; Feng, K.; Li, J.; Yu, D.; Fan, Q.; Tang, T.; Yao, X.; Wang, X. Curcumin Inhibits Apoptosis of Chondrocytes through Activation ERK1/2 Signaling Pathways Induced Autophagy. Nutrients 2017, 9, 414. [Google Scholar] [CrossRef]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef]
- Yang, K.-Y.; Lin, L.-C.; Tseng, T.-Y.; Wang, S.-C.; Tsai, T.-H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J. Chromatogr. B 2007, 853, 183–189. [Google Scholar] [CrossRef]
- Ravindranath, V.; Chandrasekhara, N. Absorption and tissue distribution of curcumin in rats. Toxicology 1980, 16, 259–265. [Google Scholar] [CrossRef]
- Ravindranath, V.; Chandrasekhara, N. In vitro studies on the intestinal absorption of curcumin in rats. Toxicology 1981, 20, 251–257. [Google Scholar] [CrossRef]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Zheng, M.; Bai, Y.; Sun, X.; Fu, R.; Liu, L.; Liu, M.; Li, Z.; Huang, X. Resveratrol Reestablishes Mitochondrial Quality Control in Myocardial Ischemia/Reperfusion Injury through Sirt1/Sirt3-Mfn2-Parkin-PGC-1α Pathway. Molecules 2022, 27, 5545. [Google Scholar] [CrossRef]
- Chupradit, S.; Bokov, D.; Zamanian, M.Y.; Heidari, M.; Hakimizadeh, E. Hepatoprotective and therapeutic effects of resveratrol: A focus on anti-inflammatory and antioxidative activities. Fundam. Clin. Pharmacol. 2022, 36, 468–485. [Google Scholar] [CrossRef]
- Deng, Z.; Li, Y.; Liu, H.; Xiao, S.; Li, L.; Tian, J.; Cheng, C.; Zhang, G.; Zhang, F. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 2019, 39, BSR20190189. [Google Scholar] [CrossRef]
- Lei, M.; Wang, J.-G.; Xiao, D.-M.; Fan, M.; Wang, D.-P.; Xiong, J.-Y.; Chen, Y.; Ding, Y.; Liu, S.-L. Resveratrol inhibits interleukin 1β-mediated inducible nitric oxide synthase expression in articular chondrocytes by activating SIRT1 and thereby suppressing nuclear factor-κB activity. Eur. J. Pharmacol. 2012, 674, 73–79. [Google Scholar] [CrossRef]
- Yi, H.; Zhang, W.; Cui, Z.-M.; Cui, S.-Y.; Fan, J.-B.; Zhu, X.-H.; Liu, W. Resveratrol alleviates the interleukin-1β-induced chondrocytes injury through the NF-κB signaling pathway. J. Orthop. Surg. Res. 2020, 15, 424. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, Q.; Gao, R.; Park, Y. AAK-2 and SKN-1 Are Involved in Chicoric-Acid-Induced Lifespan Extension in Caenorhabditis elegans. J. Agric. Food Chem. 2019, 67, 9178–9186. [Google Scholar] [CrossRef]
- Wang, N.; Li, R.; Feng, B.; Cheng, Y.; Guo, Y.; Qian, H. Chicoric Acid Prevents Neuroinflammation and Neurodegeneration in a Mouse Parkinson’s Disease Model: Immune Response and Transcriptome Profile of the Spleen and Colon. Int. J. Mol. Sci. 2022, 23, 2031. [Google Scholar] [CrossRef]
- Sun, J.; Xu, W.; Zheng, S.; Lv, C.; Lin, J.; Chen, S.; Qiu, Y.; Jiang, X.; Draz, E.; Wang, S. Icariin promotes mouse Leydig cell testosterone synthesis via the Esr1/Src/Akt/Creb/Sf-1 pathway. Toxicol. Appl. Pharmacol. 2022, 441, 115969. [Google Scholar] [CrossRef]
- Bi, Z.; Zhang, W.; Yan, X. Anti-inflammatory and immunoregulatory effects of icariin and icaritin. Biomed. Pharmacother. 2022, 151, 113180. [Google Scholar] [CrossRef]
- Liu, Y.; Mi, B.; Lv, H.; Liu, J.; Xiong, Y.; Hu, L.; Xue, H.; Panayi, A.C.; Liu, G.; Zhou, W. Shared KEGG pathways of icariin-targeted genes and osteoarthritis. J. Cell. Biochem. 2019, 120, 7741–7750. [Google Scholar] [CrossRef]
- Wang, M.; Gao, H.; Li, W.; Wu, B. Icariin and its metabolites regulate lipid metabolism: From effects to molecular mechanisms. Biomed. Pharmacother. 2020, 131, 110675. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and its derivates as antiviral potentials: A comprehensive review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Alizadeh, S.R.; Ebrahimzadeh, M.A. Quercetin derivatives: Drug design, development, and biological activities, a review. Eur. J. Med. Chem. 2022, 229, 114068. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, Y. Green Synthetic Nanoarchitectonics of Gold and Silver Nanoparticles Prepared Using Quercetin and Their Cytotoxicity and Catalytic Applications. J. Nanosci. Nanotechnol. 2020, 20, 2781–2790. [Google Scholar] [CrossRef]
- Wang, K.-L.; Yu, Y.-C.; Hsia, S.-M. Perspectives on the Role of Isoliquiritigenin in Cancer. Cancers 2021, 13, 115. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, W.-Q.; Zhang, S.-Q.; Bai, J.-X.; Liu, B.; Yung, K.K.-L.; Ko, J.K.-S. Isoliquiritigenin inhibits pancreatic cancer progression through blockade of p38 MAPK-regulated autophagy. Phytomedicine 2022, 106, 154406. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, Y.; Wang, C.; Wang, Z.; Li, Y.; Jiang, Z.; Zhao, W.; Pan, Z. Isoliquiritigenin inhibits non-small cell lung cancer progression via m6A/IGF2BP3-dependent TWIST1 mRNA stabilization. Phytomedicine 2022, 104, 154299. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Wang, X.; Zhu, Q.; Liu, L.; Qiu, S.; Zou, L.; Liu, K.; Li, G.; Miao, H.; et al. Isoliquiritigenin induces HMOX1 and GPX4-mediated ferroptosis in gallbladder cancer cells. Chin. Med. J. 2023, 136, 2210–2220. [Google Scholar] [CrossRef]
- Yushan, R.; Ying, Y.; Yujun, T.; Jingchun, Y.; Dongguang, L.; Lihong, P.; Pingping, W.; Lili, Z.; Fanhui, Z.; Zhong, L.; et al. Isoliquiritigenin inhibits mouse S180 tumors with a new mechanism that regulates autophagy by GSK-3β/TNF-α pathway. Eur. J. Pharmacol. 2018, 838, 11–22. [Google Scholar] [CrossRef]
- Lee, Y.K.; Chin, Y.-W.; Bae, J.-K.; Seo, J.S.; Choi, Y.H. Pharmacokinetics of isoliquiritigenin and its metabolites in rats: Low bioavailability is primarily due to the hepatic and intestinal metabolism. Planta Medica 2013, 79, 1656–1665. [Google Scholar] [CrossRef]
- Qiao, H.; Zhang, X.; Wang, T.; Liang, L.; Chang, W.; Xia, H. Pharmacokinetics, biodistribution and bioavailability of isoliquiritigenin after intravenous and oral administration. Pharm. Biol. 2014, 52, 228–236. [Google Scholar] [CrossRef]
- Kou, Y.; Li, Z.; Yang, T.; Shen, X.; Wang, X.; Li, H.; Zhou, K.; Li, L.; Xia, Z.; Zheng, X.; et al. Therapeutic potential of plant iridoids in depression: A review. Pharm. Biol. 2022, 60, 2167–2181. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, Y.; Zhang, J.; Li, L.; Wang, Y.-W.; Xia, C.-Y.; Lian, W.-W.; Peng, Y.; Zheng, J.; He, J.; et al. Phytochemistry, synthesis, analytical methods, pharmacological activity, and pharmacokinetics of loganin: A comprehensive review. Phytother. Res. 2022, 36, 2272–2299. [Google Scholar] [CrossRef]
- Chen, X.; Cao, G.; Jiang, J. Comparison of pharmacokinetic behavior of two iridoid glycosides in rat plasma after oral administration of crude Cornus officinals and its jiuzhipin by high performance liquid chromatography triple quadrupole mass spectrometry combined with multiple reactions monitoring mode. Pharmacogn. Mag. 2014, 10, S115–S121. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Zhang, L.; Xu, L.; Yin, W. HPLC study of tissue distribution of loganin in rats. Biomed. Chromatogr. 2006, 20, 1087–1092. [Google Scholar] [CrossRef]
- Tao, J.-H.; Zhao, M.; Jiang, S.; Pu, X.-L.; Wei, X.-Y. Comparative metabolism of two major compounds in Fructus Corni extracts by gut microflora from normal and chronic nephropathy rats in vitro by UPLC-Q-TOF/MS. J. Chromatogr. B 2018, 1073, 170–176. [Google Scholar] [CrossRef]
- Cai, Q.; Zhang, W.; Sun, Y.; Xu, L.; Wang, M.; Wang, X.; Wang, S.; Ni, Z. Study on the mechanism of andrographolide activation. Front. Neurosci. 2022, 16, 977376. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Y.; Liu, Y.; Sun, D.; Li, H.; Chen, L. Recent Progress in Andrographolide Derivatization: Structural Modification and Biological Activity. Mini Rev. Med. Chem. 2022, 22, 1559–1584. [Google Scholar] [CrossRef]
- Chung, W.-J.; Chan, K.-L.; Lee, C.-Y. Comparing the pharmacokinetics of 13α,21-dihydroeurycomanone and eurycomanone exclusively enriched in Eurycoma longifolia extracts and their spermatogenesis enhancement in andrographolide-induced oligospermia in rats. J. Pharm. Pharmacol. 2021, 73, 161–168. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Gui, B.-J.; Liu, J.; Rong, G.-X.; Deng, R.; Bu, Y.-H.; Zhang, H. Geniposide alleviates VEGF-induced angiogenesis by inhibiting VEGFR2/PKC/ERK1/2-mediated SphK1 translocation. Phytomedicine 2022, 100, 154068. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Hu, Q.; Xiao, X.; Ou, L.; Chen, Y.; Luo, S.; Cheng, Y.; Jiang, Y.; Ma, X.; et al. The emerging possibility of the use of geniposide in the treatment of cerebral diseases: A review. Chin. Med. 2021, 16, 86. [Google Scholar] [CrossRef]
- Chen, Y.; Shou, K.; Gong, C.; Yang, H.; Yang, Y.; Bao, T. Anti-Inflammatory Effect of Geniposide on Osteoarthritis by Suppressing the Activation of p38 MAPK Signaling Pathway. BioMed Res. Int. 2018, 2018, 8384576. [Google Scholar] [CrossRef]
- Wang, F.; Cao, J.; Hao, J.; Liu, K. Pharmacokinetics, bioavailability and tissue distribution of geniposide following intravenous and peroral administration to rats. Biopharm. Drug Dispos. 2014, 35, 97–103. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, T.; Tao, J.-S.; Zhang, L.-Y.; Shi, J.-R.; Ji, G. Potential hepatotoxicity of geniposide, the major iridoid glycoside in dried ripe fruits of Gardenia jasminoides (Zhi-zi). Nat. Prod. Res. 2013, 27, 929–933. [Google Scholar] [CrossRef]
- Yi, X.; Tao, J.; Qian, Y.; Feng, F.; Hu, X.; Xu, T.; Jin, H.; Ruan, H.; Zheng, H.-F.; Tong, P. Morroniside ameliorates inflammatory skeletal muscle atrophy via inhibiting canonical and non-canonical NF-κB and regulating protein synthesis/degradation. Front. Pharmacol. 2022, 13, 1056460. [Google Scholar] [CrossRef]
- Cheng, L.; Zeng, G.; Liu, Z.; Zhang, B.; Cui, X.; Zhao, H.; Zheng, X.; Song, G.; Kang, J.; Xia, C. Protein kinase B and extracellular signal-regulated kinase contribute to the chondroprotective effect of morroniside on osteoarthritis chondrocytes. J. Cell. Mol. Med. 2015, 19, 1877–1886. [Google Scholar] [CrossRef]
- Li, Z.; Chen, K.; Zhu, Y.Z. Leonurine inhibits cardiomyocyte pyroptosis to attenuate cardiac fibrosis via the TGF-β/Smad2 signalling pathway. PLoS ONE 2022, 17, e0275258. [Google Scholar] [CrossRef]
- Qi, L.; Chen, X.; Pan, Y.; Zha, Z.; Tang, M.; Shi, C.; Yang, B.; Wang, H. Leonurine exerts a protective effect in dextran sodium sulfate-induced experimental inflammatory bowel disease mice model. Gen. Physiol. Biophys. 2022, 41, 43–51. [Google Scholar] [CrossRef]
- Deng, Z.; Li, J.; Tang, X.; Li, D.; Wang, Y.; Wu, S.; Fan, K.; Ma, Y. Leonurine Reduces Oxidative Stress and Provides Neuroprotection against Ischemic Injury via Modulating Oxidative and NO/NOS Pathway. Int. J. Mol. Sci. 2022, 23, 10188. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, J.; Yang, P.; Tan, B.; Liu, X.; Zheng, Y.; Cai, W.; Zhu, Y. Characterization of metabolites of leonurine (SCM-198) in rats after oral administration by liquid chromatography/tandem mass spectrometry and NMR spectrometry. Sci. World J. 2014, 2014, 947946. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Y.; Wang, C.; Jiang, J.; Liu, S.; Bai, Q.; Li, L.; Jin, H.; Jin, Y.; Yan, G. Panax notoginseng saponin R1 attenuates allergic rhinitis through AMPK/Drp1 mediated mitochondrial fission. Biochem. Pharmacol. 2022, 202, 115106. [Google Scholar] [CrossRef]
- Pan, Y.-W.; Wu, D.-P.; Liang, H.-F.; Tang, G.-Y.; Fan, C.-L.; Shi, L.; Ye, W.-C.; Li, M.-M. Total Saponins of Panax notoginseng Activate Akt/mTOR Pathway and Exhibit Neuroprotection in vitro and in vivo against Ischemic Damage. Chin. J. Integr. Med. 2022, 28, 410–418. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Yang, Y.; Huang, S.; Zou, X.; Wei, C.; Liang, T.; Zhong, X. Panax notoginseng saponins reduces the cisplatin-induced acute renal injury by increasing HIF-1α/BNIP3 to inhibit mitochondrial apoptosis pathway. Biomed. Pharmacother. 2021, 142, 111965. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Niu, W.; Olaleye, O.E.; Du, F.-F.; Wang, F.-Q.; Huang, Y.-H.; Yuan, L.; Li, Y.-F.; Liu, G.-P.; Xu, F.; et al. Comparison of intramuscular and intravenous pharmacokinetics of ginsenosides in humans after dosing XueShuanTong, a lyophilized extract of Panax notoginseng roots. J. Ethnopharmacol. 2020, 253, 112658. [Google Scholar] [CrossRef]
- Wu, L.; Song, H.; Zhang, C.; Wang, A.; Zhang, B.; Xiong, C.; Zhuang, X.; Zang, Y.; Li, C.; Fang, Q.; et al. Efficacy and Safety of Panax notoginseng Saponins in the Treatment of Adults with Ischemic Stroke in China: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2317574. [Google Scholar] [CrossRef]
- Liu, K.-Z.; Li, J.-B.; Lu, H.-L.; Wen, J.-K.; Han, M. Effects of Astragalus and saponins of Panax notoginseng on MMP-9 in patients with type 2 diabetic macroangiopathy. Zhongguo Zhong Yao Za Zhi 2004, 29, 264–266. [Google Scholar]
- Yadav, S.; Sharma, A.; Nayik, G.A.; Cooper, R.; Bhardwaj, G.; Sohal, H.S.; Mutreja, V.; Kaur, R.; Areche, F.O.; AlOudat, M.; et al. Review of Shikonin and Derivatives: Isolation, Chemistry, Biosynthesis, Pharmacology and Toxicology. Front. Pharmacol. 2022, 13, 905755. [Google Scholar] [CrossRef]
- Rihan, M.; Sharma, S.S. Inhibition of Pyruvate kinase M2 (PKM2) by shikonin attenuates isoproterenol-induced acute myocardial infarction via reduction in inflammation, hypoxia, apoptosis, and fibrosis. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 397, 145–159. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Wang, M.; Gao, S.; Hong, L.; Hou, T.; Zhang, Y.; Zhu, Y.; Qian, F. Shikonin ameliorates lipoteichoic acid-induced acute lung injury via promotion of neutrophil apoptosis. Mol. Med. Rep. 2021, 23, 133. [Google Scholar] [CrossRef]
- Li, J.; Pang, J.; Liu, Z.; Ge, X.; Zhen, Y.; Jiang, C.C.; Liu, Y.; Huo, Q.; Sun, Y.; Liu, H. Shikonin induces programmed death of fibroblast synovial cells in rheumatoid arthritis by inhibiting energy pathways. Sci. Rep. 2021, 11, 18263. [Google Scholar] [CrossRef]
- Su, L.; Liu, L.; Wang, Y.; Yan, G.; Zhang, Y. Long-term systemic toxicity of shikonin derivatives in Wistar rats. Pharm. Biol. 2013, 52, 486–490. [Google Scholar] [CrossRef]
- Wei, Q.; Su, J.; Dong, G.; Zhang, M.; Huo, Y.; Dong, Z. Glycolysis inhibitors suppress renal interstitial fibrosis via divergent effects on fibroblasts and tubular cells. Am. J. Physiol.-Ren. Physiol. 2019, 316, F1162–F1172. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Hu, F.; Luo, P. Stevioside attenuates osteoarthritis via regulating Nrf2/HO-1/NF-κB pathway. J. Orthop. Transl. 2023, 38, 190–202. [Google Scholar] [CrossRef]
- Wan, J.; Zhu, Z.; He, Z.; Wu, H.; Chen, A.; Zhu, W.; Cheng, P. Stevioside protects primary articular chondrocytes against IL-1β-induced inflammation and catabolism by targeting integrin. Int. Immunopharmacol. 2023, 119, 110261. [Google Scholar] [CrossRef]
- Yodyingyuad, V.; Bunyawong, S. Effect of stevioside on growth and reproduction. Hum. Reprod. 1991, 6, 158–165. [Google Scholar] [CrossRef]
- Cai, E.; Cheng, Q.; Yu, S.; Ding, F. Achyranthes bidentata polypeptide k enhances the survival, growth and axonal regeneration of spinal cord motor neurons in vitro. Neuroreport 2021, 32, 518–524. [Google Scholar] [CrossRef]
- Hou, G.; Peng, W.; Wei, L.; Li, R.; Huang, X.; Yin, Y. Probiotics and Achyranthes bidentata Polysaccharides Improve Growth Performance via Promoting Intestinal Nutrient Utilization and Enhancing Immune Function of Weaned Pigs. Animals 2021, 11, 2617. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X. The genus Achyranthes: A review on traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 2017, 203, 260–278. [Google Scholar] [CrossRef]
- Wang, C.; La, L.; Feng, H.; Yang, Q.; Wu, F.; Wang, C.; Wu, J.; Hou, L.; Hou, C.; Liu, W. Aldose Reductase Inhibitor Engeletin Suppresses Pelvic Inflammatory Disease by Blocking the Phospholipase C/Protein Kinase C-Dependent/NF-κB and MAPK Cascades. J. Agric. Food Chem. 2020, 68, 11747–11757. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Xu, Y.; Wang, X.; Ren, R.; Zhu, H.; Zhang, S. Engeletin protects against cerebral ischemia/reperfusion injury by modulating the VEGF/vasohibin and Ang-1/Tie-2 pathways. Braz. J. Med. Biol. Res. 2021, 54, e11028. [Google Scholar] [CrossRef]
- Hu, Z.; Guan, Y.; Hu, W.; Xu, Z.; Ishfaq, M. An overview of pharmacological activities of baicalin and its aglycone baicalein: New insights into molecular mechanisms and signaling pathways. Iran. J. Basic Med. Sci. 2022, 25, 14–26. [Google Scholar] [CrossRef]
- Nazari-Khanamiri, F.; Ghasemnejad-Berenji, M. Cellular and molecular mechanisms of genistein in prevention and treatment of diseases: An overview. J. Food Biochem. 2021, 45, e13972. [Google Scholar] [CrossRef]
- Kim, K.H.; Dodsworth, C.; Paras, A.; Burton, B.K. High dose genistein aglycone therapy is safe in patients with mucopolysaccharidoses involving the central nervous system. Mol. Genet. Metab. 2013, 109, 382–385. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, S.; Li, Q.; Lang, W.; Li, W.; Jiang, X.; Wan, Z.; Chen, J.; Wang, H. Epigallocatechin-3-gallate: A phytochemical as a promising drug candidate for the treatment of Parkinson’s disease. Front. Pharmacol. 2022, 13, 977521. [Google Scholar] [CrossRef]
- Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]
- Farpour, H.R.; Rajabi, N.; Ebrahimi, B. The Efficacy of (Teltonal) in Patients with Knee Osteoarthritis: A Randomized Active-Controlled Clinical Trial. Evid.-Based Complement. Altern. Med. 2021, 2021, 5596892. [Google Scholar] [CrossRef]
- Huseini, H.F.; Mohtashami, R.; Sadeghzadeh, E.; Shadmanfar, S.; Hashem-Dabaghian, F.; Kianbakht, S. Efficacy and safety of oral Nigella sativa oil for symptomatic treatment of knee osteoarthritis: A double-blind, randomized, placebo-controlled clinical trial. Complement. Ther. Clin. Pr. 2022, 49, 101666. [Google Scholar] [CrossRef]
- Kakuo, S.; Fushimi, T.; Kawasaki, K.; Nakamura, J.; Ota, N. Effects of Psidium guajava Linn. leaf extract in Japanese subjects with knee pain: A randomized, double-blind, placebo-controlled, parallel pilot study. Aging Clin. Exp. Res. 2018, 30, 1391–1398. [Google Scholar] [CrossRef]
- Rondanelli, M.; Riva, A.; Morazzoni, P.; Allegrini, P.; Faliva, M.A.; Naso, M.; Miccono, A.; Peroni, G.; Degli Agosti, I.; Perna, S. The effect and safety of highly standardized Ginger (Zingiber officinale) and Echinacea (Echinacea angustifolia) extract supplementation on inflammation and chronic pain in NSAIDs poor responders. A pilot study in subjects with knee arthrosis. Nat. Prod. Res. 2017, 31, 1309–1313. [Google Scholar] [CrossRef]
- Maghsoumi-Norouzabad, L.; Alipoor, B.; Abed, R.; Eftekhar Sadat, B.; Mesgari-Abbasi, M.; Asghari Jafarabadi, M. Effects of Arctium lappa L. (Burdock) root tea on inflammatory status and oxidative stress in patients with knee osteoarthritis. Int. J. Rheum. Dis. 2016, 19, 255–261. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Farney, T.M.; McCarthy, C.G.; Lee, S.-R. Cissus quadrangularis reduces joint pain in exercise-trained men: A pilot study. Physician Sportsmed. 2013, 41, 29–35. [Google Scholar] [CrossRef]
- Cheras, P.A.; Myers, S.P.; Paul-Brent, P.-A.; Outerbridge, K.H.; Nielsen, G.V.L. Randomized double-blind placebo-controlled trial on the potential modes of action of SheaFlex70 in osteoarthritis. Phytother. Res. 2010, 24, 1126–1131. [Google Scholar] [CrossRef]
- Karimifar, M.; Soltani, R.; Hajhashemi, V.; Sarrafchi, S. Evaluation of the effect of Elaeagnus angustifolia alone and combined with Boswellia thurifera compared with ibuprofen in patients with knee osteoarthritis: A randomized double-blind controlled clinical trial. Clin. Rheumatol. 2017, 36, 1849–1853. [Google Scholar] [CrossRef]
- Panahi, Y.; Alishiri, G.H.; Bayat, N.; Hosseini, S.M.; Sahebkar, A. Efficacy of Elaeagnus Angustifolia extract in the treatment of knee osteoarthritis: A randomized controlled trial. EXCLI J. 2016, 15, 203–210. [Google Scholar] [CrossRef]
- Nikniaz, Z.; Ostadrahimi, A.; Mahdavi, R.; Ebrahimi, A.A.; Nikniaz, L. Effects of Elaeagnus angustifolia L. supplementation on serum levels of inflammatory cytokines and matrix metalloproteinases in females with knee osteoarthritis. Complement. Ther. Med. 2014, 22, 864–869. [Google Scholar] [CrossRef]
- Altman, R.D.; Marcussen, K.C. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001, 44, 2531–2538. [Google Scholar] [CrossRef]
- Rondanelli, M.; Riva, A.; Allegrini, P.; Faliva, M.A.; Naso, M.; Peroni, G.; Nichetti, M.; Gasparri, C.; Spadaccini, D.; Iannello, G.; et al. The Use of a New Food-Grade Lecithin Formulation of Highly Standardized Ginger (Zingiber officinale) and Extracts for the Treatment of Pain and Inflammation in a Group of Subjects with Moderate Knee Osteoarthritis. J. Pain Res. 2020, 13, 761–770. [Google Scholar] [CrossRef]
- Black, C.D.; Herring, M.P.; Hurley, D.J.; O’Connor, P.J. Ginger (Zingiber officinale) reduces muscle pain caused by eccentric exercise. J. Pain 2010, 11, 894–903. [Google Scholar] [CrossRef]
- Hancke, J.L.; Srivastav, S.; Cáceres, D.D.; Burgos, R.A. A double-blind, randomized, placebo-controlled study to assess the efficacy of Andrographis paniculata standardized extract (ParActin®) on pain reduction in subjects with knee osteoarthritis. Phytother. Res. 2019, 33, 1469–1479. [Google Scholar] [CrossRef]
- Ferguson, J.J.A.; Oldmeadow, C.; Bentley, D.; Garg, M.L. Antioxidant Effects of a Polyphenol-Rich Dietary Supplement Incorporating Bark Extract in Healthy Older Adults: A Two-Arm, Parallel Group, Randomized Placebo-Controlled Trial. Antioxidants 2022, 11, 1560. [Google Scholar] [CrossRef]
- Wang, Z.; Jones, G.; Winzenberg, T.; Cai, G.; Laslett, L.L.; Aitken, D.; Hopper, I.; Singh, A.; Jones, R.; Fripp, J.; et al. Effectiveness of Extract for the Treatment of Symptoms and Effusion-Synovitis of Knee Osteoarthritis: A Randomized Trial. Ann. Intern. Med. 2020, 173, 861–869. [Google Scholar] [CrossRef]
- Henrotin, Y.; Malaise, M.; Wittoek, R.; de Vlam, K.; Brasseur, J.P.; Luyten, F.P.; Jiangang, Q.; Van den Berghe, M.; Uhoda, R.; Bentin, J.; et al. Bio-optimized Curcuma longa extract is efficient on knee osteoarthritis pain: A double-blind multicenter randomized placebo controlled three-arm study. Arthritis Res. Ther. 2019, 21, 179. [Google Scholar] [CrossRef]
- Srivastava, S.; Saksena, A.K.; Khattri, S.; Kumar, S.; Dagur, R.S. Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: A four-month, double-blind, randomized, placebo-controlled trial. Inflammopharmacology 2016, 24, 377–388. [Google Scholar] [CrossRef]
- Gomes, T.P.O.; Souza, J.I.N.; Somerlate, L.C.; Mendonça, V.A.; Lima, N.M.; Carli, G.P.; Castro, S.B.R.; Andrade, T.d.J.A.S.; Dias, J.V.L.; Oliveira, M.A.L.; et al. Miconia albicans and Curcuma longa herbal medicines positively modulate joint pain, function and inflammation in patients with osteoarthritis: A clinical study. Inflammopharmacology 2021, 29, 377–391. [Google Scholar] [CrossRef]
- Madhu, K.; Chanda, K.; Saji, M.J. Safety and efficacy of Curcuma longa extract in the treatment of painful knee osteoarthritis: A randomized placebo-controlled trial. Inflammopharmacology 2013, 21, 129–136. [Google Scholar] [CrossRef]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: A randomized open-label parallel-arm study. Trials 2019, 20, 214. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Jackson-Michel, S.; Fairchild, T. An Investigation into the Effects of a Curcumin Extract (Curcugen) on Osteoarthritis Pain of the Knee: A Randomised, Double-Blind, Placebo-Controlled Study. Nutrients 2021, 14, 41. [Google Scholar] [CrossRef]
- Kuptniratsaikul, V.; Dajpratham, P.; Taechaarpornkul, W.; Buntragulpoontawee, M.; Lukkanapichonchut, P.; Chootip, C.; Saengsuwan, J.; Tantayakom, K.; Laongpech, S. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: A multicenter study. Clin. Interv. Aging 2014, 9, 451–458. [Google Scholar] [CrossRef]
- Marouf, B.H. Effect of Resveratrol on Serum Levels of Type II Collagen and Aggrecan in Patients with Knee Osteoarthritis: A Pilot Clinical Study. BioMed Res. Int. 2021, 2021, 3668568. [Google Scholar] [CrossRef]
- Marouf, B.H.; Hussain, S.A.; Ali, Z.S.; Ahmmad, R.S. Resveratrol Supplementation Reduces Pain and Inflammation in Knee Osteoarthritis Patients Treated with Meloxicam: A Randomized Placebo-Controlled Study. J. Med. Food 2018, 21, 1253–1259. [Google Scholar] [CrossRef]
- Hussain, S.A.; Marouf, B.H.; Ali, Z.S.; Ahmmad, R.S. Efficacy and safety of co-administration of resveratrol with meloxicam in patients with knee osteoarthritis: A pilot interventional study. Clin. Interv. Aging 2018, 13, 1621–1630. [Google Scholar] [CrossRef]
- Stebbings, S.; Beattie, E.; McNamara, D.; Hunt, S. A pilot randomized, placebo-controlled clinical trial to investigate the efficacy and safety of an extract of Artemisia annua administered over 12 weeks, for managing pain, stiffness, and functional limitation associated with osteoarthritis of the hip and knee. Clin. Rheumatol. 2016, 35, 1829–1836. [Google Scholar] [CrossRef]
- Felson, D.T.; Lawrence, R.C.; Dieppe, P.A.; Hirsch, R.; Helmick, C.G.; Jordan, J.M.; Kington, R.S.; Lane, N.E.; Nevitt, M.C.; Zhang, Y.; et al. Osteoarthritis: New insights. Part 1: The disease and its risk factors. Ann. Intern. Med. 2000, 133, 635–646. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Martin, J.A. Osteoarthritis. Adv. Drug. Deliv. Rev. 2006, 58, 150–167. [Google Scholar] [CrossRef]
- Bennell, K.L.; Hunter, D.J.; Paterson, K.L. Platelet-Rich Plasma for the Management of Hip and Knee Osteoarthritis. Curr. Rheumatol. Rep. 2017, 19, 24. [Google Scholar] [CrossRef]
- Wood, J.N. From plant extract to molecular panacea: A commentary on Stone (1763) ‘An account of the success of the bark of the willow in the cure of the agues’. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140317. [Google Scholar] [CrossRef]
- Lu, H.; Jia, C.; Wu, D.; Jin, H.; Lin, Z.; Pan, J.; Li, X.; Wang, W. Fibroblast growth factor 21 (FGF21) alleviates senescence, apoptosis, and extracellular matrix degradation in osteoarthritis via the SIRT1-mTOR signaling pathway. Cell Death Dis. 2021, 12, 865. [Google Scholar] [CrossRef]
| Type | Phytochemical | Plant Species/Family | Plant Sources | Model | Route of Treatment | Dosage Range | The optimal Active Concentration | Duration | Signal Pathways/Mechanisms | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Polyphenols | Curcumin | Curcuma longa L., Zingiberaceae Martinov | Curcuma longa | In vitro, human chondrocytes | Cell seeding | 1, 2, 5, and 10 μM | 5 μM | 10 days | Sox9/NF-kB | [31] |
| In vivo, ACLT and MMx surgery rats In vitro, SNP-induced rat chondrocytes | In vivo, intra-articular injection In vitro, cell seeding | In vivo, 20 and 40 μM In vitro, 1–30 μM | In vivo, 40 μM In vitro, 10 μM | In vivo, 10 weeks In vitro, 2 h | NF-κB/HIF-2α | [32] | ||||
| In vivo, surgery mice In vitro, IL-1β-induced murine chondrocytes | / In vitro, cell seeding | / In vitro, 1 and 5 μM | / / | / In vitro, 48 h | miR-124/NF-kB miR-143/ROCK1/TLR9 | [33] | ||||
| In vitro, SNP-induced rabbit chondrocytes | Cell seeding | 5, 10, and 20 μM | 20 μM | 24 h | Inhibit mitochondrial-dependent apoptosis pathway and restore the balance between synthesis and degradation of extracellular matrix. | [34] | ||||
| In vitro, human chondrocytes | Cell seeding | 0, 5, 10, 20, and 40 μmol/L | 40 μmol/L | 24 h | Downregulate the expression of MMP3 and regulate apoptosis. | [35] | ||||
| In vitro, IL-1β-induced rat chondrocytes | Cell seeding | 5, 10, 15, and 20 μM | 10 μM | 4 h | ERK1/2 | [36] | ||||
| In vivo, HFD rats | Intra-articular injection | 200 and 400 μg/kg | 200 μg/kg | 4 weeks | E2F1/PITX1, AKT/mTOR | [37] | ||||
| Resveratrol | Vitis vinifera L., Vitaceae Juss | Grape leaves | In vivo, MCL surgery rabbits | Intra-articular injection | 50, 20, and 10 μmol/kg | 50 μmol/kg | 2 weeks | Reduce the production of NO in chondrocytes | [38] | |
| In vivo, HFD mice | Oral administration | 5, 22.5, and 45 mg/kg | 45 mg/kg | 2 weeks | Reduce the degradation of type II collagen and inhibit chondrocyte apoptosis | [39] | ||||
| In vitro, IL-1β-induced murine chondrocytes | Cell seeding | 5, 10, 20, 50, and 100 μM | 10 μM | 12 h | COX-2/NF-κB | [40] | ||||
| In vitro, human chondrocytes | Cell seeding | 10 μM | 10 μM | 48 h | Sirt1, Wnt/β-catenin | [41] | ||||
| Chicoric acid | Echinacea purpurea (L.) Moench., Asteraceae Bercht. and J. Presl | Echinacea purpurea | In vivo, MIA-induced rats In vitro, TNF-α-induced human chondrocytes | In vivo, oral administration In vitro, cell seeding | In vivo, 16 and 32 mg/kg In vitro, 0, 2.5, 5, 10, 20, 40, and 80 μM | In vivo, 32 mg/kg In vitro, 20 μM | In vivo, 4 weeks In vitro, 24 h | PI3K/AKT, NF-κB | [42] | |
| Flavonoids | Epigallocatechin-3-gallate | Camellia sinensis (L.) Kuntze, Theaceae | green tea | In vitro, IL-1β-induced human chondrocytes | Cell seeding | 20 and 50 μM | 50 μM | 2 h | PTEN/miRNA-29b | [43] |
| In vivo, ACL surgery rats | Cell seeding | 40 μM | 40 μM | 5 weeks | / | [44] | ||||
| Icariin | Epimedium brevicornu Maxim., Berberidaceae Juss | Epimedium | In vitro, TNF-α-induced rat chondrocytes | Cell seeding | 0, 3, 5, 7, 10, and 20 μM | 10 μM | 24 h | NF-κB | [45] | |
| In vitro, collagenase-induced rat chondrocytes | Cell seeding | 10, 20, and 40 ng/mL | 10 ng/mL | 7 days | TDP-43 | [46] | ||||
| In vitro, oxygen-, glucose-, and serum-deprivation-induced rabbit bone-marrow-derived mesenchymal stem cells | Cell seeding | 0.1, 1, and 10 μM | 10 μM | 24 h | MAPK | [47] | ||||
| In vitro, IL-1β-induced human and rat chondrocytes | Cell seeding | 0, 10, 20, and 30 μM | 30 μM | 48 h | lncRNA CYTOR | [48] | ||||
| Quercetin | / | Plant extracts such as rutin, glycoside, isoglycoside, anisidine, luteolin, wintersweet glycoside, etc. | In vivo, DMM surgery rats In vitro, IL-1β-induced rat chondrocytes | In vivo, intraperitoneal injection In vitro, cell seeding | In vivo, 50 mg/kg In vitro, 0–100 μM | In vivo, 50 mg/kg In vitro, 25 μM | In vivo, 12 weeks In vitro, 24 h | SIRT1/AMPK | [49] | |
| In vivo, DMM surgery rats In vitro, IL-1β-induced rat chondrocytes | In vivo, intra-articular injection In vitro, cell seeding | In vivo, 8 μM In vitro, 0–16 μM | In vivo, 8 μM In vitro, 8 μM | In vivo, 6 weeks In vitro, 24 h | Regulating the polarization of synovial macrophages to M2 macrophages | [50] | ||||
| In vitro, IL-1β-induced rat chondrocytes | Cell seeding | 0–600 μM | 100 μM | 24 h | MAPK | [51] | ||||
| In vivo, ACL surgery rats In vitro, IL-1β-induced rat chondrocytes | In vivo, intraperitoneal injection In vitro, cell seeding | In vivo, 49.5 and 99 nM In vitro, 8 μM | In vivo, 99 nM In vitro, 8 μM | In vivo, 12 weeks In vitro, 2 h | IRAK1/NLRP3 | [52] | ||||
| Isoliquiritigenin | Glycyrrhiza glabra L., Fabaceae Lindl | Glycyrrhiza uralensis, Cicer arietinum, Dalbergia sericea, Dalbergia stevensonii, and Sophora tomentosa | In vivo, ACLT surgery mice In vitro, IL-1β-induced murine chondrocytes | In vivo, intraperitoneal injection In vitro, cell seeding | In vivo, 10, 20, and 40 mg/kg In vitro, 2.5, 5, 10, 20, and 40 μmol/l | In vivo, 40 mg/kg In vitro, 10 μmol/l | In vivo, 8 weeks In vitro, 1 h | NF-κB | [53] | |
| Baicalein | Scutellaria baicalensis Georgi, Lamiaceae | Scutellaria baicalensis | In vivo, DMM surgery mice | Intragastric injection | 50 mg/kg | 50 mg/kg | 8 weeks | / | [54] | |
| In vivo, DMM surgery rats In vitro, osteogenic differentiation | In vivo, intra-articular injection In vitro, cell seeding | In vivo, 1 mg In vitro, 0, 2.5, 5, 10, 20, and 50 μmol/L | In vivo, 1 mg In vitro, 10, 20, and 50 μmol/L | In vivo, 20 weeks In vitro, 24 h | / | [55] | ||||
| In vitro, IL-1β- and TNF-α-induced human chondrocytes | Cell seeding | 0, 5, 10, 25, 50, and 100 μM | 50 μM | 0, 12, 24, 36, and 48 h | NF-κB | [36] | ||||
| In vitro, IL-1β-induced murine chondrocytes | Cell seeding | 0, 5, 20, and 50 μM | 20 and 50 μM | 2 h | / | [56] | ||||
| Genistein | Genista Linn., Fabaceae | soybean | In vivo, ACL surgery rats In vitro, IL-1β-induced human chondrocytes | In vivo, intragastric injection In vitro, cell seeding | In vitro, 20 mg/kg In vitro, 25, 50, and 100 μg/mL | In vitro, 20 mg/kg In vitro, 100 μg/mL | In vitro, 6 weeks In vitro, 72 h | / | [57] | |
| Terpenoids | Loganin | Cornus officinalis Siebold and Zucc., Cornaceae Bercht. and J. Presl | Flos lonicerae, Cornus mas L., and Strychnos nux vomica | In vivo, ACL surgery rats In vitro, IL-1β-induced rat chondrocytes | In vivo, subcutaneous injection In vitro, cell seeding | In vivo, 20 mg/kg/d In vitro, 0–20 μM | In vivo, 20 mg/kg/d In vitro, 10 μM | / In vitro, 2 h | PI3K/Akt | [58] |
| Andrographolide | Andrographis paniculata (Burm.f.) Wall. Ex Nees., Acanthaceae Juss | Stems and leaves of the botanical drug andrographis paniculata | In vivo, ACLT surgery mice | Oral administration | 50 mg/kg | 50 mg/kg | 12 weeks | miR-27-3p/MMP13 | [59] | |
| Geniposide | Gardenia jasminoides J.Ellis., Rubiaceae Juss | Gardenia | In vivo, MMT surgery rats In vitro, IL-1β-induced rat chondrocytes | In vivo, intraperitoneal injection In vitro, cell seeding | In vivo, 10 mg/kg In vitro, 0, 2.5, 5, 10, and 25 μM | In vivo, 10 mg/kg In vitro, 10 μM | In vivo, 8 weeks In vitro, 24 h | PI3K/Akt, NF-κB | [60] | |
| Morroniside | Cornus officinalis Siebold and Zucc., Cornaceae Bercht. And J. Presl | Dry flower buds of Lonicera japonica Thunb. | In vivo, DMM surgery mice In vitro, IL-1β-induced murine chondrocytes | In vivo, intra-articular injection In vitro, cell seeding | In vivo, 4 and 20 μg/kg In vitro, 20 and 100 μg/mL | / | In vivo, 8 and 12 weeks In vitro, 12 h | NF-κB | [61] | |
| Small molecules compounds | Leonurine | Leonurus sibiricus L., Lamiaceae Martinov | Leaves of Leonurus sibiricus L. or whole grass of Leonurus heterophyllus Sweet. | In vitro, IL-1β-induced rat chondrocytes | Cell seeding | 0–40 μM | 5 μM | 24 h | NF-κB, MAPK | [62] |
| Panax notoginseng saponins | Panax notoginseng (Burkill) F.H.Chen., Araliaceae Juss | Panax notoginseng | In vivo, DMM surgery rats In vitro, TNF-α-induced rat chondrocytes | In vivo, intra-articular injection In vitro, cell seeding | In vivo, 100 and 200 mg/kg In vitro, 100 and 200 μg/mL | / In vitro, 200 μg/mL | In vivo, 8 weeks In vitro, 24 h | PI3K-AKT-mTOR | [63] | |
| Shikonin | Lithospermum erythrorhizon Siebold and Zucc., Boraginaceae Juss | Arnebia euchroma | In vivo, ACLT surgery rats | Intra-articular injection | 10 mg/kg | 10 mg/kg | 4 days | PI3K/Akt | [64] | |
| In vitro, IL-1β-induced rat chondrocytes | Cell seeding | 0–8 μM | 4 μM | 2 h | PI3K/Akt | [65] | ||||
| Stevioside | Stevia rebaudiana (Bertoni) Bertoni., Asteraceae Bercht. and J. Presl | Stevia | In vivo, DMM surgery mice In vitro, IL-1β-induced murine chondrocytes | In vivo, oral administration In vitro, cell seeding | In vivo, 100 mg/kg In vitro, 0–200 μg/mL | In vivo, 100 mg/kg In vitro, 100 μg/mL | In vivo, 8 weeks In vitro, 24 h | NF-κB, MAPK | [66] | |
| Achyranthes bidentata polyscharides | Achyranthes bidentata Blume., Amaranthaceae Juss | Achyranthes bidentata | In vivo, ACLT surgery mice In vitro, thapsigargin-induced murine chondrocytes | In vivo, gavage In vitro, cell seeding | In vivo, 8 mg/kg/d In vitro, 0, 10, 25, 50, 100, and 150 μg/mL | In vivo, 8 mg/kg In vitro, 100 μg/mL | In vivo, 2 weeks / | lncRNA NEAT1/miR-377-3p | [67] | |
| Engeletin | Smilax glabra Roxb., Smilacaceae Vent | Smilax glabra Roxb | In vivo, ACLT surgery rats In vitro, TNF-α-induced rat chondrocytes | In vivo, intra-articular injection In vitro, cell seeding | In vivo, 50 μg/100 μL In vitro, 0–160 μM | In vivo, 50 μg/100 μL In vitro, 10 and 20 μM | In vivo, 8 weeks In vitro, 2 h | NF-κB, MAPK | [68] |
| Plant Name | Plant Species/Family | Dosage | Duration | Control | Design | Placebo | Function | References |
|---|---|---|---|---|---|---|---|---|
| Harpagophytum procumbens | Harpagophytum procumbens (Burch.) DC. ex Meisn., Pedaliaceae R. Br. | 2 × 480 × 30 mg/d, pure compound | 4 weeks | Positive control | Double-blinded RCT | No | Relieve pain and improve function | [143] |
| Arctium lappa | Arctium lappa L., Asteraceae Bercht. and J. Presl | 3 × 2 g/d, powder | 6 weeks | Negative control | RCT | Yes | Alleviate the progression of OA | [147] |
| Psidium guajava | Psidium guajava L., Myrtaceae Juss | 1 g/d, powder | 12 weeks | Negative control | Double-blinded RCT | Yes | Relieve pain | [145] |
| Cissus quadrangularis | Cissus quadrangularis L., Vitaceae Juss | 3200 mg/d, powder | 8 weeks | Negative control | Double-blinded RCT | Yes | Relieve pain | [148] |
| Nigella sativa | Nigella sativa L., Ranunculaceae Juss | 2.5 mL/8 h, extract | 4 weeks | Negative control | Double-blinded RCT | Yes | Relieve pain | [144] |
| Vitellaria paradoxa | Vitellaria paradoxa C. F. Gaertn., Sapotaceae Juss | 3 × 750 mg/d, extract | 15 weeks | Negative control | Double-blinded RCT | Yes | Alleviate the progression of OA | [149] |
| Elaeagnus angustifolia | Elaeagnus angustifolia L., Elaeagnaceae Juss | 3 × 200 mg/d, extract | 4 weeks | Positive control | Double-blinded RCT | No | Relieve pain and improve function | [150] |
| 300 mg/d and 600 mg/d, extract | 7 weeks | Positive control | Double-blinded RCT | No | Relieve pain | [151] | ||
| 15 g/d, powder | 8 weeks | Negative control | Double-blinded RCT | Yes | Alleviate the progression of OA | [152] | ||
| Zingiber officinale | Zingiber officinale Roscoe., Zingiberaceae Martinov | 2 × 255 mg/d, extract | 6 weeks | Negative control | Double-blinded RCT | Yes | Relieve pain and improve function | [153] |
| 37.5 mg/d, extract | 4 weeks | No | One-group, pre-test–post-test, quasi-experimental pilot study | No | Relieve pain | [154] | ||
| 25 mg/d, extract | 4 weeks | No | Uncontrolled multicenter study | No | Relieve pain | [146] | ||
| 1 g/d, pure compound | 11 days | Negative control | Double-blinded RCT | Yes | Relieve pain | [155] | ||
| Andrographis paniculata | Andrographis paniculata (Burm.f.) Wall. ex Nees., Acanthaceae Juss | 300 mg/d and 600 mg/d, extract | 12 weeks | Negative control | Double-blinded RCT | Yes | Alleviate the progression of OA | [156] |
| Pinus massoniana | Pinus massoniana Lamb., Pinaceae Spreng. ex F. Rudolphi | 1322 mg/d, pure compound | 12 weeks | Negative control | Double-blinded RCT | Yes | Alleviate the progression of OA | [157] |
| Curcuma longa | Curcuma longa L., Zingiberaceae Martinov | 2 × 500 mg/d, extract | 12 weeks | Negative control | Double-blinded RCT | Yes | Relieve pain | [158] |
| 2 × 46.67 mg/d and 3 × 46.67 mg/d, extract | 12 weeks | Negative control | Double-blinded RCT | Yes | Relieve pain | [159] | ||
| 500 mg/d, extract | 4 months | Negative control | Double-blinded RCT | Yes | Alleviate the progression of OA | [160] | ||
| 1000 mg/d, extract | 4 weeks | Positive control | A clinical study | No | Relieve pain | [161] | ||
| 2 × 500 mg/d, extract | 21 days, 42 days | Negative control | Single-blinded RCT | Yes | Relieve pain | [162] | ||
| Curcumin | Curcuma longa L., Zingiberaceae Martinov | 3 × 500 mg/d, extract | 4 weeks | Positive control | RCT | No | Relieve pain | [163] |
| 2 × 500 mg/d, extract | 4 weeks | Negative control | RCT | Yes | Relieve pain | [164] | ||
| 1500 g/d, extract | 4 weeks | Positive control | A multicenter study | No | Relieve pain and improve function | [165] | ||
| Resveratrol | Vitis vinifera L., Vitaceae Juss | 500 mg/d, powder | 90 days | No | A noncontrolled clinical trial | No | Relieve pain and improve function | [166] |
| 500 mg/d, powder | 90 days | Negative control, Positive control | Double-blinded RCT | Yes | Relieve pain and inflammation | [167] | ||
| 500 mg/d, powder | 90 days | Negative control, Positive control | Double-blinded RCT | Yes | Relieve pain and improve function | [168] | ||
| Artemisia annua | Artemisia annua L., Asteraceae Bercht. and J. Presl | 150 mg/d and 300 mg/d, extract | 12 weeks | Negative control | Double-blinded RCT | Yes | Improve the pain, stiffness, and limited function of knee joint | [169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, H.; Han, J.-J.; Dmitrii, G.; Zhang, X.-a. Phytochemicals against Osteoarthritis by Inhibiting Apoptosis. Molecules 2024, 29, 1487. https://doi.org/10.3390/molecules29071487
Kong H, Han J-J, Dmitrii G, Zhang X-a. Phytochemicals against Osteoarthritis by Inhibiting Apoptosis. Molecules. 2024; 29(7):1487. https://doi.org/10.3390/molecules29071487
Chicago/Turabian StyleKong, Hui, Juan-Juan Han, Gorbachev Dmitrii, and Xin-an Zhang. 2024. "Phytochemicals against Osteoarthritis by Inhibiting Apoptosis" Molecules 29, no. 7: 1487. https://doi.org/10.3390/molecules29071487
APA StyleKong, H., Han, J.-J., Dmitrii, G., & Zhang, X.-a. (2024). Phytochemicals against Osteoarthritis by Inhibiting Apoptosis. Molecules, 29(7), 1487. https://doi.org/10.3390/molecules29071487






